Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review
Abstract
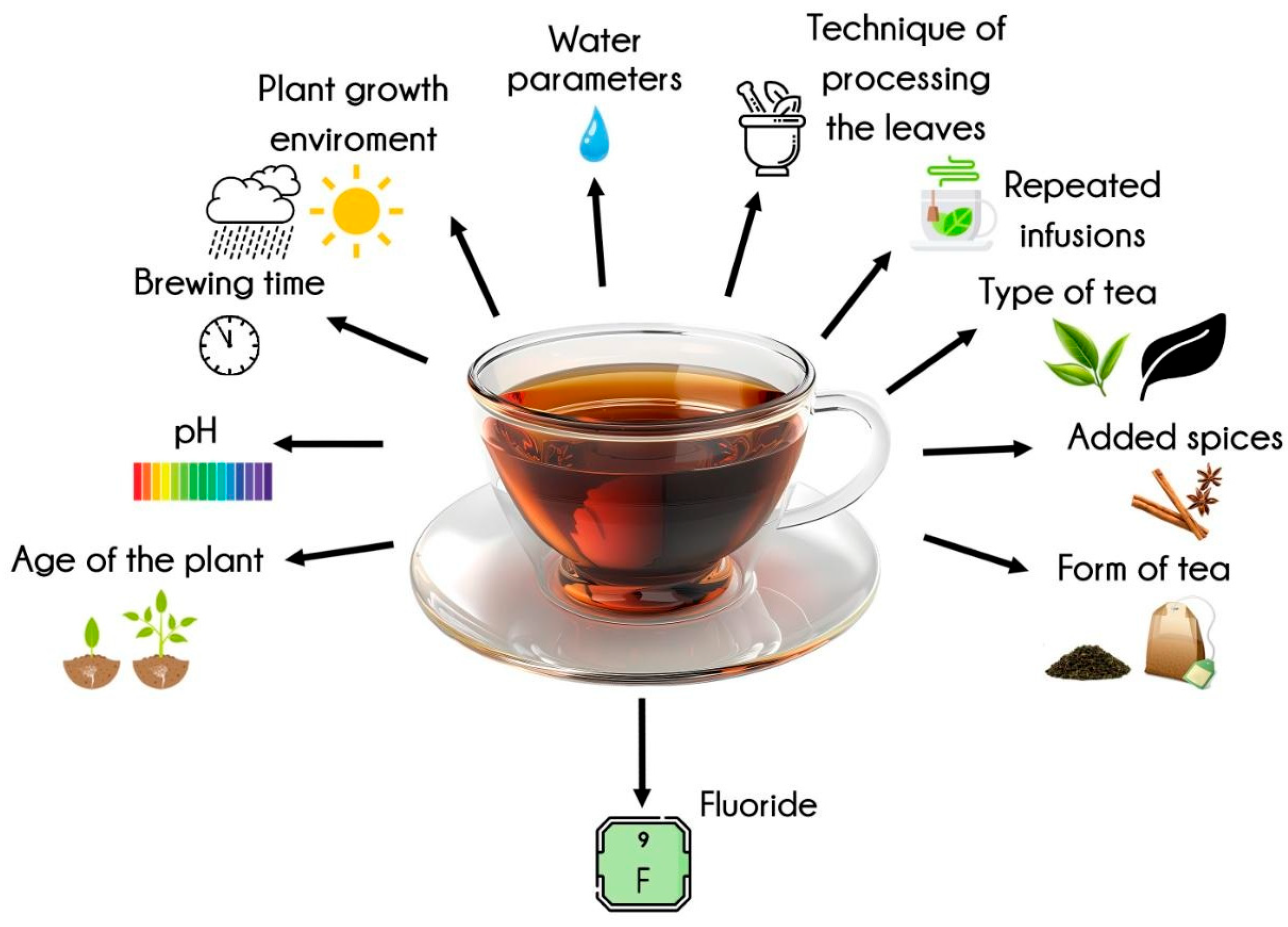
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Protocol
2.3. Eligibility Criteria
- Investigation of tea infusions;
- Fluoride release evaluation;
- In vitro studies;
- Studies in English;
- Full-text articles.
- Not a tea infusion investigation;
- Evaluation of properties other than fluoride release;
- Non-English papers;
- Systematic review articles;
- Review articles;
- Full-text not accessible;
- Duplicated publications.
2.4. Information Sources, Search Strategy, and Study Selection
2.5. Data Collection Process and Data Items
2.6. Risk of Bias and Quality Assessment
2.7. Quality Assessment and Risk of Bias
- Is it clear in the study what is the ‘cause’ and what is the ‘effect’?
- Were the participants included in any similar comparisons?
- Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest?
- Was there a control group?
- Were there multiple measurements of the outcome both before and after the intervention/exposure?
- Was a follow-up completed, and if not, were differences between groups in terms of their follow-up adequately described and analyzed?
- Were the outcomes of participants included in any comparisons measured in the same way?
- Were the outcomes measured in a reliable way?
- Was an appropriate statistical analysis used?
3. Results
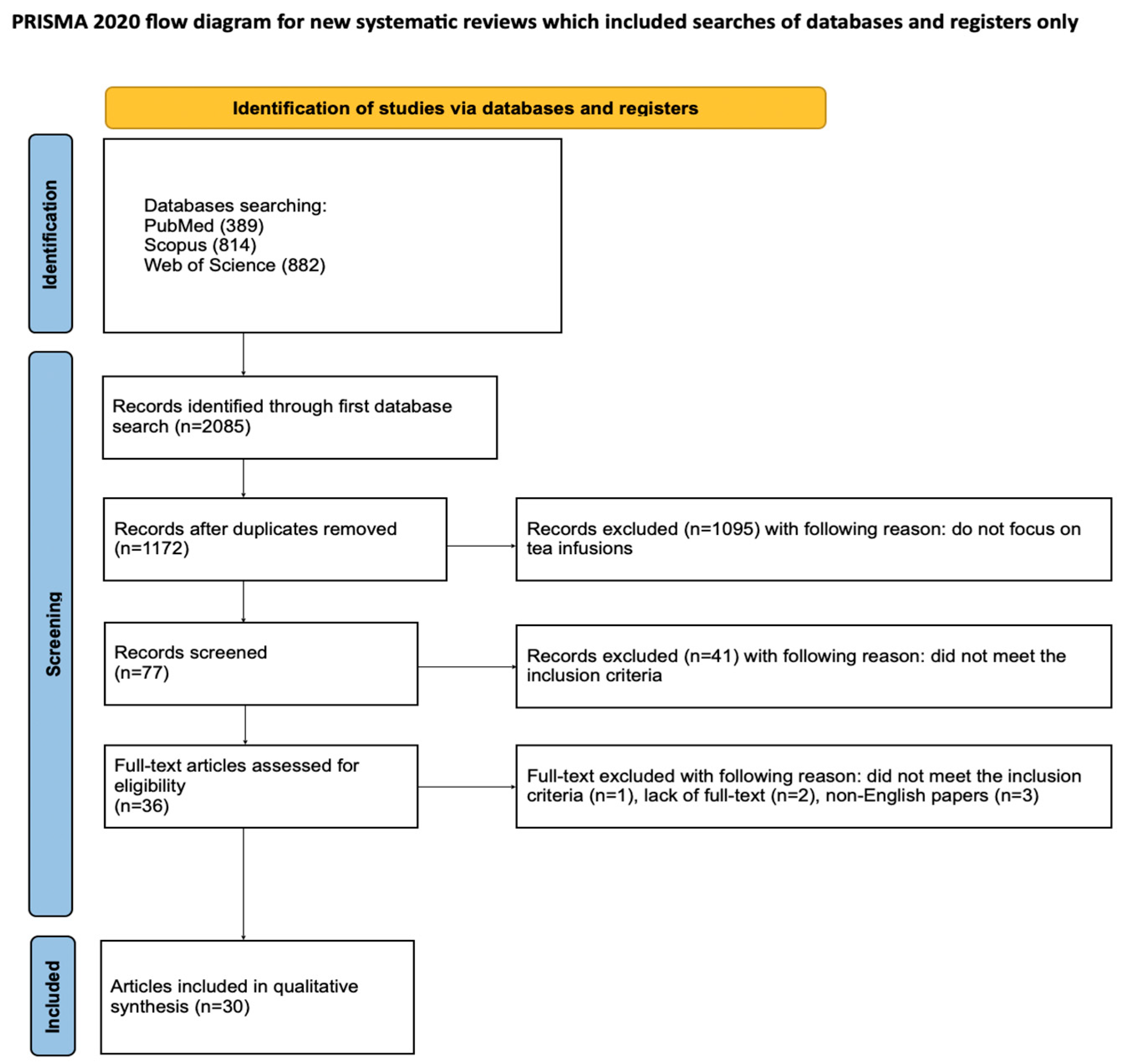
3.1. Study Selection
3.2. General Characteristics of the Qualified Articles
3.3. Main Study Outcomes
3.3.1. Variability in Fluoride Concentration Across Samples
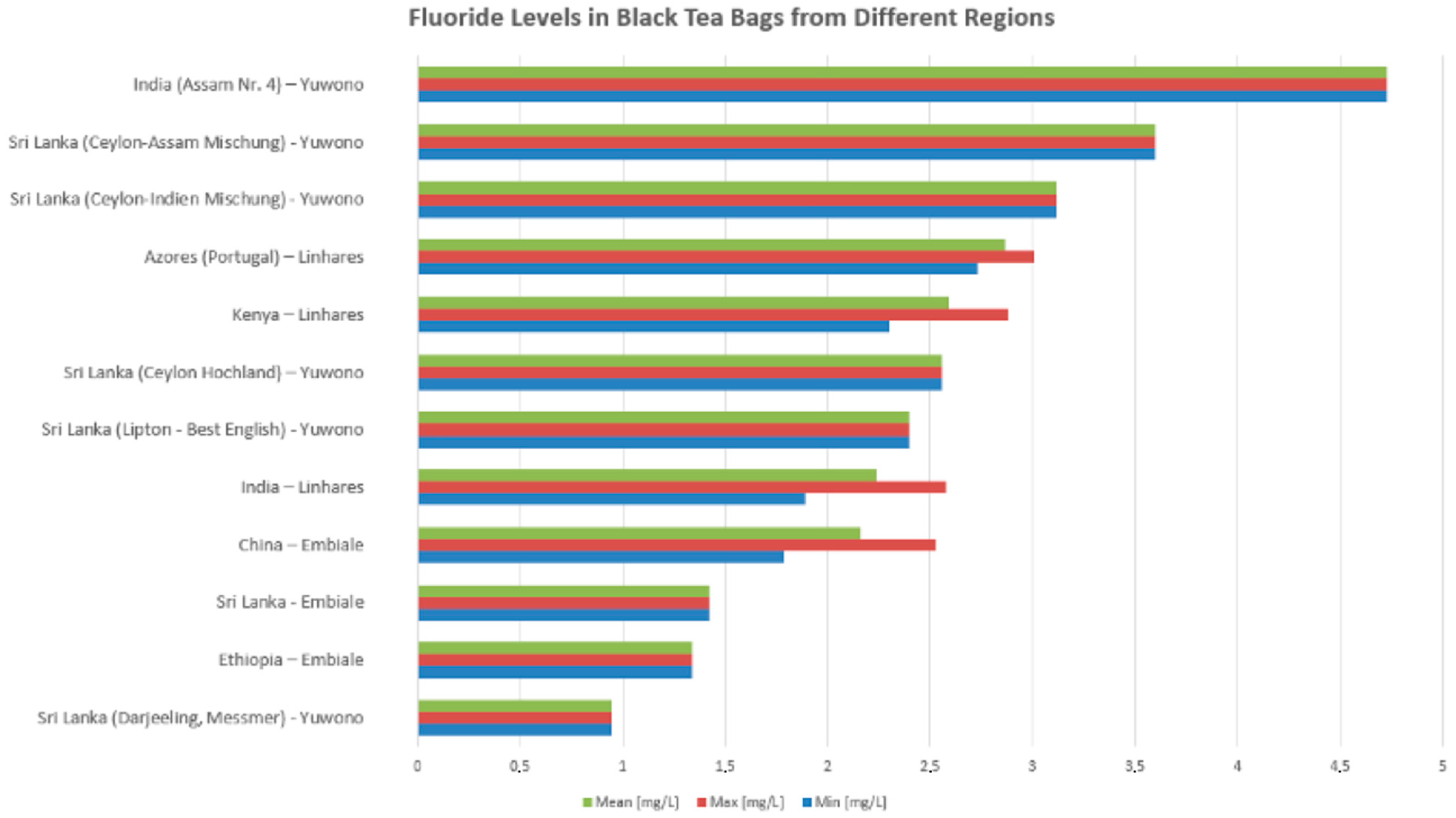
3.3.2. Fluoride Content by Tea Type and Geographic Origin
3.3.3. Effect of Brewing Time on Fluoride Release
3.3.4. Influence of Tea Mass and Leaf Fragmentation
3.3.5. Impact of Brewing Temperature and Water Composition
3.4. Quality Assessment and Risk of Bias of the Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride Contamination, Consequences and Removal Techniques in Water: A Review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Aoun, A.; Darwiche, F.; Al Hayek, S.; Doumit, J. The Fluoride Debate: The Pros and Cons of Fluoridation. Prev. Nutr. Food Sci. 2018, 23, 171–180. [Google Scholar] [CrossRef]
- Lubojanski, A.; Piesiak-Panczyszyn, D.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Mielan, B.; Wiglusz, R.J.; Watras, A.; Dobrzynski, M. The Safety of Fluoride Compounds and Their Effect on the Human Body—A Narrative Review. Materials 2023, 16, 1242. [Google Scholar] [CrossRef] [PubMed]
- Peckham, S.; Awofeso, N. Water Fluoridation: A Critical Review of the Physiological Effects of Ingested Fluoride as a Public Health Intervention. Sci. World J. 2014, 2014, 293019. [Google Scholar] [CrossRef] [PubMed]
- Olczak-Kowalczyk, D.; Mielczarek, A.; Jackowska, T.; Mielnik-Błaszczak, M.; Turska-Szybka, A.; Opydo-Szymaczek, J.; Jurczak, A.; Kaczmarek, U. Fluoride agents in the prevention and treatment of dental caries and erosion in children, adolescents and adults—Recommendations of Polish Experts. Update of recommendations: Individual fluoride prevention in children and adolescents—Recommendations of Polish Experts. Nowa Stomatol. 2022, 27, 35–59. [Google Scholar] [CrossRef]
- Herman, K.; Czajczyńska-Waszkiewicz, A.; Kowalczyk-Zając, M.; Dobrzyński, M. Assessment of the influence of vegetarian diet on the occurrence of erosive and abrasive cavities in hard tooth tissues. Postepy Hig. Med. Dosw. (Online) 2011, 65, 764–769. [Google Scholar] [CrossRef]
- Zohoori, F.V.; Buzalaf, M. Fluoride. Adv. Nutr. 2022, 13, 2679–2680. [Google Scholar] [CrossRef]
- Nassar, Y.; Brizuela, M. The Role of Fluoride on Caries Prevention. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mankar, N.; Kumbhare, S.; Nikhade, P.; Mahapatra, J.; Agrawal, P. Role of Fluoride in Dentistry: A Narrative Review. Cureus 2023, 15, e50884. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, S.; Kiryk, J.; Kensy, J.; Michalak, M.; Matys, J.; Dobrzyński, M. Fluoride Release from Two Commercially Available Dental Fluoride Gels—In Vitro Study. Gels 2025, 11, 135. [Google Scholar] [CrossRef]
- Jullien, S. Prophylaxis of Caries with Fluoride for Children under Five Years. BMC Pediatr. 2021, 21, 351. [Google Scholar] [CrossRef]
- Vasisth, D.; Mehra, P.; Yadav, L.; Kumari, V.; Bhatia, U.; Garg, R. Fluoride and Its Implications on Oral Health: A Review. J. Pharm. Bioallied Sci. 2024, 16, S49–S52. [Google Scholar] [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of Fluoride: Critical Evaluation of Evidence for Human Developmental Neurotoxicity in Epidemiological Studies, Animal Experiments and in Vitro Analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, E.; Inkielewicz, I.; Czarnowski, W.; Szefer, P. Assessment of Fluoride Concentration and Daily Intake by Human from Tea and Herbal Infusions. Food Chem. Toxicol. 2008, 46, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.T.; Potter, W.; Limeback, H.; Godfrey, M. Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and Its Implications for Public Health and Water Fluoridation. Int. J. Environ. Res. Public. Health 2016, 13, 259. [Google Scholar] [CrossRef]
- Rankin, S.J.; Levy, S.M.; Warren, J.J.; Gilmore, J.E.; Broffitt, B. Fluoride content of solid foods impacts daily intake. J. Public Health Dent. 2012, 72, 128–134. [Google Scholar] [CrossRef]
- Peng, C.; Cai, H.; Zhu, X.; Li, D.; Yang, Y.; Hou, R.; Wan, X. Analysis of Naturally Occurring Fluoride in Commercial Teas and Estimation of Its Daily Intake through Tea Consumption. J. Food Sci. 2016, 81, H235–H239. [Google Scholar] [CrossRef] [PubMed]
- Esfehani, M.; Ghasemzadeh, S.; Mirzadeh, M. Comparison of Fluoride Ion Concentration in Black, Green and White Tea. Int. J. Ayurvedic Med. 2018, 9, 263–265. [Google Scholar] [CrossRef]
- Szmagara, A.; Krzyszczak, A.; Stefaniak, E.A. Determination of Fluoride Content in Teas and Herbal Products Popular in Poland. J. Environ. Health Sci. Eng. 2022, 20, 717–727. [Google Scholar] [CrossRef]
- Emekli-Alturfan, E.; Yarat, A.; Akyuz, S. Fluoride Levels in Various Black Tea, Herbal and Fruit Infusions Consumed in Turkey. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 1495–1498. [Google Scholar] [CrossRef]
- Validandi, V.; Viswanathan, G.; Khandare, A.L. Comparison of Fluoride Levels (Total and Extracted) in Young, Old Tea Leaves and Market Tea Samples along with Impact of Tea Infusion on Dental Fluorosis in Fluoride Endemic Villages of Nalgonda District, India. Adv. Dent. Oral Health 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Liu, Q.; Wang, W.; Yang, L.; Li, Y.; Feng, F.; Zhao, X.; Hou, K.; Wang, G. Fluoride in Drinking Water, Brick Tea Infusion and Human Urine in Two Counties in Inner Mongolia, China. J. Hazard. Mater. 2009, 167, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Fluoride Contents in Tea and Soil from Tea Plantations and the Release of Fluoride into Tea Liquor during Infusion. Environ. Pollut. 1999, 104, 197–205. [Google Scholar] [CrossRef]
- Cai, H.; Zhu, X.; Peng, C.; Xu, W.; Li, D.; Wang, Y.; Fang, S.; Li, Y.; Hu, S.; Wan, X. Critical Factors Determining Fluoride Concentration in Tea Leaves Produced from Anhui Province, China. Ecotoxicol. Environ. Saf. 2016, 131, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, C.; Li, J.; Zhang, Y.; Zhu, C.; Gu, D.; Zeng, L. Critical Review of Fluoride in Tea Plants (Camellia sinensis): Absorption, Transportation, Tolerance Mechanisms, and Defluorination Measures. Beverage Plant Res. 2024, 4, e019. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Wu, X.; Lin, Y.; Li, B.; Chen, Y.; Li, X.; Shen, J.; Xiao, L.; Lu, S. Monitoring Fluorine Levels in Tea Leaves from Major Producing Areas in China and the Relative Health Risk. J. Food Compos. Anal. 2023, 118, 105205. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Li, Y.; Deng, H.J.; Yi, J.; Liu, J.W. Fluoride Levels in Various Black Tea Commodities: Measurement and Safety Evaluation. Food Chem. Toxicol. 2006, 44, 1131–1137. [Google Scholar] [CrossRef]
- Mazurek, A.; Kowalska, G.; Włodarczyk-Stasiak, M.; Wyrostek, J.; Kowalski, R. The Influence of the Preparation of Tea Infusion on the Content of Fluoride and the Assessment of Health Risk for the Consumer in Poland. Appl. Sci. 2023, 13, 5075. [Google Scholar] [CrossRef]
- Jin, C.; Yan, Z.; Jianwei, L. Processing Procedures of Brick Tea and Their Influence on Fluorine Content. Food Chem. Toxicol. 2001, 39, 959–962. [Google Scholar] [CrossRef]
- Amorello, D.; Barreca, S.; Pensato, F.; Orecchio, S. Potentiometric Analysis of Fluoride in Commonly Consumed Beverages: Method Development, Evaluation, and Risk Assessment. J. Food Compos. Anal. 2025, 137, 106836. [Google Scholar] [CrossRef]
- Giljanović, J.; Prkić, A.; Bralić, M.; Brkljača, M. Determination of Fluoride Content in Tea Infusion by Using Fluoride Ion-Selective Electrode. Int. J. Electrochem. Sci. 2012, 7, 2918–2927. [Google Scholar] [CrossRef]
- Maleki, A.; Abulmohammadi, P.; Teymouri, P.; Zand, S.; Daraei, H.; Mahvi, A.; Shahsawari, S. Effect of Brewing Time and Water Hardness on Fluoride Release from Different Iranian Teas. Fluoride 2016, 49, 263–273. [Google Scholar]
- Lu, Y.; Guo, W.-F.; Yang, X.-Q. Fluoride Content in Tea and Its Relationship with Tea Quality. J. Agric. Food Chem. 2004, 52, 4472–4476. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, I.; Gutowska, I.; Janda, K.; Jakubczyk, K.; Woźniak, K.; Siwiec, E.; Wolska, J. Does the Addition of Spices Change the Content of Fluoride and Antioxidants in Black Tea Infusions? J. Elem. 2018, 23, 599–609. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA. Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef]
- Kensy, J.; Dobrzyński, M.; Wiench, R.; Grzech-Leśniak, K.; Matys, J. Fibroblasts Adhesion to Laser-Modified Titanium Surfaces—A Systematic Review. Materials 2021, 14, 7305. [Google Scholar] [CrossRef]
- Kiryk, J.; Kiryk, S.; Kensy, J.; Świenc, W.; Palka, B.; Zimoląg-Dydak, M.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Effectiveness of Laser-Assisted Teeth Bleaching: A Systematic Review. Appl. Sci. 2024, 14, 9219. [Google Scholar] [CrossRef]
- Matys, J.; Kensy, J.; Gedrange, T.; Zawiślak, I.; Grzech-Leśniak, K.; Dobrzyński, M. A Molecular Approach for Detecting Bacteria and Fungi in Healthcare Environment Aerosols: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4154. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef]
- Oleniacz-Trawińska, M.; Kotela, A.; Kensy, J.; Kiryk, S.; Dobrzyński, W.; Kiryk, J.; Gerber, H.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Affecting Fluoride Release from Compomer Restorative Materials: A Systematic Review. Materials 2025, 18, 1627. [Google Scholar] [CrossRef]
- Tokarczuk, D.; Tokarczuk, O.; Kiryk, J.; Kensy, J.; Szablińska, M.; Dyl, T.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Fluoride Release by Restorative Materials after the Application of Surface Coating Agents: A Systematic Review. Appl. Sci. 2024, 14, 4956. [Google Scholar] [CrossRef]
- Morawska-Wilk, A.; Kensy, J.; Kiryk, S.; Kotela, A.; Kiryk, J.; Michalak, M.; Grychowska, N.; Fast, M.; Matys, J.; Dobrzyński, M. Evaluation of Factors Influencing Fluoride Release from Dental Nanocomposite Materials: A Systematic Review. Nanomaterials 2025, 15, 651. [Google Scholar] [CrossRef]
- Struzik, N.; Kensy, J.; Piszko, P.J.; Kiryk, J.; Wiśniewska, K.; Kiryk, S.; Korjat, Ł.; Horodniczy, T.; Sobierajska, P.; Matys, J.; et al. Contamination in Bone Substitute Materials: A Systematic Review. Appl. Sci. 2024, 14, 8266. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]
- Chapter 3: Systematic Reviews of Effectiveness. In JBI Manual for Evidence Synthesis; JBI: Adelide, Australia, 2020; ISBN 978-0-6488488-0-6.
- Guo, W.; Lin, X.; Jin, L.; Hu, S. Single Quadrupole Inductively Coupled Plasma-Mass Spectrometry for the Measurement of Fluorine in Tea Infusions and Its Health Risk Assessment. J. Food Compos. Anal. 2020, 86, 103378. [Google Scholar] [CrossRef]
- Morés, S.; Monteiro, G.C.; Santos, F. da S.; Carasek, E.; Welz, B. Determination of Fluorine in Tea Using High-Resolution Molecular Absorption Spectrometry with Electrothermal Vaporization of the Calcium Mono-Fluoride CaF. Talanta 2011, 85, 2681–2685. [Google Scholar] [CrossRef]
- Yuwono, M. Determination of Fluoride in Black, Green and Herbal Teas by Ionselective Electrode Using a Standard-Addition Method. Dent. J. Maj. Kedokt. Gigi 2005, 38, 91. [Google Scholar] [CrossRef]
- Gupta, P.; Sandesh, N. Estimation of Fluoride Concentration in Tea Infusions, Prepared from Different Forms of Tea, Commercially Available in Mathura City. J. Int. Soc. Prev. Community Dent. 2012, 2, 64–68. [Google Scholar] [CrossRef]
- Zhu, J.J.; Ath, T.; Matinlinna, J.P.; Jkh, T.; Hägg, U. Potentiometric Determination of Fluoride Release from Three Types of Tea Leaves. Int. J. Electrochem. Sci. 2013, 8, 11142–11150. [Google Scholar] [CrossRef]
- Chan, L.; Mehra, A.; Saikat, S.; Lynch, P. Human Exposure Assessment of Fluoride from Tea (Camellia Sinensis L.): A UK Based Issue? Food Res. Int. 2013, 51, 564–570. [Google Scholar] [CrossRef]
- Fojo, C.; Figueira, M.E.; Almeida, C.M.M. Fluoride Content of Soft Drinks, Nectars, Juices, Juice Drinks, Concentrates, Teas and Infusions Marketed in Portugal. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2013, 30, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Embiale, A.; Chandravanshi, B.S.; Zewge, F. Levels of Fluoride in the Ethiopian and Imported Black Tea (Camellia sinensis) Infusions Prepared in Tap and Fluoride-Rich Natural Waters. Int. J. Food Eng. 2014, 10, 447–455. [Google Scholar] [CrossRef]
- Das, S.; de Oliveira, L.M.; da Silva, E.; Liu, Y.; Ma, L.Q. Fluoride Concentrations in Traditional and Herbal Teas: Health Risk Assessment. Environ. Pollut. Barking Essex 1987 2017, 231, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Linhares, D.P.S.; Garcia, P.V.; Amaral, L.; Ferreira, T.; Dos Santos Rodrigues, A. Safety Evaluation of Fluoride Content in Tea Infusions Consumed in the Azores—A Volcanic Region with Water Springs Naturally Enriched in Fluoride. Biol. Trace Elem. Res. 2017, 179, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, H.; Ye, S.; Tan, H. A New Method for Determination of Fluoride Ion in Commodity Tea by Ion-Exclusion Chromatography. CyTA-J. Food 2018, 16, 637–641. [Google Scholar] [CrossRef]
- Satou, R.; Oka, S.; Sugihara, N. Risk Assessment of Fluoride Daily Intake from Preference Beverage. J. Dent. Sci. 2021, 16, 220–228. [Google Scholar] [CrossRef]
- Akhdhar, A.; Schneider, M.; Hellmann, S.; Orme, A.; Carasek, E.; Krupp, E.M.; Feldmann, J. The Use of Microwave-Induced Plasma Optical Emission Spectrometry for Fluorine Determination and Its Application to Tea Infusions. Talanta 2021, 227, 122190. [Google Scholar] [CrossRef]
- Essebbahi, I.; Ouazzani, C.; Moustaghfir, A.; Er-ramly, A.; El Baroudi, Y.; Dami, A.; Balouch, L. Analysis of the Fluoride Levels of Well Water and Tea Consumed by the Moroccan Population in Different Rural Areas. Mater. Today Proc. 2023, 72, 3347–3350. [Google Scholar] [CrossRef]
- Aktuğ, Ü.; Duydu, Y. The Effects of Color and Brightness of Brewed Black Tea on Its Fluoride Concentration in Türkiye. Ank. Univ. Eczaci. Fak. Derg. 2023, 47, 10. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Zazoli, M.A.; Younecian, M.; Esfandiari, Y. Fluoride Content of Iranian Black Tea and Tea Liquor. Fluoride 2006, 39, 266. [Google Scholar]
- Chandrajith, R.; Bhagya, S.; Diyabalanage, S.; Wimalasiri, S.; Ranatunga, M.A.B.; Barth, J.A.C. Exposure Assessment of Fluoride Intake Through Commercially Available Black Tea (Camellia sinensis L.) from Areas with High Incidences of Chronic Kidney Disease with Undetermined Origin (CKDu) in Sri Lanka. Biol. Trace Elem. Res. 2022, 200, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Edussuriya, R.; Hettithanthri, O.; Rajapaksha, A.U.; Jayasinghe, C.; Vithanage, M. Intake of Fluoride and Other Hofmeister Ions from Black Tea Consumption in CKDu Prevalent Areas, Sri Lanka. Environ. Sci. Pollut. Res. 2023, 30, 41900–41909. [Google Scholar] [CrossRef]
- Rajiv, D.; Prem, D.; Ramesh, M.; Jacob, M.; Indrapriyadharshini, K. Fluoride Content in Various Types of Tea Used by Tea Stalls in Salem District—An in Vitro Cross Sectional Study. J. Oral Maxillofac. Pathol. JOMFP 2023, 27, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.-P.; Lin, Z.; Tan, J.-F.; Guo, L. Contents of Fluoride, Lead, Copper, Chromium, Arsenic and Cadmium in Chinese Pu-Erh Tea. Food Res. Int. 2013, 53, 938–944. [Google Scholar] [CrossRef]
- Erdemoglu, S.B.; Türkdemir, H.; Gücer, S. Determination of Total and Fluoride Bound Aluminium in Tea Infusions by Ion Selective Electrode and Flame Atomic Absorption Spectrometry. Anal. Lett. 2000, 33, 1513–1529. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, B.; Wang, B.; Wang, M. The Characteristics of Dissolution of Fluoride and Aluminum from Brick Tea. Chin. J. Geochem. 2005, 24, 382–385. [Google Scholar] [CrossRef]
- Kanrar, B.; Kundu, S.; Sengupta, S.; Yeasin, M.; Paul, R.K.; Karak, T. Assessment and Health Risk of Fluoride from Northeast Indian Tea (Camellia sinensis L.): Fixing up the Maximum Residue Level of Fluoride in Tea. J. Food Compos. Anal. 2024, 127, 105928. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, Y.; Wang, W.; Gong, H.; Guo, M.; Zhao, S.; Liu, X.; Yu, B.; Sun, D. Prevalence of Brick Tea-Type Fluorosis in the Tibet Autonomous Region. J. Epidemiol. 2016, 26, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jasim, S.; Wenger, D.; Wermers, R.A. Skeletal fluorosis related to habitual tea consumption: Long-term follow-up after reduction and discontinuation of tea. AACE Clin. Case Rep. 2018, 4, 98–103. [Google Scholar] [CrossRef]
- Johnson, J.E.H.; Kearns, A.E.; Doran, P.M.; Khoo, T.K.; Wermers, R.A. Fluoride-related bone disease associated with habitual tea consumption. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2007; pp. 719–724. [Google Scholar] [CrossRef]
- Whyte, M.P.; Totty, W.G.; Lim, V.T.; Whitford, G.M. Skeletal fluorosis from instant tea. J. Bone Miner. Res. 2008, 23, 759–769. [Google Scholar] [CrossRef]
- Izuora, K.; Twombly, J.G.; Whitford, G.M.; Demertzis, J.; Pacifici, R.; Whyte, M.P. Skeletal fluorosis from brewed tea. J. Clin. Endocrinol. Metab. 2011, 96, 2318–2324. [Google Scholar] [CrossRef] [PubMed]


| Study | Aim of the Study | Materials and Methods | Results | Conclusions |
|---|---|---|---|---|
| Erdemoglu [67] | To assess how pH and time affect Al-F salt formation and how other metals influence free F content in the infusion. | The infusion was prepared by brewing 2 g tea in 100 mL deionized water, then filtered after 10 min at pH = 5. Al was measured via FAAS while free and total F were determined using FISE with ALCOA buffer to release F from complexes. | Higher pH increases free F in tea infusion. Elevated Al and F(III) concentrations reduce free F. Fe(II) ions react negligibly with F. Fe(III) ions increasingly bind F as pH decreases. Mn2+ and Mg2+ have statistically insignificant reactions with F due to low tea concentrations. | The amount of free fluoride in the infusion is influenced by its pH and the concentration of Al, Fe(III) ions present. |
| Liu [68] | To evaluate the effect of temperature, brewing time, and tea-to-water ratio on the release of F and Al in the infusion. | Tea cubes were ground, dried, and 1 g brewed in appropriate amounts of deionized water. To 5 mL of centrifuged tea liqueur, either 10 mL TISAB buffer (for F quantification) or 0.1 ml HNO3 (for Al quantification) was added. | F and Al release decreases with successive brewings but increases with higher temperatures. F release peaks at 40 min of brewing. Greater water volume reduces F release. | Brewing parameters: time, temperature, and amount of water influence the amount of F released into the infusion. |
| Mahvi [62] | To compare the amount of F released into the infusion depending on the tea brand. | 60 black Iranian tea samples (10 brands, 2 g each) were brewed in 50 mL water for 10 min at 80 °C, then diluted to 100 mL with distilled water. F content was measured using FISE method. | The amount of fluoride released into the infusion ranged from 0.57 mg/L to 2.6 mg/L. | The amount of F released varies depending on the brand of tea. |
| Li [22] | To examine the fluoride content in drinking water, tea infusion, and urine of inhabitants of a specific region of China. | F content was measured with a CSBF-1 fluoride electrode in tap water and with an Orion 868 Electrode in urine and brick tea infusion samples. All samples were diluted with TISAB buffer. | One of three tested water sources exceeded the F norm (1 mg/L); 82% of infusions contained above-normal F levels. Higher drinking water F correlated with increased urinary F. | The sum of fluoride from drinking water and tea infusion may reach values that pose a risk of fluorosis in the studied regions of China. |
| Morés [48] | To develop a simple tea fluorine detection method using molecular absorption spectrometry with high-resolution continuum source AAS and electrothermal vaporization. | 10 tea samples were prepared by three methods: microwave HNO3 digestion, TMAH alkaline solubilization, and 90 °C/5-min infusion. Fluoride was measured via CaF molecular absorption using high-resolution continuum source AAS with a graphite furnace. All preparations were performed in triplicate, with measurements repeated at least 3 times. | Fluorine in tea was determined via CaF molecular absorption at 606.440 nm, yielding 42–87 µg/g in leaves and 21–56 µg/g in infusions, with 48–74% extraction rates. Method validation showed comparable results between acid digestion and alkaline solubilization. | The method proved accurate and reliable, with acid digestion and alkaline solubilization yielding comparable results, offering a simple, effective approach for routine tea fluorine analysis. |
| Yuwono [49] | To establish a technique for measuring fluoride concentrations in black, green, and herbal tea infusions. | Fluoride in 12 tea brands was analyzed using a fluoride ion-selective electrode after 5 min hot water infusion, filtration, and dilution. Potentiometry with Gran’s plot was used for concentration determination. | After a 5 min infusion, fluoride concentrations varied from 0.95 to 4.73 mg/L in black tea, 0.70 to 1.00 mg/L in green tea, and 0.26 to 0.27 mg/L in herbal tea. | Black and green teas are major sources of daily fluoride intake, and children at risk of dental fluorosis should refrain from consuming black teas with high fluoride levels. |
| Giljanović [31] | To analyze fluoride content in various teas from markets in Split, Croatia. | Fluoride content was analyzed in 43 tea infusions, including mint, lemon balm, green tea, and pomegranate from tea bags, bottled beverages, and bulk samples. Fluoride concentration was measured using FISE. | Average fluoride: 0.116 ± 0.211 mg/L. Highest in green tea (0.393 ± 0.23 mg/L), lowest in pomegranate (0.008 ± 0.002 mg/L) and mint (0.011 ± 0.004 mg/L). Tea bags contained more fluoride than bulk tea, with green tea bags showing the highest levels (0.558 ± 0.12 mg/L vs. 0.161 ± 0.12 mg/L bottled). Packaging had no effect on mint tea fluoride levels. | Fluoride content varies by tea type and packaging, highest in green tea and tea bags/bottled drinks. |
| Gupta [50] | Assess fluoride variation in tea infusions across different tea forms prepared in 3 different methods. | 16 tea brands (3 dip, 2 leafy, 11 granulated) were analyzed for fluoride using an ion-selective electrode after preparing infusions via 3 methods: hot water steeping, water boiling, and boiling with milk and sugar. | Tea infusions had mean fluoride concentrations of 1.437, 3.375, and 3.437 mg/L, depending on the preparation method, with tea granules showing statistically significant fluoride levels regardless of preparation. | Fluoride in tea can contribute to dietary intake, potentially aiding in cavity prevention or increasing the risk of fluorosis. |
| Zhu [51] | Fluoride release properties of 3 common tea types consumed in Hong Kong and Hangzhou were investigated. | 72 water samples (Hong Kong, Hangzhou, deionized) were used to brew pu-erh, black, and green teas via repeated and continuous infusion methods, with fluoride measured by selective electrode. | Fluoride was highest in Hong Kong’s drinking water versus Hangzhou’s. Black tea bags released the most fluoride, followed by pu-erh and green tea leaves. Repeated infusions increased fluoride concentration over time. | Fluoride release from tea depends on water composition, brewing time, and infusions, but not on pH. |
| Chan [52] | To examine the fluoride intake from tea consumption in the UK. | 38 tea products in 5 groups (Black, Green, Pure, Oolong/Pu’er, Economy) were analyzed with FISE after brewing 2 g in 100 mL boiling water for 2, 10, and 30 min. | Fluoride in tea infusions ranged from 0.43 to 8.85 mg/L. The fluoride release order was Economy > Green > Black > Pure > Oolong/Pu’er teas. Fluoride concentration increased with time brewing. | Economy tea contains high fluoride levels (3.60–7.96 mg/L), with 1 L providing 75–150% of the recommended daily intake. |
| Lv [66] | To assess the safety of Chinese Pu-erh tea consumption by analyzing the levels of microelements (fluoride, lead, copper, chromium, arsenic, cadmium). | 56 Pu-erh teas (26 loose, 30 compressed) from 5 Yunnan regions were analyzed for fluoride and heavy metals after drying, grinding, and sieving. Infusions from 20 selected samples were prepared by brewing 5 g in 250 mL boiled water for 5 min twice, combined, freeze-dried, and analyzed. | Highest fluoride in Dali and Dehong samples; lowest in Lincang. Compressed tea had 30% less lead than loose tea. Infusions contained mean concentrations: fluoride (523.86 μg/L), copper (43.18 μg/L), chromium (13.67 μg/L), lead (5.70 μg/L), arsenic (0.43 μg/L), and cadmium (0.17 μg/L), with dissolving rates of 24.6–45.8%. | Regional variations were significant except for copper. Fluoride had the highest dissolving rate (45.8%). All elements were below safety thresholds, suggesting no health risk from Pu-erh tea infusions, though chromium warrants monitoring due to high contribution (92.2%). |
| Fojo [53] | To determine the fluoride levels in various drinks marketed in Portugal for children. | 183 samples were collected from markets (106 soft drinks, 23 juices, 37 nectars, 6 juice drinks, 5 concentrates, 3 teas, and 3 infusions), then FISE was used. | Extract-based soft drinks contained the highest fluoride levels (0.86 mg/L), while tea (0.16 mg/L) and tea infusions had the lowest (0.12 mg/L). Tea and tea infusion had higher pH values (6.0 and 5.7) compared to other pH values around 3.4. | All drinks contain fluoride levels below toxic thresholds, with typical consumption contributing only 7.4% of a child’s recommended daily intake. |
| Embiale [54] | To assess how water fluoride levels affect fluoride release from tea leaves during infusion and evaluate potential fluoride absorption by leaves. | Water from 5 fluoride-rich Ethiopian Rift Valley locations and Addis Ababa University tap water was used. 9 tea brands (3 green tea bags, 3 black tea powders, 3 black tea bags) were tested by adding 1 g to 100 mL boiling water (92 °C) for 3, 5, and 10 min. Fluoride was measured using FISE. | Fluoride ranged from 0.254–30.2 mg/L in water to 0.51–20.57 mg/L in tea infusions. Moderately fluoride-rich water (12.5 mg/L) decreased to <5 mg/L when used for tea, while highly fluoride-rich water (30.2 mg/L) reduced to <10 mg/L in most infusions after 3 min brewing. Ethiopian teas contained higher fluoride than imported ones. | Tea brands showed varying fluoride absorption capacities (30–80%), demonstrating that tea leaves can reduce fluoride in high-fluoride water. Absorption rates varied by tea brand and brewing time. Drinking tea prepared with fluoride-rich water could lower daily fluoride intake compared to direct water consumption. |
| Maleki [32] | To assess fluoride levels in various Iranian tea leaves and analyze how brewing duration and water hardness impact the fluoride release. | 100 tea samples (white, green, oolong, black) from one Iranian manufacturer in leaf and bag forms were prepared. 1 g of each sample was steeped in 100 mL boiling water in Teflon teapots at 80 °C for 3–120 min. Water hardness varied from 0 to 350 mg CaCO3/L. Fluoride, phosphate, sulfate, nitrate, and chloride concentrations were measured using a Metrohm 882 compact IC plus ion chromatography system. | Tea bags released more fluoride than loose leaves. Black tea contained the highest fluoride levels, and oolong the lowest. Fluoride release increased with brewing time. Water hardness reduced fluoride extraction, especially above 100 mg CaCO3/L. | Brewing time and water hardness significantly influence fluoride release from tea, with longer brewing increasing and harder water decreasing fluoride extraction. |
| Fan [70] | Investigate the frequency and severity of brick-tea fluorosis in Tibet, and assess fluoride exposure from varying sources. | 416 child and 1287 adult urine samples, 1227 brick tea infusions, 29 drinking water samples, and 107 brick tea samples were analyzed for fluoride concentration using FISE. | 106/107 samples exceeded national fluoride standards (median: 732.81 mg/kg). Median daily brick tea consumption was 3.2 L, providing 24.73 mg fluoride daily—7× the national safety standard (3.5 mg). Dental fluorosis affected 33.57% of children, while skeletal fluorosis affected 46.06% of adults. | Tibetans have high fluoride exposure due to their consumption of brick tea. The study identified altitude and occupation as key risk factors. |
| Das [55] | Evaluation of potential health risk factors associated with fluoride exposure from tea consumption. | 47 tea samples (15 herbal mixes, 15 black, 9 green, 4 oolong, 3 pu-erh, 1 white) from 13 countries were analyzed. 500 mg tea powder was infused in 50 mL boiling water for 5 min, filtered, and adjusted to pH 4.25–4.75 before fluoride analysis. | Herbal teas had the lowest fluoride (0.06–0.69 mg/L). Traditional teas contained higher concentrations: green (2.43–6.94 mg/L), black (1.47–5.45 mg/L), oolong (3.08–5.63 mg/L), pu-erh (2.87–4.96 mg/L), white (5.39 mg/L). Traditional teas released 18–99% of fluoride during brewing versus 6–96% for herbal teas. All infusions were acidic (pH 3.5–5.5). | 10 traditional teas exceeded recommended daily intake limits, though no teas showed immediate health risks (HQ < 1). Combined with other fluoride sources, regular consumption of high-fluoride teas may increase fluorosis risk. |
| Linhares [56] | To compare fluoride content in commercial teas and analyze release rates based on brewing time and water type used. | F content was determined using the FISE method in 30 drinking water samples from three Azores locations and 450 tea samples (black and green) from three commercial brands. Infusions were prepared using 5 brewing times (1, 2, 3, 5, and 10 min). | F- concentration in water samples ranged from 0.29 to 1.56 ppm. F- concentration in tea infusions increased with brewing time. A negative correlation existed between tea F- content and water pH used for infusion preparation. F- in infusions was significantly associated with baseline F- present in the water. | In regions with naturally high F- levels in water supplies and prevalent tea consumption, rigorous monitoring of F- concentrations in both water sources and commercial tea brands is advisable. |
| Janda [34] | To determine polyphenol, F- content, and antioxidant potential in black tea infusions with added spices. | Infusions of black tea with selected spices were prepared at 80 °C. Polyphenol content and antioxidant activity were determined spectrophotometrically, while F- concentration was measured using an ion-selective electrode. | The highest polyphenol content and strongest antioxidant properties were observed in tea infused with cloves. The highest F- content was detected in tea with turmeric, and the lowest in the clove infusion. The addition of spices and F- content influenced the antioxidant properties and polyphenol levels in tea infusions. High F- concentrations were associated with decreased antioxidant capacity of tea infusions. | Antioxidant activity is a significant determinant of black tea infusions’ health-related quality. Adding spices influences the infusion’s composition and properties. Elevated F- levels diminish black tea infusions’ antioxidant capacity. |
| Zhou [57] | Development and evaluation of a novel ion-exclusion chromatography method for determining F- ions in tea infusions. | Ion-exclusion chromatography was performed using 2 connected Metrosep organic acid columns. The method was validated with standard samples and Chinese tea samples, with accuracy confirmed through recovery tests and comparison with ion-selective electrode measurements. | Two columns in series provided better F- separation than a single column. The method showed good sensitivity and a wide linearity range, with results matching those from ion-selective electrode measurements. | Ion-exclusion chromatography offers a novel, rapid, accurate, and direct method for F- determination in tea infusions, effectively separating fluoride ions from interfering organic acids. |
| Guo [47] | Development of a method for total F determination (both F- and covalently bonded F) in tea infusions using single quadrupole ICP-MS, with assessment of associated health risks. | ICP-MS measured BaF+ ions generated from fluorine and barium in plasma. 100 tea samples of various types were analyzed, with chronic daily intake (CDI) and target hazard quotient (THQ) of F- assessed for adults. | The developed ICP-QMS method accurately determined total F- with a low detection limit. Brick tea showed the highest F- concentration. Estimated chronic daily intake (CDI) and target hazard quotient (THQ) values indicated that F- exposure from tea consumption is within safe levels for adults. | ICP-QMS coupled with online aerosol dilution and mathematical correction provides a viable method for determining total F- in tea infusions. |
| Satou [58] | Estimation of daily fluoride intake from beverages. | 31 tea infusions were prepared by extracting 1.0 g of tea leaves with 100 mL of distilled water at 80 °C for 5 min. After cooling to 25 °C, fluoride levels were measured using a fluoride ion-selective electrode. | The concentration of fluoride ions in green tea infusion was the highest: 0.26–4.09 mg/L, while in herbal tea it was 0.07–0.17 mg/L. | Regular consumption of certain beverages requires management of the risk of dental fluorosis. |
| Akhdhar [59] | To evaluate using CaF for determining total fluorine in tea infusion without sample preparation using microwave-induced plasma optical emission spectrometry (MIP-OES). | 7 samples were prepared by brewing 0.15 g of tea in 15 mL of water at 90 °C for 5 min. Fluoride levels were measured using MIP-OES and a high-resolution continuum source atomic absorption spectrometer (HR GF-MAS). | Fluoride concentrations in the tea samples tested ranged from <3.8 to 7.8 mg/L and were similar between both methods. | The MIP-OES method is sufficiently sensitive, and the results obtained are comparable to those obtained with the reference method. |
| Szmagara [19] | Determining which brand/type of tea and tea products release the highest levels of fluoride and how brewing time affects this. | The samples were prepared by brewing 2 g of tea in 200 mL of distilled water at 100 °C for 5 or 15 min. After cooling the samples to 18–20 °C, the fluorine content was measured using an ion-selective electrode. | The highest fluoride levels were obtained from black tea infusions, with more infusions prepared from tea bags than from leaves. Green tea followed, and white tea and rooibos had the lowest levels. In each case, longer brewing times were associated with higher fluoride content. | Even if tea is brewed with a high fluoride content or brewed longer than necessary, the public’s exposure to fluoride is negligible. |
| Chandrajith [63] | To assess F- exposure via commercially available tea in regions affected by kidney disease with undetermined origin (CKDu) and the effect of different brewing times and repeated tea brewing. | Samples were prepared by brewing 2 g of black tea in 100 mL of distilled water at a temperature of >95 °C for 2, 5, 10, or 15 min. Some infusions were prepared by brewing the tea 4 times for 5 min. An ion-selective electrode was used to assess the amount of fluoride. | There is a significant difference in the amount of fluoride in the infusion prepared from tea leaves and tea bags, and with each subsequent brewing, the amount of fluoride in the infusion decreases. However, the effect of brewing time on the amount of fluoride has not been demonstrated. | The study found elevated levels of F- in tea infusions. The effect is increased when using water with a higher F- content. |
| Mazurek [28] | Assessment of fluoride content in tea infusion based on tea type, leaf fragmentation, brewing time, and evaluation of health risks for Polish consumers. | Samples were prepared by brewing covered 2 g of tea leaves between 1.5 and 2 mm in 100 mL of deionized water at 100 °C for 6 min. Fluoride levels were measured using the potentiometric method, according to Shyu. | The fluoride level increases with the degree of leaf fragmentation and the lengthening of the brewing time. The most fluoride is found in the infusion of black tea, then green, and the least in pu-erh. | There is a potential for negative health effects from fluoride consumption, particularly from black and green tea infusions purchased in tea bags. |
| Edussurya [64] | To investigate the content of selected ions, including fluoride, in black tea consumed in areas of Sri Lanka where CKDu occurs, and to assess the risk of dietary exposure. | Samples were prepared by adding 200 mL of boiling deionized water to 2 g of black tea and brewing for 5, 10, 20, 30, 45, 60, or 120 min, maintaining the temperature between 80 and 85 °C. Fluoride levels were assessed using an ion-selective electrode. | The amount of fluoride increased with brewing time. | Frequent drinking of black tea contributes to increasing the daily intake of fluoride. |
| Rajiv [65] | To assess fluoride levels in tea after different brewing times and compare them between different tea brands. | Samples were prepared by brewing 1 g of black tea powder in 100 mL of boiling demineralized water or Mettur water. Fluoride concentration was assessed by spectrophotometry. | Higher amounts of fluoride were released into the infusion brewed with Mettur water than with demineralized water. | The fluoride content of tea depends on the fluoride content of the water that is used to brew it. |
| Aktug [61] | Determining the effect of tea brewing time on its color, brightness, and fluoride ion concentration in the infusion. | Samples were prepared by brewing 6 g of tea in 250 mL of boiling water, heating for 15 to 18 min. Fluoride concentration was determined using an ion-selective electrode. | At 15 min, the fluoride ion concentration reaches a maximum and remains constant, but the color of the infusion continues to darken. | The fluoride concentration in tea infusions reaches a plateau after 20 min of brewing using the Turkish method. |
| Essebbahi [60] | To determine the fluoride content in green and black tea and well water consumed by the population of seven rural regions of Morocco. | A total of 12 samples of green and black tea were prepared. Each was brewed for 10 and 30 min. Fluoride levels were determined using the Belcher–West colorimetric method. | Fluoride levels tested after 30 min of brewing are higher than those after 10 min of brewing. The fluoride content of tea and the way it is consumed pose a risk of dental fluorosis. | The fluoride content of boiled teas is higher than that of teas brewed for 30 or 10 min. Three types of green tea and one black tea have fluoride levels above recommended standards. |
| Kanrar [69] | Evaluation of fluoride content in Indian tea produced in north-eastern India. | Samples were prepared by brewing 3 g of tea in 150 mL of boiling deionized water for 5 min. Fluorine content was measured using an ion-selective electrode. | The highest fluoride concentration was found in Upper Assam samples, followed by Dooars, South Bank, Cachar, Darjeeling, and Tripura. | The concentration increased with the brewing time and decreased with each subsequent brewing. |
| Author | Tea Mass/Form | Granules and Tea Bags Had the Highest Fluoride Levels. |
|---|---|---|
| Mazurek et al. [28] | 2 g; leaves, bags, granules | Granulated and tea bag samples had higher fluoride content than loose-leaf tea. |
| Giljanović et al. [31] | 2 g (leaves and bags); bottled teas analyzed directly | Tea bags contained higher fluoride concentrations than loose-leaf and bottled tea. |
| Maleki et al. [32] | 1 g; leaves and bags | Tea bags released more fluoride than loose leaves; extraction efficiency reached up to 73.5%. |
| Yuwono et al. [49] | 1 g powder, 1.75–2.25 g bags | Highest fluoride concentrations were found in black tea, followed by green and herbal teas. |
| Chan et al. [52] | 2 g; leaves and bags | Fluoride content was highest in economy blends, followed by green and black teas. |
| Embiale et al. [54] | 1 g; powder and bags | Black tea powder released more fluoride than tea bags; values varied with water fluoride content. |
| Satou et al. [58] | 1 g; leaves and bags | Loose green teas showed higher fluoride levels than bagged herbal teas. |
| Chandrajith et al. [63] | 2 g; loose vs. packed tea | Loose tea had higher fluoride concentrations than bagged tea. |
| Gupta et al. [50] | 2 g; bags, leaves, granules | Granules > leaves > bags in fluoride content. |
| Szmagara et al. [19] | 2 g; leaves and bags | Fluoride content was highest in black tea bags and increased with brewing time. |
| Das et al. [55] | 500 mg powder | Traditional powdered teas showed elevated fluoride levels (up to 6.9 mg/L); herbal teas contained significantly less. |
| Linhares et al. [56] | 2.0 ± 0.5 g bags | Fluoride levels varied by tea origin and were negatively correlated with the pH of brewing water. |
| Author | Parameter(s) | Effect on Fluoride | Results |
|---|---|---|---|
| Liu et al. [68] | Temperature (60–95 °C), repeated brewing | ↑ with temperature, ↓ with each brew | Detailed temperature profile and brewing ratios studied |
| Zhu et al. [51] | Water type (DI vs. drinking), repeated brewing | ↑ with time, variation by water type | Fluoride release higher in drinking water |
| Embiale et al. [54] | Water fluoride content (0.25–30.2 mg/L) | Complex—sometimes ↓ due to absorption | Tea leaves absorb fluoride from highly fluoridated water |
| Chandrajith et al. [63] | Repeated brewing, fermentation | ↓ with each brew, ↑ with fermentation | Loose vs. packed tea also compared |
| Linhares et al. [56] | Water source (different pH/fluoride levels) | ↓ fluoride release with ↑ pH | Water origin matters |
| Rajiv et al. [65] | Water type (distilled vs. natural) | ↑ in natural water | Higher fluoride release in mineralized water |
| Maleki et al. [32] | Water hardness (CaCO3 0–350 mg/L) | ↑ hardness = ↓ fluoride release | Significant reduction in extraction with hard water |
| Morés et al. [48] | Calcium presence | ↓ fluoride extraction | Tea with calcium-rich solution released less fluoride |
| Erdemoglu et al. [67] | pH, Al, and Fe3+ ion concentration | ↑ pH = ↑ fluoride; ↑ Al/Fe = ↓ fluoride | Ion competition affects free fluoride |
| Essebbahi et al. [60] | Brewing time (10 vs. 30 min) | ↑ fluoride with longer time | Limited temperature data |
| Kanrar et al. [69] | Repeated infusion | ↓ fluoride with more brews | Region of origin also noted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małyszek, A.; Kiryk, S.; Kensy, J.; Kotela, A.; Michalak, M.; Kiryk, J.; Janeczek, M.; Matys, J.; Dobrzyński, M. Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review. Appl. Sci. 2025, 15, 5974. https://doi.org/10.3390/app15115974
Małyszek A, Kiryk S, Kensy J, Kotela A, Michalak M, Kiryk J, Janeczek M, Matys J, Dobrzyński M. Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review. Applied Sciences. 2025; 15(11):5974. https://doi.org/10.3390/app15115974
Chicago/Turabian StyleMałyszek, Agata, Sylwia Kiryk, Julia Kensy, Agnieszka Kotela, Mateusz Michalak, Jan Kiryk, Maciej Janeczek, Jacek Matys, and Maciej Dobrzyński. 2025. "Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review" Applied Sciences 15, no. 11: 5974. https://doi.org/10.3390/app15115974
APA StyleMałyszek, A., Kiryk, S., Kensy, J., Kotela, A., Michalak, M., Kiryk, J., Janeczek, M., Matys, J., & Dobrzyński, M. (2025). Identification of Factors Influencing Fluoride Content in Tea Infusions: A Systematic Review. Applied Sciences, 15(11), 5974. https://doi.org/10.3390/app15115974









