Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach
Abstract
1. Introduction
2. Materials and Methods
- The study evaluated antioxidant potential using spectrophotometric assays;
- The samples analyzed were derived from plant-based foods.
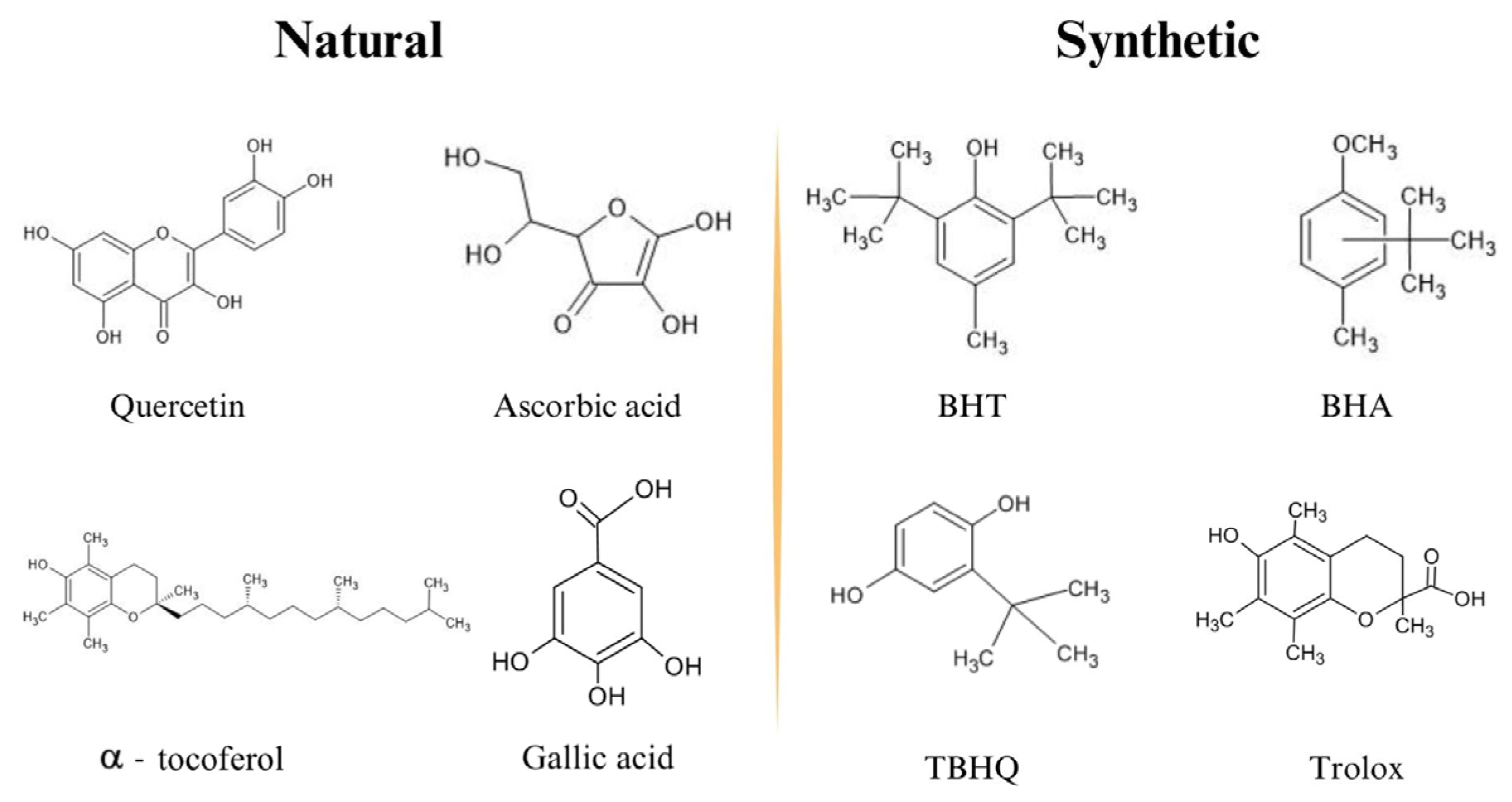
3. Plant-Derived Antioxidants in In Vitro Assays
4. Electron Transfer-Based Assays
4.1. Ferric-Reducing Antioxidant Power
4.1.1. Working Principle of FRAP Assay
4.1.2. A Comparative Analysis of Methodological Approaches in Studies on Plant Materials
4.2. Cupric Reducing Antioxidant Capacity (CUPRAC)
4.2.1. Working Principle of CUPRAC Assay
4.2.2. Comparison of Methodological Approaches in Previous Studies of Plant Materials
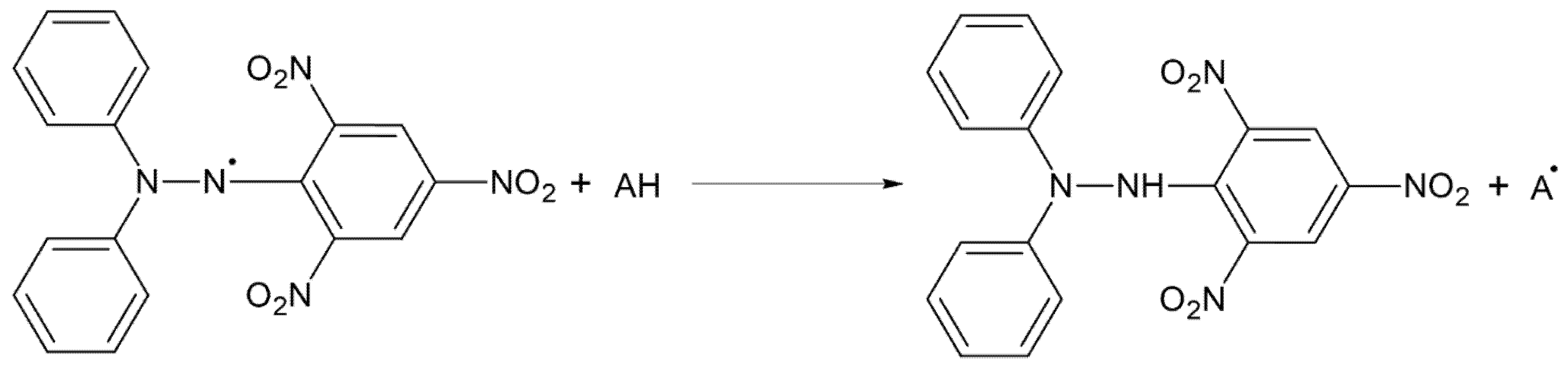
4.3. DPPH
4.3.1. Operating Principle
4.3.2. Comparison of Methodological Approaches Used in Previous Studies of Plant Materials
| Test Material | Standard | Substrates | Concentration of DPPH Working Solution | Type and Time of Incubation | Units | Wavelength | Source |
|---|---|---|---|---|---|---|---|
| Beetroot | Nd | 3.9 mL DPPH, 0.1 mL of extract | 60 μM | 30 min, darkness, 37 °C | % | 515 | [76] |
| Carrot-orange juice | Nd | 2 mL of juice sample, 2 mL DPPH | 60 μM | 30 min, darkness, room temperature | % | 517 | [78] |
| Edible roots | BHT | 2.5 mL DPPH, | 100 μM | 30 min | %, IC50 | 517 | [77] |
| Carrot | TBHQ | 2 mL DPPH, 0.5 mL of extract | 1.5 mM | 30 min, darkness, room temperature | %, IC50 | 517 | [79] |
| Cocoa beans | Trolox | 3.9 mL DPPH, 0.1 mL extract | 60 μM | 30 min | TEC | 515 | [80] |
| Grape | Ascorbic acid | 2.97 mL DPPH, 0.03 mL of extract | 15 μM | 4 h, room temperature | AAE ascorbic acid eq | 515 | [81] |
| Garlic | Ascorbic acid | 0.5 mL extract, 0.5 mL DPPH | 250 μM | 30 min, darkness, room temperature | AAE | 517 | [82] |
| Grape | Trolox | 0.01 mL extract, 3 mL DPPH | 150 μM | 60 min, darkness, room temperature | TE | 517 | [83] |
| Tea fruit | BHT | 1 mL extract, 3 mL DPPH | 100 μM | 30 min, 30 °C, darkness | %, IC50 | 517 | [84] |
| Wine | Trolox | 0.075 mL extract, 1.425 mL DPPH | 100 μM | 60 min, darkness, room temp | TE | 515 | [49] |
| Tea | Ascorbic acid | 1 mL extract, 1 mL DPPH | 200 μM | 30 min | % | 517 | [85] |
| Tea | Quercetin, EGCG | 0.1 mL extract, 2.4 mL DPPH | 60 μM | 30 min, darkness | % | 516, 525 | [86] |
| Tomato juice and carrot juice | α-tocopherol | 1.0 mL extract, 0.5 mL DPPH | 300 μM | 15 min | mol α-TE/mol | 540 | [86] |
| Chlorella vulgaris | Nd | 25 μL of extract, 975 μL DPPH | 60 μM | 30 min, darkness | %, IC50 | 517 | [87] |
4.4. ABTS+ Assay
4.4.1. Mechanism of Action
4.4.2. Comparison of Methodological Approaches in Previous Research on Plant Materials
4.5. Folin–Ciocalteu Method
4.5.1. Principle of the Method
4.5.2. Comparison of Analytical Approaches Plant-Based Studies
5. Hydrogen Atom Transfer-Based Assays
5.1. Oxygen Radical Absorbance Capacity (ORAC)
5.1.1. Method Principle
5.1.2. Comparative Analysis of Methodological Approaches in Previous Studies on Plant Materials
6. Summary and Future Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta 2017, 1863, 1066. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Huang, M.Z.; Li, J.Y. Physiological Regulation of Reactive Oxygen Species in Organisms Based on Their Physicochemical Properties. Acta Physiol. 2020, 228, e13351. [Google Scholar] [CrossRef]
- Tan, Y.; Duan, Y.; Chi, Q.; Wang, R.; Yin, Y.; Cui, D.; Li, S.; Wang, A.; Ma, R.; Li, B.; et al. The Role of Reactive Oxygen Species in Plant Response to Radiation. Int. J. Mol. Sci. 2023, 24, 3346. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Cardoso, M.A.; Gonçalves, H.M.R.; Davis, F. Reactive Oxygen Species in Biological Media Are They Friend or Foe? Major In Vivo and In Vitro Sensing Challenges. Talanta 2023, 260, 124648. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of Natural Antioxidants in Neurodegenerative Disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Podsedek, A. Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review. LWT–Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Rasines-Perea, Z.; Teissedre, P.L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Olasunkanmi, A.A.; Fadahunsi, O.S.; Adegbola, P.I. Gas Chromatography-Mass Spectroscopic, High Performance Liquid Chromatographic and In-Silico Characterization of Antimicrobial and Antioxidant Constituents of Rhus Longipes (Engl). Arab. J. Chem. 2022, 15, 103601. [Google Scholar] [CrossRef]
- Meng, M.; Wan, H.; Bao, Y.; He, Y.; Li, C.; Wan, H. Rapid Identification, Quantitation, and Antioxidant Activity Evaluation of the Components in Guanxin Shutong Capsule with Liquid Chromatography and Mass Spectrometry. J. Pharm. Biomed. Anal. 2023, 224, 115194. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound Assisted Extraction of Selected Edible Macroalgae: Effect on Antioxidant Activity and Quantitative Assessment of Polyphenols by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Luo, J.; Liang, L.; Xie, Q.; Qiu, Y.; Jiang, S.; Yang, Y.; Zhu, L.; Fu, Y.; Chen, S.; Wang, W.; et al. Differential Analysis of Phytochemistry and Antioxidant Activity in Five Citrus By-Products Based on Chromatography, Mass Spectrometry, and Spectrum-Effect Relationships. Food Chem. X 2023, 20, 101010. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Jimoh, M.A.; Kerebba, N.; Bakare, O.O.; Senjobi, C.T.; Saheed, S.A.; Kadye, R.; Prinsloo, E.; Laubscher, C.P. Liquid Chromatography–Mass Spectrometry and XCELLigence Real Time Cell Analyzer Revealed Anticancer and Antioxidant Metabolites in Trianthema portulacastrum L. (Aizoaceae). Phytomed. Plus 2024, 4, 100550. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Shao, Q.; Li, Z.; Chun, Z.; Wang, Y.; Zhou, Y.; Chen, R. Screening, Fingerprinting, and Identification of Phenolic Antioxidants in Persicaria chinensis (L.) H. Gross by Liquid Chromatography–Electrochemical Detection and Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2025, 1250, 124387. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Reprint of “Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.B.L.; Lim, Y.Y. Critical Analysis of Current Methods for Assessing the in Vitro Antioxidant and Antibacterial Activity of Plant Extracts. Food Chem. 2015, 172, 814–822. [Google Scholar] [CrossRef]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an Essential Micronutrient for Human Health: Common, Novel, and Unexplored Dietary Sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- See, X.Z.; Yeo, W.S.; Saptoro, A. A Comprehensive Review and Recent Advances of Vitamin C: Overview, Functions, Sources, Applications, Market Survey and Processes. Chem. Eng. Res. Des. 2024, 206, 108–129. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D.; Maramag, C.C.; Tengco, L.W.; Dolnikowski, G.G.; Blumberg, J.B.; Solon, F.S. Carotene-Rich Plant Foods Ingested with Minimal Dietary Fat Enhance the Total-Body Vitamin A Pool Size in Filipino Schoolchildren as Assessed by Stable-Isotope-Dilution Methodology. Am. J. Clin. Nutr. 2007, 85, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Charlton, N.C.; Mastyugin, M.; Török, B.; Török, M. Structural Features of Small Molecule Antioxidants and Strategic Modifications to Improve Potential Bioactivity. Molecules 2023, 28, 1057. [Google Scholar] [CrossRef] [PubMed]
- Igielska-Kalwat, J.; Gościańska, J.; Nowak, I. Carotenoids as Natural Antioxidants. Postep. Hig. Med. Dosw. 2015, 69, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Evolution of Food Antioxidants as a Core Topic of Food Science for a Century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive Peptides as Natural Antioxidants in Food Products—A Review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Du, B.; Xu, B. Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) of β-Glucans from Different Sources with Various Molecular Weight. Bioact. Carbohydr. Diet. Fibre 2014, 3, 11–16. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (ATEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Hayes, W.A.; Mills, D.S.; Neville, R.F.; Kiddie, J.; Collins, L.M. Determination of the Molar Extinction Coefficient for the Ferric Reducing/Antioxidant Power Assay. Anal. Biochem. 2011, 416, 202–205. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A. Antioxidant Activity of Water Soluble Vitamins in the TEAC (Trolox Equivalent Antioxidant Capacity) and the FRAP (Ferric Reducing Antioxidant Power) Assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Avelino, F. Ferric Reducing Antioxidant Power (FRAP). In Antioxidant Methods; Academic Press: New York, NY, USA, 2024; pp. 123–133. [Google Scholar] [CrossRef]
- van Wonderen, D.; Melse-Boonstra, A.; Gerdessen, J.C. Iron Bioavailability Should Be Considered When Modeling Omnivorous, Vegetarian, and Vegan Diets. J. Nutr. 2023, 153, 2125–2132. [Google Scholar] [CrossRef]
- Tomova, T.; Petkov, V.; Slavova, I.; Stoyanov, P.; Argirova, M. Naturally Present Metal Ions in Plants Could Interfere with Common Antioxidant Assays. MethodsX 2020, 7, 100995. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent Effects on the Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants Measured by CUPRAC, ABTS/Persulphate and FRAP Methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef]
- Samid, I.; Khouya, T.; Elbouny, H.; Sellam, K.; Alem, C. Quantification of Phytochemical and Antioxidant Properties of Aqueous Apple Extracts from the High Atlas Mountains in Morocco and Their Anti-Inflammatory Effect on Wistar Rats. Sci. Afr. 2023, 22, 01897. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant Activities of Peel, Pulp and Seed Fractions of Common Fruits as Determined by FRAP Assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, P.; Ma, W.; Zeng, X.A.; Fang, Z. Physicochemical Properties, Antioxidant Activities and Comprehensive Phenolic Profiles of Tea-Macerated Chardonnay Wine and Model Wine. Food Chem. 2024, 436, 137748. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Deng, H.; Yuan, H.; Fan, X.; Yang, H.; Tan, S. In Vitro and in Vivo Bioaccessibility, Antioxidant Activity, and Color of Red Radish Anthocyanins as Influenced by Different Drying Methods. Food Chem. X 2023, 18, 100633. [Google Scholar] [CrossRef]
- Adrar, N.; Gulsunoglu-Konuskan, Z.; Ceylan, F.D.; Capanoglu, E. Overview and Trends in Electrochemical Sensors, Biosensors and Cellular Antioxidant Assays for Oxidant and Antioxidant Determination in Food. Talanta 2025, 283, 127058. [Google Scholar] [CrossRef]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction Conditions Can Greatly Influence Antioxidant Capacity Assays in Plant Food Matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and Recovery of Phytochemical Components and Antioxidative Properties in Fruit Parts of Dacryodes Rostrata Influenced by Different Solvents. J. Food Sci. Technol. 2018, 55, 2523. [Google Scholar] [CrossRef]
- Orosz-Tóth, M.; Nemes-Kun, A.; Lowy, D.A.; Csihon, Á.; Sándor, Z.; Kincses, I.; Holb, I.J. Comparison and Intercorrelation of Extraction Methods for Polyphenol Content and Antioxidant Capacity of Scab-Resistant Apple Cultivars. Agronomy 2022, 12, 289. [Google Scholar] [CrossRef]
- Berlic, M.; Jug, U.; Battelino, T.; Levart, A.; Dimitrovska, I.; Albreht, A.; Korošec, M. Antioxidant-Rich Foods and Nutritional Value in Daily Kindergarten Menu: A Randomized Controlled Evaluation Executed in Slovenia. Food Chem. 2023, 404, 134566. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, J.; Hua, M.Z.; Lu, X. Rapid Determination of Total Phenolic Content and Antioxidant Capacity of Maple Syrup Using Raman Spectroscopy and Deep Learning. Food Chem. 2025, 463, 141289. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.; Gomez, M.; Frontana, C. Electrochemical Method to Quantify Antioxidants Employing Cupric Reducing Antioxidant Capacity, CUPRAC. Procedia Chem. 2014, 12, 62–65. [Google Scholar] [CrossRef][Green Version]
- Özyürek, M.; Güçlü, K.; Apak, R. The Main and Modified CUPRAC Methods of Antioxidant Measurement. Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Emu, S.A.; Dulal, M.A.; Kali, T.D.; Chadni, M.S.; Rasul, M.G.; Mondal, M.N.; Ahsan, M.E.; Khan, M.; Shah, A.K.M.A. Effects of Extracting Solvents on Phytochemical, Antioxidant, and Antibacterial Activity of Some Seaweeds from the Bay of Bengal Offshore Island. Food Humanit. 2023, 1, 1157–1166. [Google Scholar] [CrossRef]
- Granato, D.; Fidelis, M.; Haapakoski, M.; dos Santos Lima, A.; Viil, J.; Hellström, J.; Rätsep, R.; Kaldmäe, H.; Bleive, U.; Azevedo, L.; et al. Enzyme-Assisted Extraction of Anthocyanins and Other Phenolic Compounds from Blackcurrant (Ribes nigrum L.) Press Cake: From Processing to Bioactivities. Food Chem. 2022, 391, 133240. [Google Scholar] [CrossRef] [PubMed]
- Krylova, E.; Gavrilenko, N.; Saranchina, N.; Gavrilenko, M. Novel Colorimetric Sensor for Cupric Reducing Antioxidant Capacity (CUPRAC) Measurement. Procedia Eng. 2016, 168, 355–358. [Google Scholar] [CrossRef]
- Özyürek, M.; Bektaşoǧlu, B.; Güçlü, K.; Güngör, N.; Apak, R. Simultaneous Total Antioxidant Capacity Assay of Lipophilic and Hydrophilic Antioxidants in the Same Acetone–Water Solution Containing 2% Methyl-β-Cyclodextrin Using the Cupric Reducing Antioxidant Capacity (CUPRAC) Method. Anal. Chim. Acta 2008, 630, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Güçlü, K.; Altun, M.; Özyürek, M.; Karademir, S.E.; Apak, R. Antioxidant Capacity of Fresh, Sun- and Sulphited-Dried Malatya Apricot (Prunus Armeniaca) Assayed by CUPRAC, ABTS/TEAC and Folin Methods. Int. J. Food Sci. Technol. 2006, 41, 76–85. [Google Scholar] [CrossRef]
- Bayrakçeken Güven, Z.; Alshehri, O.; Yüce, N.; Bakan, E.; Demirci, B.; Yilmaz, M.A.; Ertas, A.; Basaran, A.A. Chemical Composition, Nutritional Values, Elemental Analysis and Biological Properties of Prunus Mahaleb L.: From Waste to New Potential Sources for Food, Cosmetic and Drug Industry. Food Biosci. 2023, 53, 102632. [Google Scholar] [CrossRef]
- Puangbanlang, C.; Sirivibulkovit, K.; Nacapricha, D.; Sameenoi, Y. A Paper-Based Device for Simultaneous Determination of Antioxidant Activity and Total Phenolic Content in Food Samples. Talanta 2019, 198, 542–549. [Google Scholar] [CrossRef]
- Brzezińska-Rojek, J.; Sagatovych, S.; Malinowska, P.; Gadaj, K.; Prokopowicz, M.; Grembecka, M. Antioxidant Capacity, Nitrite and Nitrate Content in Beetroot-Based Dietary Supplements. Foods 2023, 12, 1017. [Google Scholar] [CrossRef]
- Knez, E.; Hałasa, R.; Turecka, K.; Ośko, J.; Kadac-Czapska, K.; Waleron, K.; Grembecka, M. Microbiological Safety and Health Properties of Marketed Fermented Root Vegetables. Appl. Sci. 2024, 15, 121. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- de Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; da Rosa, M.B. A Critical Examination of the DPPH Method: Mistakes and Inconsistencies in Stoichiometry and IC50 Determination by UV–Vis Spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Mota, J.C.; Almeida, P.P.; Freitas, M.Q.; Stockler-Pinto, M.B.; Guimarães, J.T. Far from Being a Simple Question: The Complexity between in Vitro and in Vivo Responses from Nutrients and Bioactive Compounds with Antioxidant Potential. Food Chem. 2023, 402, 134351. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lin, L.; Su, G.; Zhao, Q.; Zhao, M. Pitfalls of Using 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Assay to Assess the Radical Scavenging Activity of Peptides: Its Susceptibility to Interference and Low Reactivity towards Peptides. Food Res. Int. 2015, 76, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Ghanbarzadeh, B.; Hajibonabi, F.; Hojabri, Z.; Ganbarov, K.; Kafil, H.S.; Hamishehkar, H.; Yousefi, M.; Mokarram, R.R.; Kamounah, F.S.; et al. Turmeric Extract Loaded Nanoliposome as a Potential Antioxidant and Antimicrobial Nanocarrier for Food Applications. Food Biosci. 2019, 29, 110–117. [Google Scholar] [CrossRef]
- Ravichandran, K.; Ahmed, A.R.; Knorr, D.; Smetanska, I. The Effect of Different Processing Methods on Phenolic Acid Content and Antioxidant Activity of Red Beet. Food Res. Int. 2012, 48, 16–20. [Google Scholar] [CrossRef]
- Moulick, S.P.; Jahan, F.; Islam, M.B.; Bashera, M.A.; Hasan, M.S.; Islam, M.J.; Ahmed, S.; Karmakar, D.; Ahmed, F.; Saha, T.; et al. Nutritional Characteristics and Antiradical Activity of Turmeric (Curcuma longa L.), Beetroot (Beta vulgaris L.), and Carrot (Daucus carota L.) Grown in Bangladesh. Heliyon 2023, 9, e21495. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ma, T.; Tian, C.; Luo, J.; Zhou, R.; Sun, X.; Ma, J. Influence of Technical Processing Units on Polyphenols and Antioxidant Capacity of Carrot (Daucus carrot L.) Juice. Food Chem. 2013, 141, 1637–1644. [Google Scholar] [CrossRef]
- Azusena Castillejos-Mijangos, L.; Gabriela Meza-Márquez, O.; Osorio-Revilla, G.; Jiménez-Martínez, C.; Gallardo-Velázquez, T.; Massieu, W.; Cda Miguel Stampa, E.; Unidad Profesional Adolfo López Mateos, C.; Gustavo Madero, A.A.; de México, C.C.; et al. Identification of Variety and Prediction of Chemical Composition in Cocoa Beans (Theobroma cacao L.) by FT-MIR Spectroscopy and Chemometrics. Foods 2023, 12, 4144. [Google Scholar] [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic Profile and Antioxidant Activity of Green Extracts from Grape Pomace Skins and Seeds of Italian Cultivars. Foods 2023, 12, 3880. [Google Scholar] [CrossRef]
- Farhat, Z.; Scheving, T.; Aga, D.S.; Hershberger, P.A.; Freudenheim, J.L.; Hageman Blair, R.; Mammen, M.J.; Mu, L. Antioxidant and Antiproliferative Activities of Several Garlic Forms. Nutrients 2023, 15, 4099. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Viskelis, P.; Viskelis, J.; Bernatoniene, J. Citrus × Paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential. Nutrients 2023, 15, 1276. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Shao, S.; Qian, L.; Xu, P. Studies on Bioactivities of Tea (Camellia sinensis L.) Fruit Peel Extracts: Antioxidant Activity and Inhibitory Potential against α-Glucosidase and α-Amylase in Vitro. Ind. Crops Prod. 2012, 37, 520–526. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Wei, X. Antioxidant Activities Potential of Tea Polysaccharide Fractions Obtained by Ultra Filtration. Int. J. Biol. Macromol. 2012, 50, 558–564. [Google Scholar] [CrossRef]
- Pekal, A.; Pyrzynska, K. Effect of PH and Metal Ions on DPPH Radical Scavenging Activity of Tea. Int. J. Food Sci. Nutr. 2015, 66, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Tomassi, E.; Arouna, N.; Caruso, M.G.; Girgenti, A.; Picone, P.; Nuzzo, D.; Pucci, L. Fermentation of Chlorella Vulgaris and Aphanizomenon Flos-Aquae Biomass Improves the Antioxidant Profile. LWT 2025, 215, 117183. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Schaich, K.M. Effects of Molecular Structure on Kinetics and Dynamics of the Trolox Equivalent Antioxidant Capacity Assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. The Importance of Solvent Type in Estimating Antioxidant Properties of Phenolic Compounds by ABTS Assay. Eur. Food Res. Technol. 2013, 236, 1099–1105. [Google Scholar] [CrossRef]
- Ramírez-Anaya, J.D.P.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; De La Serrana, H.L.G. Phenols and the Antioxidant Capacity of Mediterranean Vegetables Prepared with Extra Virgin Olive Oil Using Different Domestic Cooking Techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Jastrzebski, Z.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Park, Y.S.; Heo, B.G.; Cho, J.Y.; Bae, J.H. Comparative Control of the Bioactivity of Some Frequently Consumed Vegetables Subjected to Different Processing Conditions. Food Control 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Siddhuraju, P. The Antioxidant Activity and Free Radical-Scavenging Capacity of Phenolics of Raw and Dry Heated Moth Bean (Vigna aconitifolia) (Jacq.) Marechal Seed Extracts. Food Chem. 2006, 99, 149–157. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Skibsted, L.H. Antioxidative Capacity of Rhizome Extract and Rhizome Knot Extract of Edible Lotus (Nelumbo nuficera). Food Chem. 2002, 76, 327–333. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Anthocyanins from Black Sorghum and Their Antioxidant Properties. Food Chem. 2005, 90, 293–301. [Google Scholar] [CrossRef]
- Larrosa, M.; Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A. Increase of Antioxidant Activity of Tomato Juice Upon Functionalisation with Vegetable Byproduct Extracts. LWT–Food Sci. Technol. 2002, 35, 532–542. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J.A. Phenolic Compounds and Antioxidant Activity of Spanish Commercial Grape Juices. J. Food Compos. Anal. 2015, 38, 19–26. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant Activity and Phenolic Content of Selected Fruit Seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic Acids, Flavonoids and Total Antioxidant Capacity of Selected Leafy Vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Di Matteo, A.; Lavorgna, M.; Russo, C.; Orlo, E.; Isidori, M. Natural Plant-Derived Terpenes: Antioxidant Activity and Antibacterial Properties against Foodborne Pathogens, Food Spoilage and Lactic Acid Bacteria. Appl. Food Res. 2024, 4, 100528. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Gutiérrez-González, J.A. A Comprehensive and Critical Review of the Unstandardized Folin-Ciocalteu Assay to Determine the Total Content of Polyphenols: The Conundrum of the Experimental Factors and Method Validation. Talanta 2024, 272, 125771. [Google Scholar] [CrossRef]
- Kaluza, W.Z.; McGrath, R.M.; Roberts, T.C.; Schroder, H.H. Separation of Phenolics of Sorghum bicolor (L.) Moench Grain. J. Agric. Food Chem. 1980, 28, 1191–1196. [Google Scholar] [CrossRef]
- Pękal, A.J. Wpływ Doboru Procedury Analitycznej Na Wyznaczanie Właściwości Antyutleniających Próbek Żywności; Uniwersytet Warszawski Wydział Chemii: Warszawa, Poland, 2014. [Google Scholar]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Prakash Jain, B.; Pandey, S.; Goswami, S.K. Protein Estimation by Folin-Ciocalteau (Lowry) Method. In Protocols in Biochemistry and Clinical Biochemistry; Academic Press: New York, NY, USA, 2025; pp. 93–95. [Google Scholar] [CrossRef]
- Insanu, M.; Nayaka, N.M.D.M.W.; Solihin, L.; Wirasutisna, K.R.; Pramastya, H.; Fidrianny, I. Antioxidant Activities and Phytochemicals of Polar, Semi-Polar, and Nonpolar Extracts of Used and Unused Parts of Carica Papaya Fruit. Biocatal. Agric. Biotechnol. 2022, 39, 102270. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielińska, M. Effect of the Solvent on the Extraction of Polyphenols from Distillery Stillage and on Their Antioxidant Activity. Acta Univ. Lodziensis. Folia Biol. Oecol. 2021, 17, 54–62. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, L.; Xu, Z.; Kong, Q.; Feng, S.; Chen, T.; Zhou, L.; Yang, H.; Xiao, Y.; Ding, C. Solvent Effects on the Phenolic Compounds and Antioxidant Activity Associated with Camellia Polyodonta Flower Extracts. ACS Omega 2024, 9, 27192–27203. [Google Scholar] [CrossRef] [PubMed]
- Elnour, A.A.; Mirghani, M.E.S.; Kabbashi, N.A.; Musa, K.H. Effect of Solvent Types on Phenolics Content and Antioxidant Activities of Acacia polyacantha gum. Int. Food Res. J. 2017, 24, 369–377. [Google Scholar]
- Ammar, A.F.; Baeshen, M.; Aqlan, F.M.; Siddeeg, A.; Chamba, M.V.M.; Afifi, M.; Almulaiky, Y.Q.; Alayafi, A. Solvent Effects on Antioxidant Activities and Phenolic Contents of the Alhydwan (Boerhavia Elegana Choisy) Seed Flour. J. Food Meas. Charact. 2018, 12, 2121–2127. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cheng, C.W.; Liang, J.Y. Effect of Esterification Condensation on the Folin–Ciocalteu Method for the Quantitative Measurement of Total Phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative Analysis of Strawberry Total Phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay Interference by Ascorbic Acid. J. Food Compos. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Effects of Extraction Solvents on Concentration and Antioxidant Activity of Black and Black Mate Tea Polyphenols Determined by Ferrous Tartrate and Folin–Ciocalteu Methods. Food Chem. 2006, 99, 835–841. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the Total Antioxidant Capacity and Total Polyphenol Content of 23 Commercially Available Vegetable Juices before and after in Vitro Digestion Measured by FRAP, DPPH, ABTS and Folin–Ciocalteu Methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Zhang, J.; Zhang, Y.T.; Sun, J.Y.; Prieto, M.A.; Simal-Gandara, J.; Putnik, P.; Li, N.Y.; Liu, C. The Link between the Phenolic Composition and the Antioxidant Activity in Different Small Berries: A Metabolomic Approach. LWT 2023, 182, 114853. [Google Scholar] [CrossRef]
- Shaghaghi, M.; Manzoori, J.L.; Jouyban, A. Determination of Total Phenols in Tea Infusions, Tomato and Apple Juice by Terbium Sensitized Fluorescence Method as an Alternative Approach to the Folin–Ciocalteu Spectrophotometric Method. Food Chem. 2008, 108, 695–701. [Google Scholar] [CrossRef]
- Alim, M.A.; Karim, A.; Shohan, M.A.R.; Sarker, S.C.; Khan, T.; Mondal, S.; Esrafil, M.; Linkon, K.M.M.R.; Rahman, M.N.; Akther, F.; et al. Study on Stability of Antioxidant Activity of Fresh, Pasteurized, and Commercial Fruit Juice during Refrigerated Storage. Food Humanit. 2023, 1, 1117–1124. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C.; Liu, L.; Xu, K.; Li, B.; Zhang, L.; Xu, H.; Lei, H. Antioxidant Activities and Volatile Compounds of Chinese Cabbage Sauce Prepared by the Combination of Lactobacillus Plantarum and Functional Oligosaccharides. Food Biosci. 2023, 54, 102854. [Google Scholar] [CrossRef]
- Parada, R.B.; Marguet, E.; Campos, C.; Vallejo, M. Improved Antioxidant Capacity of Three Brassica Vegetables by Two-Step Controlled Fermentation Using Isolated Autochthone Strains of the Genus leuconostoc spp. and Lactiplantibacillus spp. Food Chem Mol. Sci. 2023, 6, 100163. [Google Scholar] [CrossRef]
- Waweru, D.M.; Arimi, J.M.; Marete, E.; Jacquier, J.C.; Harbourne, N. Chemical and Antioxidant Characterization of Dovyalis Caffra and Dovyalis Abyssinica Fruits in Kenya. Heliyon 2022, 8, 11064. [Google Scholar] [CrossRef] [PubMed]
- Bani, C.; Peñas, E.; Baron, G.; Martínez-Villaluenga, C.; Mercogliano, F.; Aldini, G.; Piazza, S.; Di Lorenzo, C.; Restani, P. Characterization of the Phenolic Profile and in Vitro Antioxidant Potential of Different Varieties of Common Buckwheat (Fagopyrum esculentum Moench) and Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn.). LWT 2025, 215, 117261. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-Radical Absorbance Capacity Assay for Antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC): New Horizons in Relating Dietary Antioxidants/Bioactives and Health Benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Esfandi, R.; Willmore, W.G.; Tsopmo, A. Structural Characterization of Peroxyl Radical Oxidative Products of Antioxidant Peptides from Hydrolyzed Proteins. Heliyon 2024, 10, 30588. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods. In American Institute for Cancer Research Launch Conference; Nutrient Data Laboratory, Beltsville Human Nutrition Research Center: Beltsville, MD, USA, 2007. [Google Scholar]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Matencio, A.; Rodríguez-Bonilla, P.; García-Carmona, F.; López-Nicolás, J.M. The Use of Cyclodextrins as Solubility Enhancers in the ORAC Method May Cause Interference in the Measurement of Antioxidant Activity. Talanta 2022, 243, 123336. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Hayashi, S.; Ochiai, Y.; Shirakawa, H.; Wu, H.; Endo, H.; Yu, H. Modification of the Oxygen Radical Absorbance Capacity Assay and Its Application in Evaluating the Total Antioxidative State in Fish. Adv. Redox Res. 2022, 6, 100049. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Jullian, C.; Speisky, H.; Olea-Azar, C. Antioxidant Activity of Inclusion Complexes of Tea Catechins with β-Cyclodextrins by ORAC Assays. Food Res. Int. 2010, 43, 2039–2044. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Alarcón, E.; Campos, A.M.; Edwards, A.M.; Lissi, E.; López-Alarcón, C. Antioxidant Capacity of Herbal Infusions and Tea Extracts: A Comparison of ORAC-Fluorescein and ORAC-Pyrogallol Red Methodologies. Food Chem. 2008, 107, 1114–1119. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Ishimoto, H.; Tai, A.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Hatano, T.; Ito, H. Antioxidative Properties of Functional Polyphenols and Their Metabolites Assessed by an ORAC Assay. Biosci. Biotechnol. Biochem. 2012, 76, 395–399. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.; Hua, M.Z.; Singh, K.; Lu, X. Determination of Antioxidant Capacity and Phenolic Content of Haskap Berries (Lonicera caerulea L.) by Attenuated Total Reflectance-Fourier Transformed-Infrared Spectroscopy. Food Chem. 2025, 463, 141283. [Google Scholar] [CrossRef]
- Vargas, V.; Saldarriaga, S.; Sánchez, F.S.; Cuellar, L.N.; Paladines, G.M. Effects of the Spray-Drying Process Using Maltodextrin on Bioactive Compounds and Antioxidant Activity of the Pulp of the Tropical Fruit Açai (Euterpe oleracea Mart.). Heliyon 2024, 10, 33544. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, Antiradical, and Antimicrobial Activities of Polysaccharides Obtained by Microwave-Assisted Extraction Method: A Review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, M.; Radha; Lorenzo, J.M.; Sharma, D.; Puri, S.; Pundir, A.; Dhumal, S.; Bhuyan, D.J.; Jayanthy, G.; et al. Onion and Garlic Polysaccharides: A Review on Extraction, Characterization, Bioactivity, and Modifications. Int. J. Biol. Macromol. 2022, 219, 1047–1061. [Google Scholar] [CrossRef]
- SMPR, NEW AOAC. AOAC SMPR 2011.011 Standard Method Performance Requirements for in Vitro Determination of Total Antioxidant Activity in Foods, Beverages, Food Ingredients, and Dietary Supplements. J. AOAC Int. 2012, 95, 1557. [Google Scholar] [CrossRef] [PubMed]
- Kılınçer, M.; Çiçek, G.; Özyürek, M.; Apak, R. Uncertainty Estimation for Total Antioxidant Capacity Measurement of Apple Juice Using Main CUPRAC Method. J. Chem. Metrol. 2022, 16, 28–37. [Google Scholar] [CrossRef]
- Yeo, J.D.; Shahidi, F. Critical Re-Evaluation of DPPH Assay: Presence of Pigments Affects the Results. J. Agric. Food Chem. 2019, 67, 7526–7529. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the DPPH Assay for Evaluating the Antioxidant Capacity of Food Additives—Inter-Laboratory Evaluation Study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]


| Tested Material | Standard | Substrates | Type and Time of Incubation | Wavelength | Source |
|---|---|---|---|---|---|
| Various kinds of fruits | FeSO4 | 1.8 mL FRAP reagent (2.5 mL of a 10 mM TPTZ solution in 40 mM HCl, 2.5 mL of 20 mM FeCl3, 25 mL of 300 mM acetate buffer (pH = 3.6), 40 μL of tested sample, 0.2 mL MQ | 10 min, 37 °C | 593 | [48] |
| Apple | FeSO4 | 2 mL of FRAP reagent (10 mL of 10 mM TPTZ in HCl, 10 mL of 20 mM FeCl3, 100 mL of acetate buffer pH = 3.6), 50 μL of tested sample | 10 min, room temperature | 593 | [47] |
| Oat β-glucans | millimolar Fe2+ | 3 mL of FRAP reagent (1 mL of 20 mM FeCl3⋅6H2O, 1 mL of 10 mM TPTZ in 40 mM HCl, 10 mL of 300 mM acetate buffer, pH = 3.6), 0.1 mL of sample, 0.3 mL of MQ | 4 min, 37 °C | 593 | [38] |
| Wine | FeSO4⋅7H2O | 1 mL of FRAP reagent (1 mL of 20 mM FeCl3⋅6H2O, 1 mL of 10 mM TPTZ in 40 mM HCl, 10 mL of 300 mM acetate buffer, pH = 3.6), 50 μL of tested sample, 0.45 mL of MQ | 5 min, darkness, 37 °C | 593 | [49] |
| Radish | Ascorbic acid | 0.19 mL of FRAP reagent (2.5 mL of 20 mM FeCl3, 2.5 mL of 10 mM TPTZ, 2.5 mL of 10 mM acetate buffer pH = 3.6), 10 μL of tested sample | 10 min, 37 °C | 593 | [50] |
| Tomato juice and carrot juice | BHT | 0.6 mL of FRAP reagent (50 μL of 20 mM FeCl3, 50 μL of 10 mM TPTZ, 0.5 mL of 300 mM acetate buffer pH = 3.6), 100 μL of extract | 6 min, 25 °C | 595 | [39] |
| Various meals in kindergarten | Ascorbic acid | 3 mL of FRAP reagent (20 mM FeCl3, 10 mM TPTZ in 40 mM HCl, 300 mM acetate buffer pH = 3.6), 100 μL of extract | 10 min, room temperature | 593 | [56] |
| Maple syrup | FeSO4 | 210 μL of FRAP reagent (20 mM FeCl3, 10 mM TPTZ in 40 mM HCl, 300 mM acetate buffer pH = 3.6), 30 μL of extract | 8 min, 37 °C | 593 | [57] |
| Tested Material | Standard | Substrates | Type and Time of Incubation | Wavelength | Source |
|---|---|---|---|---|---|
| Synthetic mixture of plant antioxidants | Trolox | 1 mL of aqueous solution of CuCl2 (10 mM), 1 mL of ethanolic solution of neocuproine (7.5 mM), 1 mL of ammonium acetate buffer at pH = 7, 1.1 mL of tested sample | 20 min, 50 °C in water bath | 450 nm | [58] |
| Synthetic mixture of plant antioxidants/green tea extracts | Trolox | 1 mL of aqueous solution of CuCl2 (10 mM), 1 mL of ethanolic solution of neocuproine (7.5 mM), 1 mL of ammonium acetate buffer at pH = 7, 1.1 mL of tested sample | 30 min, room temperature | 450 nm | [46] |
| Blackcurrant | Ascorbic acid | 1 mL of aqueous solution of CuCl2 (10 mM), 1 mL of ethanolic solution of neocuproine (7.5 mM), 1 mL of ammonium acetate buffer at pH = 7, 1.1 mL of tested sample | 20 min, 50 °C in water bath | 450 nm | [62] |
| Beetroot | Trolox | 1 mL of aqueous solution of CuCl2 (10 mM), 1 mL of ethanolic solution of neocuproine (7.5 mM), 1 mL of ammonium acetate buffer at pH = 7, 1.1 mL of tested sample | 30 min, room temperature | 450 nm | [68] |
| Tea extracts | Ascorbic acid | 2 mL of 1 g/L CuSO4 · 5H2O, 2 mL of 0.25% solution of neocuproine in ethanol, 6 mL of H2O | Mixed in rotator for 20 min | 450 nm | [63] |
| Tea, wine, and fruit juices | Gallic acid | 5 μL of CuCl2 (150 mM), 5 μL of neocuproine (600 mML), 5 μL of ammonium acetate at pH = 7, 25 μL of examined sample | 30 min, room temperature | 450 nm | [67] |
| Apricot | Trolox | 1 mL of aqueous solution of CuCl2 (10 mM), 1 mL of ethanolic solution of neocuproine (7.5 mM), 1 mL of ammonium acetate buffer at pH = 7, 1.1 mL of tested sample | 30 min, room temperature | 450 nm | [65] |
| Tested Material | Standard | Substrates | Type and Time of Incubation | Wavelength | Source |
|---|---|---|---|---|---|
| Apricot | Trolox | 1 mL of 10% ABTS radical diluted in ethanol, 4 mL of ethanol, 0.1–0.5 mL of sample extract, | 6 min, room temperature | 734 nm | [65] |
| Various plant beverages | Gallic acid | 1.425 mL of ABTS working solution [equal volumes of ABTS solution (mM/L) and potassium persulfate solution (2.45 mM) were mixed, and after 16–20 h in the dark, it was diluted with water at the ratio 1:30], 0.075 mL of sample | 10 min, room temperature | 734 nm | [67] |
| Meals in kindergarten | Ascorbic acid | 3 mL of ABTS working solution [equal volumes of ABTS solution (7 mM) and potassium persulfate solution (2.45 mM) were mixed and after 16 h in the dark at 5 °C, it was diluted with water at the ratio 1:50], 0.1 mL of sample | 30 min in darkness, room temperature | 734 nm | [56] |
| Various fruits and vegetables | Ascorbic acid | 0.98 mL of ABTS working solution (equal volumes of: 2.5 mM of ABTS solution, 1 mM of 2,2-azobis(2-amidinopropane)dihydrochloride), and 10 mM PBS were mixed and incubated in a water bath at 68 °C for 40 min, then cooled to room temperature and diluted with PBS until an absorbance of 0.65 ± 0.02 was achieved | 10 min, 37 °C, water bath | 734 nm | [72] |
| Synthetic mixture of plant antioxidants | Trolox | 227 μL of ABTS working solution [equal volumes of ABTS solution (7 mM) and potassium persulfate solution (145 mM) were mixed, and after 16 h in the dark at room temperature, it was diluted with PBS until absorbance of 0.70 ± 0.02], 22.7 μL of sample | 6 min, room temperature | 734 nm | [104] |
| Tested Material | Standard | Substrates | Type and Time of Incubation | Wavelength | Source |
|---|---|---|---|---|---|
| Soybean, green tea extracts | Gallic acid | 0.25 mL of extract, 0.25 mL FCR and 4.5 mL 20% Na2CO3 | 25 min, room temperature | 730 nm | [115] |
| Strawberries | Gallic acid | 0.05 mL of extract, 0.43 mL MQ, 0.02 mL FCR, 0.05 mL 20% Na2CO3, 0.45 mL MQ | 60 min, room temperature | 725 nm | [116] |
| Black tea | Gallic acid | 1 mL of diluted extract, 1 mL of 3-fold-diluted FCR, 2 mL of 35% Na2CO3 | 30 min, room temperature | 700 nm | [117] |
| Vegetable juices | Gallic acid | 0.2 mL of extract, 1.5 mL of 10-fold-diluted FCR, 1.5 mL of 6% Na2CO3 | 90 min, room temperature | 725 nm | [118] |
| Berries | Gallic acid | 0.5 mL of extract, 2.5 mL of FCR, 2 mL 7.5% Na2CO3 | 60 min, room temperature | 765 nm | [119] |
| Tomato and apple juices | Quercetin | 0.5 mL of extract, 2.4 mL MQ, 0.1 mL FCR, 2 mL 2% Na2CO3 | 60 min, room temperature | 750 nm | [120] |
| Fruit juices | Gallic acid | 0.1 mL of extract, 0.5 mL MQ, 0.1 mL FCR, 1 mL of 7% Na2CO3, 0.5 MQ | 90 min | 760 nm | [121] |
| Pickled Chinese cabbage | Gallic acid | 0.5 mL of extract, 2.5 mL FCR, 2 mL of 7.5% Na2CO3, | 60 min, darkness | 760 nm | [122] |
| Cabbage: white, Chinese, and red | Gallic acid | 0.05 mL of extract, 0.1 FCR, 2 mL 1% Na2CO3, | 90 min, room temperature | 750 nm | [123] |
| Dovyalis caffra | Gallic acid | 0.3 mL of extract, 0.5 mL of FCR, 5.8 mL of MQ, 1.5 mL of 20% Na2CO3 | 2 h, room temperature | 760 nm | [124] |
| Tea extracts | Ascorbic acid | 0.1–0.3 mL of tea sample, 0.5 mL of FCR, 0.5 mL of 30% NaOH | 30 min, room temperature | 630 nm | [63] |
| Buckwheat | Gallic acid | 0.3 mL of sample, 1.5 mL of FCR, 1.2 mL of 7.5% Na2CO3 | 30 min, room temperature | 765 nm | [125] |
| Tested Material | Standard | Substrates | Type and Time of Incubation | Wavelength | Source |
|---|---|---|---|---|---|
| Various plants extracts | Trolox | 0.3 mL of extract, 75 mM phosphate buffer (pH 7.4), 1.8 mL of fluorescein (70 nM), 0.9 mL of AAPH (12 mM) | 60 min, 27 °C | 530 nm | [138] |
| Polyphenols | Trolox | 20 μL of sample (75 mM phosphate buffer (pH 7.0) mixed with 10 mg of test sample), 0.2 mL of fluorescein (94.4 nM), 75 μL of AAPH (31.7 mM) | 10 min, 37 °C | 528 nm | [139] |
| Oranges | Trolox | 50 μL of extract, 75 mM phosphate buffer (pH 7.0), 50 μL of fluorescein (78 nM), 25 μL of AAPH (221 mM) | 15 min, 37 °C | 535 nm | [136] |
| Berries | Trolox | 100 μL of extract, 75 mM phosphate buffer (pH 7.4), 60 μL of fluorescein (200 nM), 40 μL of AAPH (60 mM) | 10 min, 37 °C | 527 nm | [140] |
| Maple syrup | Trolox | 120 μL of extract, 75 mM phosphate buffer (pH 7.4), 30 μL of fluorescein (400 nM), 50 μL of AAPH (200 mM) | 15 min, 37 °C | 527 nm | [57] |
| Chlorella vulgaris | Trolox | 100 μL of extract, 75 mM phosphate buffer (pH 7.4), 800 μL of fluorescein (40 nM), 100 μL of AAPH (400 mM) | nd | 520 nm | [87] |
| Acai pulp | Trolox | 100 μL of extract, 75 mM phosphate buffer (pH 7.0), 2.6 mL of fluorescein (40 nM), 300 μL of AAPH (200 mM) | 30 min, 37 °C | 520 nm | [141] |
| Method | Feature | Advantage | Limitation | Explanation |
|---|---|---|---|---|
| FRAP | pH | X | pH = 3.6 below physiological pH | |
| Wavelength | X | 593 nm Repeatability | ||
| Biological molecules | X | The method includes Fe ions, which naturally occur in the body | ||
| Substrates used in the method | X | Various molar ratios between FRAP reagent and the sample | ||
| CUPRAC | pH | X | pH = 7 similar to physiological pH | |
| Wavelength | X | 450 nm Repeatability | ||
| Biological molecules | X | There is a lack of biologically relevant molecules | ||
| Substrates used in the method | X | Repeatability of substrates and the molar ratio among reagents | ||
| DPPH | pH | X | pH = 5.0–6.5 below physiological pH | |
| Wavelength | X | Various e.g., 515, 516, 517, 525, 540 | ||
| Biological molecules | X | There is a lack of biologically relevant molecules | ||
| Substrates used in the method | X | Lack of a standardized concentration of DPPH solution | ||
| ABTS+ | pH | X | pH ~ 4.5–7.0 (near neutral): however, large pH deviations may affect the reaction | |
| Wavelength | X | 734 nm Repeatability | ||
| Biological molecules | X | There is a lack of biologically relevant molecules | ||
| Substrates used in the method | X | Variability in the concentrations of ABTS radical in the working solution | ||
| FC | pH | X | pH = 10 above physiological pH | |
| Wavelength | X | 700–760 nm—results can differ significantly (high discrepancy) | ||
| Biological molecules | X | There is a lack of biologically relevant molecules | ||
| Substrates used in the method | X | Lack of unification FCR can interact with hydroxyl groups present in various compounds, not just phenols | ||
| ORAC | pH | X | pH = 7.0–7.4 similar to physiological pH | |
| Wavelength | X | e.g., 528, 530, 535 nm High discrepancy | ||
| Biological molecules | X | It allows for the assessment of the scavenging activity against peroxyl radicals | ||
| Substrates used in the method | X | Divergent concentrations of AAPH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knez, E.; Kadac-Czapska, K.; Grembecka, M. Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach. Appl. Sci. 2025, 15, 5925. https://doi.org/10.3390/app15115925
Knez E, Kadac-Czapska K, Grembecka M. Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach. Applied Sciences. 2025; 15(11):5925. https://doi.org/10.3390/app15115925
Chicago/Turabian StyleKnez, Eliza, Kornelia Kadac-Czapska, and Małgorzata Grembecka. 2025. "Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach" Applied Sciences 15, no. 11: 5925. https://doi.org/10.3390/app15115925
APA StyleKnez, E., Kadac-Czapska, K., & Grembecka, M. (2025). Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach. Applied Sciences, 15(11), 5925. https://doi.org/10.3390/app15115925






