18F-Fluorodeoxyglucose Uptake in Cerebrospinal Fluid Reflects Both Brain Glucose Demand and Impaired Blood–Brain Barrier Transport in Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Enrolment and Study Design
2.2. CSF Collection and Biomarker Analysis
2.3. F-FDG-PET Data
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
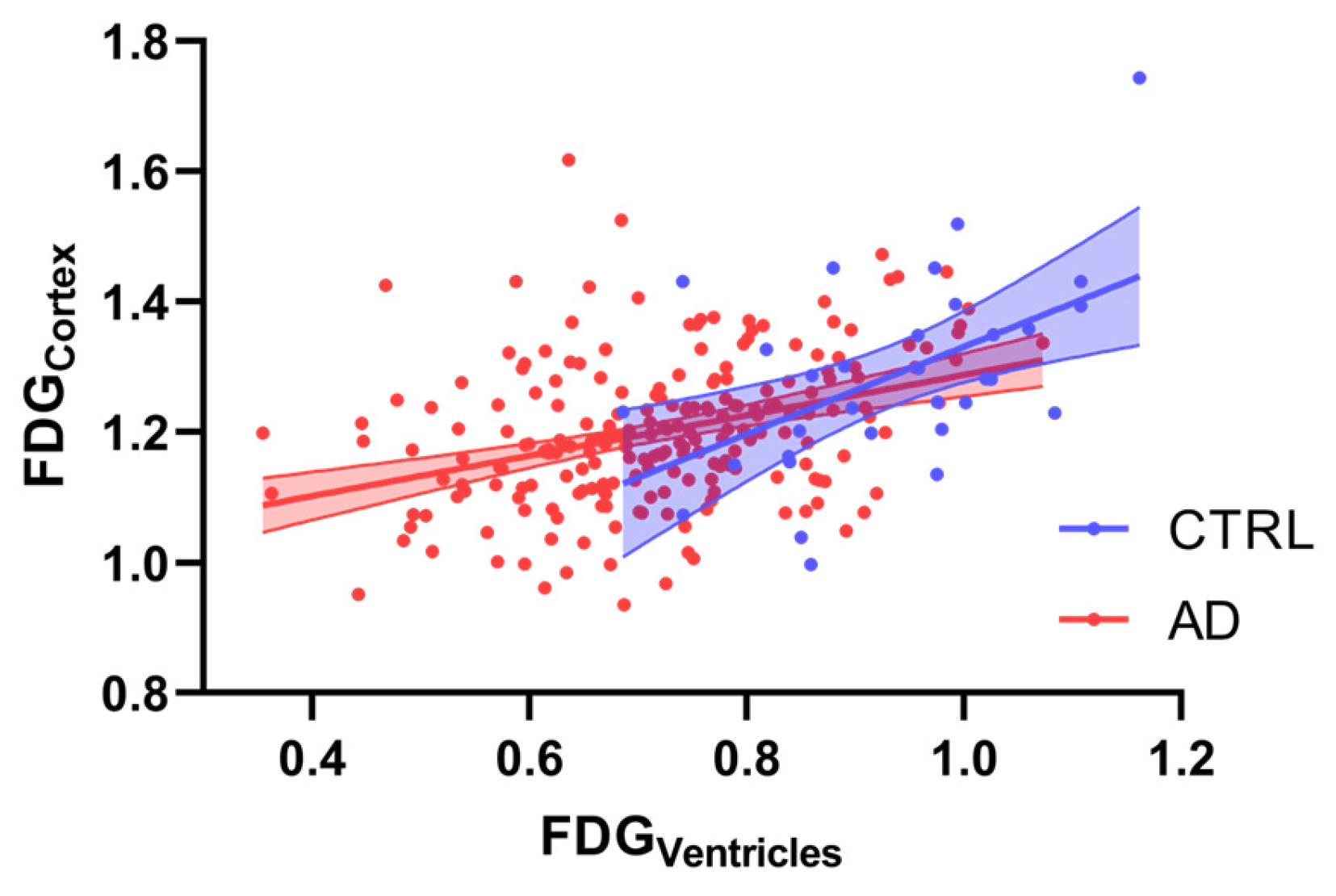
3.2. Correlation Analysis Between 18F-FDG Parameters
3.3. Regression Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| APOE | Apolipoprotein E |
| Aβ42 | amyloid-β 1–42 |
| CSF | cerebrospinal fluid |
| F | female |
| FDG | fluorodeoxyglucose |
| GLUT | glucose transporter |
| CTRL | control |
| n | number |
| p | p-value |
| p-tau | phosphorylated-tau |
| Qalb | Albumin Quotient |
| t-tau | total tau |
| 18F-FDG-PET | positron emission tomography with [18F]fluoro-2-deoxyglucose |
References
- Sweeney, M.D.; Montagne, A.; Sagare, A.P.; Nation, D.A.; Schneider, L.S.; Chui, H.C.; Harrington, M.G.; Pa, J.; Law, M.; Wang, D.J.J.; et al. Vascular Dysfunction-The Disregarded Partner of Alzheimer’s Disease. Alzheimers Dement. 2019, 15, 158–167. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Salmon, E.; Collette, F.; Bastin, C. Cerebral Glucose Metabolism in Alzheimer’s Disease. Cortex 2024, 179, 50–61. [Google Scholar] [CrossRef]
- Mosconi, L.; Berti, V.; Glodzik, L.; Pupi, A.; De Santi, S.; De Leon, M.J. Pre-Clinical Detection of Alzheimer’s Disease Using FDG-PET, with or without Amyloid Imaging. J. Alzheimers Dis. 2010, 20, 843–854. [Google Scholar] [CrossRef]
- Nordberg, A.; Rinne, J.O.; Kadir, A.; Lngström, B. The Use of PET in Alzheimer Disease. Nat. Rev. Neurol. 2010, 6, 78–87. [Google Scholar] [CrossRef]
- Hunt, A.; Schönknecht, P.; Henze, M.; Seidl, U.; Haberkorn, U.; Schröder, J. Reduced Cerebral Glucose Metabolism in Patients at Risk for Alzheimer’s Disease. Psychiatry Res. 2007, 155, 147–154. [Google Scholar] [CrossRef]
- Ibáñez, V.; Pietrini, P.; Alexander, G.E.; Furey, M.L.; Teichberg, D.; Rajapakse, J.C.; Rapoport, S.I.; Schapiro, M.B.; Horwitz, B. Regional Glucose Metabolic Abnormalities Are Not the Result of Atrophy in Alzheimer’s Disease. Neurology 1998, 50, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Patching, S.G. Glucose Transporters at the Blood-Brain Barrier: Function, Regulation and Gateways for Drug Delivery. Mol. Neurobiol. 2017, 54, 1046–1077. [Google Scholar] [CrossRef]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R.; et al. GLUT1 Reductions Exacerbate Alzheimer’s Disease Vasculo-Neuronal Dysfunction and Degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, Z.; Sagare, A.P.; Wu, Y.; Wang, M.; Owens, N.C.; Verghese, P.B.; Herz, J.; Holtzman, D.M.; Zlokovic, B.V. Blood-Brain Barrier-Associated Pericytes Internalize and Clear Aggregated Amyloid-Β42 by LRP1-Dependent Apolipoprotein E Isoform-Specific Mechanism. Mol. Neurodegener. 2018, 13, 57. [Google Scholar] [CrossRef]
- Kalaria, R.H.N.; Harik, S.I. Reduced Glucose Transporter at the Blood-Brain Barrier and in Cerebral Cortex in Alzheimer Disease. J. Neurochem. 1989, 53, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Patching, S. Roles of Facilitative Glucose Transporter GLUT1 in [18F]FDG Positron Emission Tomography (PET) Imaging of Human Diseases. J. Diagn. Imaging Ther. 2015, 2, 30–102. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, T.; Gardus, J.; Wen, Q.; Feng, Y.; DeLorenzo, C.; Parsey, R.; Huang, C. Quantifying Ventricular CSF Clearance in the Human Brain Using Dynamic 18F-FDG PET: Insights into Age-Related Glymphatic Impairment. medRxiv 2025. [Google Scholar] [CrossRef]
- Yao, J.; Rettberg, J.R.; Klosinski, L.P.; Cadenas, E.; Brinton, R.D. Shift in Brain Metabolism in Late Onset Alzheimer’s Disease: Implications for Biomarkers and Therapeutic Interventions. Mol. Asp. Med. 2011, 32, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Johnson, L.A. APOE and Metabolic Dysfunction in Alzheimer’s Disease. Int. Rev. Neurobiol. 2020, 154, 131–151. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.; Zhao, L. Human ApoE Isoforms Differentially Modulate Brain Glucose and Ketone Body Metabolism: Implications for Alzheimer’s Disease Risk Reduction and Early Intervention. J. Neurosci. 2018, 38, 6665–6681. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Guedj, E.; Varrone, A.; Boellaard, R.; Albert, N.L.; Barthel, H.; van Berckel, B.; Brendel, M.; Cecchin, D.; Ekmekcioglu, O.; Garibotto, V.; et al. EANM Procedure Guidelines for Brain PET Imaging Using [18F]FDG, Version 3. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 632–651. [Google Scholar] [CrossRef]
- D’Agostino, E.; Maes, F.; Vandermeulen, D.; Suetens, P. Atlas-to-Image Non-Rigid Registration by Minimization of Conditional Local Entropy. Inf. Process. Med. Imaging 2007, 20, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, J.C.; Toga, A.W.; Evans, A.; Fox, P.; Lancaster, J. A Probabilistic Atlas of the Human Brain: Theory and Rationale for Its Development. Neuroimage 1995, 2, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Maldjian, J.A.; Laurienti, P.J.; Kraft, R.A.; Burdette, J.H. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-Based Interrogation of FMRI Data Sets. Neuroimage 2003, 19, 1233–1239. [Google Scholar] [CrossRef]
- Verger, A.; Doyen, M.; Campion, J.Y.; Guedj, E. The Pons as Reference Region for Intensity Normalization in Semi-Quantitative Analysis of Brain 18FDG PET: Application to Metabolic Changes Related to Ageing in Conventional and Digital Control Databases. EJNMMI Res. 2021, 11, 31. [Google Scholar] [CrossRef]
- Minoshima, S.; Mosci, K.; Cross, D.; Thientunyakit, T. Brain [F-18]FDG PET for Clinical Dementia Workup: Differential Diagnosis of Alzheimer’s Disease and Other Types of Dementing Disorders. Semin. Nucl. Med. 2021, 51, 230–240. [Google Scholar] [CrossRef]
- Demetrius, L.A.; Magistretti, P.J.; Pellerin, L. Alzheimer’s Disease: The Amyloid Hypothesis and the Inverse Warburg Effect. Front. Physiol. 2015, 5, 522. [Google Scholar] [CrossRef]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.L.; Stella, N.; Magistretti, P.J. Evidence Supporting the Existence of an Activity-Dependent Astrocyte-Neuron Lactate Shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Peters, A.; Sprengell, M.; Kubera, B. The Principle of “brain Energy on Demand” and Its Predictive Power for Stress, Sleep, Stroke, Obesity and Diabetes. Neurosci. Biobehav. Rev. 2022, 141, 104847. [Google Scholar] [CrossRef]
- Cornford, E.M.; Hyman, S.; Swartz, B.E. The Human Brain GLUT1 Glucose Transporter: Ultrastructural Localization to the Blood-Brain Barrier Endothelia. J. Cereb. Blood Flow Metab. 1994, 14, 106–112. [Google Scholar] [CrossRef]
- Lee, W.H.; Bondy, C.A. Ischemic Injury Induces Brain Glucose Transporter Gene Expression. Endocrinology 1993, 133, 2540–2544. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. Cellular Mechanisms of Brain Energy Metabolism and Their Relevance to Functional Brain Imaging. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Chiaravalloti, A.; Sancesario, G.; Stefani, A.; Sancesario, G.M.; Mercuri, N.B.; Schillaci, O.; Pierantozzi, M. Cerebrospinal Fluid Lactate Levels and Brain [18F]FDG PET Hypometabolism within the Default Mode Network in Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Redzic, Z. Molecular Biology of the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers: Similarities and Differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [CrossRef]
- Chiaravalloti, A.; Fiorentini, A.; Francesco, U.; Martorana, A.; Koch, G.; Belli, L.; Torniolo, S.; Di Pietro, B.; Motta, C.; Schillaci, O. Is Cerebral Glucose Metabolism Related to Blood-Brain Barrier Dysfunction and Intrathecal IgG Synthesis in Alzheimer Disease? A 18 F-FDG PET/CT Study. Medicine 2016, 95, e4206. [Google Scholar] [CrossRef]
- Rhea, E.M.; Leclerc, M.; Yassine, H.N.; Capuano, A.W.; Tong, H.; Petyuk, V.A.; Macauley, S.L.; Fioramonti, X.; Carmichael, O.; Calon, F.; et al. State of the Science on Brain Insulin Resistance and Cognitive Decline Due to Alzheimer’s Disease. Aging Dis. 2024, 15, 1688–1725. [Google Scholar] [CrossRef]
- Onos, K.; Lin, P.B.; Pandey, R.S.; Persohn, S.A.; Burton, C.P.; Miner, E.W.; Eldridge, K.; Kanyinda, J.N.; Foley, K.E.; Carter, G.W.; et al. Assessment of Neurovascular Uncoupling: APOE Status Is a Key Driver of Early Metabolic and Vascular Dysfunction. Alzheimer’s Dement. 2024, 20, 4951–4969. [Google Scholar] [CrossRef]
- Williams, H.C.; Farmer, B.C.; Piron, M.A.; Walsh, A.E.; Bruntz, R.C.; Gentry, M.S.; Sun, R.C.; Johnson, L.A. APOE Alters Glucose Flux through Central Carbon Pathways in Astrocytes. Neurobiol. Dis. 2020, 136, 104742. [Google Scholar] [CrossRef]

| CTRL (n = 35) | All AD (n = 224) | p | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| MMSE | 28.60 ± 1.22 | 29.00 (2.22) | 22.73 ± 2.79 | 23.50 (3.00) | <0.001 |
| CSF Aβ42 (pg/mL) | 1028.22 ± 290.34 | 1005.00 (334.00) | 391.57 ± 140.25 | 390.50 (182.20) | <0.001 |
| CSF p-tau (pg/mL) | 35.75 ± 15.19 | 32.35 (23.00) | 84.57 ± 44.37 | 73.50 (53.00) | <0.001 |
| CSF t-tau (pg/mL) | 212.05 ± 133.80 | 169.00 (158.95) | 616.98 ± 351.76 | 555.81 (457.65) | <0.001 |
| CSF p-tau/Aβ42 | 0.036 ± 0.018 | 0.034 (0.020) | 0.252 ± 0.201 | 0.200 (0.147) | <0.001 |
| Qalb | 7.21 ± 2.15 | 6.98 (2.42) | 6.72 ± 2.87 | 6.05 (3.54) | <0.001 |
| CSF lactates (pg/mL) | 1.63 ± 0.40 | 1.50 (0.69) | 1.79 ± 0.35 | 1.80 (0.40) | <0.001 |
| Age (y) | 67.09 ± 7.33 | 67.00 (11.50) | 70.25 ± 6.83 | 71.00 (9.00) | 0.085 |
| % | % | ||||
| Sex (F) | 40.0 | 53.5 | 0.007 | ||
| APOE (ε4) | 14.3 | 44.3 | 0.014 | ||
| mean ± SD | mean ± SD | ||||
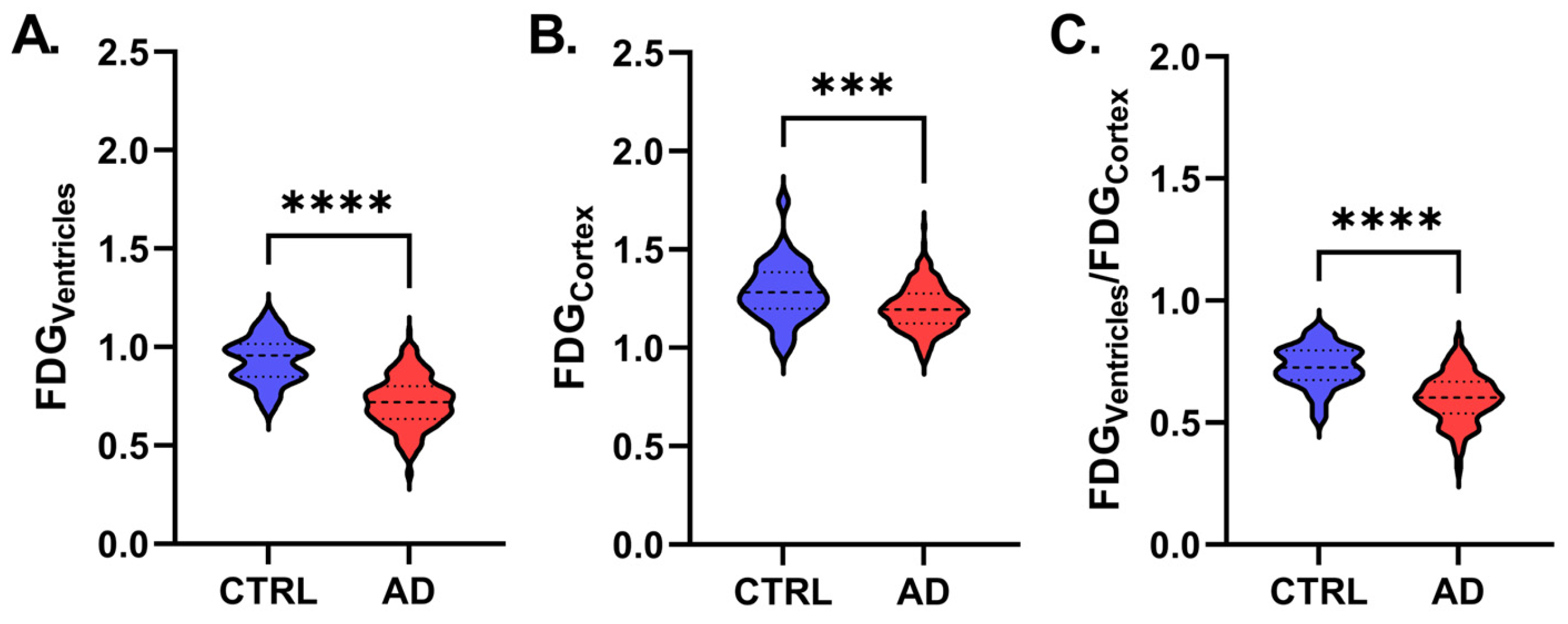
| FDGVentricles | 0.94 ± 0.22 | 0.72 ± 0.13 | <0.001 | ||
| FDGCortex | 1.28 ± 0.15 | 1.20 ± 0.11 | <0.001 | ||
| FDGVentricles/FDGCortex | 0.71 ± 0.11 | 0.60 ± 0.10 | <0.001 | ||
| CTRL | All AD | |||
|---|---|---|---|---|
| β | p | β | p | |
| FDGCortex | ||||
| Age | −0.455 | 0.044 | −0.013 | 0.854 |
| Sex | n.a. | 0.532 | n.a. | 0.480 |
| Plasma glucose (mg/dL) | −0.080 | 0.689 | −0.147 | 0.036 |
| Qalb | 0.098 | 0.588 | −0.158 | 0.033 |
| CSF p-tau/Aβ42 | 0.037 | 0.869 | −0.071 | 0.295 |
| CSF lactates | 0.082 | 0.698 | 0.011 | 0.876 |
| Adjusted R2 | 0.019 | 0.036 | ||
| FDGVentricles | ||||
| Age | −0.608 | 0.004 | −0.255 | <0.001 |
| Sex | n.a. | 0.188 | n.a. | 0.053 |
| Plasma glucose (mg/dL) | 0.013 | 0.940 | −0.002 | 0.978 |
| Qalb | 0.226 | 0.173 | −0.067 | 0.333 |
| CSF p-tau/Aβ42 | 0.254 | 0.207 | 0.075 | 0.238 |
| CSF lactates | 0.026 | 0.893 | −0.207 | 0.002 |
| Adjusted R2 | 0.212 | 0.148 | ||
| FDGVentricles/FDGCortex | ||||
| Age | −0.200 | 0.397 | −0.264 | <0.001 |
| Sex | n.a. | 0.481 | n.a. | 0.100 |
| Plasma glucose (mg/dL) | 0.081 | 0.707 | 0.082 | 0.213 |
| Qalb | 0.152 | 0.439 | 0.010 | 0.885 |
| CSF p-tau/Aβ42 | 0.213 | 0.375 | 0.136 | 0.034 |
| CSF lactates | −0.047 | 0.838 | −0.214 | 0.002 |
| Adjusted R2 | −0.139 | 0.144 | ||
| APOE ε3 | APOE ε4 | |||
|---|---|---|---|---|
| β | p | β | p | |
| FDGCortex | ||||
| Age | −0.126 | 0.900 | −0.032 | 0.747 |
| Plasma glucose (mg/dL) | −0.059 | 0.544 | −0.268 | 0.010 |
| Qalb | −0.138 | 0.166 | −0.240 | 0.019 |
| CSF p-tau/Aβ42 | −0.096 | 0.311 | −0.071 | 0.468 |
| CSF lactates | 0.141 | 0.151 | −0.085 | 0.431 |
| Adjusted R2 | −0.001 | 0.126 | ||
| FDGVentricles | ||||
| Age | −0.247 | 0.008 | −0.289 | 0.002 |
| Plasma glucose (mg/dL) | 0.061 | 0.511 | −0.080 | 0.395 |
| Qalb | −0.032 | 0.742 | −0.218 | 0.020 |
| CSF p-tau/Aβ42 | −0.057 | 0.531 | 0.253 | 0.006 |
| CSF lactates | −0.201 | 0.035 | −0.142 | 0.155 |
| Adjusted R2 | 0.069 | 0.261 | ||
| FDGVentricles/FDGCortex | ||||
| Age | −0.238 | 0.010 | −0.303 | 0.002 |
| Plasma glucose (mg/dL) | 0.089 | 0.341 | 0.071 | 0.457 |
| Qalb | 0.041 | 0.669 | −0.120 | 0.202 |
| CSF p-tau/Aβ42 | 0.015 | 0.866 | 0.334 | <0.001 |
| CSF lactates | −0.260 | 0.006 | −0.118 | 0.243 |
| Adjusted R2 | 0.086 | 0.248 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, C.; Bonomi, C.G.; Poli, M.; Mercuri, N.B.; Martorana, A.; Chiaravalloti, A. 18F-Fluorodeoxyglucose Uptake in Cerebrospinal Fluid Reflects Both Brain Glucose Demand and Impaired Blood–Brain Barrier Transport in Alzheimer’s Disease. Appl. Sci. 2025, 15, 5677. https://doi.org/10.3390/app15105677
Motta C, Bonomi CG, Poli M, Mercuri NB, Martorana A, Chiaravalloti A. 18F-Fluorodeoxyglucose Uptake in Cerebrospinal Fluid Reflects Both Brain Glucose Demand and Impaired Blood–Brain Barrier Transport in Alzheimer’s Disease. Applied Sciences. 2025; 15(10):5677. https://doi.org/10.3390/app15105677
Chicago/Turabian StyleMotta, Caterina, Chiara Giuseppina Bonomi, Martina Poli, Nicola Biagio Mercuri, Alessandro Martorana, and Agostino Chiaravalloti. 2025. "18F-Fluorodeoxyglucose Uptake in Cerebrospinal Fluid Reflects Both Brain Glucose Demand and Impaired Blood–Brain Barrier Transport in Alzheimer’s Disease" Applied Sciences 15, no. 10: 5677. https://doi.org/10.3390/app15105677
APA StyleMotta, C., Bonomi, C. G., Poli, M., Mercuri, N. B., Martorana, A., & Chiaravalloti, A. (2025). 18F-Fluorodeoxyglucose Uptake in Cerebrospinal Fluid Reflects Both Brain Glucose Demand and Impaired Blood–Brain Barrier Transport in Alzheimer’s Disease. Applied Sciences, 15(10), 5677. https://doi.org/10.3390/app15105677








