The Impact of Drying Method on the Physicochemical, Bioactive Compounds and Antioxidant Properties of Common Quince Fruit (Cydonia oblonga Mill.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Drying Methods
2.3. Measurement of Quantitative and Qualitative Characteristics
2.3.1. Shrinkage
2.3.2. Rehydration Ratio
2.3.3. Bulk Density
2.4. Colour Measurements
2.5. Determination of Total Acidity
2.6. Bioactive Properties
2.6.1. Determination of Tannin Content
- V1—volume of 0.1 M thiosulfate consumed to titrate the control sample;
- V2—volume of 0.1 M thiosulfate consumed to titrate the control sample;
- 0.01039—the number of grams of tannins corresponding to 1 mL of 0.1 M thiosulfate.
2.6.2. Bioactive Compounds Extraction
2.6.3. Total Polyphenol Content (TPC)
2.6.4. Total Flavonoid Content (TFC)
2.6.5. ABTS Radical Scavenging Activity
2.6.6. DPPH Radical Scavenging Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Quantitative and Qualitative Characteristics
3.2. Colour Measurements
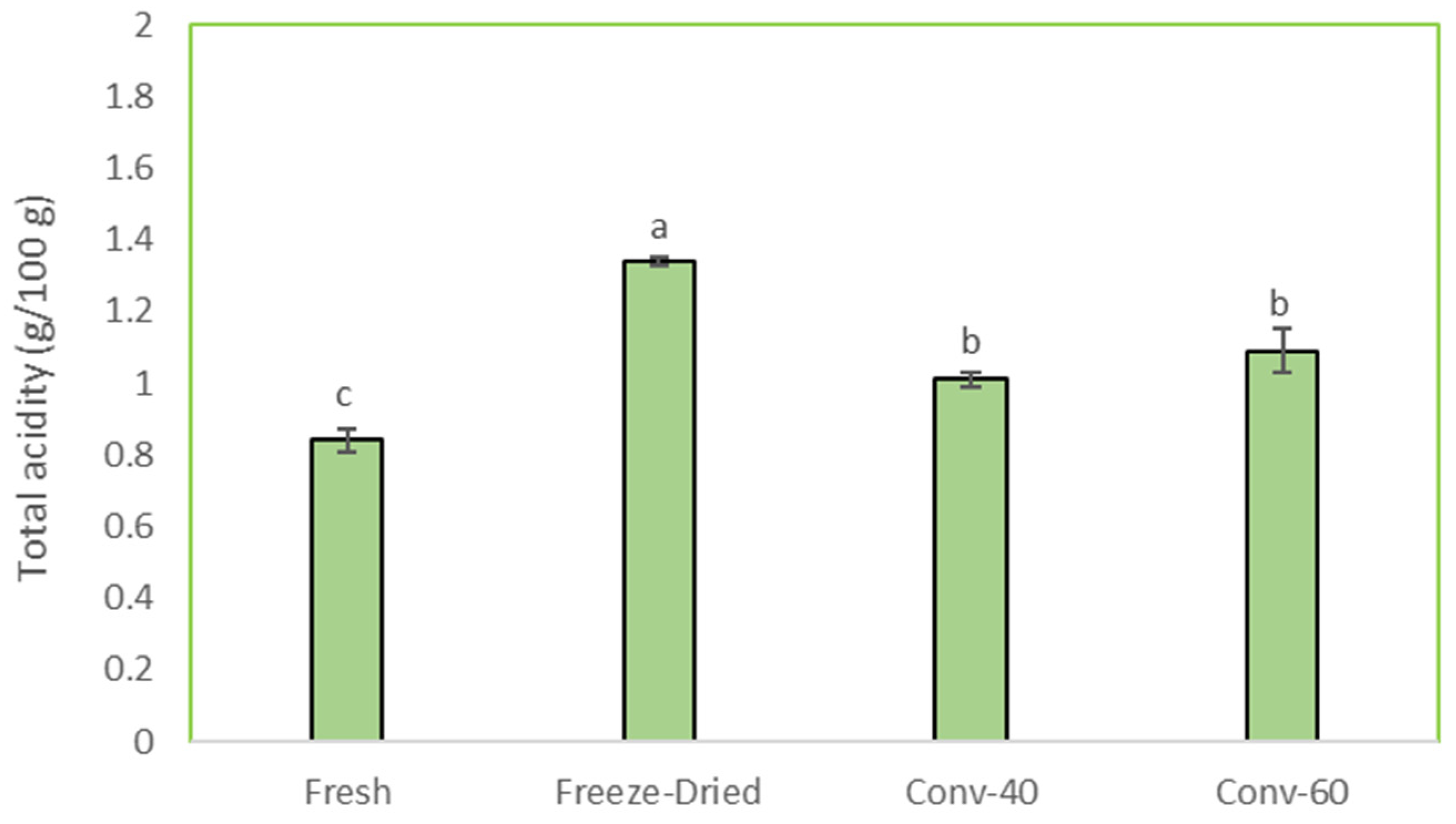
3.3. Total Acidity
3.4. Bioactive Properties
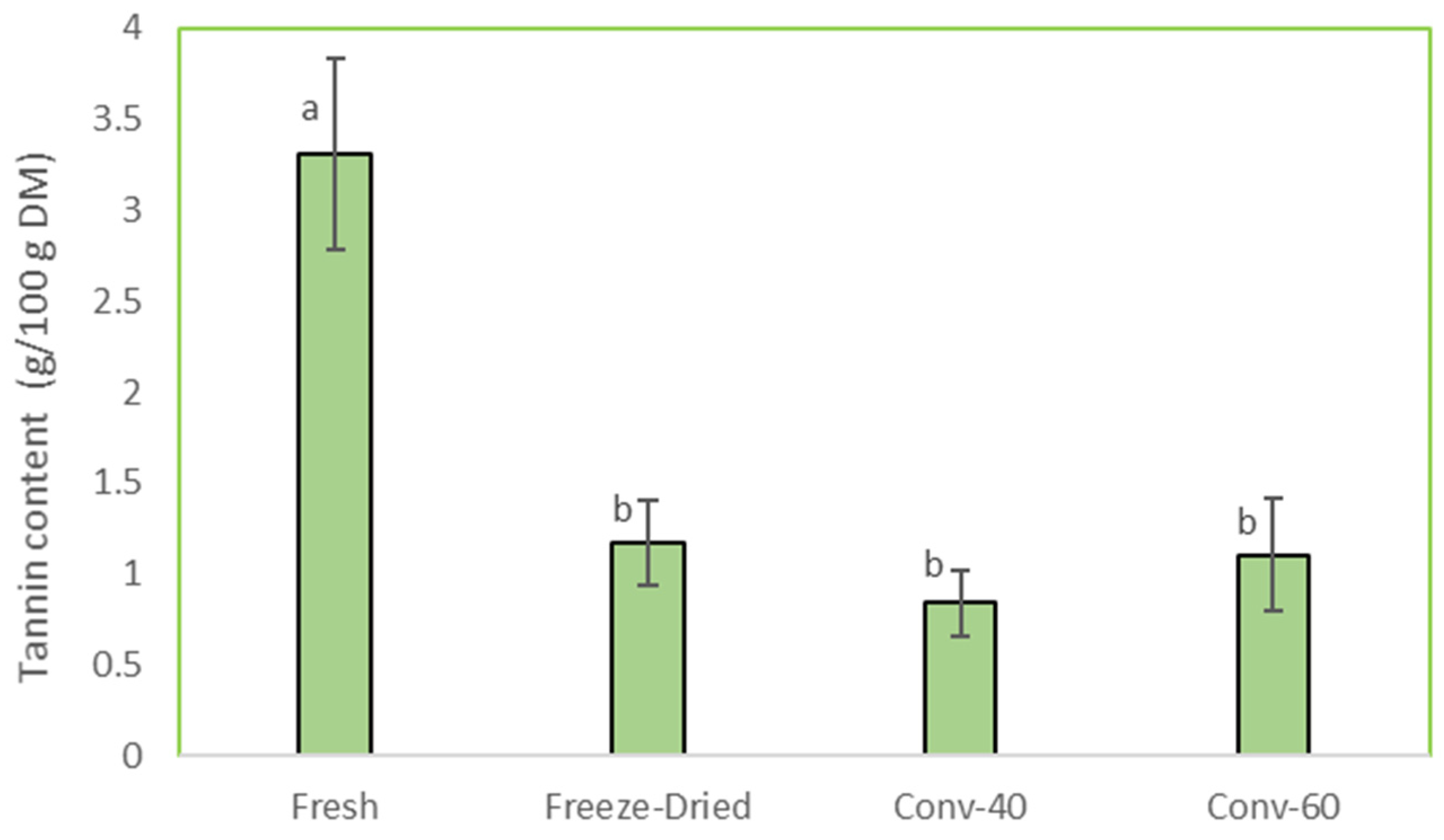
3.4.1. Tannin Content
3.4.2. TPC and TFC
3.4.3. Antioxidant Activity Against ABTS and DPPH
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Zughbi, I.; Krayem, M. Quince Fruit Cydonia Oblonga Mill Nutritional Composition, Antioxidative Properties, Health Benefits and Consumers Preferences towards Some Industrial Quince Products: A Review. Food Chem. 2022, 393, 133362. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Dall’Acqua, S.; Poloniato, G.; Maggi, F.; Malagoli, M. Preliminary Evaluation of Quince (Cydonia oblonga Mill.) Fruit as Extraction Source of Antioxidant Phytoconstituents for Nutraceutical and Functional Food Applications. J. Sci. Food Agric. 2019, 99, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Rop, O.; Balík, J.; Řezníček, V.; Juríková, T.; Škardová, P.; Salaš, P.; Sochor, J.; Mlček, J.; Kramářová, D. Chemical Characteristics of Fruits of Some Selected Quince (Cydonia oblonga Mill.) Cultivars. Czech J. Food Sci. 2011, 29, 65–73. [Google Scholar] [CrossRef]
- Koç, B.; Eren, I.; Kaymak Ertekin, F. Modelling Bulk Density, Porosity and Shrinkage of Quince during Drying: The Effect of Drying Method. J. Food Eng. 2008, 85, 340–349. [Google Scholar] [CrossRef]
- Caliskan, G.; Dirim, S.N. Drying Characteristics of Pumpkin (Cucurbita moschata) Slices in Convective and Freeze Dryer. Heat Mass Transf. 2017, 53, 2129–2141. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Boonyapranai, K.; Laokuldilok, T.; Utama-ang, N.; Nutprem, A.; Kaewprasit, K.; Tatongjai, K. Impact of Different Dehydration Methods on Physicochemical and Functional Properties of Guava (Psidium guajava L.) Powder Prepared from White and Pink Pomaces. Appl. Food Res. 2025, 5, 100696. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A. Quinces (Cydonia oblonga, Chaenomeles Sp., and Pseudocydonia sinensis) as Medicinal Fruits of the Rosaceae Family: Current State of Knowledge on Properties and Use. Antioxidants 2024, 13, 71. [Google Scholar] [CrossRef]
- de Almeida Lopes, M.M.; Guimarães Sanches, A.; de Souza, K.O.; de Oliveira Silva, E. Quince—Cydonia oblonga. In Exotic Fruits Reference Guide; Academic Press: Cambridge, MA, USA, 2018; pp. 363–368. [Google Scholar] [CrossRef]
- Romero, J.; Díez Méndez, A.; Castro-Alija, M.J.; Poveda, J.; Albertos, I. Quince (Cydonia oblonga Mill.) Waste By-Product Characterization as a Potential Functional Ingredient. Sustainability 2024, 16, 8596. [Google Scholar] [CrossRef]
- Yang, Q.; Yi, X.; Xiao, H.; Wang, X.; Liu, L.; Tang, Z.; Hu, C.; Li, X. Effects of Different Drying Methods on Drying Characteristics, Microstructure, Quality, and Energy Consumption of Apricot Slices. Foods 2024, 13, 1295. [Google Scholar] [CrossRef]
- Dehghannya, J.; Hosseinlar, S.H.; Heshmati, M.K. Multi-Stage Continuous and Intermittent Microwave Drying of Quince Fruit Coupled with Osmotic Dehydration and Low Temperature Hot Air Drying. Innov. Food Sci. Emerg. Technol. 2018, 45, 132–151. [Google Scholar] [CrossRef]
- Saricoban, C.; Tahsin Yilmaz, M. Modelling the Effects of Processing Factors on the Changes in Colour Parameters of Cooked Meatballs Using Response Surface Methodology. World Appl. Sci. J. 2010, 9, 14–22. [Google Scholar]
- Polish Standard PN-EN 12147:2000; Fruit and Vegetable Juices—Determination of Titratable Acidity. Polish Committee for Standardization. Department of Standardization Publishing: Warsaw, Poland, 2000.
- Ciszewska, R.; Przeszlakowska, M.; Sykut, A.; Szynal, J. Przewodnik Do Ćwiczeń z Biochemii; Wydawnictwo Akademii Rolniczej w Lubline: Lublin, Poland, 1975; pp. 126–129. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant Activity of Selected Fruits and Vegetables Grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hasbullah, R.; Putra, N.S. Study on the Vacuum Pressure and Drying Time of Freeze-Drying Method to Maintain the Quality of Strawberry (Fragaria virginiana). J. Tek. Pertan. Lampung 2022, 11, 279–291. [Google Scholar] [CrossRef]
- Izli, N.; Polat, A. Freeze and Convective Drying of Quince (Cydonia oblonga Miller.): Effects on Drying Kinetics and Quality Attributes. Heat Mass Transf. 2019, 55, 1317–1326. [Google Scholar] [CrossRef]
- Zalewska, M.; Otreszko-Arski, A.; Zalewski, M. Wpływ Suszenia Konwekcyjnego i Liofilizacji Na Barwę Wybranych Owoców. Apar. Badaw. I Dydakt. 2016, 21, 141–145. [Google Scholar]
- Maskan, M. Kinetics of Colour Change of Kiwifruits during Hot Air and Microwave Drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Barroca, M.J. Quantification of Browning Kinetics and Colour Change for Quince (Cydonia oblonga Mill.) Exposed to Atmospheric Conditions. Agric. Eng. Int. CIGR J. 2014, 16, 285–298. [Google Scholar]
- Orenes-Piñero, E.; García-Carmona, F.; Sánchez-Ferrer, A. Latent Polyphenol Oxidase from Quince Fruit Pulp (Cydonia oblonga): Purification, Activation and Some Properties. J. Sci. Food Agric. 2006, 86, 2172–2178. [Google Scholar] [CrossRef]
- Ali, N.; Abbas, Y.; Ali, A.; Shahnawaz, M.; Hussain, N.; Hussain, A. Physio—Chemical Nutritional and Sensory Evaluation of Local Quince Fruit of Nomal Village, Gilgit—Baltistan, Pakistan. Int. J. Nutr. Food Sci. 2015, 4, 600–608. [Google Scholar] [CrossRef][Green Version]
- Curi, P.N.; Coutinho, G.; Matos, M.; Pio, R.; Albergaria, F.C.; Rios De Souza, V. Characterization and Marmelade Processing Potential of Quince Cultivars Cultivated in Tropical Regions. Rev. Bras. Frutic. 2018, 40, e-986. [Google Scholar] [CrossRef]
- Rasheed, M.; Hussain, I.; Rafiq, S.; Hayat, I.; Qayyum, A.; Ishaq, S.; Awan, M.S. Chemical Composition and Antioxidant Activity of Quince Fruit Pulp Collected from Different Locations. Int. J. Food Prop. 2018, 21, 2320–2327. [Google Scholar] [CrossRef]
- Ionica, M.E.; Corbu, A.R. The Evolution of Some Chemical Compounds of Quince (Cydonia oblonga Mill.) During Growth and Ripening. South West. J. Hortic. Biol. Environ. 2022, 13, 59–66. [Google Scholar]
- Szychowski, P.J.; Munera-Picazo, S.; Szumny, A.; Carbonell-Barrachina, Á.A.; Hernández, F. Quality Parameters, Bio-Compounds, Antioxidant Activity and Sensory Attributes of Spanish Quinces (Cydonia oblonga Miller). Sci. Hortic. 2014, 165, 163–170. [Google Scholar] [CrossRef]
- Sharma, R.; Joshi, V.K.; Rana, J.C. Nutritional Composition and Processed Products of Quince (Cydonia oblonga Mill.). Indian J. Nat. Prod. Resour. 2011, 2, 354–357. [Google Scholar]
- Najman, K.; Adrian, S.; Hallmann, E.; Sadowska, A.; Buczak, K.; Waszkiewicz-Robak, B.; Szterk, A. Effect of Various Drying Methods on Physicochemical and Bioactive Properties of Quince Fruit (Cydonia oblonga Mill.). Agriculture 2023, 13, 446. [Google Scholar] [CrossRef]
- Mir, S.A.; Wani, S.M.; Wani, T.A.; Ahmad, M.; Gani, A.; Masoodi, F.A.; Nazir, A. Comparative Evaluation of the Proximate Composition and Antioxidant Properties of Processed Products of Quince (Cydonia oblonga Miller). Int. Food Res. J. 2016, 23, 816–821. [Google Scholar]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Gonçalves, B. A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules 2025, 30, 800. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Phenolic, Volatile, and Sensory Profiles of Beer Enriched by Macerating Quince Fruits. LWT 2019, 103, 139–146. [Google Scholar] [CrossRef]
- Benahmed Djilali, A.; Mehraz, R.; Bouacem, K.; Benseddik, A.; Moualek, I.; Nabiev, M.; Benzara, A. Bioactive Substances of Cydonia Oblonga Fruit: Insecticidal Effect of Tannins on Tribuliumm confusum. Int. J. Fruit Sci. 2021, 21, 721–731. [Google Scholar] [CrossRef]
- Ňorbová, M.; Vollmannová, A.; Fedorková, S.; Musilová, J.; Lidiková, J. The Forgotten Fruit (Cydonia oblonga Mill.) and Its Chemical Composition: A Review. Eur. Food Res. Technol. 2024, 250, 2093–2102. [Google Scholar] [CrossRef]
- Umar, A.; Iskandar, G.; Aikemu, A.; Yiming, W.; Zhou, W.; Berké, B.; Begaud, B.; Moore, N. Effects of Cydonia oblonga Miller Leaf and Fruit Flavonoids on Blood Lipids and Anti-Oxydant Potential in Hyperlipidemia Rats. J. Ethnopharmacol. 2015, 169, 239–243. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Tkacz, K.; Nowicka, P.; Michalska-Ciechanowska, A.; Lech, K.; Wojdyło, A. Physicochemical Characterization and Biological Potential of Japanese Quince Polyphenol Extract Treated by Different Drying Techniques. LWT—Food Sci. Technol. 2021, 152, 112247. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Dek, M.S.P.; Hairuddin, M.R. Effect of Freeze-Drying on the Antioxidant Compounds and Antioxidant Activity of Selected Tropical Fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef]
- Bayram, H.M.; Ozkan, K.; Ozturkcan, A.; Sagdic, O.; Gunes, E.; Karadag, A. Effect of Drying Methods on Free and Bound Phenolic Compounds, Antioxidant Capacities, and Bioaccessibility of Cornelian Cherry. Eur. Food Res. Technol. 2024, 250, 2461–2478. [Google Scholar] [CrossRef]
- Stamenković, Z.; Pavkov, I.; Radojčin, M.; Horecki, A.T.; Kešelj, K.; Kovačević, D.B.; Putnik, P. Convective Drying of Fresh and Frozen Raspberries and Change of Their Physical and Nutritive Properties. Foods 2019, 8, 251. [Google Scholar] [CrossRef]
- Donno, D.; Neirotti, G.; Fioccardi, A.; Razafindrakoto, Z.R.; Tombozara, N.; Mellano, M.G.; Beccaro, G.L.; Gamba, G. Freeze-Drying for the Reduction of Fruit and Vegetable Chain Losses: A Sustainable Solution to Produce Potential Health-Promoting Food Applications. Plants 2025, 14, 168. [Google Scholar] [CrossRef]
- Antoniewska, A.; Rutkowska, J.; Pineda, M.M. Antioxidative, Sensory and Volatile Profiles of Cookies Enriched with Freeze-Dried Japanese Quince (Chaenomeles japonica) Fruits. Food Chem. 2019, 286, 376–387. [Google Scholar] [CrossRef] [PubMed]
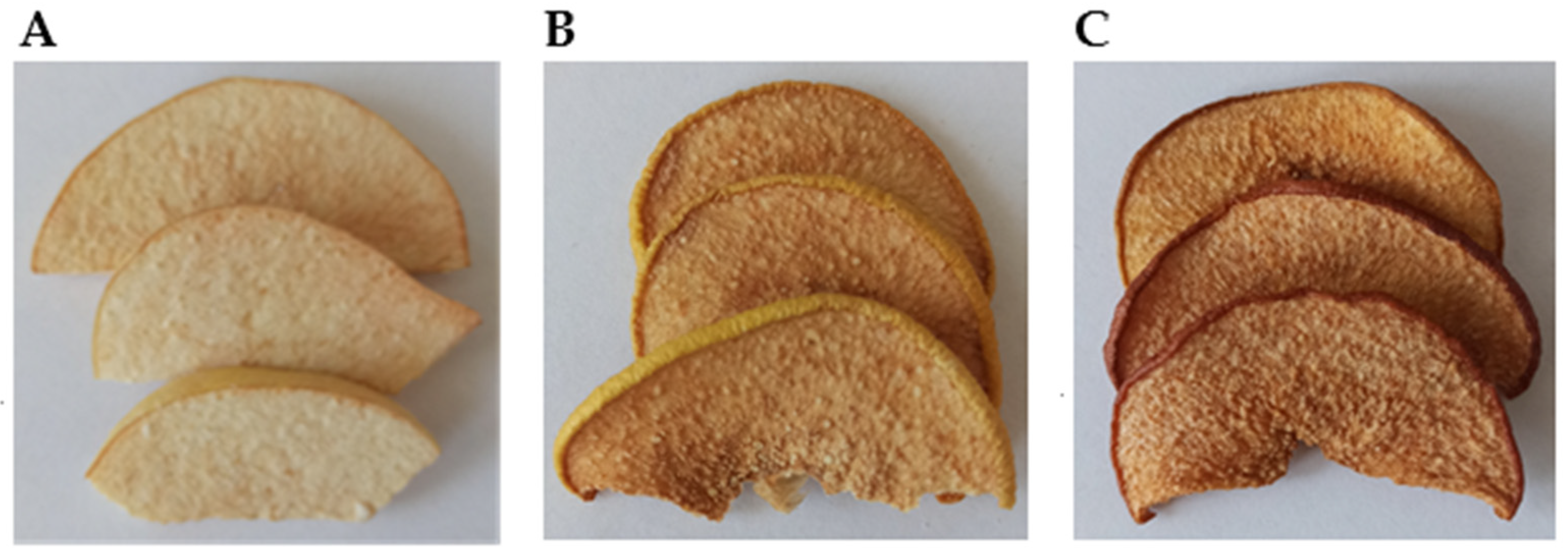
| Characteristics | Freeze-Dried | Conv-40 | Conv-60 |
|---|---|---|---|
| rehydration ratio | 3.53 ± 0.04 a | 2.58 ± 0.06 b | 1.84 ± 0.04 c |
| shrinkage rate [%] | 9.87 ± 0.29 c | 57.33 ± 0.55 b | 66.03 ± 0.8 a |
| bulk density [g/cm3] | 0.41 ± 0.01 c | 0.63 ± 0.02 b | 0.71 ± 0.02 a |
| Parameter | Fresh | Freeze-Dried | Conv-40 | Conv-60 |
|---|---|---|---|---|
| L* | 75.70 ± 1.71 b | 79.66 ± 1.85 a | 57.56 ± 1.82 c | 58.45 ± 3.78 c |
| a* | 5.21 ± 0.95 d | 10.48 ± 1.80 c | 17.42 ± 0.86 b | 18.92 ± 1.14 a |
| b* | 27.26 ± 1.77 d | 30.28 ± 2.22 c | 33.91 ± 2.22 b | 37.85 ± 1.84 a |
| ΔE | - | 7.25 | 22.85 | 24.25 |
| BI | 5.03 | 9.40 | 22.28 | 20.90 |
| Fresh | Freeze-Dried | Conv-40 | Conv-60 | |
|---|---|---|---|---|
| Total polyphenol content (mg GEA/100 g DM) | 259.68 ± 23.26 a | 233.56 ± 5.96 ab | 205.87 ± 12.90 b | 141.79 ± 9.83 c |
| Total flavonoid content (mg EPI/100 g DM) | 40.17 ± 0.48 a | 36.79 ± 0.74 b | 25.99 ± 0.21 c | 22.41 ± 0.70 d |
| ABTS scavenging activity (µM Trolox/100 g DM) | 448.62 ± 4.23 a | 364.51 ± 9.12 b | 283.56 ± 8.26 c | 232.12 ± 11.47 d |
| DPPH scavenging activity (µM Trolox/100 g DM) | 328.85 ± 16.98 a | 258.78 ± 5.16 b | 172.85 ± 15.51 c | 144.24 ± 5.83 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgórska-Kryszczuk, I.; Pankiewicz, U. The Impact of Drying Method on the Physicochemical, Bioactive Compounds and Antioxidant Properties of Common Quince Fruit (Cydonia oblonga Mill.). Appl. Sci. 2025, 15, 6122. https://doi.org/10.3390/app15116122
Podgórska-Kryszczuk I, Pankiewicz U. The Impact of Drying Method on the Physicochemical, Bioactive Compounds and Antioxidant Properties of Common Quince Fruit (Cydonia oblonga Mill.). Applied Sciences. 2025; 15(11):6122. https://doi.org/10.3390/app15116122
Chicago/Turabian StylePodgórska-Kryszczuk, Izabela, and Urszula Pankiewicz. 2025. "The Impact of Drying Method on the Physicochemical, Bioactive Compounds and Antioxidant Properties of Common Quince Fruit (Cydonia oblonga Mill.)" Applied Sciences 15, no. 11: 6122. https://doi.org/10.3390/app15116122
APA StylePodgórska-Kryszczuk, I., & Pankiewicz, U. (2025). The Impact of Drying Method on the Physicochemical, Bioactive Compounds and Antioxidant Properties of Common Quince Fruit (Cydonia oblonga Mill.). Applied Sciences, 15(11), 6122. https://doi.org/10.3390/app15116122







