Preventive Capacity of Citrus paradisi Juice for Male Reproductive Damage Induced by Cadmium Chloride in Mice
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals, Grapefruit Juice, and Mice
2.2. Experimental Design
2.3. Spermatic Concentration and Quality
2.4. Sperm DNA and Micronuclei Evaluation
2.4.1. Sperm Comet Assay
2.4.2. Spermatid Micronucleus Test
2.5. Oxidative Assays
2.5.1. Testicular Protein Content
2.5.2. Testicular Protein Oxidation
2.5.3. Testicular Malondialdehyde Content
2.5.4. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Test
2.6. Statistical Analysis
3. Results
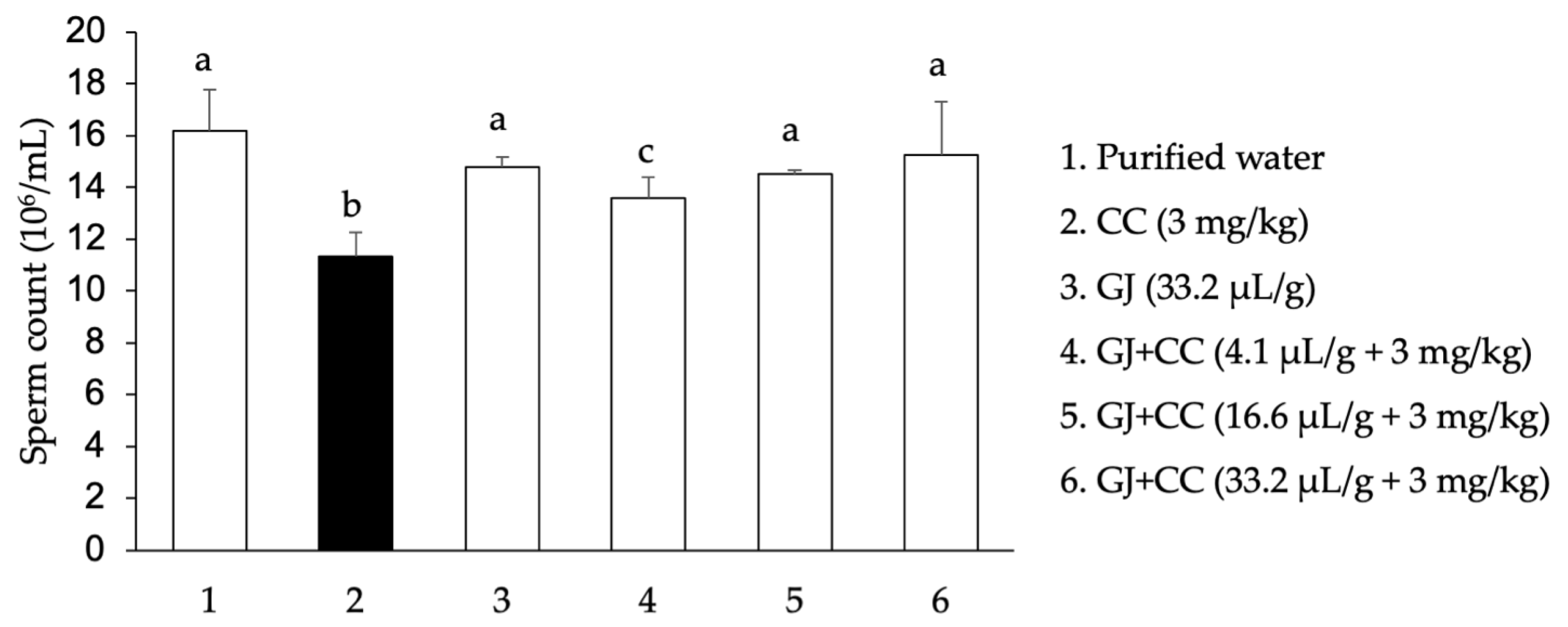
3.1. Sperm Concentration and Quality
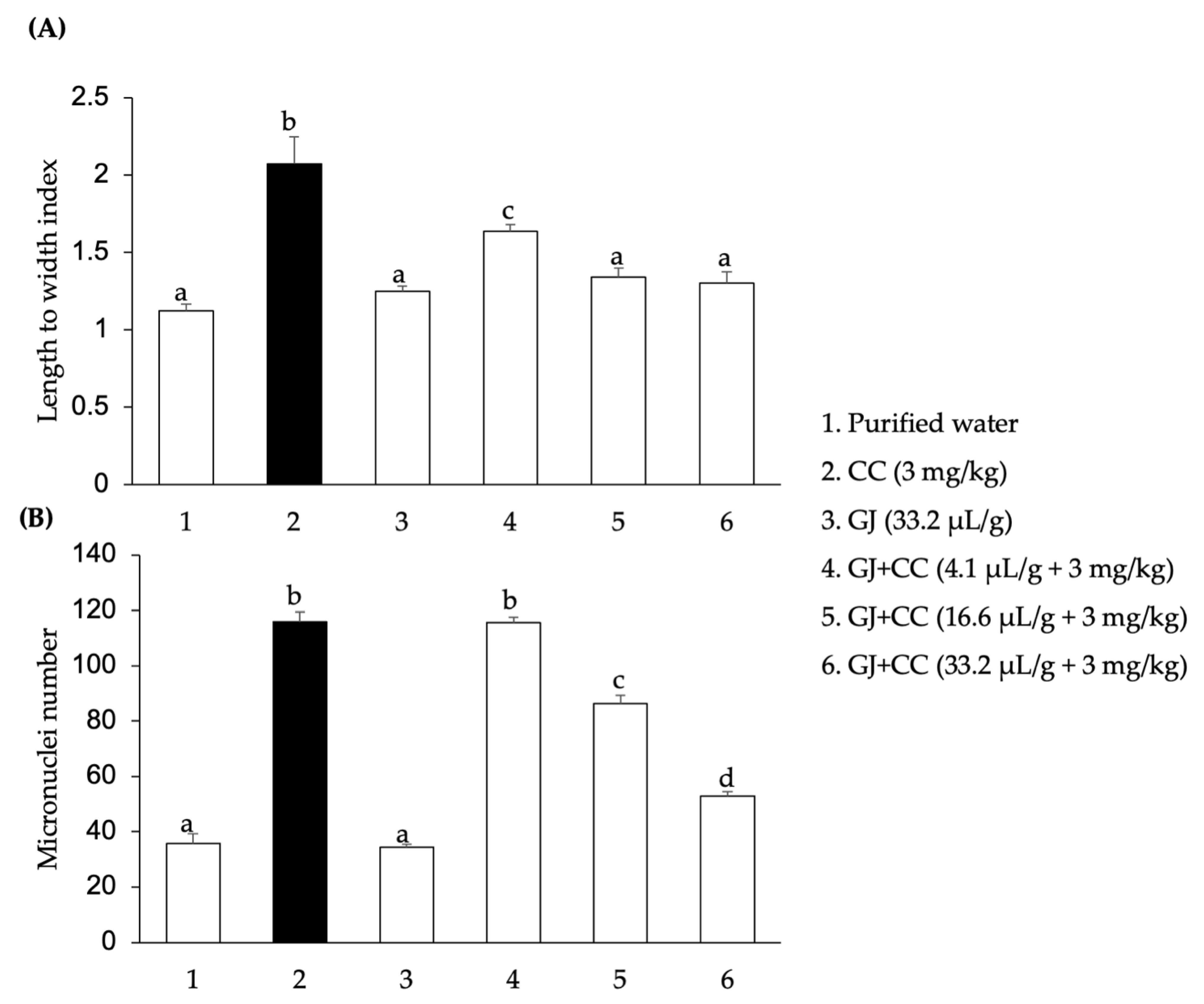
3.2. DNA Damage and Micronuclei in Spermatids
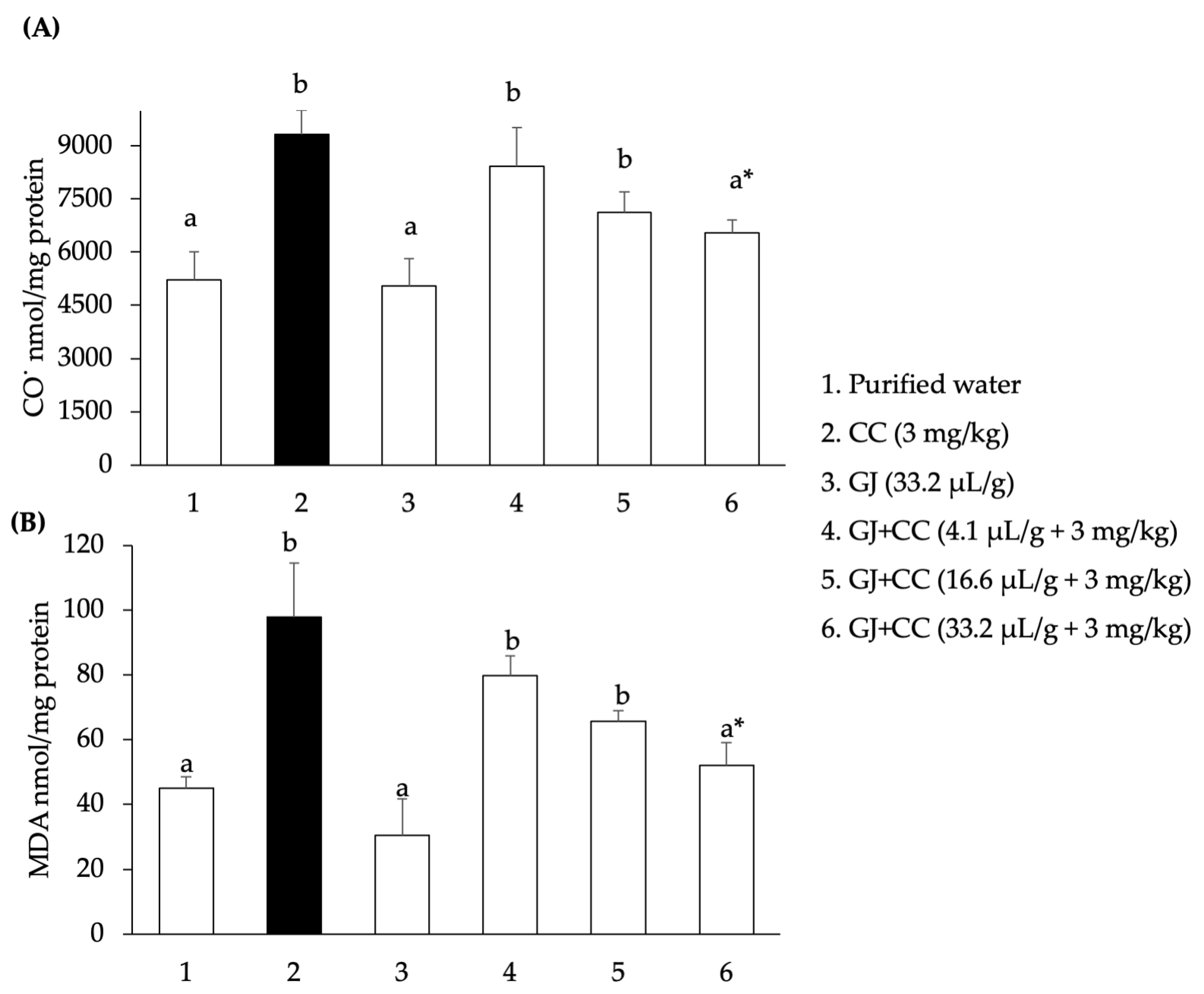
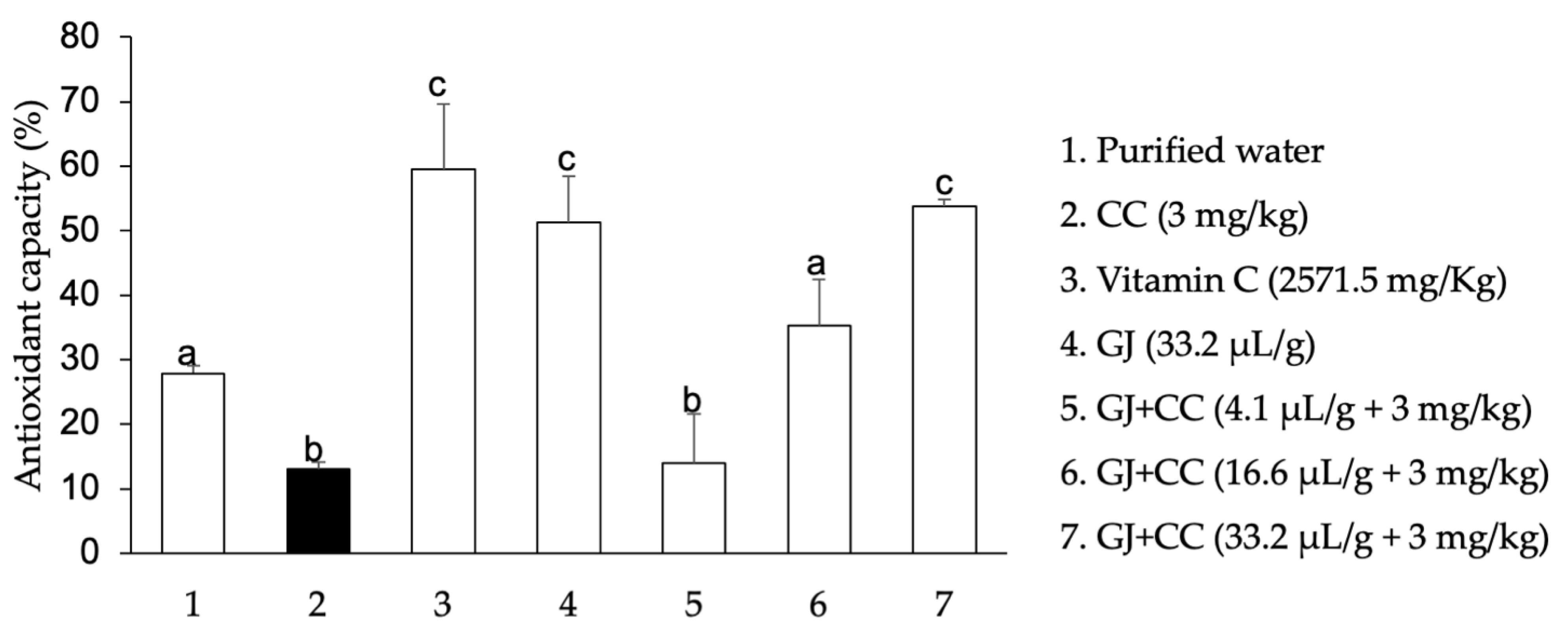
3.3. Oxidation of Biomolecules in Mouse Testes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Badar, A.; Khilwani, B.; Lohiya, N.K.; Ansari, A.S. Etiology of male infertility; A review. Int. J. Reprod. Contracept. Obstet. Gynecol. 2022, 11, 2320–2328. [Google Scholar] [CrossRef]
- Bellver, J.; Donnez, J. Introduction: Infertility etiology and offspring health. Fertil. Steril. 2019, 111, 1033–1035. [Google Scholar] [CrossRef]
- Babakhanzadeh, E.; Nazari, M.; Ghasemifar, S.; Khodadadian, A. Some of the factors involved in male infertility: A prospective review. Int. J. Gen. Med. 2020, 13, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy-Siraudin, C.; Loundou, A.D.; Romain, F.; Achard, V.; Courbière, B.; Perrard, M.H.; Durand, P.; Guichaoua, M.R. Decline of semen quality among 10 932 males consulting for couple infertility over a 20-year period in Marseille, France. Asian J. Androl. 2012, 14, 584–590. [Google Scholar] [CrossRef]
- Marić, T.; Fučić, A.; Aghayanian, A. Environmental and occupational exposures associated with male infertility. Arh. Hig. Rada Toksikol. 2021, 72, 101–113. [Google Scholar] [CrossRef]
- López-Botella, A.; Velasco, I.; Acién, M.; Sáez-Espinosa, P.; Todolí-Torró, J.L.; Sánchez-Romero, R.; Gómez-Torres, M.J. Impact of heavy metals on human male fertility-an overview. Antioxidants 2021, 10, 1473. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Yao, W.; Ba, Q.; Wang, H. Effects of cadmium exposure on the immune system and immunoregulation. Front. Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.R.; Flannery, B.M.; Crosby, L.; Jones-Dominic, O.E.; Punzalan, C.; Middleton, K. A systematic review of adverse health effects associated with oral cadmium exposure. Regul. Toxicol. Pharmacol. 2022, 134, 105243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, X.; Ge, R.S. Toxicological effects of cadmium on mammalian testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ahedo, B.A.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Izquierdo-Vega, J.A.; Morales-González, J.A.; Madrigal-Santillán, E.O.; Álvarez-González, I. Potential protective effect of beta-caryophyllene against cadmium chloride-induced damage to the male reproductive system in mouse. Reprod. Toxicol. 2022, 110, 19–30. [Google Scholar] [CrossRef]
- Cristóbal-Luna, J.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G. Grapefruit and its biomedical, antigenotoxic and chemopreventive properties. Food Chem. Toxicol. 2018, 112, 224–234. [Google Scholar] [CrossRef]
- Álvarez-González, I.; Madrigal-Bujaidar, E.; Martino-Roaro, L.; Espinosa-Aguirre, J.J. Antigenotoxic and antioxidant effect of grapefruit juice in mice treated with daunorubicin. Toxicol. Lett. 2004, 152, 203–211. [Google Scholar] [CrossRef]
- Miyata, M.; Takano, H.; Guo, L.Q.; Nagata, K.; Yamazoe, Y. Grapefruit juice intake does not enhance but rather protects against aflatoxin B1-induced liver DNA damage through a reduction in hepatic CYP3A activity. Carcinogenesis 2004, 25, 203–209. [Google Scholar] [CrossRef]
- Álvarez-González, I.; Madrigal-Bujaidar, E.; Sánchez-García, V.Y. Inhibitory effect of grapefruit juice on the genotoxic damage induced by ifosfamide in mouse. Plant Foods Hum. Nutr. 2010, 65, 369–373. [Google Scholar] [CrossRef]
- Argüelles, N.; Álvarez-González, I.; Chamorro, G.; Madrigal-Bujaidar, E. Protective effect of grapefruit juice on the teratogenic and genotoxic damage induced by cadmium in mice. J. Med. Food 2012, 15, 887–893. [Google Scholar] [CrossRef]
- Diab, K.A. In vitro studies on phytochemical content, antioxidant, anticancer, immunomodulatory, and antigenotoxic activities of lemon, grapefruit, and mandarin citrus peels. Asian Pac. J. Cancer Prev. 2016, 17, 3559–3567. [Google Scholar]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Albus, U. Guide for the care and use of laboratory animals (8th ed.). Lab. Anim. 2011, 46, 267–268. [Google Scholar] [CrossRef]
- ARRIVE Guidelines, Animal Research: Reporting of In Vivo Experiments. 2021. Available online: https://arriveguidelines.org/ (accessed on 3 January 2025).
- Álvarez-Gonzalez, I.; Mojica, R.; Madrigal-Bujaidar, E.; Camacho-Carranza, R.; Escobar-García, D.; Espinosa-Aguirre, J.J. The antigenotoxic effects of grapefruit juice on the damage induced by benzo(a)pyrene and evaluation of its interaction with hepatic and intestinal Cytochrome P450 (Cyp) 1a1. Food Chem. Toxicol. 2011, 49, 807–811. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Urióstegui-Acosta, M.; Hernández-Ochoa, I.; Solís-Heredia, M.d.J.; Martínez-Aguilar, G.; Quintanilla-Vega, B. Comparative effect of technical and commercial formulations of methamidophos on sperm quality and DNA integrity in mice. Environ. Toxicol. 2014, 29, 942–949. [Google Scholar] [CrossRef]
- Álvarez-González, I.; Camacho-Cantera, S.; Gómez-González, P.; Rendon-Barrón, M.J.; Morales-González, J.A.; Madrigal-Santillán, E.O.; Paniagua-Pérez, R.; Madrigal-Bujaidar, E. Genotoxic and oxidative effect of duloxetine on mouse brain and liver tissues. Sci. Rep. 2021, 11, 6897. [Google Scholar] [CrossRef] [PubMed]
- Russo, A. In vivo cytogenetics: Mammalian germ cells. Mutat. Res. 2000, 455, 167–189. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 138, 673–680. [Google Scholar] [CrossRef]
- Chrzczanowicz, J.; Gawron, A.; Zwolinska, A.; de Graft-Johnson, J.; Krajewski, W.; Krol, M.; Markowski, J.; Kostka, T.; Nowak, D. Simple method for determining human serum 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity—Possible application in clinical studies on dietary antioxidants. Clin. Chem. Lab. Med. 2008, 46, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Arıcan, E.Y.; Gökçeoğlu Kayalı, D.; Ulus Karaca, B.; Boran, T.; Öztürk, N.; Okyar, A.; Ercan, F.; Özhan, G. Reproductive effects of subchronic exposure to acetamiprid in male rats. Sci. Rep. 2020, 10, 8985. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Ola-Mudathir, K.F.; Suru, S.M.; Fafunso, M.A.; Obioha, U.E.; Faremi, T.Y. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3604–3611. [Google Scholar] [CrossRef]
- El-Neweshy, M.S.; El-Maddawy, Z.K.; El-Sayed, Y.S. Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia 2013, 45, 369–378. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Akanni, O.O. Protective effects of Artocarpus altilis (Moraceae) on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Andrologia 2016, 48, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Olaniyan, O.T.; Kunle-Alabi, O.T.; Raji, Y. Protective effects of methanol extract of Plukenetia conophora seeds and 4H-Pyran-4-One 2,3-Dihydro-3,5-Dihydroxy-6-Methyl on the reproductive function of male Wistar rats treated with cadmium chloride. JBRA Assist. Reprod. 2018, 22, 289–300. [Google Scholar] [CrossRef]
- Elblehi, S.S.; El Euony, O.I.; El-Nahas, A.F. Partial ameliorative effect of Moringa leaf ethanolic extract on the reproductive toxicity and the expression of steroidogenic genes induced by subchronic cadmium in male rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 23306–23318. [Google Scholar] [CrossRef]
- Akinola, A.O.; Oyeyemi, A.W.; Daramola, O.O.; Raji, Y. Effects of the methanol root extract of Carpolobia lutea on sperm indices, acrosome reaction, and sperm DNA integrity in cadmium-induced reproductive toxicity in male Wistar rats. JBRA Assist. Reprod. 2020, 24, 454–465. [Google Scholar] [CrossRef]
- Iqbal, T.; Jahan, S.; Ain, Q.U.; Ullah, H.; Li, C.; Chen, L.; Zhou, X. Ameliorative effects of morel mushroom (Morchella esculenta) against cadmium-induced reproductive toxicity in adult male rats. Braz. J. Biol. 2021, 82, e250865. [Google Scholar] [CrossRef] [PubMed]
- Daramola, J.O.; Adekunde, E.O.; Onagbesan, O.M.; Oke, O.E.; Ladokun, A.O.; Abiona, J.A.; Abioja, M.O.; Oyewusi, I.K.; Oyewusi, J.A.; Isah, O.A.; et al. Protective effects of fruit juices on sperm viability of West African Dwarf goat bucks during cryopreservation. Anim. Reprod. 2016, 13, 7–13. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Nwachukwu, C.U. Efficacy of soursop juice extender on rooster semen quality, oxidative activity and spermatozoa kinematics. Transl. Anim. Sci. 2022, 6, txac046. [Google Scholar] [CrossRef] [PubMed]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M.; Abdel Moneim, A.E. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement. Altern. Med. 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Lamas, C.A.; Gollücke, A.P.; Dolder, H. Grape juice concentrate (G8000(®)) intake mitigates testicular morphological and ultrastructural damage following cadmium intoxication. Int. J. Exp. Pathol. 2015, 96, 301–310. [Google Scholar] [CrossRef]
- Lamas, C.A.; Cuquetto-Leite, L.; do Nascimento da Silva, E.; Thomazini, B.F.; Cordeiro, G.D.S.; Predes, F.S.; Gollücke, A.P.B.; Dolder, H. Grape juice concentrate alleviates epididymis and sperm damage in cadmium-intoxicated rats. Int. J. Exp. Pathol. 2017, 98, 86–99. [Google Scholar] [CrossRef]
- Sakr, S.A.; Zoil, M.E.S.; El-Shafey, S.S. Ameliorative effect of grapefruit juice on amiodarone-induced cytogenetic and testicular damage in albino rats. Asian Pac. J. Trop. Biomed. 2013, 3, 573–579. [Google Scholar] [CrossRef]
- Duester, K.C. Avocado fruit is a rich source of beta-sitosterol. J. Am. Diet. Assoc. 2001, 101, 404–405. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, A.A.; Başaran, N. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef]
- Sarker, S.K.; Tun, K.D.; Eva, E.O.; Paul, R. Grapefruit juice: Nutritional values and drug interactions. Int. J. Integr. Med. Sci. 2015, 2, 186–189. [Google Scholar] [CrossRef]
- Singh, Z.; Sharma, S.; Kaur, A. Antitoxic effects of naringin: A flavonoid with diverse biological activities. World J. Pharm. Res. 2018, 7, 484–489. [Google Scholar] [CrossRef]
- Ko, J.H.; Arfuso, F.; Sethi, G.; Ahn, K.S. Pharmacological utilization of bergamottin, derived from grapefruits, in cancer prevention and therapy. Int. J. Mol. Sci. 2018, 19, 4048. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Khalil, M.N.A.; Farghal, H.H.; Farag, M.A. Outgoing and potential trends of composition, health benefits, juice production and waste management of the multi-faceted Grapefruit Citrus x paradisi: A comprehensive review for maximizing its value. Crit. Rev. Food Sci. Nutr. 2022, 62, 935–956. [Google Scholar] [CrossRef]
- Martins, R.V.L.; Silva, A.M.S.; Duarte, A.P.; Socorro, S.; Correia, S.; Maia, C.J. Natural products as protective agents for male fertility. BioChem 2021, 1, 122–147. [Google Scholar] [CrossRef]
- Zargar, S.; Al-Majed, A.A.; Wani, T.A. Potentiating and synergistic effect of grapefruit juice on the antioxidant and anti-inflammatory activity of aripiprazole against hydrogen peroxide induced oxidative stress in mice. BMC Complement. Altern. Med. 2018, 18, 106. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male infertility and oxidative stress: A focus on the underlying mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M. Oxidative stress in male infertility: Causes, effects in assisted reproductive techniques, and protective support of antioxidants. Biology 2020, 9, 77. [Google Scholar] [CrossRef]

| Group | Treatments | Normal Morphology | Progressive Motility | Viability |
|---|---|---|---|---|
| 1 | Purified water/10 µL/g | 16 ± 1.41 a | 51.4 ± 2.7 a | 94 ± 1.58 a |
| 2 | CC/3 mg/kg | 2.8 ± 0.84 b | 13 ± 2.83 b | 14.8 ± 1.64 b |
| 3 | GJ/33.28 µL/g | 15.6 ± 1.52 a | 50.4 ± 6.42 a | 89 ± 1.58 a |
| 4 |
GJ + CC 4.16 µL/g 3 mg/kg | 6.8 ± 0.83 c | 12.8 ± 2.05 b | 66.2 ± 2.59 c |
| 5 |
GJ + CC 16.64 µL/g 3 mg/kg | 12.6 ± 1.67 d | 39 ± 4.30 c | 77.8 ± 4.92 d |
| 6 |
GJ + CC 33.28 µL/g 3 mg/kg | 14 ± 1 e | 46.6 ± 2.88 a | 83.4 ± 3.21 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-González, I.; García-García, J.D.; Espinosa-Ahedo, B.A.; Muñoz-Carrillo, L.S.; Morales-González, J.A.; Madrigal-Santillán, E.O.; Carrillo-Romo, F.d.J.; García-Murillo, A.; Paniagua-Pérez, R.; Madrigal-Bujaidar, E. Preventive Capacity of Citrus paradisi Juice for Male Reproductive Damage Induced by Cadmium Chloride in Mice. Appl. Sci. 2025, 15, 6071. https://doi.org/10.3390/app15116071
Álvarez-González I, García-García JD, Espinosa-Ahedo BA, Muñoz-Carrillo LS, Morales-González JA, Madrigal-Santillán EO, Carrillo-Romo FdJ, García-Murillo A, Paniagua-Pérez R, Madrigal-Bujaidar E. Preventive Capacity of Citrus paradisi Juice for Male Reproductive Damage Induced by Cadmium Chloride in Mice. Applied Sciences. 2025; 15(11):6071. https://doi.org/10.3390/app15116071
Chicago/Turabian StyleÁlvarez-González, Isela, José David García-García, Beatriz A. Espinosa-Ahedo, Luis S. Muñoz-Carrillo, José A. Morales-González, Eduardo O. Madrigal-Santillán, Felipe de Jesús Carrillo-Romo, Antonieta García-Murillo, Rogelio Paniagua-Pérez, and Eduardo Madrigal-Bujaidar. 2025. "Preventive Capacity of Citrus paradisi Juice for Male Reproductive Damage Induced by Cadmium Chloride in Mice" Applied Sciences 15, no. 11: 6071. https://doi.org/10.3390/app15116071
APA StyleÁlvarez-González, I., García-García, J. D., Espinosa-Ahedo, B. A., Muñoz-Carrillo, L. S., Morales-González, J. A., Madrigal-Santillán, E. O., Carrillo-Romo, F. d. J., García-Murillo, A., Paniagua-Pérez, R., & Madrigal-Bujaidar, E. (2025). Preventive Capacity of Citrus paradisi Juice for Male Reproductive Damage Induced by Cadmium Chloride in Mice. Applied Sciences, 15(11), 6071. https://doi.org/10.3390/app15116071








