Brazilian Propolis: Nature’s Liquid Gold with Anti-Inflammatory and Anticancer Potential
Abstract
1. Introduction
2. Experimental Paper Selection Criteria (Study Design)
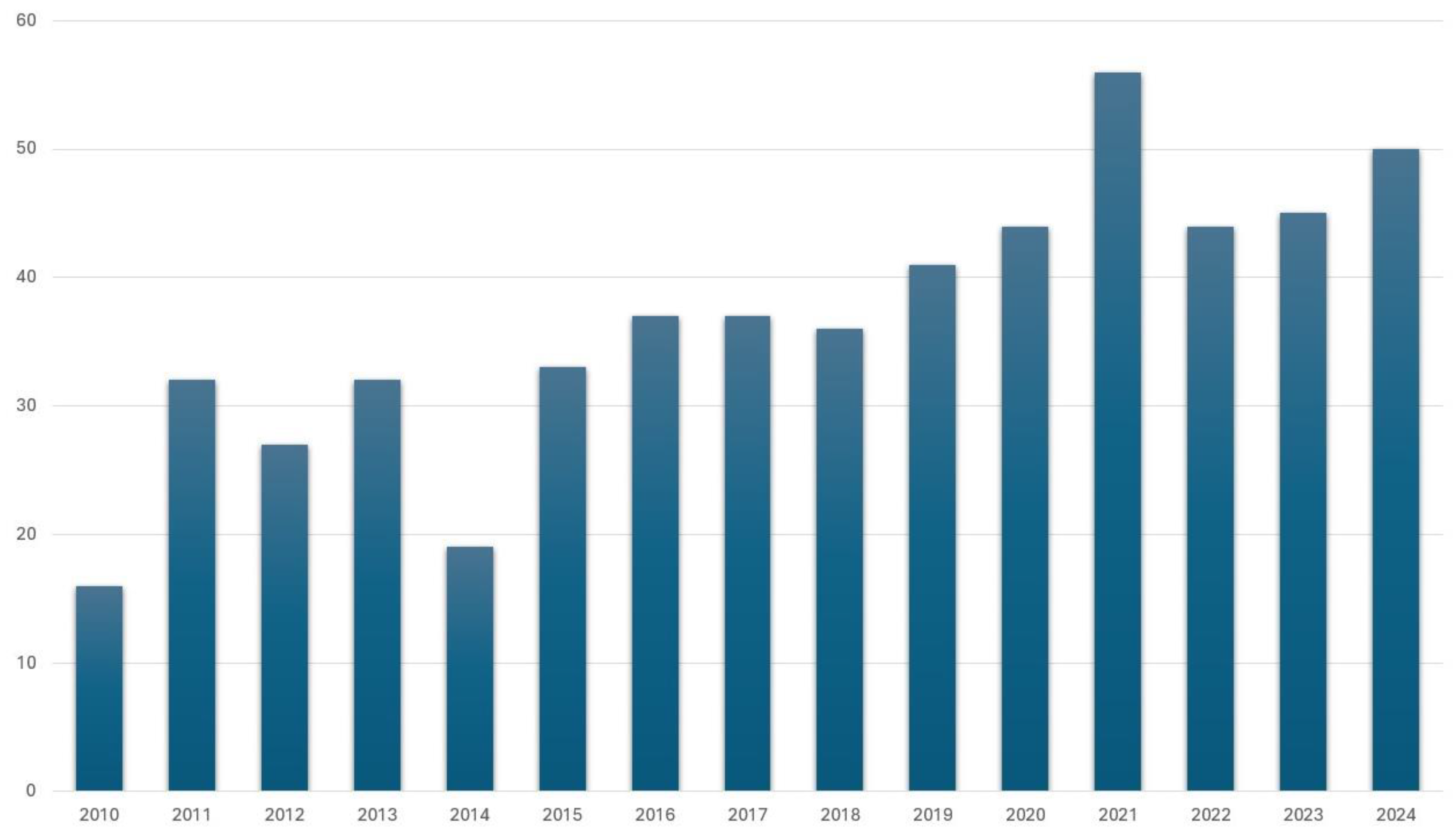
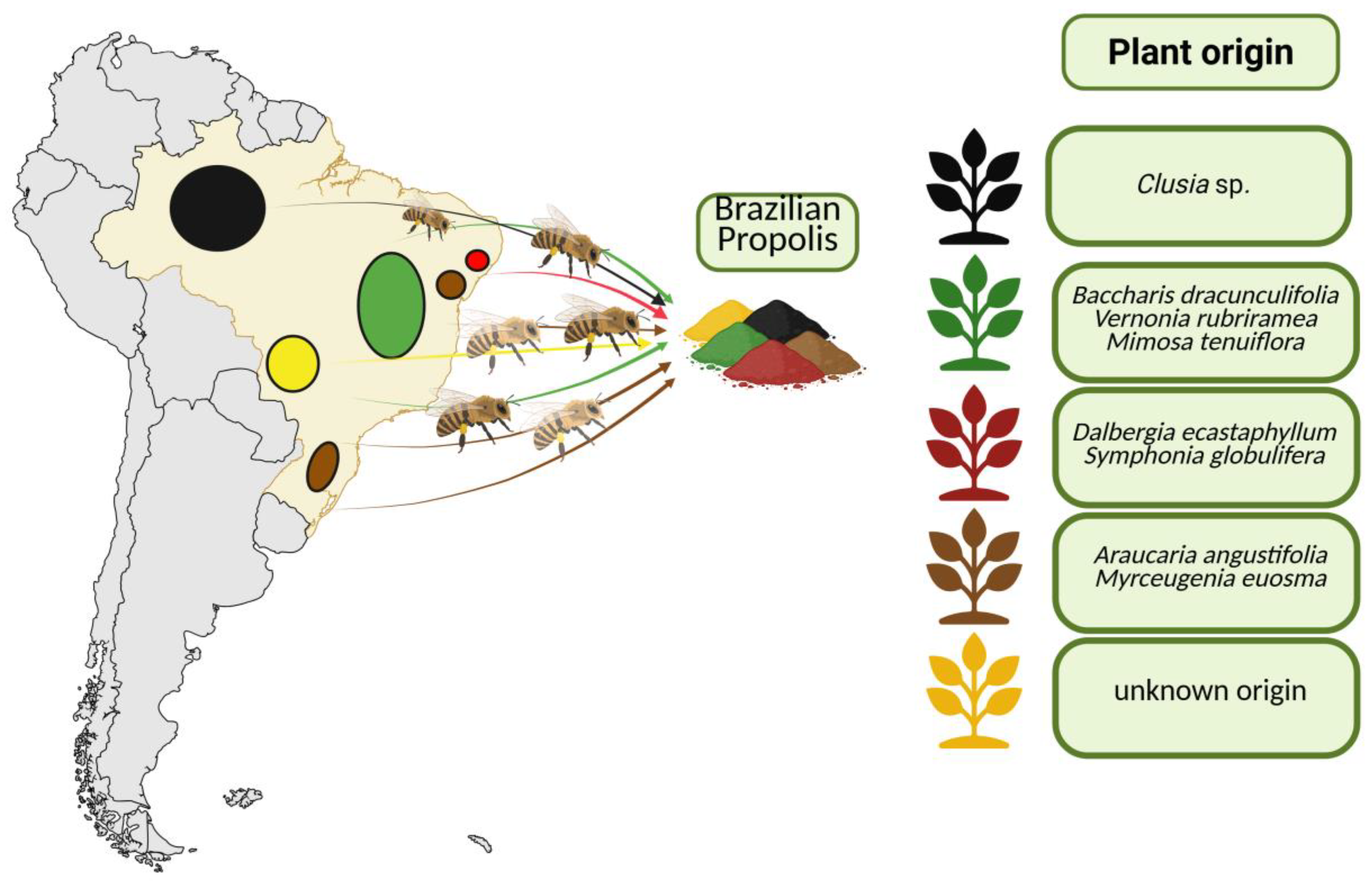
3. Brazilian Propolis: Unique Properties and Economic Significance
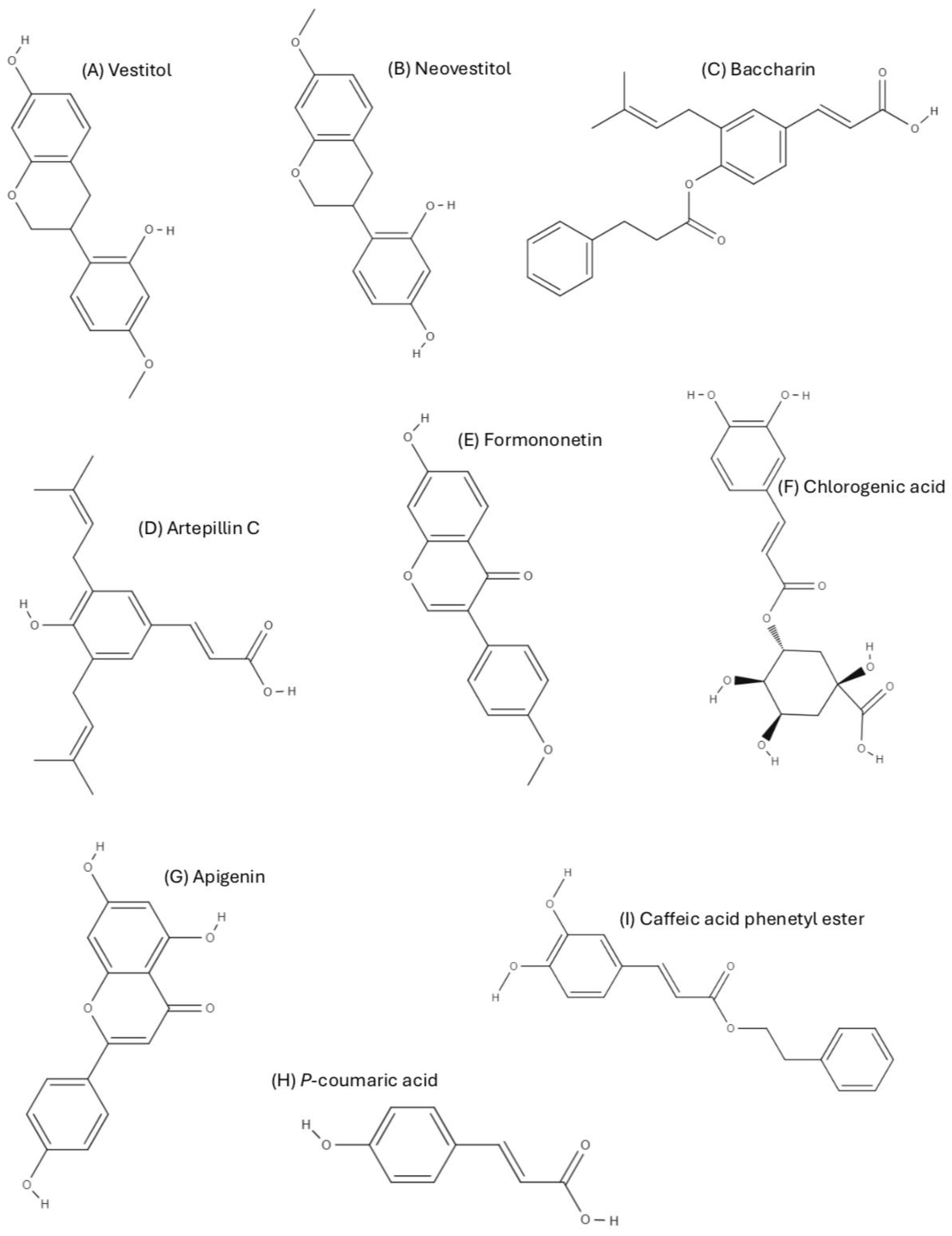
4. Secondary Metabolites of Brazilian Propolis
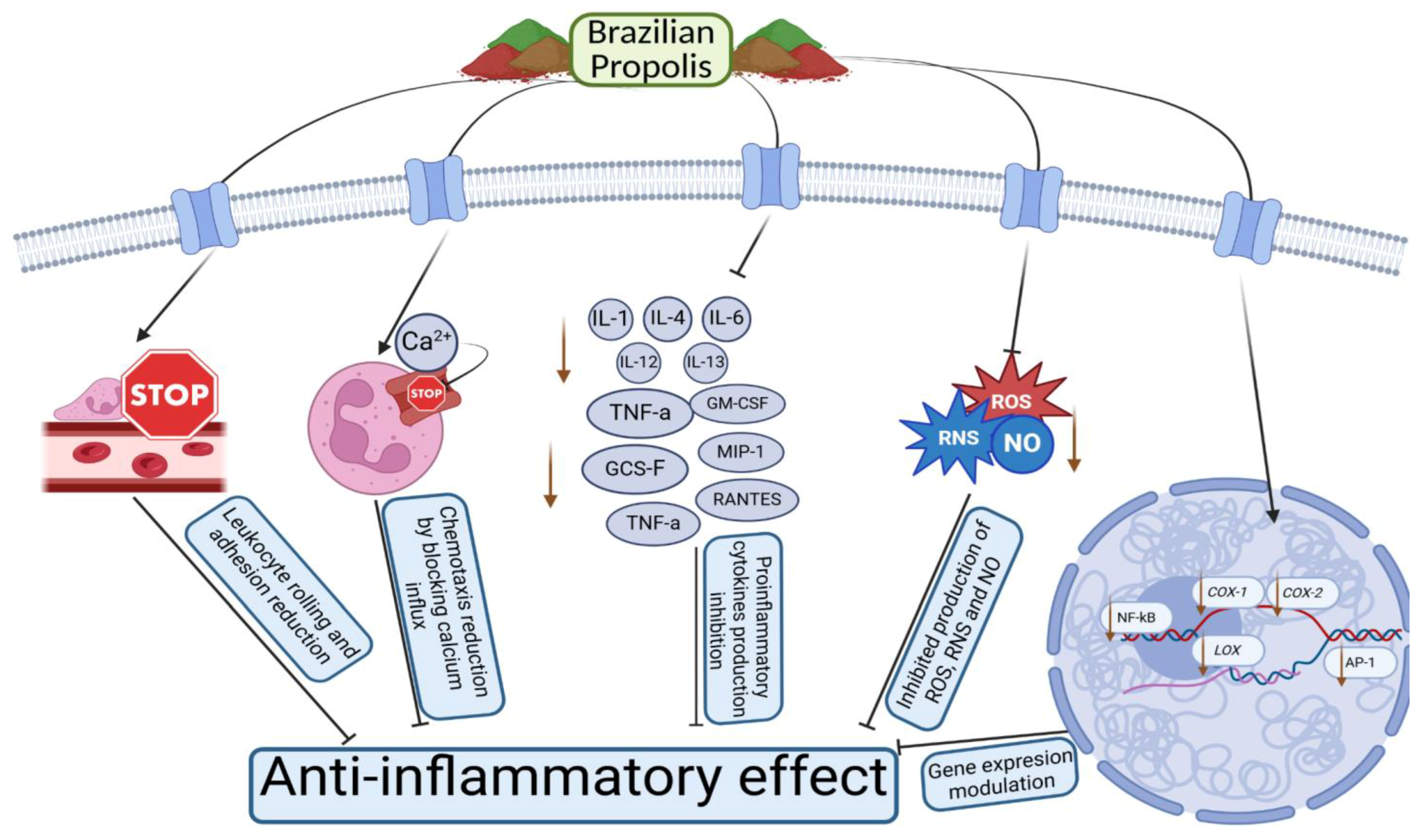
5. Molecular Mechanism and In Vitro and In Vivo Anti-Inflammatory Activity of Different Brazilian Propolis Extracts
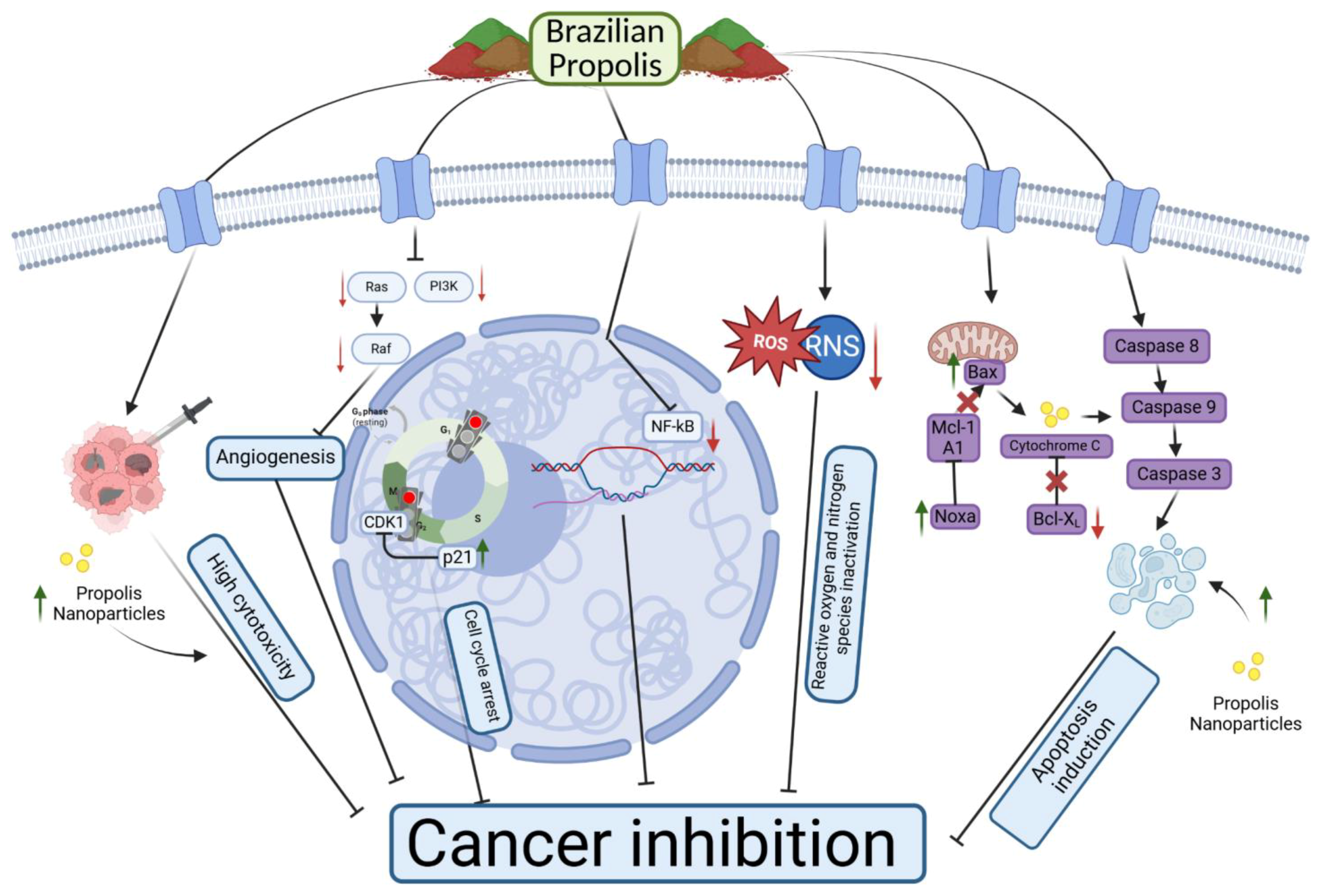
6. Molecular Mechanism and In Vitro and In Vivo Anticancer Effects of Various Brazilian Propolis Extracts
7. Use of Nanotechnology in Research on Brazilian Propolis
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Propolis: A Natural Substance with Multifaceted Properties and Activities. Int. J. Mol. Sci. 2025, 26, 1519. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Siheri, W.; Alenezi, S.; Tusiimire, J.; Watson, D.G. The Chemical and Biological Properties of Propolis. In Bee Products—Chemical and Biological Properties; Springer: Cham, Switzerland, 2017; pp. 137–178. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.; Umsza-Guez, M.A.; Barbosa, J.D.; Portela, R.D.; Machado, B.A. Propolis: Types, Composition, Biological Activities, and Veterinary Product Patent Prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef]
- Moise, A.R.; Bobiş, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- Machado, C.S.; Mokochinski, J.B.; Lira, T.O.; de Oliveira, F.D.; Cardoso, M.V.; Ferreira, R.G.; Sawaya, A.C.; Ferreira, A.G.; Pessoa, C.; Cuesta-Rubio, O.; et al. Comparative Study of Chemical Composition and Biological Activity of Yellow, Green, Brown, and Red Brazilian Propolis. Evid.-Based Complement. Alternat. Med. 2016, 2016, 6057650. [Google Scholar] [CrossRef]
- Nani, B.D.; Franchin, M.; Lazarini, J.G.; Freires, I.A.; da Cunha, M.G.; Bueno-Silva, B.; de Alencar, S.M.; Murata, R.M.; Rosalen, P.L. Isoflavonoids from Brazilian Red Propolis Down-Regulate the Expression of Cancer-Related Target Proteins: A Pharmacogenomic Analysis. Phytother. Res. 2018, 32, 750–754. [Google Scholar] [CrossRef]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid.-Based Complement. Alternat. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef]
- Reis, J.H.O.; Barreto, G.A.; Cerqueira, J.C.; Anjos, J.P.; Andrade, L.N.; Padilha, F.F.; Druzian, J.I.; Machado, B.A.S. Evaluation of the Antioxidant Profile and Cytotoxic Activity of Red Propolis Extracts from Different Regions of Northeastern Brazil Obtained by Conventional and Ultrasound-Assisted Extraction. PLoS ONE 2019, 14, e0219063. [Google Scholar] [CrossRef]
- Dantas Silva, R.P.; Machado, B.A.S.; Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, Antimicrobial, Antiparasitic, and Cytotoxic Properties of Various Brazilian Propolis Extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Cancer Tomorrow; Global Cancer Observatory. Available online: https://gco.iarc.who.int/tomorrow/en/dataviz/isotype?years=2050 (accessed on 20 March 2025).
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How Diverse Is the Chemistry and Plant Origin of Brazilian Propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative Chemistry of Propolis from Eight Brazilian Localities. Evid.-Based Complement. Alternat. Med. 2013, 2013, 267878. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- da Costa Silva, V.; do Nascimento, T.G.; Mergulhão, N.L.; Freitas, J.D.; Duarte, I.F.B.; de Bulhões, L.C.G.; Dornelas, C.B.; de Araújo, J.X.; Dos Santos, J.; Silva, A.C.; et al. Development of a polymeric membrane impregnated with poly-lactic acid (PLA) nanoparticles loaded with red propolis (RP). Molecules 2022, 27, 6959. [Google Scholar] [CrossRef]
- Justino, I.A.; Furlan, J.P.R.; Ferreira, I.R.S.; Marincek, A.; Aldana-Mejía, J.A.; Tucci, L.F.F.; Bastos, J.K.; Stehling, E.G.; Marzocchi-Machado, C.M.; Marcato, P.D. Antimicrobial, Antioxidant, and Anticancer Effects of Nanoencapsulated Brazilian Red Propolis Extract: Applications in Cancer Therapy. Processes 2024, 12, 2856. [Google Scholar] [CrossRef]
- Kasote, D.M. Propolis: A Neglected Product of Value in the Indian Beekeeping Sector. Bee World 2017, 94, 80–83. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, e1800080. [Google Scholar] [CrossRef]
- Huang, X.Y.; Guo, X.L.; Luo, H.L.; Fang, X.W.; Zhu, T.G.; Zhang, X.L.; Chen, H.W.; Luo, L.P. Fast Differential Analysis of Propolis Using Surface Desorption Atmospheric Pressure Chemical Ionization Mass Spectrometry. Int. J. Anal. Chem. 2015, 2015, 176475. [Google Scholar] [CrossRef]
- Bankova, V. Recent Trends and Important Developments in Propolis Research. Evid.-Based Complement. Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Yuan, M.; Yuan, X.J.; Pineda, M.; Liang, Z.Y.; He, J.; Sun, S.W.; Pan, T.L.; Li, K.P. A Comparative Study between Chinese Propolis and Brazilian Green Propolis: Metabolite Profile and Bioactivity. Food Funct. 2020, 11, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Mountford-McAuley, R.; Prior, J.; Clavijo McCormick, A. Factors Affecting Propolis Production. J. Apic. Res. 2021, 62, 162–170. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, Antifungal and Antiviral Activity of Propolis of Different Geographic Origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian Red Propolis: Botanical Origin and Comparative Analysis by High-Performance Liquid Chromatography-Photodiode Array Detection/Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef]
- Conceição, M.; Gushiken, L.F.S.; Aldana-Mejía, J.A.; Tanimoto, M.H.; Ferreira, M.V.S.; Alves, A.C.M.; Miyashita, M.N.; Bastos, J.K.; Beserra, F.P.; Pellizzon, C.H. Histological, Immunohistochemical and Antioxidant Analysis of Skin Wound Healing Influenced by the Topical Application of Brazilian Red Propolis. Antioxidants 2022, 11, 2188. [Google Scholar] [CrossRef]
- Boeing, T.; Monteiro Magalhães de Oliveira, B.; Aldana-Mejía, J.A.; Vidal Ccana-Ccapatinta, G.; Venzon, L.; Judah Cury, B.; Santos França, T.C.; de Souza, P.; Roman Junior, W.A.; Mota da Silva, L.; et al. Brazilian Red Propolis Accelerates Gastric Healing and Reduces Gastric Submucosal Layer Inflammation in Ultrasound-Monitored Rats. Chem. Biodivers. 2023, 20, e202200992. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef]
- IBGE. Map of Geographic Indications 2019 Brings Four New Products. Agência de Notícias IBGE. Available online: https://agenciadenoticias.ibge.gov.br/en/agencia-press-room/2185-news-agency/releases-en/25224-map-of-geographic-indications-2019-brings-four-new-products (accessed on 20 March 2025).
- de Amorim, F.R.; Alves, M.R.; Silva, S.A.; Pigatto, G. Sustainable Performance of Honey and Propolis Production in the Countryside of the State of São Paulo, Brazil. CEP 2022, 17602, 660. [Google Scholar] [CrossRef]
- IBGE. Beekeepers Are Nearly One-Fourth of Landless Producers in Minas Gerais. Agência de Notícias IBGE. Available online: https://agenciadenoticias.ibge.gov.br/en/agencia-news/2184-news-agency/news/26410-beekeepers-are-nearly-one-fourth-of-landless-producers-in-minas-gerais (accessed on 20 March 2025).
- Pires, L.; Castro, R. Isolation and Quantification of the Main Prenylated Compounds from Brazilian Green Propolis with Antioxidant Properties. Rev. Virtual Química 2022, 15, 713–721. [Google Scholar] [CrossRef]
- Sun, S.; Liu, M.; He, J.; Li, K.; Zhang, X.; Yin, G. Identification and Determination of Seven Phenolic Acids in Brazilian Green Propolis by UPLC-ESI-QTOF-MS and HPLC. Molecules 2019, 24, 1791. [Google Scholar] [CrossRef]
- Fernandes-Silva, C.C.; Salatino, A.; Salatino, M.L.F.; Breyer, E.D.H.; Negri, G. Chemical Profiling of Six Samples of Brazilian Propolis. Quím. Nova 2013, 36, 237–240. [Google Scholar] [CrossRef]
- de Figueiredo, S.M.; Binda, N.S.; Almeida, B.M.; Lemos Abreu, S.R.; Silva de Abreu, J.A.; Pastore, G.M.; Sato, H.H.; Toreti, V.C.; Tapia, E.V.; Park, Y.K.; et al. Green Propolis: Thirteen Constituents of Polar Extract and Total Flavonoids Evaluated During Six Years through RP-HPLC. Curr. Drug Discov. Technol. 2015, 12, 229–239. [Google Scholar] [CrossRef]
- Santiago, M.B.; Tanimoto, M.H.; Ambrosio, M.A.L.V.; Veneziani, R.C.S.; Bastos, J.K.; Sabino-Silva, R.; Martins, C.H.G. The Antibacterial Potential of Brazilian Red Propolis against the Formation and Eradication of Biofilm of Helicobacter pylori. Antibiotics 2024, 13, 719. [Google Scholar] [CrossRef]
- Machado, B.A.; Silva, R.P.; Barreto, G.d.A.; Costa, S.S.; Silva, D.F.; Brandão, H.N.; Rocha, J.L.; Dellagostin, O.A.; Henriques, J.A.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.; Alenzi, N.D. Evaluation of the Antitrypanosomal Activity, Cytotoxicity and Phytochemistry of Red Brazilian Propolis. PLoS ONE 2024, 19, e0313987. [Google Scholar] [CrossRef]
- Gomes, K.O.; Messias da Silva, L.C.F.; Dos Santos, R.D.; Prado, B.A.; da Silva Montes, P.; Silva Rodrigues, L.F.; de Araújo, M.O.; Bilac, C.A.; Freire, D.O.; Gris, E.F.; et al. Chemical Characterization and Antibacterial Activities of Brazilian Propolis Extracts from Apis mellifera Bees and Stingless Bees (Meliponini). PLoS ONE 2024, 19, e0307289. [Google Scholar] [CrossRef]
- de Oliveira Dembogurski, D.S.; Silva Trentin, D.; Boaretto, A.G.; Rigo, G.V.; da Silva, R.C.; Tasca, T.; Macedo, A.J.; Carollo, C.A.; Silva, D.B. Brown Propolis—Metabolomic Innovative Approach to Determine Compounds Capable of Killing Staphylococcus aureus Biofilm and Trichomonas vaginalis. Food Res. Int. 2018, 111, 661–673. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.U. An Overview of Inflammation: Mechanism and Consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, R.K.; Shukla, S.S.; Pandey, R.K. Current and Future Molecular Mechanisms in Inflammation and Arthritis. J. Pharmacopunct. 2020, 23, 54–61. [Google Scholar] [CrossRef]
- Wu, Y.; Antony, S.; Meitzler, J.L.; Doroshow, J.H. Molecular Mechanisms Underlying Chronic Inflammation-Associated Cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L. Complexity of Danger: The Diverse Nature of Damage-Associated Molecular Patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef] [PubMed]
- Castellheim, A.; Brekke, O.L.; Espevik, T.; Harboe, M.; Mollnes, T.E. Innate Immune Responses to Danger Signals in Systemic Inflammatory Response Syndrome and Sepsis. Scand. J. Immunol. 2009, 69, 479–491. [Google Scholar] [CrossRef]
- Harvanová, G.; Duranková, S.; Bernasovská, J. The Role of Cytokines and Chemokines in the Inflammatory Response. Alergol. Pol.-Pol. J. Allergol. 2023, 10, 210–219. [Google Scholar] [CrossRef]
- Quintino, R.L.; Reis, A.C.; Fernandes, C.C.; Martins, C.H.G.; Colli, A.C.; Crotti, A.E.M.; Squarisi, I.S.; Ribeiro, A.B.; Tavares, D.C.; Miranda, M.L.D. Brazilian Green Propolis: Chemical Composition of Essential Oil and Their In Vitro Antioxidant, Antibacterial and Antiproliferative Activities. Braz. Arch. Biol. Technol. 2020, 63, e20190408. [Google Scholar] [CrossRef]
- Fernandes-Silva, C.C.; Lima, C.A.; Negri, G.; Salatino, M.L.; Salatino, A.; Mayworm, M.A. Composition of the Volatile Fraction of a Sample of Brazilian Green Propolis and Its Phytotoxic Activity. J. Sci. Food Agric. 2015, 95, 3091–3095. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Ivanova, D.; Popova, M.; Bankova, V. Insights into the Essential Oil Compositions of Brazilian Red and Taiwanese Green Propolis. Nat. Prod. Commun. 2017, 12, 197–200. [Google Scholar] [CrossRef]
- de Lima, V.H.M.; Almeida, K.C.R.; Alves, C.C.F.; Rodrigues, M.L.; Crotti, A.E.M.; Souza, J.M.; Ribeiro, A.B.; Squarisi, I.S.; Tavares, D.C.; Martins, C.H.G.; et al. Biological Properties of Volatile Oil from Brazilian Brown Propolis. Rev. Bras. Farmacogn. 2019, 29, 807–810. [Google Scholar] [CrossRef]
- Oršolić, N. Allergic Inflammation: Effect of Propolis and Its Flavonoids. Molecules 2022, 27, 6694. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Chang, H.; Liu, X.; Wang, S.; Liu, H.; Xuan, H. Brazilian Green Propolis Inhibits Ox-LDL-Stimulated Oxidative Stress in Human Umbilical Vein Endothelial Cells Partly through PI3K/Akt/mTOR-Mediated Nrf2/HO-1 Pathway. Evid.-Based Complement. Altern. Med. 2019, 2019, 5789574. [Google Scholar] [CrossRef]
- Corrêa, F.R.S.; Schanuel, F.S.; Moura-Nunes, N.; Monte-Alto-Costa, A.; Daleprane, J.B. Brazilian Red Propolis Improves Cutaneous Wound Healing Suppressing Inflammation-Associated Transcription Factor NF-κB. Biomed. Pharmacother. 2017, 86, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.D.; Andrade, S.P.; Campos, P.P.; Barcelos, L.S.; Soriani, F.M.; Moura, S.A.; Ferreira, M.A. Brazilian Green Propolis Modulates Inflammation, Angiogenesis, and Fibrogenesis in Intraperitoneal Implant in Mice. BMC Complement. Altern. Med. 2014, 14, 177. [Google Scholar] [CrossRef]
- Pereira, P.M.; de Almeida-Junior, S.; Taveira, N.N.d.M.; de Melo, E.M.; Santos, M.F.C.; Nascimento, L.C.G.D.; Rodrigues, M.A.; Aldana-Mejía, J.A.; e Silva, M.L.A.; Ambrósio, S.R.; et al. Therapeutic Efficacy of Brown Propolis from Araucaria sp. in Modulating Rheumatoid Arthritis. Inflammopharmacology 2025, 33, 799–807. [Google Scholar] [CrossRef]
- Hori, J.I.; Zamboni, D.S.; Carrão, D.B.; Goldman, G.H.; Berretta, A.A. The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF). Evid.-Based Complement. Altern. Med. 2013, 2013, 418508. [Google Scholar] [CrossRef]
- de Miranda, M.B.; de Lana, M.F.; de Nascimento, A.L.B.; de Paula, C.A.; de Souza, M.E.; Felipetto, M.; de Barcelos, L.S.; de Moura, S.A.L. Hydroalcoholic Extract of Brazilian Green Propolis Modulates Inflammatory Process in Mice Submitted to a Low Protein Diet. Biomed. Pharmacother. 2019, 109, 610–620. [Google Scholar] [CrossRef]
- Santini, A.T.; Pinto, R.A.O.; Lazarini, J.G.; de Morais, D.V.; de Piloto Fernandes, A.M.A.; Franchin, M.; de Carvalho, P.L.N.; Pressete, C.G.; Rosalen, P.L.; de Alencar, S.M.; et al. Bioactives of Melipona rufiventris Propolis: Exploring Its Antimicrobial, Anti-Inflammatory, and Antioxidant Activities. Chem. Biodivers. 2024, 21, e202302084. [Google Scholar] [CrossRef]
- Shi, B.; Zhao, Y.; Yuan, X. Effects of MTA and Brazilian Propolis on the Biological Properties of Dental Pulp Cells. Braz. Oral Res. 2020, 33, e117. [Google Scholar] [CrossRef]
- Conte, F.L.; Pereira, A.C.; Brites, G.; Ferreira, I.; Silva, A.C.; Sebastião, A.I.; Matos, P.; Pereira, C.; Batista, M.T.; Sforcin, J.M.; et al. Exploring the Antioxidant, Anti-Inflammatory, and Antiallergic Potential of Brazilian Propolis in Monocytes. Phytomedicine Plus 2022, 2, 100231. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Kawamoto, D.; Ando-Suguimoto, E.S.; Alencar, S.M.; Rosalen, P.L.; Mayer, M.P. Brazilian Red Propolis Attenuates Inflammatory Signaling Cascade in LPS-Activated Macrophages. PLoS ONE 2015, 10, e0144954. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Franchin, M.; Alves, C.d.F.; Denny, C.; Colón, D.F.; Cunha, T.M.; Alencar, S.M.; Napimoga, M.H.; Rosalen, P.L. Main Pathways of Action of Brazilian Red Propolis on the Modulation of Neutrophils Migration in the Inflammatory Process. Phytomedicine 2016, 23, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, S.M.; de Oliveira Sartori, A.G.; Dag, D.; Batista, P.S.; Rosalen, P.L.; Ikegaki, M.; Kong, F. Dynamic Gastrointestinal Digestion/Intestinal Permeability of Encapsulated and Nonencapsulated Brazilian Red Propolis: Active Compounds Stability and Bioactivity. Food Chem. 2023, 411, 135469. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.A.; Lemos, C.N.; Dalmolin, L.F.; Arruda, C.; Brait, Í.S.C.; Cazarim, M.d.S.; da Cruz-Cazarim, E.L.C.; Bueno, P.C.P.; Júnior, M.P.; Pereira, L.R.L.; et al. A New Approach to Atopic Dermatitis Control with Low-Concentration Propolis-Loaded Cold Cream. Pharmaceutics 2021, 13, 1346. [Google Scholar] [CrossRef]
- Kłósek, M.; Sędek, Ł.; Lewandowska, H.; Czuba, Z.P. The Effect of Ethanolic Extract of Brazilian Green Propolis and Artepillin C on aFGF-1, E-Selectin, and CD40L Secreted by Human Gingival Fibroblasts. Cent. Eur. J. Immunol. 2021, 46, 438–445. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef]
- Piñeros, A.R.; de Lima, M.H.; Rodrigues, T.; Gembre, A.F.; Bertolini, T.B.; Fonseca, M.D.; Berretta, A.A.; Ramalho, L.N.; Cunha, F.Q.; Hori, J.I.; et al. Green Propolis Increases Myeloid Suppressor Cells and CD4⁺Foxp3⁺ Cells and Reduces Th2 Inflammation in the Lungs after Allergen Exposure. J. Ethnopharmacol. 2020, 252, 112496. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Rosalen, P.L.; Franchin, M.; Lacerda, R.C.C.; Bueno-Silva, B.; Benso, B.; Denny, C.; Ikegaki, M.; de Alencar, S.M. Chemical Characterization and Antioxidant, Antimicrobial, and Anti-Inflammatory Activities of South Brazilian Organic Propolis. PLoS ONE 2016, 11, e0165588. [Google Scholar] [CrossRef] [PubMed]
- Cury, B.J.; Jerônimo, D.T.; Silva, T.F.d.Q.e.; França, T.C.S.; Dos Santos, A.C.; Andriolo, I.R.L.; Santin, J.R.; Benvenutti, L.; Vaz, C.R.; Santos, M.F.C.; et al. Hydroalcoholic Extract of Araucaria sp. Brown Propolis Alleviates Ulcerative Colitis Induced by TNBS in Rats by Reducing Inflammatory Cell Infiltration and Oxidative Damage. J. Pharm. Pharmacol. 2024, 76, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Sartori, G.; Pesarico, A.P.; Pinton, S.; Dobrachinski, F.; Roman, S.S.; Pauletto, F.; Rodrigues, L.C.; Prigol, M. Protective Effect of Brown Brazilian Propolis against Acute Vaginal Lesions Caused by Herpes Simplex Virus Type 2 in Mice: Involvement of Antioxidant and Anti-Inflammatory Mechanisms. Cell Biochem. Funct. 2012, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, K.S.; da Silva, L.H.D.; Squarisi, I.S.; Oliveira, L.T.d.S.; Ribeiro, A.B.; Alves, B.S.; Esperandim, T.R.; de Melo, M.R.S.; Ozelin, S.D.; Lemes, D.C.; et al. Red Propolis Exhibits Chemopreventive Effect Associated with Antiproliferative and Anti-Inflammatory Activities. Toxicol. Res. 2022, 11, 750–757. [Google Scholar] [CrossRef]
- Batista, C.; Alves, A.; Queiroz, L.; Lima, B.; Filho, R.; Araújo, A.; Júnior, R.d.A.; Cardoso, J. The Photoprotective and Anti-Inflammatory Activity of Red Propolis Extract in Rats. J. Photochem. Photobiol. B Biol. 2018, 180, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; Carvalho, J.C.A.S.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Capcha, J.M.C.; Sanches, T.R.; Moreira, R.d.S.; Garnica, M.S.; Shimizu, M.H.; Berretta, A.; Teles, F.; Noronha, I.L.; Andrade, L. Green Propolis Extract Attenuates Acute Kidney Injury and Lung Injury in a Rat Model of Sepsis. Sci. Rep. 2021, 11, 5925. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sakai, H.; Hirata, A.; Yanai, T. Brazilian Green Propolis Suppresses Acetaminophen-Induced Hepatocellular Necrosis by Modulating Inflammation-Related Factors in Rats. J. Toxicol. Pathol. 2018, 31, 275–282. [Google Scholar] [CrossRef]
- Machado, J.L.; Assunção, A.K.M.; da Silva, M.C.P.; dos Reis, A.S.; Costa, G.C.; Arruda, D.d.S.; Rocha, B.A.; Vaz, M.M.d.O.L.L.; Paes, A.M.d.A.; Guerra, R.N.M.; et al. Brazilian Green Propolis: Anti-Inflammatory Property by an Immunomodulatory Activity. Evid.-Based Complement. Altern. Med. 2012, 2012, 157652. [Google Scholar] [CrossRef]
- Schuler, M.; Green, D.R. Mechanisms of p53-Dependent Apoptosis. Biochem. Soc. Trans. 2001, 29, 684–688. [Google Scholar] [CrossRef]
- Wu, F.; Song, X.M.; Qiu, Y.L.; Zheng, H.Q.; Hu, F.L.; Li, H.L. Unique Dynamic Mode between Artepillin C and Human Serum Albumin Implies the Characteristics of Brazilian Green Propolis Representative Bioactive Component. Sci. Rep. 2020, 10, 17277. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.B.; Rodrigues, J.C.Z.; de Oliveira Cardoso, E.; Conte, F.L.; Tasca, K.I.; Romagnoli, G.G.; Aldana-Mejía, J.A.; Bastos, J.K.; Sforcin, J.M. Brazilian Red Propolis Exerts a Cytotoxic Action Against Prostate Cancer Cells and Upregulates Human Monocyte Functions. Phytother. Res. 2023, 37, 399–409. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Díaz-García, A.; Ruiz-Fuentes, J.; Rodríguez-Sánchez, H.; Sforcin, J.M. Brazilian Green Propolis Induced Apoptosis in Human Lung Cancer A549 Cells through Mitochondrial-Mediated Pathway. J. Pharm. Pharmacol. 2015, 67, 1448–1456. [Google Scholar] [CrossRef]
- Ziyad, S.; Iruela-Arispe, M.L. Molecular Mechanisms of Tumor Angiogenesis. Genes Cancer 2011, 2, 1085–1096. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of Survival, Proliferation, Invasion, Angiogenesis, and Metastasis of Tumor Cells through Modulation of Inflammatory Pathways by Nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Muninathan, N.; Megalatha, S.T.; Suresh, A.; Kumar, K.S.; Jhansi, N.; Kalaivani, K.; Krishnamoorthy, G. An Insight into Anticancer Effect of Propolis and Its Constituents: A Review of Molecular Mechanisms. Evid.-Based Complement. Alternat. Med. 2022, 2022, 5901191. [Google Scholar] [CrossRef] [PubMed]
- Altabbal, S.; Athamnah, K.; Rahma, A.; Wali, A.F.; Eid, A.H.; Iratni, R.; Al Dhaheri, Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 2023, 16, 450. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Núñez, O.; Torres, A.; Guzmán, L.; Wehinger, S. Antitumor Activity of Propolis: Recent Advances in Cellular Perspectives, Animal Models and Possible Applications. Food Rev. Int. 2019, 36, 429–455. [Google Scholar] [CrossRef]
- Buitrago, D.M.; Perdomo, S.J.; Silva, F.A.; Cely-Veloza, W.; Lafaurie, G.I. Physicochemical Characterization, Antioxidant, and Proliferative Activity of Colombian Propolis Extracts: A Comparative Study. Molecules 2024, 29, 1643. [Google Scholar] [CrossRef]
- Vendruscolo, I.; Berton, G.H.; Biffi, M.T.; Bressiani, P.A.; Oliveira, A.K.G.; Berti, A.P.; Concato-Lopes, V.M.; Pavanelli, W.R.; Simon, A.P.; Oldoni, T.L.C.; et al. Antiproliferative Effect of Hydroalcoholic Brown Propolis Extract on Tumor and Non-Tumor Cells. Braz. J. Biol. 2025, 84, e287297. [Google Scholar] [CrossRef]
- Frozza, C.O.D.S.; Santos, D.A.; Rufatto, L.C.; Minetto, L.; Scariot, F.J.; Echeverrigaray, S.; Pich, C.T.; Moura, S.; Padilha, F.F.; Borsuk, S.; et al. Antitumor Activity of Brazilian Red Propolis Fractions Against Hep-2 Cancer Cell Line. Biomed. Pharmacother. 2017, 91, 951–963. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Cardoso, E.; Santiago, K.B.; Conti, B.J.; Conte, F.L.; Tasca, K.I.; Romagnoli, G.G.; de Assis Golim, M.; Rainho, C.A.; Bastos, J.K.; Sforcin, J.M. Brazilian Green Propolis: A Novel Tool to Improve the Cytotoxic and Immunomodulatory Action of Docetaxel on MCF-7 Breast Cancer Cells and on Women Monocyte. Phytother. Res. 2022, 36, 448–461. [Google Scholar] [CrossRef]
- dos Santos, D.A.; Munari, F.M.; Frozza, C.O.S.; Moura, S.; Barcellos, T.; Henriques, J.A.P.; Roesch-Ely, M. Brazilian Red Propolis Extracts: Study of Chemical Composition by ESI-MS/MS (ESI⁺) and Cytotoxic Profiles Against Colon Cancer Cell Lines. Biotechnol. Res. Innov. 2019, 3, 120–130. [Google Scholar] [CrossRef]
- de Mendonça, I.C.G.; Porto, I.C.C.M.; do Nascimento, T.G.; Oliveira, A.E.M.F.M.; Lima, E.O.; Capella, M.A.M.; Albuquerque-Júnior, R.L.C.; Souto, R.N.P.; Basílio-Júnior, I.D.; Guerra, G.C.B. Brazilian Red Propolis: Phytochemical Screening, Antioxidant Activity and Effect Against Cancer Cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef]
- Falcão, S.I.; Duarte, D.; Diallo, M.; Santos, J.; Ribeiro, E.; Vale, N.; Vilas-Boas, M. Improvement of the In Vitro Cytotoxic Effect on HT-29 Colon Cancer Cells by Combining 5-Fluorouracil and Fluphenazine with Green, Red or Brown Propolis. Molecules 2023, 28, 3393. [Google Scholar] [CrossRef]
- Bhargava, P.; Grover, A.; Nigam, N.; Kaul, A.; Doi, M.; Ishida, Y.; Kakuta, H.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer Activity of the Supercritical Extract of Brazilian Green Propolis and Its Active Component, Artepillin C: Bioinformatics and Experimental Analyses of Its Mechanisms of Action. Int. J. Oncol. 2018, 52, 925–932. [Google Scholar] [CrossRef]
- de Carvalho, F.M.A.; Schneider, J.K.; de Jesus, C.V.F.; de Andrade, L.N.; Amaral, R.G.; David, J.M.; Krause, L.C.; Severino, P.; Soares, C.M.F.; Bastos, E.C.; et al. Brazilian Red Propolis: Extracts Production, Physicochemical Characterization, and Cytotoxicity Profile for Antitumor Activity. Biomolecules 2020, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Ishii, N.; Fujioka, M.; Doi, K.; Gi, M.; Wanibuchi, H. Ethanol-Extracted Brazilian Propolis Exerts Protective Effects on Tumorigenesis in Wistar Hannover Rats. PLoS ONE 2016, 11, e0158654. [Google Scholar] [CrossRef]
- Ribeiro, D.R.; Alves, Â.V.; dos Santos, E.P.; Padilha, F.F.; Gomes, M.Z.; Rabelo, A.S.; Cardoso, J.C.; Massarioli, A.P.; de Alencar, S.M.; de Albuquerque-Júnior, R.L. Inhibition of DMBA-Induced Oral Squamous Cells Carcinoma Growth by Brazilian Red Propolis in Rodent Model. Basic Clin. Pharmacol. Toxicol. 2015, 117, 85–95. [Google Scholar] [CrossRef]
- Pelegrini, B.B.; Becker, A.A.; Ferreira, C.A.; Machado, G.R.; Gauer, M.A.; Mazarin, S.R.; Dembogurski, D.S.O.; Kaneshima, A.M.S.; Da Silva, D.B.; Becker, T.C.A. Antineoplastic Activity Evaluation of Brazilian Brown Propolis and Artepillin C in Colorectal Area of Wistar Rats. Asian Pac. J. Cancer Prev. 2024, 25, 563–573. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Berretta, A.; Arruda, C.; Miguel, F.; Baptista, N.; Nascimento, A.; Marquele-Oliveira, F.; Hori, J.; Barud, H.; Damaso, B.; Ramos, C.; et al. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market. In Propolis: Properties, Application and Its Potential; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Sorita, G.D.; Caicedo Chacon, W.D.; Strieder, M.M.; Rodriguez-García, C.; Fritz, A.M.; Verruck, S.; Ayala Valencia, G.; Mendiola, J.A. Biorefining Brazilian Green Propolis: An Eco-Friendly Approach Based on a Sequential High-Pressure Extraction for Recovering High-Added-Value Compounds. Molecules 2025, 30, 189. [Google Scholar] [CrossRef]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The Use of Brazilian Propolis for Discovery and Development of Novel Anti-Inflammatory Drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Justino, I.A.; Marincek, A.; Ferreira, I.R.S.; Amaral, R.L.F.; Fontanezi, B.B.; Aldana-Mejía, J.A.; Bastos, J.K.; Marcato, P.D. Brazilian Red Propolis Extract Free and Encapsulated into Polymeric Nanoparticles against Ovarian Cancer: Formulation, Characterisation and Biological Assays in 2D and 3D Models. J. Pharm. Pharmacol. 2023, 75, 806–818. [Google Scholar] [CrossRef]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and Characterization of Gold Nanoparticles Using Brazilian Red Propolis and Evaluation of Its Antimicrobial and Anticancer Activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Botteon, C.; Amaral, R.; Silva, L.; Gaspari, P. Assessment of Cytotoxicity of Gold Nanoparticles Functionalized with Brazilian Red Propolis in 2D and 3D Models of Urological Cancers. In Proceedings of the International Conference on Nanomedicine, Nanobiotechnology and Functional Coatings (ICNNFC 2023), Lisbon, Portugal, 23–25 March 2023. [Google Scholar] [CrossRef]
- Justino, I.A.; Ferreira, I.R.S.; Botteon, C.A.; Tucci, L.F.F.; Marincek, A.; Amaral, R.; Aldana-Mejia, J.A.; Gonçalves, Y.G.; Bastos, J.K.; Marzocchi-Machado, C.M.; et al. Cytotoxic Potential of Polymeric Nanoparticles Loaded with Brazilian Red Propolis in Breast Cancer. J. Drug Deliv. Sci. Technol. 2025, 105, 106663. [Google Scholar] [CrossRef]

| Type of Propolis | Class of Compounds | Cell Line | Dose | Activity/Mechanism/Effect | Ref. |
|---|---|---|---|---|---|
| Brazilian Propolis | Polyphenols | RAW 264.7 macrophages transfected with the NF-kB-pLUC gene | 1, 3, 10, 30, 100, and 200 μg/mL | Treatment with an ethanolic extract of Brazilian propolis resulted in a decrease in NF-kB activation and a considerable reduction in the levels of TNF-α. These results confirm the potential use of propolis as an anti-inflammatory compound. | [65] |
| Brazilian Propolis | - | Human dental pulp cells (hDPCs) | 10, 20, 40, 80, and 160 μg/mL | An ethanolic extract of Brazilian propolis caused notable improvement of osteogenic potential and suppressed expression of IL-1β and IL-6 was observed after exposure to LPS. It also had a significant anti-inflammatory and mineralizing effect on hDPCs. | [66] |
| Brazilian Propolis | - | THP-1 human monocytic cell line | 1, 5, 10, 20 and 50 µg/mL | Propolis exerted an anti-inflammatory/antiallergic effect. In the presence of LPS, it induced a higher HMOX-1 expression but inhibited CD86 expression stimulated by DNFB. | [67] |
| Red Brazilian Propolis | Isoflavonoids (e.g., vestitol and neovestitol) Flavonoids | RAW 264.7 murine macrophages | 40–100 μg/mL | An ethanolic extract of red Brazilian propolis inhibited multiple signalling pathways in macrophages involved in the inflammatory process activated by LPS. | [68] |
| Red Brazilian Propolis | Daidzein, Formononetin, Biochanin | Neutrophils isolated from male, SPF (specific-pathogen free), BALB/c mouse bone marrow | 0.01, 0.1 and 1 μg/mL | An ethanolic extract of red Brazilian propolis at 1 μg/mL reduced calcium influx in neutrophils under CXCL2/MIP-2 stimulation. Blocking calcium influx reduced neutrophil chemotaxis. | [69] |
| Red Brazilian Propolis | Isoflavonoids (e.g., vestitol, neovestitol), Chalcones, Flavanones | Transgenic RAW 264.7 macrophages transfected with the nuclear factor ĸB (NF-ĸB) luciferase | 10, 20, and 30 µg/mL | An ethanolic extract of Brazilian red propolis at 30 μg/mL considerably decreased NF-κB activation and TNF-α. The LPS-stimulated macrophages treated with EEBRP showed no reduction in viability at concentrations of up to 30 μg/mL. | [70] |
| Green Brazilian Propolis | Artepillin C | AMJ-2 macrophages | 34, 60, and 120 ng/mL | A green Brazilian propolis hydroalcoholic extract reduced the TNF-α level in LPS-stimulated macrophage culture. | [71] |
| Green Brazilian Propolis | - | Human gingival fibroblasts (HGF-1 cells) | 1, 10, and 20 μg/mL | An ethanolic extract of green Brazilian propolis (EEBP) stimulated human gingival fibroblasts to secrete high levels of acidic FGF-1 and caused a decrease in E-selectin secretion by HGF-1 cells. Artepillin C and other various compounds (such as flavonoids) in EEBP may have an anti-inflammatory effect and accelerate the process of wound healing. | [72] |
| Green Brazilian Propolis | Phenolics (mainly artepillin C, kaempferide and their derivatives), Flavonoids | Murine macrophage J774A.1 cell line | 5–50 μg/mL | An ethanolic extract of green Brazilian propolis exerted intense antioxidant activity and considerably inhibited the production of ROS, RNS, NO, cytokine IL-1 α, IL-1 β, IL-4, IL-6, IL-12p40, IL-13, TNF-α, G-CSF, GM-CSF, MCP-1, MIP-1 α, MIP-1 β, and RANTES in stimulated J774A.1 macrophages. | [9] |
| Green Brazilian Propolis | Polyphenols (e.g., flavonoids), Cinnamic acids, Triterpenes | Mouse aortic endothelial cells | 5, 10 and 20 μg/mL | An ethanolic extract of green Brazilian propolis showed strong anti-inflammatory effects by inhibiting the levels of TNF-α and IL-6 cytokines and protected MAECs through regulation of the expression of ICAM-1, VCAM-1 and MCP-1. | [73] |
| Green Brazilian Propolis | Polyphenols (e.g., flavonoids), Caffeic acid, p-Coumaric acid, 3,5-Dicafeoyl quinic, 4,5-DCQ, Aromadendrin-4-O-methyl-ether, Drupanin, Artepillin C and Baccharin | Bone marrow cell precursors, naive T cells (CD4+CD44−CD62L+) [C57BL/6 naive female mice] | 50 μg/mL | An ethanolic extract of green Brazilian propolis increased PMN-MDSC and Treg cells differentiation in vitro, which indicates that leukocytes decrease asthma associated with Th2 inflammation. The propolis-induced anti-inflammatory effect depends on suppressor myeloid cells and regulatory T cells. | [74] |
| South Brazilian Propolis | Phenolic acids (gallic acid, caffeic acid, and coumaric acid), Prenylated derivative of cinnamic acid (artepillin C), Flavonoids (pinocembrin) | RAW 264.7 macrophages | 0.1, 1, 10, and 100 μg/mL | An ethanolic extract of Brazilian propolis showed anti-inflammatory activity by downregulating NF-kB activation and TNF-α release in RAW 264.7 macrophages, and regulated the expression of a NF-κB-luciferase reporter gene. | [75] |
| Type of Propolis | Class of Compounds | Organism | Dose | N | Exposure Time | Activity/Mechanism/Effect | Ref. |
|---|---|---|---|---|---|---|---|
| Brown Brazilian Propolis | - | Female Wistar rats | Hydroalcoholic extract of Araucaria Brazilian Propolis (HEABP) (30, 100, and 300 mg/kg b.w.) | 5 groups of 6 rats + 1 naive group of 6 rats | 5 days | The extract of brown Brazilian propolis at doses of 100 and 300 mg/kg prevented TNBS-induced colon damage, prevented GSH depletion, reduced MDA, and restored antioxidant enzyme activity to levels found in the colons of healthy animals. HEBPA can be used in mitigating TNBS-induced colitis in rats. | [76] |
| Brown Brazilian Propolis | Phenolic compounds | Female adult BALB/c mice | Brown Brazilian hydroalcoholic propolis extract (HPE) (50 mg/kg b.w.) | Control group (n = 4), HCV-2-infected (n = 8), HPE-treated (n = 5), HPE-treated and HSV-2 infected mice (n = 5) | 10 days | The hydroalcoholic extract of brown Brazilian propolis treatment reduced extravaginal lesions and the histological damage caused by HSV-2 infection in vaginal tissues of animals. HPE had a protective effect on HSV-2 infected animals by acting on inflammatory and oxidative processes | [77] |
| Red Brazilian Propolis | Phenolic compounds (liquiritigenin, formononetin, vestitol, neovestitol, medicarpin, 7-O-methylvestitol, and guttiferone E, xanthochymol, and oblongifolin B) | Male Swiss mice (Mus musculus), Wistar Hannover rats (Rattus norvegicus) | Red Brazilian propolis hydroalcoholic extract (12, 24, and 48 mg/kg b.w.) | 5 groups of 5 animals | - | The red propolis hydroalcoholic extract exhibited a chemoprotective effect in vivo on DXR-induced genomic instability and on DMH-induced colon carcinogenesis. This effect is related to the anti-inflammatory and antiproliferative activities of red propolis. | [78] |
| Red Brazilian Propolis | - | SPF (specific pathogen free) BALB/c mice | Red Brazilian propolis alcoholic extract (1, 3 and 10 mg/kg b.w.) | Groups of 5 animals | 4 h | The Brazilian red propolis extract is a promising anti-inflammatory natural product whose mechanism seems to act by reducing leukocyte rolling and adhesion; TNF-α, IL-1β, CXCL1/KC and CXCL2/MIP-2 release; and CXCL2/MIP-2-induced chemotaxis and calcium influx. | [69] |
| Red Brazilian Propolis | Dadzein, Formononetin, Biochanin A | Male Wistar rats | Hydroalcoholic extract (HERP) [x] (topical treatment) | 7 groups of 5 animals | 6 days | Application of HERP (3.5%) suppressed the clinical signs of inflammation (erythema). HERP has photoprotective activity in a murine model and the mechanisms of protection can be related to the antioxidant and anti-inflammatory characteristics of HERP. | [79] |
| Red Brazilian Propolis | Flavanones, Isoflavones, Isoflavanes, Polyprenylated acylphloroglucinols | Male Wistar rats | Hydroalcoholic extract (HERP) (100 mg/kg b.w.) | Groups of 6–8 animals | Twice a day for 7 days | The hydroalcoholic extract of red Brazilian propolis displayed a gastric healing effect via reducing oxidative stress and inflammation. Treatment with HERP reduced MPO activity. | [80] |
| Red Brazilian Propolis | Male Wistar rats | Brazilian red propolis hydroalcoholic solution at 1%, paste containing Brazilian red propolis at 1% | Three experimental groups of six animals | No significant differences in IL-10 or TNF-α levels between collagenase and propolis treatments were observed, suggesting that propolis might act as an anti-inflammatory by maintaining adequate levels of both cytokines, such as collagenase. | [30] | ||
| Green Brazilian Propolis | - | Male Wistar rats | Green propolis extract in the form of a powder (500 mg/kg BW, diluted in 5 mL of saline) | Three survival study groups, control group, cecal ligation and puncture groups, metabolic cage study groups | - | The extract reduced inflammation and decreased oxidative stress. It also reduced mortality and protected against the inflammatory response in the kidneys and lungs. That protection might be regulated by decreased expression of the TLR4/NF-κB pathway and consequent attenuation of the inflammatory process. | [81] |
| Green Brazilian Propolis | Phenolics (e.g., flavonoids) | Male C57BL/6J mice | 0.1 mL of green Brazilian propolis ethanolic extract in 0.5% CMC-Na | Control group (n = 8) LPS group (n = 8) LPS + Chinese propolis (n = 12) LPS + green Brazilian propolis (n = 12) | 3 days | Prophylactic administration of the green Brazilian propolis ethanol extract reduced the LPS-induced expression of TNF-α, IL-6 and IL-1β, which means it reduced inflammation. | [25] |
| Green Brazilian Propolis | - | Male Wistar/ST rats | Ethanolic extract of green Brazilian propolis (EEGB) 291 mg/kg per day | Control group (4 animals) EEGBP group (5 animals) | 7 days | Administration of 0.3% EEBGP in the diet for 7 days reduced centrilobular hepatocellular necrosis with inflammatory cell infiltration induced by oral administration of APAP (800 mg/kg) and significantly reduced the area of necrosis. | [82] |
| Green Brazilian Propolis | Polyphenolic compounds | Male Sprague-Dawley rats | 300 mg/kg b.w. | 4 groups of 8 animals | 17 days | BP significantly reduced the colitis disease activity index, prevented significant DSS-induced colonic tissue damage and increased resistance to DSS-induced colonic oxidative stress. | [21] |
| Green Brazilian Propolis | p-Coumaric acid, Caffeic acid, Cinnamic, Aromadendrin, Isosakuranetin, Artepillin C | Swiss and BALB/c mice | 5 mg/kg b.w. Directly extracted or obtained from the concentrated and alkaline hydrolysis of the standardized propolis extract (EPP-AF) solubilized in purified water. | 6 groups of 6 animals | 6 days | The extract has anti-inflammatory properties via inhibition of proinflammatory cytokines and increasing anti-inflammatory cytokines, preventing amplification of the inflammatory process in the pulmonary site, suggesting an immunomodulatory activity. | [83] |
| Type of Propolis | Class of Compounds | Cell Lines | Concentration/ Ic50 | Activity/Mechanism/Effect | Ref |
|---|---|---|---|---|---|
| Hydroalcoholic extract of brown propolis | Gallic acid, catechin, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, cinnamic acid, crisin | HuH7.5, A549, LLC-MK2 | 0.25, 0.5, 1.0, 2.5, 5.0, 10, 25, 50, and 100 μg/mL | Hydroalcoholic extract of brown propolis shows selective cytotoxic and antiproliferative activity against human liver carcinoma cells with no cytotoxic effect on normal cells. | [94] |
| Ethanolic extract of green propolis | - | A549 | 6.25, 12.5, 25, 50, and 100 μg/mL Ic50—69.17 ± 11.28 μg/mL | Propolis suppressed the proliferation of human lung cancer cells by inducing apoptosis via the intrinsic pathway. The expression of Bcl-XL decreased while Bax and Noxa increased. The upregulation of p21 without p53 was also observed. | [87] |
| Hydroalcoholic extract of red propolis and fractions | - | Hep-2 | 5–175 μg/mL Ic50 24 h: Hydroalcoholic red propolis: J fraction: 60.96 ± 4.06 L fraction: 74.60 ± 2.39 Ic50 48 h: Hydroalcoholic red propolis: 57.54 ± 0.98 J fraction: 30.71 ± 3.54 L fraction: 43.73 ± 2.84 | Fractions induced apoptosis in Hep-2 cells. Many cells were found in the sub-G1 apoptotic phase. | [95] |
| Ethanolic extract of red propolis | Liquiritigenin, calycosin, formononetin, isoliquiritigenin, medicarpin, vestitol, neovestitol, 7-O-methylvestitol, oblongifolin B, guttiferone E, xanthochymol | LNCaP, PC-3 | 1.0, 2.5, 5.0, 10, 20, 25, 50, 75, and 100 μg/mL Ic50 (PC-3 cell line): 53.0 μg/mL | The extract of Brazilian red propolis showed cytotoxic activity against prostate cancer cells (especially against the LNCaP cell line). | [86] |
| Ethanolic extract of green propolis | Caffeic acid, dihydrocinnamic acid, benzoic acid, flavonoids, triterpenes, prenyl-p-coumaric acid, artepillin C | MCF-7 | 25, 50, 75, and 100 μg/mL | Green propolis extract, in combination with DTX (docetaxel), showed cytotoxic activity against breast cancer cells. It also decreased MCF-7 cell migration. | [96] |
| Ethyl acetate extract of red propolis | Methoxyeugenol, cis-asarone, trans isoelemicin, (2S)-7 hydroxyflavanone, chrysin, liquiritigenin, formononetin, medicarpin, vestitol, isovestitol, biochanin A, homopterocarpin, (3S) vestitone, 7-O-methylvestitol, (3S) violanone, guttiferone E, xanthochymol | HT-29, HCT-116, Vero cells | 5–150 μg/mL Ic50: Ht-29: 75.15 ± 3.35 HCT-116: 70.81 ± 4.18 Vero: 68.52 ± 4.72 | Red propolis extracts and its fractions showed cytotoxic activity against colon cancer cell lines. | [97] |
| Ethanolic extract of red propolis | Phlobaphene tannins, flavones, flavonols, xanthones, chalcones, aurones, catechins, triterpenoids | SF-295, OVCAR-8, HCT-116 | 0.09, 0.19, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25, and 50 μg/mL Ic50: SF-295: 34.27 μg/mL OVCAR-8: 28.76 μg/mL HCT-116: 25.26 μg/mL | At higher concentrations, the extract showed high cytotoxicity against human tumour cell lines. | [98] |
| Hydroalcoholic extracts of red and green propolis | - | HT-29 | 6.25, 12.5, 25, 50, and 100 μg/mL | The combination of propolis extract and 5-FU (5-fluorouracil) enhanced the cytotoxic effect, especially at higher concentrations. The combination of 5-FU and green propolis showed higher cytotoxicity at all concentrations compared to green propolis alone. For the red propolis extract, the effect was promising, especially in the case of the 100 μg/mL concentration. | [99] |
| Supercritical extract of green propolis | Artepillin C | HT1080, A549, U2OS | 0.01%, 0.1%, 0.5%, and 1% Ic50: 0.2–0.5% | The green propolis extract, containing 9.6% artepillin C, showed high cytotoxicity against cancer cell lines. Green propolis showed anti-migratory activity. | [100] |
| Hexane-based extract of red propolis | - | HCT-116, PC3 | 1.56, 3.13, 6.25, 12.5, 25, 50, and 100 μg/mL Ic50 HCT-116: 31.53 μg/mL | Most extracts showed moderate cytotoxic activity. An extract obtained at 70 °C in one 10-min cycle exhibited the strongest cytotoxic effect against tumour cell lines. | [101] |
| Supercritical extracts of red, green and brown propolis | Artepillin C, p-coumaric acid | B16F10 | 50 and 100 μg/mL | All the extracts showed significant inhibition of cell proliferation. The best results were observed in red propolis extracts from northeastern regions of Brazil. | [41] |
| Type of Propolis | Class of Compounds | Organism | Dose | N | Exposure Time | Activity/Mechanism/Effect | Ref |
|---|---|---|---|---|---|---|---|
| Ethanolic extract of green propolis | p-Coumaric acid, artepillin C, baccharin, drupanin | Wistar Hannover rats | 0.5% and 2.5% | Six groups of 50 rats; 150 female and 150 male rats | 104 weeks | The incidents of pituitary tumours were significantly lower than in the control group. Also, malignant lymphoma/leukaemia were significantly decreased in both male and female rats receiving treatment. Green propolis extract showed antitumourigenic effects on rats. | [102] |
| Hydroalcoholic extract of red propolis | Daidzein, formononetin, biochanin A, propyl gallate, catechin, epicatechin | Male Swiss mice | 10, 50, and 100 mg/kg | Six groups of five mice | 26 weeks | The progression of DMBA-induced cancer was retarded by red propolis extract. Red propolis showed chemopreventive activity. | [103] |
| Alcoholic extract of brown propolis | - | Male Wistar rats | 80 mg/kg | Ten groups of five rats | 16 weeks | Brown propolis, due to its antioxidant capacity, inactivated reactive nitrogen and oxygen species, which modified colon carcinogenesis in rats. Also, brown propolis prevented crypt cell clonal expansion. | [104] |
| Supercritical extract of green propolis | Artepillin C | BALB/c nude mice | 100 mg/kg | Three groups of three mice | 3 weeks | The complex of green propolis and γ cyclodextrin displayed antitumour activity in mice. | [100] |
| Type of Propolis | Nanoparticles | Dose | Disease Entity/Type of Cells | Activity/Mechanism/Effect | Ref. |
|---|---|---|---|---|---|
| Red Brazilian Propolis | Polymeric nanoparticles (nanocapsuled Brazilian red propolis extract NC-BRPE) | 66–320 µg/mL | OVCAR-3 (ovarian cancer) | NC-BRPE enhanced the antitumour activity of propolis in ovarian cancer cells, both in 2D and 3D models. | [112] |
| Red Brazilian Propolis | Gold nanoparticles | 200 μg/mL | Bladder cancer cells (T24) and prostate cancer cells (PC-3) | The cytotoxicity of nanoparticles with the hydroethanolic extract of Brazilian red propolis was induced by mechanisms associated with apoptosis. | [113] |
| Red Brazilian Propolis | Polymeric nanoparticles (nanocapsuled Brazilian red propolis extract NCBRPE) | 2–1024 mg/L (2–1024 μg/mL) | OVCAR-3 cells | NCBRPE effectively limited the proliferation of cancer cells with long-term survival capacity. The antioxidant properties of NCBRPE were demonstrated by its ability to reduce ROS generation in neutrophils, thereby mitigating the oxidative stress that is commonly associated with tumorigenesis and immune suppression. | [19] |
| Red Brazilian Propolis | Gold nanoparticles (BRP-AuNPs) | BRP-AuNPs IC50 values were 27.32 µg/mL (RT4) and 53 µg/mL (PC3) | PC3 (prostate cancer) and RT4 (bladder cancer) cell lines | BRP-AuNPs exhibited a dose-dependent antitumour effect. In 3D viability assays, a superior antitumour effect of BRP-AuNPs was found when compared to free BRP extract. | [114] |
| Red Brazilian Propolis | Polymeric nanoparticles | - | Normal breast cells (MCF-10) Breast cancer cells (MCF-7) | Nanoparticle-encapsulated BRPE (NCBRPE) showed reduced toxicity to normal breast cells (MCF-10) but increased toxicity to breast cancer cells (MCF-7) compared to free BRPE, which indicates targeted therapeutic potential. | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, T.; Sikora, J.; Śpiewak, I.; Kowalski, M.; Wieczfińska, J.; Brčić Karačonji, I.; Kolska, M.; Sitarek, P. Brazilian Propolis: Nature’s Liquid Gold with Anti-Inflammatory and Anticancer Potential. Appl. Sci. 2025, 15, 5994. https://doi.org/10.3390/app15115994
Kowalczyk T, Sikora J, Śpiewak I, Kowalski M, Wieczfińska J, Brčić Karačonji I, Kolska M, Sitarek P. Brazilian Propolis: Nature’s Liquid Gold with Anti-Inflammatory and Anticancer Potential. Applied Sciences. 2025; 15(11):5994. https://doi.org/10.3390/app15115994
Chicago/Turabian StyleKowalczyk, Tomasz, Joanna Sikora, Igor Śpiewak, Maciej Kowalski, Joanna Wieczfińska, Irena Brčić Karačonji, Monika Kolska, and Przemysław Sitarek. 2025. "Brazilian Propolis: Nature’s Liquid Gold with Anti-Inflammatory and Anticancer Potential" Applied Sciences 15, no. 11: 5994. https://doi.org/10.3390/app15115994
APA StyleKowalczyk, T., Sikora, J., Śpiewak, I., Kowalski, M., Wieczfińska, J., Brčić Karačonji, I., Kolska, M., & Sitarek, P. (2025). Brazilian Propolis: Nature’s Liquid Gold with Anti-Inflammatory and Anticancer Potential. Applied Sciences, 15(11), 5994. https://doi.org/10.3390/app15115994










