Abstract
This work presents the results of a multi-technique comparative investigation aimed at assessing the mineralogical composition and radioactivity levels of two stone fragments from different areas of the archaeological site of Halaesa (Sicily, Italy). The analysis employed an integrated approach combining μ-energy-dispersive X-ray fluorescence (μ-EDXRF) spectroscopy, µ-Raman spectroscopy, X-Ray Diffraction (XRD), ion chromatography (IC), High-Purity Germanium (HPGe) gamma spectrometry, and E-PERM electret ion chamber methods. By examining the stone composition at both the elemental and molecular scales, with support from ion chromatography data, potential degradation patterns linked to post-depositional weathering and external decay agents were identified. Moreover, the specific activity of radionuclides (226Ra, 232Th, and 40K) and the 222Rn exhalation rates were measured, enabling the estimation of a set of radiological indices that assess potential health hazards associated with prolonged exposure to these lithic materials. The findings highlight how a multidisciplinary approach can foster the assessment of stone deterioration mechanisms, supporting the design of optimized conservation strategies aimed at preserving the archaeological heritage of Halaesa and ensuring the safety of both the public and onsite personnel.
1. Introduction
The archaeological site of Halaesa, situated in Northern Sicily (Italy), is one of the most significant centers from the Hellenistic–Roman period on the island. Founded in 403 BCE, the site features a wide range of buildings and artifacts that offer valuable insights into the historical, architectural, and cultural evolution of ancient Sicily.
Archaeological excavations at Halaesa began in the late 19th century, when Antonio Salinas discovered the so-called colombario and part of the southern necropolis in 1899. Systematic excavations started in 1942 under Luigi Bernabò Brea, with further campaigns conducted between 1952 and 1956 by Gianfilippo Carettoni, who explored sections of the fortifications, the Sanctuary of Apollo, and other key areas of the city [1,2]. Research resumed in the 1970s with Giacomo Scibona’s excavation of the monumental agora. In 2003–2004, the Superintendency of Messina expanded the excavation area to include the Cardo Maximus and the entire southern sector of the city. These studies revealed Halaesa’s monumental character, featuring an orthogonal layout with parallel and perpendicular streets, as well as large public buildings [3].
Since 2016, archaeological research at Halaesa has intensified, with multiple teams exploring different areas of the site. While the joint Italian–English mission from the Universities of Messina and Oxford has focused on the Northern Acropolis at the Sanctuary of Apollo [4], the University of Palermo has investigated the eastern fortifications and public baths [5], while the French team from the University of Amiens has concentrated on the Southern Acropolis and the theatre area [6]. These excavations have shown that argillite, a schistose stone abundantly found in the Halaisos River valley, was the primary material used in the public and private buildings of the ancient Halaesa. This locally sourced argillite was widely employed, particularly in the construction of the Sanctuary of Apollo’s walls, built during the Hellenistic–Roman period. Previous archaeological test pits have revealed that these blocks are in a state of severe deterioration, characterized by heavy fragmentation and crumbling, especially on upper surfaces subjected to weathering and chemical pollutants.
As a matter of fact, the long-term weathering behavior of archaeological masonry materials is influenced by various environmental stress factors, including air pollution, moisture infiltration, salt crystallization, biological colonization, and thermal fluctuations, depending on the particular burial/laying conditions to which the material has been subjected over time [7,8,9,10,11,12,13,14]. These factors interact with the materials’ inherent properties, leading to surface erosion, loss of cohesion, discoloration, and efflorescence, strongly affecting the durability and vulnerability of ancient structures [15,16,17,18]. Moreover, recent studies have also shown that buildings can act as reservoirs for metal pollutants, which may pose risks to both human health and the environment, especially during restoration activities involving contaminated materials [19].
In this context, the analysis of archaeological remains through scientific methods represents a well-established approach in the view of developing optimized conservation strategies and preventive restoration plans. The possibility of retrieving the material composition of archaeological matrices—at both the elemental and molecular scales—along with the identification of the primary decay processes and external pollutants, provides a fundamental building block for assessing the vulnerability and long-term usability of an archaeological site [20,21,22,23]. These analyses often require a multidisciplinary approach involving mineralogical and physicochemical techniques [24,25,26,27,28,29,30], bringing together experts from various fields. Beyond material characterization, the integration of spectroscopic data with information on the natural activity concentration of radionuclides (e.g., uranium-238 (238U), thorium-232 (232Th), and potassium-40 (40K)) is gaining increasing importance for both environmental safety and heritage conservation purposes [31,32,33]. Continuous monitoring of radioactivity levels is crucial to ensure compliance with radiological protection standards, especially in popular tourist destinations.
In this context, the integration of the mineralogical data with activity concentration analyses offers a novel approach to conservation. This method supports the development of comprehensive preservation strategies that not only enhance the quality of restoration efforts but also ensure the safety of workers and the surrounding environment.
As part of ongoing efforts to study and preserve the material heritage of Halaesa, this work reports the results of a comparative analysis of the mineralogical composition and radioactivity levels of two stone fragments (approx. 50 × 30 × 30 cm3) sampled from different areas of the ancient city of Halaesa Archonidea. One fragment is representative of stones used in the construction of the archaeological structures (specifically the Sanctuary of Apollo at the Northern Acropolis), while the other serves as a “reference”, having been collected from the surrounding outcrop. For this purpose, a combined approach involving μ-energy-dispersive X-ray fluorescence (μ-EDXRF) spectroscopy, µ-Raman spectroscopy, X-Ray Diffraction (XRD), ion chromatography (IC), High-Purity Germanium (HPGe) gamma spectrometry, and E-PERM electret ion chamber methods was adopted, with the aim of (i) obtaining insights into the stone composition at both the elemental and molecular scales, with particular regards to potential compositional variations between the two fragments due to weathering, mineral heterogeneity, and environmental exposure; (ii) assessing any degradation phenomena, including weathering agents and potential chemical contamination; and (iii) monitoring the specific activity concentrations of 226Ra, 232Th, and 40K, as well as of the mean specific 222Rn exhalation rate, to assess potential radiological hazards to public health. All of the aforementioned aspects aimed to shed light on a series of open research questions, including to what extent the composition of stones used for the Sanctuary of Apollo differs from that of the surrounding outcrop materials, in order to assess whether the construction materials were locally sourced, and what are the principal weathering processes or degradation phenomena affecting the investigated fragments, to quantify any post-depositional changes that could affect their potential impact on the long-term preservation of the archaeological site. At the same time, to address any potential health and safety concerns associated with prolonged exposure to these materials in humans, especially in the context of site accessibility and conservation efforts, a monitoring of the levels of natural radioactivity and radon exhalation turned out to be crucial, thereby strengthening the interpretive framework for both archaeological analysis and radioprotection/conservation plans.
It is worthy of note that the obtained results not only provide valuable data for addressing the aforementioned open questions related to the investigated archaeological record, including the understanding of the geological setting and stone–soil interactions, but also furnish a solid background for the accurate evaluation of the radiological risk associated with natural stone materials.
2. Archaeological and Geological Context
The ancient city of Halaesa Archonidea is located on the eastern slope of a hill overlooking the Tyrrhenian Sea (see Figure 1), along the lower valley of the Tusa stream, known in antiquity as the Halaisos River.
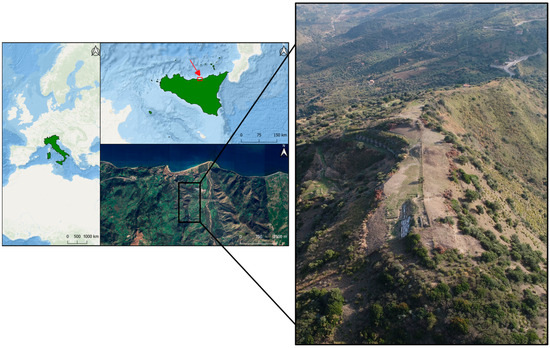
Figure 1.
Geolocation map of the archaeological site of Halaesa (Sicily, Italy), with a drone view of the ancient city of Halaesa Archonidea (from the north).
According to ancient sources, the city was founded in 403 BC by Archonides, lord of Herbita, on a hill approximately 8 stadia (about 1500 m) from the sea [34]. During the Hellenistic–Roman period, Halaesa was renowned for its great economic prosperity, largely driven by maritime trade. However, it was particularly notable for its privileged relationship with Rome [35]. Along with four other cities in Sicily, it was granted significant privileges, including tax immunity. Cicero even listed Halaesa among the civitates immunes ac liberae [36], cities that enjoyed both tax exemption and self-governance. In the Augustan era, Halaesa attained the status of municipium, and throughout the imperial age, it remained one of the most significant cities on Sicily’s northern coast. It was regarded as the principal port where agricultural products from the island’s interior were gathered before being shipped to Rome [35].
Most of the buildings in the city, many of which remain partially visible, were constructed during the Hellenistic–Roman period, a time of significant architectural development. For their construction, the inhabitants primarily used locally sourced materials, e.g., argillite, ensuring both availability and practicality.
To date, archaeological investigations of the Sanctuary of Apollo have focused solely on its accessible areas, as the western half remains on private property. Despite this limitation, excavations have revealed a large square, with a rectangular platform over 40 m long in the northern half. On top of this platform stood three Roman–Italic-type temples, all facing east. Only the massive foundations of these temples, built from squared blocks of local argillite, remain in situ (Figure 2).
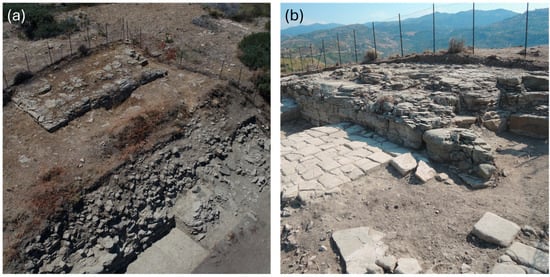
Figure 2.
(a) Aerial and (b) eastern views of a rectangular platform over 40 m long in the northern half of the archaeological site of Halaesa (Sicily, Italy).
In the largest central temple, remnants of the cella floor, made of white tesserae, are still visible [4,37]. The platform’s perimeter walls were constructed using a mixed technique, combining argillite and sandstone blocks. These walls cover a natural argillite outcrop, still visible in some areas, which was probably leveled in antiquity to create a more regular platform shape. The same rock outcrop was also encountered in recent excavations south of the square, conducted between 2023 and 2024. These excavations have uncovered the southern boundary of the terrace, marked by a wall made entirely of argillite blocks, along with a structure made using a mixed technique, identified as a monumental stepped altar [38].
From a geological point of view, the investigated archaeological area falls into the Nebrodi Mountains, which are geologically part of the Apennine–Maghrebian Chain, one of the three main orogenic domains in the central Mediterranean [39]. The hill has a maximum height of 250 m and is located on the hydrographic left of the Tusa stream. In particular, the outcrop area falls within the varied formation called “Tusa Tufites” [40], which constitute an arenaceous pelite unit with a variable sandstone/pelite ratio (see Figure 3).
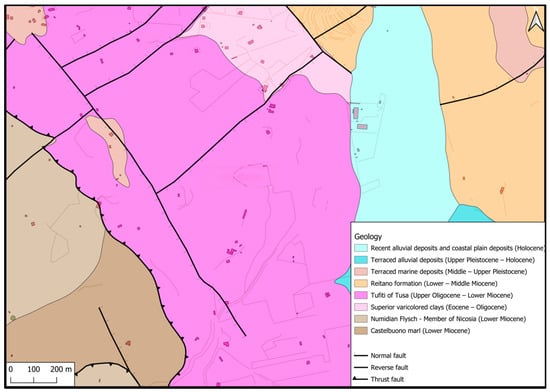
Figure 3.
Geological map of the investigated area.
The lithofacies present in the area are made up of an alternation of micaceous arenites and grey-green tuffites, generally poorly cemented, in layers of 15–40 cm up to banks of 1–2 m, with thin clayey-siltsomarly interlayers and, subordinately, whitish marly limestones with conchoidal fractures and medium coarse-grained calcarenites in thin layers [41].
3. Materials and Methods
3.1. Sampling
The investigated samples consisted of two large stone pieces (approx. 50 × 30 × 30 cm3) collected from different areas of the hill where the ruins of Halaesa Archonidea are located. The first sample, referred to as “SP1”, is representative of the stones used in the construction of the archaeological structures at the site of Halaesa. The second sample, labeled “SP2”, was collected from the surrounding outcrop and served as the reference material.
Specifically, SP1 was sampled near the ancient city buildings, unearthed during the 2017 excavation, directly from the Sanctuary of Apollo (GPS coordinates: 38°0′1.69″ N and 14°15′41.10″ E). SP2, on the other hand, was collected from the natural argillite bed incorporated into the city’s defensive walls, located in the southern sector, along the road connecting the Church of S. Maria delle Palate to the southern necropolis (GPS coordinates: 37°59′40.06″ N and 14°15′39.17″ E).
A preliminary visual inspection revealed that sample SP1 was in a poor state of conservation, with a surface scattered with uniformly distributed, shining grains spread over the bulk structure, suffering loss of the cementitious matrix. Conversely, sample SP2 appeared to be macroscopically well preserved; however, it exhibited a tendency to experience surface exfoliation upon exposure, leading to rapid flaking and crumbling.
The topographic map of the investigated area, together with the position of the sampling areas, is shown in Figure 4.
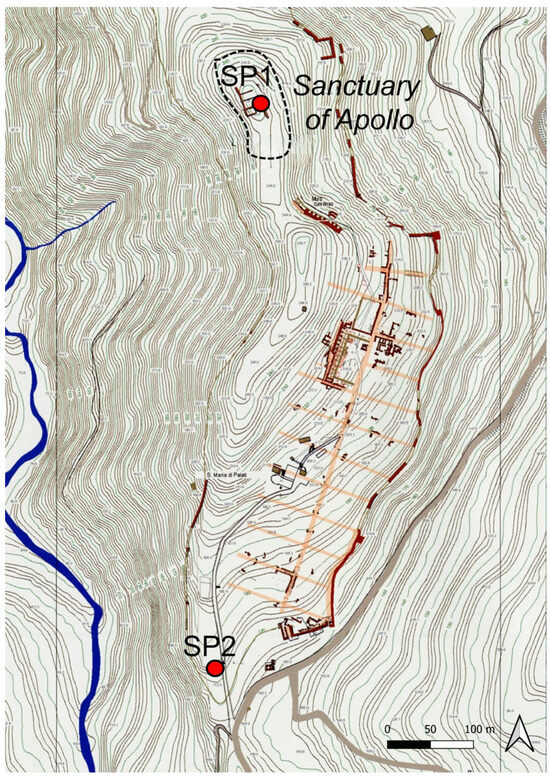
Figure 4.
Topographic map of the investigated area, together with the position of the sampling areas.
For laboratory analyses, multiple aliquots were prepared from the larger stone fragments collected in the field. These aliquots were selected as representative of different regions within each sample, with the aim of capturing potential compositional variations due to weathering, mineral heterogeneity, or environmental exposure. Prior to analysis, each aliquot was subjected to gentle cleaning to remove loose surface particles, and no chemical treatments or abrasive cleaning procedures were applied. No additional sample treatment was performed, in order to preserve the integrity of the weathered surfaces and maintain their potential textural or mineralogical heterogeneities. Then, μ-EDXRF and µ-Raman measurements were carried out on the samples as such, whereas, prior to carrying out the XRD and IC investigations, the representative lithic fragments were finely ground.
3.2. μ-Energy-Dispersive X-Ray Fluorescence (μ-EDXRF) Spectrometry
μ-EDXRF measurements were performed on aliquots representative of both the bulk matrix and surface areas of the SP1 and SP2 samples, labeled as SP#/bulk and SP#/surf (where # = 1 or 2), using a dual M4 TORNADO EDXRF spectrometer (Bruker Nano GmbH, Berlin, Germany). This instrument is equipped with two micro-focus side-window Rh X-ray tubes, powered by low-power HV generators and cooled by air. The first tube operates within a range of 10–50 kV and 100–600 μA, and it is connected to a polycapillary optic system, enabling lateral/spatial resolutions of 25 μm measured for Mo-Kα (around 17 mm at 2.3 keV to 32 μm at 18.3 keV). The second X-ray tube, capable of operating at a maximum of 50 kV and 700 μA, is connected to a mechanical collimator, which allows for X-ray spot sizes of 1 mm. Fluorescence radiation was detected using an XFlash® silicon drift detector with a 30 mm2 sensitive area and an energy resolution of 145 eV for Mn-Kα. For each acquisition, a live time of 150 s was employed. The average elemental compositions were calculated from 5 to 10 measurements per sample, using the polycapillary systems.
To enhance the detection of the light elements (Z > 11), measurements were acquired under vacuum conditions (20 mbar), achieved using an MV 10 N VARIO-B diaphragm pump. Data collection and processing were performed using the M4 TORNADO software (ver. 1.5.2.48, Bruker Nano GmbH, Berlin, Germany), whereas the semi-quantitative analyses were carried out using the M-Quant deconvolution software (ver. 1.6.0.286). All results were normalized with respect to carbon (C) and are presented in weight percent (wt.%).
3.3. µ-Raman Measurements
An inVia confocal Renishaw Raman microscope was used to perform the molecular characterization. The spectrometer was equipped with a Peltier-cooled CCD detector coupled with a DMLM Leica microscope, featuring a motorized XYZ positioning stage with integrated position sensors on the X and Y axes. The measurements were carried out using the 20× and 50× objectives, together with a 785 nm excitation laser. The measurement conditions were adjusted as needed for each sample to minimize fluorescence interference and prevent photothermal degradation. Instrumental calibration was performed daily using the characteristic silicon band at 520.5 cm−1. Raman spectra were acquired and processed using the Wire 3.0 Renishaw software. For spectral interpretation and phase identification, reference data from the RUFF spectral database were employed [42].
3.4. X-Ray Diffraction (XRD) Measurements
XRD measurements were carried out using a X’pert PRO diffractometer equipped with a copper tube (λCuKαmean = 1.5418 Å, λCuKα1 = 1.54060 Å and λCuKα2 = 1.54439 Å), a vertical goniometer (Bragg–Brentano geometry), a programmable divergence aperture, automatic interchange of samples, a secondary graphite monochromator, and a PixCel detector. The operating conditions for the copper tube were 40 kV and 40 mA, with an angular range (2θ) scanned between 5° and 70°. The resulting diffractograms were processed and interpreted using PANalytical X’pert HighScore software, in combination with the Power Diffraction Filedatabase (PDF-2) provided by the International Centre for Diffraction Data (ICDD) [43].
3.5. Ion Chromatography (IC) Measurements
Soluble salt determination was conducted using an ultrasound-assisted extraction method [44], which is an effective alternative to the standard EN 16455/2014 [45] procedure. A single representative aliquot was used for each of SP1 and SP2. These were obtained by combining material from both the surface and bulk portions of the respective stones, to provide an integrated overview of their ionic contents. The lithic fragments were finely ground, homogenized, and dried. Then, 0.1 g of each powder was suspended in 100 mL of Milli-Q water. The samples were introduced in an ultrasound bath for 2 h with a frequency of 40 kHz. For the extract filtration, Millipore 0.45 μm PTFE disk filters were used, and the solutions were stored at 4 °C in polypropylene tubes until the analysis.
A modular chromatographic and titrimetric system was used for the simultaneous quantitative analysis of dissolved cations, anions, carbonate, and bicarbonate in the prepared solutions. The Metrohm analytical system consisted of two 930 Compact IC Flex systems configured as a dual-channel ion chromatograph, with IC conductivity detectors, an 888 Titrando automatic titrator, and an 815 Robotic USB Sample Processor for high-throughput automation.
In the case of anions, a Metrosep A Supp 7–250/4.0 column was used for the separation, and 3.6 mM Na2CO3 was used as the mobile phase, at a flow rate of 0.8 mL/min, with an MSM (Metrohm Suppressor Module) for chemical suppression to achieve a baseline of 12 µS/cm. Moreover, an MCS (Metrohm CO2 Suppressor) was also used to obtain a baseline below 2 µS/cm and, therefore, to improve the limits of detection. The quantification of cations was conducted using a Metrosep C 6-150/4.0 column from Metrohm. HNO3 1.7 mM/dipicolinic acid 1.7 mM was used as the mobile phase at a flow rate of 1 mL/min. The system has inline ultrafiltration technology, which filters the samples down to 0.2 µm particle size, with generic cellulose filters to protect the modular system before the injection. Once filtered, samples were injected into the two injectors, for anion and cation analysis, respectively, each with an injection volume of 20 µL. For the carbonate/bicarbonate analysis, an 888 Titrando Titrator with an 801 magnetic stirrer, 20 mL exchange unit, and a combined “Ecotrode Plus” pH glass electrode and AgCl/Ag reference electrode were used. Moreover, an automatic dosing system that makes highly accurate dosing possible (down to 10 µL additions) was used. The software used was MagIC 3.2 and Tiamo 2.5 for the IC and titration systems, respectively. The measurements were performed in triplicate, and the presented data correspond to the average values.
3.6. High-Purity Germanium (HPGe) Gamma Spectrometry
For HPGe analysis, 5 cube-shaped aliquots (approx. 5 × 5 × 5 cm3) were prepared from each rock by cutting the larger stone fragments using a circular saw, each treated in the laboratory as reported in [46,47].
A live time of 70,000 s was employed, and the activity concentrations were evaluated as follows:
- (i)
- 226Ra: based on the 295.21 and 351.92 keV (214Pb) and 1120.29 keV (214Bi) gamma-ray lines;
- (ii)
- 232Th: using the 911.21 and 968.97 keV (228Ac) gamma-ray lines;
- (iii)
- 40K: evaluated from its 1460.8 keV gamma-ray line [48].
Gamma spectrometry analysis was performed using an electrically cooled Ortec HPGe detector housed within lead wells to minimize background radiation, i.e., a direct biased semiconductor with 1.85 keV FWHM resolution, 40% relative efficiency, 64:1 peak-to-Compton ratio, bias voltage of 4000 V, and an energy range of 50 keV–2 MeV. The Eckert and Zigler Nuclitec GmgH (Braunschweig, Germany) traceable multinuclide radioactive standard, number BC4464, covering the energy range 59.54 keV–1836.09 keV, was employed to perform efficiency and energy calibrations [49]. The precise geometry of the sample was replicated by this calibration standard in an epoxy resin matrix water equivalent. Data collection and analysis were performed using Ortec Gamma Vision software (ver. 8).
The activity concentration (Bq kg−1 dry weight, d.w.) of each radioisotope was quantified as follows:
with NE, εE, γd, M, and t representing the net area, efficiency for energy E, decay probability of the gamma photon, dry mass of the sample (kg), and acquisition time (s), respectively.
For the assessment of the combined standard measurement uncertainty at coverage factor k = 2, the counting statistics, nuclear data library, calibration efficiency, sample quantity, and self-absorption correction were addressed [50]. The detection limit evaluation was accomplished through the RISO method [50]. Moreover, in order to take into account self-absorption (SA) processes, it is important to underline that, for photons with energy greater than 100 keV, SA depends almost exclusively on the density of the sample, while for photons characterized by energy less than 100 keV, it is also necessary to consider the effect of the chemical composition of the investigated matrix. Accordingly, the sample’s bulk density must be quantified to check whether it is within the range of acceptability defined by the laboratory [50]. In cases where it is also necessary to consider the effect of the chemical composition of the sample, the same should be estimated at least approximately (also using the literature data), and it should be verified that it is compatible with the chemical composition of the matrix used for calibration or used as input data in the application of numerical correction methods. In our case, SA corrections were carried out using Gamma Vision software (ver. 8) [51].
Finally, the elevated quality of HPGe analysis outcomes was formally certified by the Italian Accreditation Body (ACCREDIA) [52], which implies the continuous verification (with an annual periodicity) of the maintenance of the performance characteristics of the gamma spectrometry method. In particular, the repeatability of the results was verified over time using the double test method. A certified reference material (also containing the radionuclides 40K and 137Cs) as analyzed twice, with the active concentration of the radionuclide of interest being defined as (first measurement) and (second measurement). The probability level p = 0.95 was considered. The following formula was used:
where t is the Student variable, while sr is the standard deviation of repeatability obtained in the validation phase.
3.7. Radiological Health Hazards
3.7.1. Absorbed Gamma Dose Rate
The absorbed gamma dose rate, D (nGy h−1), for indoor exposure, was quantified as follows [53]:
where CRa, CTh, and CK correspond to the mean specific activities (average value of the five analyzed aliquots) of 226Ra, 232Th, and 40K in the analyzed rocks, respectively.
D = 0.92CRa + 1.1CTh + 0.08CK,
3.7.2. Annual Effective Dose Equivalent
To evaluate the annual effective dose equivalent (AEDE) (mSv y−1), the following formula was applied, incorporating an 80% employment factor to account for indoor exposure, on the basis of the standard room model reported in [53]:
AEDE = (D − 50) × 8760 h × 0.7 Sv Gy−1 × 0.8 × 10−6.
This value should be below 1 mSv y−1 to guarantee a negligible radiological hazard [46,54].
3.7.3. Activity Concentration Index
The European Commission has established an activity concentration index (ACI) to assess whether the dose criterion is met [53]:
ACI = (CRa/300 + CTh/200 + CK/3000).
This index is associated with the reference level for external exposure to gamma radiation released by building materials indoors (AEDE), in addition to outdoor exposure, i.e., 1 mSv y−1 [46,54]. In this sense, the ACI serves primarily as a tool to identify materials that may pose a risk in building applications, suggesting avoiding materials with ACI > 1, i.e., corresponding to AEDE values higher than 1 mSv y−1.
3.7.4. Alpha Index
The alpha index was evaluated as follows [55]:
Iα = CRa/200.
This index provides a measure of the exposure to alpha radiation from indoor radon released by building materials. To ensure minimal exposure to radon indoors, the specific activity must be kept below 200 Bq m−3. This can be achieved by guaranteeing that the activity concentration of 226Ra remains under 200 Bq kg−1. Consequently, for a very low risk of radiation exposure, the alpha index Iα must be less than 1.
3.8. 222Rn Exhalation Rate
To determine the radon exhalation rate from each aliquot of the analyzed stones, measurements of the 222Rn activity that accumulated in a vessel after a specified build-up time were carried out through E-PERM electret ion chambers. During each run, temperature and humidity factors were constantly monitored and controlled. This method is referred to as the “accumulator method” [56] (see Figure 5).
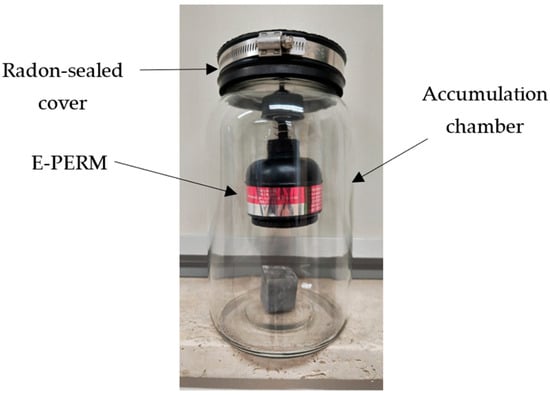
Figure 5.
A schematic drawing of the experimental setup.
The variation in 222Rn specific activity, CRn (Bq m−3), over time, in a sealed accumulation vessel, can be expressed as follows:
where E is the specific exhalation rate (Bq kg−1 h−1) from the sample, λRn is the decay constant for 222Rn (h−1), VA is the volume of the accumulation vessel (m3), m is the sample mass (kg), and C0Rn (Bq m−3) is the 222Rn activity concentration at the start of the accumulation time (t = 0) [57].
The E-PERM system operates by leveraging the ions generated during the radon decay process, thereby decreasing the electrical potential of a Teflon disk integrated in an electrostatically charged chamber. The measurement of radon concentration was accomplished through a combination of techniques, employing a 210 mL chamber configured with a short-term electret. Specifically, the radon activity concentration, CRn (Bq m−3), was determined by following the established relation:
with Vi and Vf equal to the initial and final electret voltages, respectively, CF the calibration factor (V per Bq m3 d), T the exposure interval (days), and BG the background-related equivalent radon concentration of natural gamma radiation. In particular, CF was determined as follows:
According to [58], the total volume for the E-PERM chamber, after subtracting the excluded volume, was found to be 3.8 L. Accordingly, the “back diffusion” effect had no impact on the measured radon exhalation rates, since the sample volume was more than ten times smaller than the chamber’s volume.
According to [55], the specific exhalation rate, E (Bq h−1 kg−1), is given by
In this study, the net 222Rn concentration was determined by subtracting the blank reading, which was obtained by placing E-PERM chambers into the empty jar utilized for the experiments and exposing it for the same duration as the experimental setup.
4. Results and Discussion
4.1. μ-EDXRF Analysis
Table 1 presents the average elemental composition obtained through μ-EDXRF analysis for both the bulk matrix and the external surface of the SP1 and SP2 stone samples.

Table 1.
μ-EDXRF elemental data (wt.%) of the bulk matrix and the external parts of the SP1 and SP2 stones. Values are reported as the mean ± standard deviation.
Both SP1 and SP2 showed a similar elemental composition in their bulk matrix (i.e., SP1/bulk and SP2/bulk), with a predominant calcareous phase, as indicated by the high Ca content (79.77 wt.% for SP1/bulk and 71.74 wt.% for SP2/bulk). For these areas, significant amounts of Si (i.e., approximately 12 wt.%), Fe (i.e., approximately 5 wt.%), and Al (i.e., approximately 3.6 wt.%) were also detected, in conjunction with traces of other elements, such as Ti, Mn, Mg, Cr, Ni, Rb, Sr, S, Cu, and Zn (for which a wt.% < 1 was observed). As a general consideration, the comparable wt.% values, especially those associated with trace elements, indicate that the stone used in the construction of the Sanctuary of Apollo (SP1) may have been sourced from the natural argillite bed represented by SP2. Interestingly, the slight differences in Ca content observed between SP1/bulk and SP2/bulk can be reasonably attributed to diagenetic recrystallization and soil–stone interactions, in agreement with the literature [59,60]. These processes likely occurred at different rates due to variations in environmental conditions between the two sampling locations. Furthermore, the simultaneous detection of high amounts of Si, Fe, and Al is likely related to the presence of silicate and clay minerals in the stone matrix, such as quartz and aluminosilicates.
A comparison of the elemental compositions between the bulk and the external surface of both SP1 and SP2 provided insights into the potential degradation processes affecting the investigated stones. First of all, a significant decrease in Ca content was observed on the external surface of SP1/surf (55.67 wt.%) compared to its bulk matrix (79.77 wt.%), suggesting surface decay or leaching due to environmental phenomena. In contrast, the bedrock SP2 sample showed minimal differences between its surface and bulk matrix, indicating a stable carbonate composition with negligible leaching. Interestingly, the notable increase in Si and Fe contents from the bulk (SP1/bulk) to the surface (SP1/surf) likely reflects the presence, on the external surface of the stone, of insoluble residue-rich grains of both silicate and oxide types. Such a pattern was not observed in SP2, where the elemental composition remained consistent between the bulk and the surface, indicating limited surface alteration. The sulfur content on the external surfaces of SP1 (0.07 wt.%) and SP2 (0.03 wt.%) was comparable to their respective bulk compositions, reasonably excluding the presence of sulfation from environmental exposure. This aspect is corroborated by the absence of gypsum (CaSO4·2H2O), which would result from sulfuric acid–calcite reactions, as confirmed both by molecular characterization and ion chromatography, as discussed later in the text.
μ-EDXRF elemental maps enabled us to highlight the distribution of the major and some trace elements.
Figure 6 displays the μ-EDXRF distribution maps of some main elements (Ca, Si, Fe, Al, K, and Ti) present in the bulk matrix (SP1/bulk) and over the external surface (SP1/surf) of the SP1 sample.
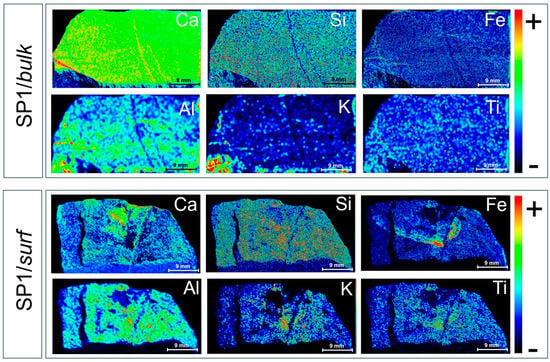
Figure 6.
μ-EDXRF maps for Ca, Si, Fe, Al, K, and Ti detected in the bulk (cross-section, SP1/bulk) and external surface (SP1/surf) of SP1. The rainbow color scale relates to the intensity of the main XRF transition line of the corresponding element.
A first inspection of the μ-EDXRF images acquired for the bulk matrix of SP1 revealed that Ca was widespread in the rock body, with two well-visible calcium-filled veins likely originating from stylolites and/or recrystallization bands. A uniform distribution was also observed for Si and Fe, although they were only visible in low amounts, representing the main constituents of the rocky matrix, likely due to the presence of quartz, clay minerals, and iron oxides. A general inspection of the maps reveals evident changes from a uniform distribution in the case of Al, K, and Ti, which appear to be mainly concentrated in specific regions. In particular, both Al and K appear to be clustered in the bottom-left region of the stone, likely due to the presence of mineral deposits and Al-/K-rich inclusions within the bulk matrix. In the case of Ti, a spotty distribution pattern, such as that typically originated by Ti-bearing accessory minerals, can be observed, suggesting localized fractions of rutile and/or anatase (TiO2).
As far as the external surface of the SP1 sample is concerned (SP1/surf), the presence of Ca-poor regions, clearly visible in the SP1/surf Ca distribution map, supports the hypothesis of carbonate leaching due to environmental phenomena and burial conditions. Worthy of note is the Ca-rich cluster clearly visible in the top-central region of the image, corresponding to a white crust upon the SP1 surface, allowing us to attribute the observed surface crusts likely to calcite rather than gypsum. Moreover, Si appears to be uniformly distributed, while Fe follows divergent pathways likely originating from biological activity, with Fe being essential for bacterial growth and biofilm formation. Finally, the obtained distribution for Al reveals a uniform distribution of clay-rich zones, whereas in the case of K and Ti, a clustering in the central part of the image that gradually thins out towards the edge can be observed, suggesting K-bearing clays and detrital heavy minerals localized in the central area of the surface.
For comparison, Figure 7 shows the Ca, Si, Fe, Al, K, and Ti μ-EDXRF distribution maps from the bulk matrix (SP2/bulk) and over the external surface (SP2/surf) of the “reference” SP2 sample.
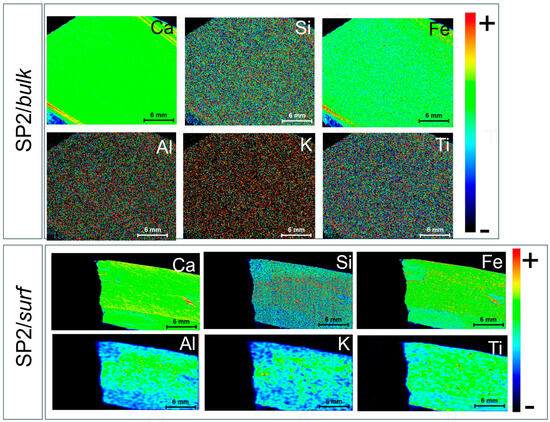
Figure 7.
μ-EDXRF maps for Ca, Si, Fe, Al, K, and Ti detected in the bulk matrix (cross-section, SP2/bulk) and external surface (SP2/surf) of SP2. The rainbow color scale relates to the intensity of the main XRF transition line of the corresponding element.
As far as the bulk matrix is concerned (SP2/bulk), the obtained Ca, K, and Al distribution maps appear to be consistent with a typical limestone, with aluminosilicates spread over the whole cross-section. Interestingly, the observation of two parallel high Ca-rich veins might reflect recrystallized zones, possibly from fluid interaction or weathering. Even in this case, a fine dispersion of clay minerals and iron oxides can be reasonably hypothesized from the distribution maps of Si and Fe. However, the slight enrichments in Fe content might correspond to oxidation rims fostered by recrystallization. Accessory minerals like Ti-bearing phases are minor but consistent with an argillaceous lithology.
Concerning the external surface of SP2 (SP2/surf), Ca, Si, and Fe appear to be quite widespread across the surface, with minor surface variations reflecting the presence of crusts originating from superficial processes. In particular, the presence of a crust on the right-hand side of the map can be considered to be responsible for the red spot observed in both the Ca and Fe distribution maps. The distributions of Al and K appear to be less finely dispersed compared with previous observations, most likely due to the presence of medium-sized aluminosilicates and K-bearing clay aggregates. Interestingly, highly concentrated Ti spots can be clearly distinguished.
4.2. Molecular/Mineralogical Characterization
μ-Raman spectroscopy was employed as a screening tool to confirm the presence of compounds previously hypothesized through μ-EDXRF, and to identify potential alteration products indicative of chemical weathering and degradation processes affecting the investigated stones. Figure 8 shows the µ-Raman spectra, and corresponding photomicrographs, collected on blackish (Figure 8a), translucent (Figure 8b), red (Figure 8c), and white (Figure 8d) areas of the SP1 sample.
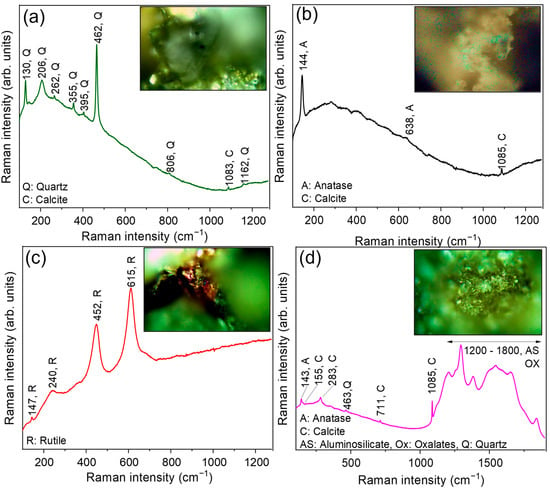
Figure 8.
µ-Raman spectra, and corresponding photomicrographs, collected on (a) blackish, (b) translucent, (c) red, and (d) white areas of the SP1 sample.
The spectrum acquired from the blackish area (Figure 8a) shows the characteristic bands of quartz (SiO2), centered at ~130 cm−1, ~206 cm−1, ~262 cm−1, ~355 cm−1, ~395 cm−1, ~462 cm−1, ~806 cm−1, and ~1162 cm−1, along with a minor contribution falling at ~1083 cm−1, ascribable to calcite, the most stable polymorph of calcium carbonate (CaCO3). This is consistent with an argillitic rock composed of silicate phases (including quartz, feldspar, and phyllosilicates) intermixed with carbonate components. Moreover, the µ-Raman analysis of the translucent area (Figure 8b) provided evidence of the presence of anatase (a metastable TiO2 polymorph), through the detection of its major contribution centered at ~144 cm−1 and a minor feature at ~638 cm−1, in conjunction with calcite (~1085 cm−1). The presence of anatase, often associated with low-temperature oxidizing conditions, typical of burial environments, can be considered to be a weathering product likely derived from the alteration of Ti-bearing minerals. In contrast, the µ-Raman spectrum (Figure 8c) acquired from the red area reveals all of the Raman features of rutile, the thermodynamically stable TiO2 polymorph, at ~147 cm−1, ~240 cm−1, ~452 cm−1, and ~615 cm−1 [61]. Rutile is commonly present as a primary accessory mineral in metamorphic and igneous rocks, typically forming reddish-brown inclusions or fine-grained aggregates [62]. The simultaneous presence of anatase and rutile can be reasonably explained by their distinct origin: rutile as a relict sedimentary component, and anatase as a secondary weathering phase. A direct transformation of rutile to anatase is unlikely, due to their different thermodynamic stabilities [63]. Finally, in the µ-Raman spectrum of the white area (Figure 8d), collected on a white crust of the surface, the presence of calcite and anatase can be distinguished, together with aluminosilicates (i.e., feldspar or kaolinite) and calcium oxalates (i.e., whewellite or weddellite) from the broad bands between 1200 and 1800 cm−1 [64,65].
Interestingly, in all of the investigated areas, no signals related to gypsum (CaSO4·2H2O) were observed, indicating that the SP1 argillite sample has not been affected by sulfate-related weathering or by severe exposure to sulfate-rich environments (e.g., coastal areas) [66].
The Raman spectra of the SP2 sample closely resemble those of SP1, indicating a similar molecular composition between the two samples.
The XRD analysis fully corroborated and expanded the findings obtained through µ-Raman spectroscopy. For the SP1 sample, the identified mineral phases included quartz (SiO2), calcite (CaCO3), plagioclase (albite, NaAlSi3O8), muscovite (KAl2(Si3Al)O10(OH)3), and clinochlore ((Mg, Fe)5(AlSi3O10)(OH)8), while in the case of SP2, quartz (SiO2), calcite (CaCO3), muscovite (KAl2(Si3Al)O10(OH)3), and clinochlore ((Mg, Fe)5(AlSi3O10)(OH)8) were identified (see Figure 9). It is worthy of note that, in the case of SP2, the presence of kaolinite (Al2Si2O5(OH)4)-type phyllosilicates cannot be ruled out, with their signal being partially “hidden” by the even basal reflections of the clinochlore.
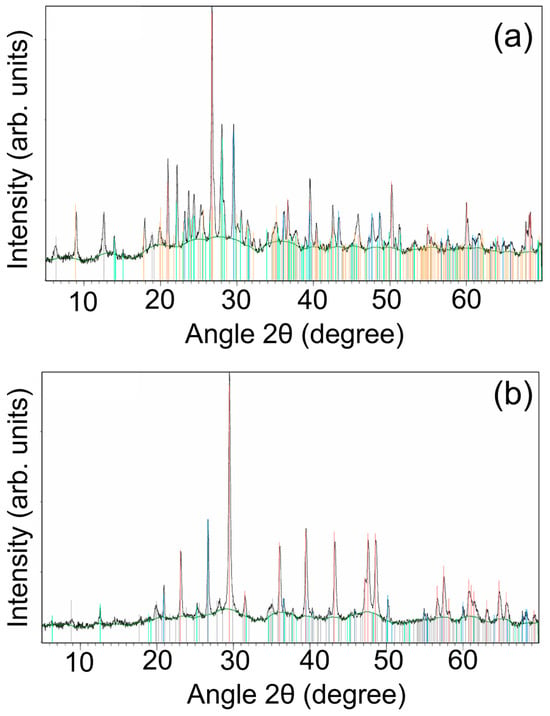
Figure 9.
X-Ray Diffraction patterns from samples (a) SP1 (quartz (marked in red), calcite (marked in blue), plagioclase (marked in light blue), muscovite (marked in orange), and clinochlore (marked in grey); see text for details) and (b) SP2 (quartz (marked in blue), calcite (marked in red), muscovite (marked in grey), and clinochlore (marked in green)).
Overall, both SP1 and SP2 exhibited similar mineralogical compositions, primarily consisting of quartz, calcite, muscovite, and clinochlore. This mineral assemblage is consistent with a common sedimentary origin, dominated by silicates, carbonates, and phyllosilicates, in agreement with the µ-Raman results. However, a key distinction was observed in SP1, i.e., the stone collected at the Sanctuary of Apollo, where the presence of plagioclase suggested a detrital or burial-related input of feldspar, potentially linked to anthropogenic or diagenetic influences within the archaeological context. Conversely, the potential presence of kaolinite in SP2, i.e., the “reference” outcrop stone taken from the natural argillite bed, would reflect a higher degree of hydrolytic weathering in the bare bedrock, consistent with prolonged surface alterations typical of an uncovered, naturally weathered lithology.
4.3. Ion Chromatography Analysis
Ion chromatography (IC) analyses were performed on both the SP1 and SP2 argillite samples. Significantly, the concentration of soluble salts in both samples (Table 2) does not suggest salt-induced deterioration as a significant decay pathway [66,67]. Sulfate (SO42−), commonly associated with salt crystallization damage and gypsum formation, was below the detection limit in both cases. Similarly, fluoride (F−) and nitrite (NO2−) were absent, indicating minimal influence from anthropogenic pollution sources.

Table 2.
pH and soluble salts (ppb) for argillite samples. Values are reported as the mean ± standard deviation.
Although SP2 exhibited a higher overall ionic load—particularly with respect to calcium (5763.5 ppb), potassium (915.5 ppb), and sodium (213.8 ppb)—these values may reflect the original geochemical signature of the bedrock. Such findings may arise from the mineralogical composition and subsurface geochemical processes, rather than surface weathering. The presence of both carbonate (CO32−, 72.6 ppb) and bicarbonate (HCO3−, 125.1 ppb) in SP2 contrasts with SP1, which exhibited only bicarbonate (HCO3−, 179.6 ppb), suggesting leaching or transformation of carbonate phases during prolonged burial for the latter. This divergence is also mirrored in the pH values of the two samples (SP1: 7.8; SP2: 9.38), further indicating that SP1’s burial context may have contributed to slight acidification.
Chloride (Cl−), another salt of potential concern in crystallization-induced weathering (e.g., from marine aerosols), was present in moderate concentrations (i.e., SP1: 150.2 ppb; SP2: 208.3 ppb), albeit well below the thresholds typically associated with aggressive decay phenomena. Nitrate (NO3−) and phosphate (PO43−), likely introduced via soil interaction or biological activity, were also detected at comparably low levels in both samples.
These findings strongly support the conclusion that salt weathering is not the dominant degradation process affecting the argillite stones at the Sanctuary of Apollo. This is consistent with field observations, which indicate that physical weathering—rather than salt-driven decay—is the prevalent deterioration mechanism. Of particular relevance, SP1 was derived from a monument that remained buried for centuries before excavation, a condition that could have mitigated its exposure to environmental salts but may simultaneously have fostered other weathering dynamics.
In general, the deterioration of lithic materials is often closely associated with the mobilization of soluble salts, typically produced by acid-induced transformation of primary mineral phases [68]. Such acids may originate from atmospheric pollution, infiltrating water, or acidic soils. Once formed, these salts undergo repeated cycles of dissolution and crystallization in response to environmental fluctuations in temperature and relative humidity. The resulting volumetric changes exert mechanical stress on the stone’s pore structure, leading to microfracturing and facilitating the penetration of reactive agents. Over time, this feedback loop may result in significant structural degradation, particularly if the stone’s cementitious matrix becomes chemically altered or dissolved. The loss of this binding phase weakens the granular cohesion of the material, which can lead to surface-scale and, in the advanced stages, structural instability. Salt-induced deterioration can often be mitigated, if identified early, through preventive conservation strategies, such as reducing exposure to acidic agents, applying desalinization treatments, and reinforcing cohesion using compatible consolidants [69,70]. However, in the case of the argillitic materials studied here, the observed deterioration appeared to stem from intrinsic mineralogical weakness rather than salt-related decay. The XRD analyses confirmed that both SP1 and SP2 consist predominantly of quartz, calcite, muscovite, and clinochlore, with SP1 also containing plagioclase. This mineral assemblage, typical of laminar argillites, suggests a fine-grained, sheeted microstructure in which felspathic and micaceous phases act as binding agents among clay-rich and siliceous layers. Of particular concern is the presence of plagioclase and other felspathic components, which are notably susceptible to acid hydrolysis, even under relatively mild conditions, such as those induced by carbonic acid formed from atmospheric CO2 [71]. The progressive dissolution of these phases leads to a reduction in structural cohesion, ultimately promoting laminar exfoliation and surface detachment. Unlike salt-driven decay, this mechanism reflects an inherent lithological vulnerability, which cannot be effectively counteracted using conventional consolidation methods. Rather, a more comprehensive and integrated conservation approach is required. Although SP2 likely represents the source lithotype used in the construction of the Sanctuary of Apollo, from which SP1 was taken, as suggested by their shared sedimentary nature and mineralogical similarity, differences emerge when comparing their respective alteration profiles. In SP1, there is evidence of partial loss of carbonate phases and a notable depletion of feldspars, features that are not observed to the same extent in SP2. These differences suggest that SP1 has undergone post-depositional weathering, likely driven by prolonged burial and exposure to soil-derived acidic conditions. These processes appear to have enhanced its mineral instability, resulting in leaching and dissolution of vulnerable phases such as carbonates and feldspar. In contrast, SP2 retains a more complete suite of original minerals, suggesting limited alteration and a relatively stable geochemical environment, although it is macroscopically prone to scaling. Taken together, these findings highlight the importance of considering not only external decay factors, such as air pollution or salt contamination, but also intrinsic material vulnerabilities and the influence of the burial environment on long-term durability.
4.4. Radioactivity and Radon Exhalation Analysis
A radioactivity and radon exhalation analysis was carried out not only for a more complete characterization of the shale unearthed within the investigated geological setting, but also considering that the site is quite populated throughout the year. In particular, the number of visitors varies annually based on the events held. In addition to occasional tourists, the Municipality of Tusa organizes at least two events per year at the site, each attracting approximately 50 to 60 attendees. Additionally, archaeologists from Italian–British, Palermo, and French teams conduct research at the site for a minimum of 30 days annually (62 archaeologists in 2023; 71 in 2024). Furthermore, staff from the Sicily Region—including 16 caretakers, 2 administrators, and 4 additional caretakers for holidays—are present daily.
To give a quantitative idea of the impact of the site in terms of tourism, Table 3 presents data on the annual numbers of visitors to the site.

Table 3.
Halaesa Archonidea’s annual visitors.
It should be noted that the total number of visitors (i.e., occasional tourists) for the years 2021–2024 (up to June) is based on an estimated calculation, while the number provided for July–December 2024 is derived from the actual count of issued entrance tickets. Moreover, in 2021, the number of archaeologists reflects only the Messina and Oxford (Italian–British) and Palermo missions, as the French mission did not participate in the excavation campaign due to COVID-19.
Table 4 reports the mean (averaged from the five aliquots of the analyzed rocks) activity concentrations of 226Ra, 232Th, and 40K for the investigated samples, as well as the mean specific 222Rn exhalation rate.

Table 4.
Mean activity concentrations of 226Ra, 232Th, and 40K for the analyzed samples, together with the mean specific 222Rn exhalation rate (E).
First of all, the obtained values were found to be consistent with the natural radioactive levels observed in building materials used in Italy and elsewhere [72,73].
In particular, as far as the natural activity concentration is concerned, it is important to point out that the specific activities of 226Ra, 232Th, and 40K, for both investigated natural stones, are lower than the world average values of 35 Bq kg−1, 30 Bq kg−1, and 400 Bq kg−1, respectively, for this environmental matrix [46], taking into account that, as also frequently described in the literature, the activity concentrations of 226Ra, 232Th, and 40K are strictly dependent on the chemical composition and mineralogical phases of the samples under study [74,75]. In particular, the presence of muscovite (KAl2(Si3Al)O10(OH)3) in both SP1 and SP2, as determined through XRD, can be considered to represent the main radioisotope-bearing mineral present in the analyzed stones. Additional contributions may arise from plagioclase feldspars and clay minerals, which host variable amounts of potassium and trace uranium/thorium substitutions. The higher 40K activity in SP2 (233 ± 28 Bq/kg) compared to SP1 (101 ± 13 Bq/kg) likely indicates better preservation of K-bearing phases in the outcrop. In SP1, weathering and burial processes, potentially involving leaching in a mildly acidic environment, may have induced partial dissolution of feldspars and degradation of muscovite, consistent with the µ-Raman and IC results.
As far as the radon exhalation rate from the analyzed natural stones is concerned, it shows considerable variation, with values spreading over several orders of magnitude, although the obtained mean values closely resemble those observed worldwide [76]. In more detail, the 222Rn exhalation rate was unexpectedly higher in SP1 despite its lower Ra content. This can be attributed to SP1’s degraded microstructure, where increased physical disaggregation and porosity enhance radon emanation. This highlights that deterioration not only impacts the mechanical properties of lithic materials but also modifies their radiological behavior, with direct implications for site safety. Similar trends of weathering-modulated mineralogical integrity and radioactivity distribution have been reported at other archaeological sites in Southern Italy, including those in Catania and Pantelleria, where degradation of feldspars and phyllosilicates affects both the structural stability and radiological behavior of ancient materials [77,78].
4.5. Evaluation of the Radiological Hazard
The D, AEDE, ACI, and Iα for the investigated natural stones, as calculated by using Equations (3)–(6), are reported in Table 5.

Table 5.
D, AEDE, ACI, and Iα for the investigated natural stones.
It is noteworthy to highlight that D is related to the lithological component of the sampling location, as extensively documented in the literature [79]. The gamma dose rates were found to be lower than the natural background value of 50 nGy h−1 in all cases. Thus, AEDE due to the activities of 226Ra, 232Th, and 40K in the investigated samples fell short of the established threshold value of 1 mSv y−1 in all cases, thus assuring a negligible radiation hazard to the public. The ACI was found to be 0.15 and 0.18 for SP1 and SP2, respectively. Both of these values are below 1, indicating negligible radiological hazard from gamma radiation exposure [80]. Finally, concerning Iα, it was found to be 0.09 and 0.07 for SP1 and SP2, respectively, always less than 1 even in this case [81].
5. Conclusions
In this work, two stone fragments from the archaeological site of Halaesa (Sicily, Italy)—one sampled from an archaeological structure unearthed during recent excavations, and the other from the surrounding outcrop (used as a reference)—underwent a comparative systematic investigation of their mineralogical composition and radioactivity levels. In particular, the compositional analysis at the elemental and molecular scales, as achieved by μ-EDXRF spectroscopy, µ-Raman spectroscopy, and XRD techniques, provided, on the one hand, the identification of the mineralogical composition of the investigated argillites used in the construction of archaeological buildings, and on the other hand, useful insights for the discrimination of the degradation products originating from soil–environmental–stone interactions.
For both investigated fragments, the levels of soluble salts detected by IC were found to be quite low, clearly indicating that salt crystallization was not the primary deterioration mechanism affecting the investigated stones. Due to their mineralogical composition, these lithotypes feature a laminated structure where feldspars and clays are responsible for interlayer cohesion; however, these components are prone to dissolution under mild acidic conditions, such as those induced by atmospheric carbon dioxide. This can weaken the internal cohesion of the stone over time, potentially leading to surface flaking or detachment of laminar layers. This is an inherent vulnerability of this kind of lithic material, rather than a pathology caused by external contaminants. Therefore, conventional conservation approaches, such as desalinization or standard surface consolidation, may not be effective. Further research is needed to assess the feasibility and to prove the efficacy of restoration interventions for these specific lithic substrates.
Finally, the natural activity concentration and radon exhalation of the sampled argillite rocks, determined through HPGe spectrometry and E-PERM electret ion chamber methods, respectively, were quantified. The obtained radiological parameters, such as D, AEDE, ACI, and Iα, were found to be less than the reference levels set by the Italian legislation for population members, indicating no substantial radiological hazard for the public and workers in the area. From a practical standpoint, in principle, the integrated dataset can provide both site-specific conservation protocols tailored to the specific stone material and environmental conditions, and a broader heritage management policy by offering a replicable methodological framework applicable to similar cultural heritage contexts. In this sense, future investigations are planned, to explore the weathering patterns and the long-term response of these lithotypes to environmental stressors under real field conditions, in view of defining new targeted conservation treatments based on novel, durable, and efficient consolidating treatments. Additionally, extending the analysis to a larger sample set from different structures within the archaeological site could contribute to a more comprehensive understanding of the material provenance, construction phases, and long-term decay trends, supporting the development of site-specific conservation plans to be applied. Such aspects would not only contribute to enhancing the promotion, dissemination, and accessibility of the investigated archaeological records but also represent fundamental pinpoints for the definition of safety guidelines for archaeologists, conservators, and visitors, aimed at prioritizing protective measures for stone materials with high susceptibility to weathering and/or radiological activity.
Author Contributions
Conceptualization, V.V., F.C., G.P., O.G.-L. and P.C.; methodology, V.V., F.C., G.P., L.P., O.G.-L. and P.C.; validation, V.V., P.C. and D.M.; formal analysis, F.C., G.P., L.P., P.C., G.L., A.F.M. and O.G.-L.; investigation, V.V., F.C., G.P., L.P., F.G., S.L., P.C., G.L., A.F.M. and O.G.-L.; data curation, F.C., G.P. and P.C.; writing—original draft preparation, V.V., F.C., G.P. and P.C.; writing—review and editing, V.V., F.C., G.P., P.C., G.L. and M.M.; visualization, V.V., P.C., G.L., D.M., O.G.-L. and M.M.; supervision, V.V., P.C. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Archaeological Park of Tindari and the Superintendence of Cultural Heritage of Messina for their demonstrated liberality.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carettoni, G. Tusa (Messina). Scavi di Halaesa. Prima relazione. In Notizie Degli Scavi di Antichità; Accademia Nazionale dei Lincei: Roma, Italy, 1959; Volume 16, pp. 293–349. [Google Scholar]
- Carettoni, G. Tusa (Messina). Scavi di Halaesa. Seconda relazione. In Notizie Degli Scavi di Antichità; Accademia Nazionale dei Lincei: Roma, Italy, 1961; Volume 15, pp. 266–321. [Google Scholar]
- Scibona, G.; Tingano, G. Alaisa-Halaesa. In Scavi e Ricerche (1970–2007); Sicania: Messina, Italy, 2009. [Google Scholar]
- Campagna, L.; Prag, J.R.W. La collina settentrionale ed il cd. Santuario di Apollo di Alesa. Le ricerche dell’Università di Messina e di Oxford negli anni 2017–2021. In Supplementi a «Kokalos», in Halaesa, du Site à la Cité, de la Cité au Site, Actes du Colloque International, Amiens; Fabrizio Serra Editore: Roma, Italy, 2023; pp. 19–41. [Google Scholar]
- Burgio, A.; De Domenico, R.; Marino, G.; Modica, F.S.; Polizzi, G.; Randazzo, M.; Schepis, L. Halaesa Arconidea (Tusa, Messina). Primi risultati dagli scavi delle fortificazioni, settore nord-est. FOLD&R 2023, 1–43. [Google Scholar]
- Costanzi, M.; Ferreira, F.; Gerber, F. Les travaux de la Misson Archéologique de l’UPJV à Halaesa (Campagne Juillet 2021): Le Projet Scientifique et les Enjeux de la Fouille du Théâtre. In Supplementi a «Kokalos», in Halaesa, du Site à la Cité, de la Cité au Site; Fabrizio Serra Editore: Roma, Italy, 2023; pp. 97–136. [Google Scholar]
- Spoto, S.E.; Paladini, G.; Caridi, F.; Crupi, V.; D’Amico, S.; Majolino, D.; Venuti, V. Multi-Technique Diagnostic Analysis of Plasters and Mortars from the Church of the Annunciation (Tortorici, Sicily). Materials 2022, 15, 958. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, G.; Robles Marín, P.; Guerrera, F.; Martín Martín, M.; Alcalá-García, F.J.; Amadori, M.L.; Asebriy, L.; El Amrani, I.-E.; Tejera de León, J. Archaeometric study of a typical medieval fortified granary (Amtoudi Agadir, Anti-Atlas Chain, southern Morocco): A key case for the maintenance and restoration of historical monuments. Ital. J. Geosci. 2016, 135, 280–299. [Google Scholar] [CrossRef]
- Columbu, S.; Antonelli, F.; Sitzia, F. Origin of Roman worked stones from St. Saturno christian Basilica (south Sardinia, Italy). Mediterr. Archaeol. Archaeom. 2018, 18, 17–36. [Google Scholar] [CrossRef]
- Lisci, C.; Sitzia, F.; Pires, V.; Aniceto, M.; Mirão, J. Stone Endurance: A Comparative Analysis of Natural and Artificial Weathering on Stone Longevity. Heritage 2023, 6, 4593–4617. [Google Scholar] [CrossRef]
- Kunz, A.; Groh, M.; Braun, F.; Brüggerhoff, S.; Orlowsky, J. Durability assessment of differently orientated surfaces of treated long-term weathered natural stones. J. Cult. Herit. 2022, 53, 176–183. [Google Scholar] [CrossRef]
- Siegesmund, S.; Wiese, F.; Klein, C.; Huster, U.; Pötzl, C. Weathering phenomena, rock physical properties and long-term restoration intervention: A case study from the St. Johannis Chapel of Lütgenrode (Lower Saxony, Germany). Environ. Earth Sci. 2022, 81, 389. [Google Scholar] [CrossRef]
- Eyssautier-Chuine, S.; Franco-Castillo, I.; Misra, A.; Hubert, J.; Vaillant-Gaveau, N.; Streb, C.; Mitchell, S.G. Evaluating the durability and performance of polyoxometalate-ionic liquid coatings on calcareous stones: Preventing biocolonisation in outdoor environments. Sci. Total Environ. 2023, 884, 163739. [Google Scholar] [CrossRef]
- Gaylarde, C.; Little, B. Biodeterioration of stone and metal—Fundamental microbial cycling processes with spatial and temporal scale differences. Sci. Total Environ. 2022, 823, 153193. [Google Scholar] [CrossRef]
- Fontenele, A.; Campos, V.; Matos, A.M.; Mesquita, E. A vulnerability index formulation for historic facades assessment. J. Build. Eng. 2023, 64, 105552. [Google Scholar] [CrossRef]
- Damas Mollá, L.; Sagarna, M.; Zabaleta, A.; Aranburu, A.; Antiguedad, I.; Uriarte, J.A. Methodology for assessing the vulnerability of built cultural heritage. Sci. Total Environ. 2022, 845, 157314. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgio, V.; Uva, G.; Adam, J.M. Integrated Seismic Vulnerability Assessment of Historical Masonry Churches Including Architectural and Artistic Assets Based on Macro-element Approach. Int. J. Archit. Herit. 2021, 15, 1609–1622. [Google Scholar] [CrossRef]
- Ferrante, A.; Schiavoni, M.; Bianconi, F.; Milani, G.; Clementi, F. Influence of Stereotomy on Discrete Approaches Applied to an Ancient Church in Muccia, Italy. J. Eng. Mech. 2021, 147, 04021103. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Ibarrondo, I.; Gómez-Laserna, O.; Martinez-Arkarazo, I.; Olazabal, M.A.; Madariaga, J.M. Buildings as repositories of hazardous pollutants of anthropogenic origin. J. Hazard. Mater. 2013, 248–249, 451–460. [Google Scholar] [CrossRef]
- Liritzis, I.; Laskaris, N.; Vafiadou, A.; Karapanagiotis, I.; Volonakis, P.; Papageorgopoulou, C.; Bratitsi, M. Archaeometry: An Overview. Sci. Cult. 2020, 6, 49. [Google Scholar] [CrossRef]
- Crupi, V.; La Russa, M.F.; Venuti, V.; Ruffolo, S.; Ricca, M.; Paladini, G.; Albini, R.; Macchia, A.; Denaro, L.; Birarda, G.; et al. A combined SR-based Raman and InfraRed investigation of pigmenting matter used in wall paintings: The San Gennaro and San Gaudioso Catacombs (Naples, Italy) case. Eur. Phys. J. Plus 2018, 133, 369. [Google Scholar] [CrossRef]
- Miliani, C.; Rosi, F.; Brunetti, B.G.; Sgamellotti, A. In Situ Noninvasive Study of Artworks: The MOLAB Multitechnique Approach. Acc. Chem. Res. 2010, 43, 728–738. [Google Scholar] [CrossRef]
- Attanasio, D.; Bultrini, G.; Ingo, G.M. The Possibility of Provenancing A Series of Bronze Punic Coins Found At Tharros (Western Sardinia) Using the Literature Lead Isotope Database. Archaeometry 2001, 43, 529–547. [Google Scholar] [CrossRef]
- Alberti, R.; Crupi, V.; Frontoni, R.; Galli, G.; La Russa, M.F.; Licchelli, M.; Majolino, D.; Malagodi, M.; Rossi, B.; Ruffolo, S.A.; et al. Handheld XRF and Raman equipment for the in situ investigation of Roman finds in the Villa dei Quintili (Rome, Italy). J. Anal. At. Spectrom. 2017, 32, 117–129. [Google Scholar] [CrossRef]
- Gheris, A. New dating approach based on the petrographical, mineralogical and chemical characterization of ancient lime mortar: Case study of the archaeological site of Hippo, Annaba city, Algeria. Herit. Sci. 2023, 11, 103. [Google Scholar] [CrossRef]
- Singh, M.; Vinodh Kumar, S. Mineralogical, Chemical, and Thermal Characterizations of Historic Lime Plasters of Thirteenth–Sixteenth-century Daulatabad Fort, India. Stud. Conserv. 2018, 63, 482–496. [Google Scholar] [CrossRef]
- Ion, R.-M.; Fierascu, R.-C.; Fierascu, I.; Senin, R.-M.; Ion, M.-L.; Leahu, M.; Turcanu-Carutiu, D. Influence of Fântâniṭa Lake (Chalk Lake) Water on the Degradation of Basarabi–Murfatlar Churches. In Engineering Geology for Society and Territory—Volume 8; Springer International Publishing: Cham, Switzerland, 2015; pp. 543–546. ISBN 9783319094083. [Google Scholar]
- Ben Chobba, M.; Weththimuni, M.; Messaoud, M.; Urzi, C.; Licchelli, M. Recent Advances in the Application of Metal Oxide Nanomaterials for the Conservation of Stone Artefacts, Ecotoxicological Impact and Preventive Measures. Coatings 2024, 14, 203. [Google Scholar] [CrossRef]
- Crupi, V.; D’Amico, S.; Denaro, L.; Donato, P.; Majolino, D.; Paladini, G.; Persico, R.; Saccone, M.; Sansotta, C.; Spagnolo, G.V.; et al. Mobile spectroscopy in archaeometry: Some case study. J. Spectrosc. 2018, 2018, 8295291. [Google Scholar] [CrossRef]
- Bottari, C.; Crisci, G.M.; Crupi, V.; Ignazzitto, V.; La Russa, M.F.; Majolino, D.; Ricca, M.; Rossi, B.; Ruffolo, S.A.; Teixeira, J.; et al. SANS investigation of the salt-crystallization- and surface-treatment-induced degradation on limestones of historic–artistic interest. Appl. Phys. A 2016, 122, 721. [Google Scholar] [CrossRef]
- Bendavid, J.; Modesto, A. Radiation therapy and antiangiogenic therapy: Opportunities and challenges. Cancer/Radiothérapie 2022, 26, 962–967. [Google Scholar] [CrossRef]
- Caridi, F.; Paladini, G.; Gregorio, F.; Lanza, F.; Lando, G.; Sfacteria, M.; Tuccinardi, S.; Venuti, M.; Cardiano, P.; Majolino, D.; et al. Natural Radioactivity Content and Radon Exhalation Rate Assessment for Building Materials from the Archaeological Park of Tindari, Sicily, Southern Italy: A Case Study. Int. J. Environ. Res. Public Health 2025, 22, 379. [Google Scholar] [CrossRef]
- Caridi, F.; Paladini, G.; Marguccio, S.; D’Agostino, M.; Belvedere, A.; Crupi, V.; Majolino, D.; Venuti, V. Radioactivity content in construction materials of assets of particular historical-artistic interest. In Proceedings of the 2023 IMEKO TC4 International Conference on Metrology for Archaeology and Cultural Heritage, Rome, Italy, 19–21 October 2023; pp. 877–881. [Google Scholar]
- Siculo, D. Biblioteca Storica; libro XIV, Chapter 16; Sellerio: Palermo, Italy, 1986; pp. 1–4. [Google Scholar]
- Facella, A. Alesa Arconidea. In Ricerche su Un’antica Città Della Sicilia Tirrenica; Edizioni Della Normale: Pisa, Italy, 2006. [Google Scholar]
- Cicerone. Verrine; libro III, Chapter 6; Carlo Signorelli: Milano, Italy, 1942; p. 13. [Google Scholar]
- Campagna, L.; Prag, J.R.W.; Miano, M.; Dalglish, D. Il Santuario di Apollo ad Alesa Arconidea: Gli scavi della Missione delle Università di Messina e di Oxford (2022–2023). In L’Isola dei Tesori. Ricerca Archeologica e Nuove Acquisizioni, Atti del Convegno Internazionale (Agrigento, Museo Archeologico Regionale “P. Griffo”, December 14-17, 2023); Ante Quem: Pianoro, Italy, 2024; pp. 135–242. [Google Scholar]
- Campagna, L.; Dalglish, D.; Giuliano, F.; Miano, M.; Prag, J.R.W. La missione congiunta delle università di Messina e Oxford. Il Santuario di Apollo: Ricerche topografiche e archeologiche nel triennio 2022–2024. In Halaesa Archonidea Dallo Scavo Della Città All’Analisi dei Contesti, A. Burgio (a Cura di), Atti del Convegno di Studi (Palermo, Tusa, Castel di Lucio, December 5–7, 2024); in press; 2024; Available online: https://www.unipa.it/dipartimenti/cultureesocieta/.content/documenti/Programma-Convegno-HALAESA-ARCHONIDEA.pdf (accessed on 13 March 2025).
- Lentini, F.; Carbone, S. Geology of Sicily. Mem. Descr. Cart. Geol. Ital. 2014, 95, 7–30. [Google Scholar]
- Servizio Geologico d’Italia. Carta Geologica d’Italia Alla Scala 1:50.000, Fogli 597 e 610 Cefalù-Castelbuono; ISPRA: Roma, Italy, 2012.
- Carbone, S.; Grasso, M.; Lentini, F.; Maniscalco, R. Note Illustrative Della Carta Geologica D’italia Alla Scala 1:50.000, F. 597-610 Cefalù-Castelbuono, Servizio Geologico d’Italia—ISPRA; ISPRA: Roma, Italy, 2023. [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. ISBN 9783110417104. [Google Scholar]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Prieto-Taboada, N.; Gómez-Laserna, O.; Martinez-Arkarazo, I.; Olazabal, M.A.; Madariaga, J.M. Optimization of two methods based on ultrasound energy as alternative to European standards for soluble salts extraction from building materials. Ultrason. Sonochem. 2012, 19, 1260–1265. [Google Scholar] [CrossRef]
- EN 16455/2014; Conservation of Cultural Heritage—Extraction and Determination of Soluble Salts in Natural Stone and Related Materials Used in and from Cultural Heritage. Slovenski inštitut za standardizacijo: Ljubljana, Slovenia. Available online: https://standards.iteh.ai/catalog/standards/cen/183d6740-886c-42fb-a619-87e47b0173e6/en-16455-2014 (accessed on 21 May 2025).
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation: Report to the General Assembly, with Scientific Annexes; UNSCEAR: New York, NY, USA, 2000; Volume I, ISBN 92-1-142238-8. [Google Scholar]
- Caridi, F.; Marguccio, S.; Durante, G.; Trozzo, R.; Fullone, F.; Belvedere, A.; D’Agostino, M.; Belmusto, G. Natural radioactivity measurements and dosimetric evaluations in soil samples with a high content of NORM. Eur. Phys. J. Plus 2017, 132, 56. [Google Scholar] [CrossRef]
- Agarwal, C.; Chaudhury, S.; Goswami, A.; Gathibandhe, M. True coincidence summing corrections in point and extended sources. J. Radioanal. Nucl. Chem. 2011, 289, 773–780. [Google Scholar] [CrossRef]
- Eckert-Ziegler Calibration Sources. 2020. Available online: https://www.ezag.com/products/isotope-products/ (accessed on 21 March 2025).
- Fabri, P.J. Measurement and Uncertainty. In Measurement and Analysis in Transforming Healthcare Delivery; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–77. [Google Scholar]
- Ortec Gamma Vision Software User Manual. Available online: https://www.ortec-online.com/products/software/gammavision (accessed on 13 March 2025).
- ACCREDIA. Available online: https://www.accredia.it/ (accessed on 13 March 2025).
- European Commission. Radiation Protection 112: Radiological protection principles concerning the natural radioactivity of building materials. Office for official publications of the European Communities. 1999. Available online: https://op.europa.eu/en/publication-detail/-/publication/988f3243-5259-43a5-b621-fbff662deeb0/language-en (accessed on 21 February 2025).
- Italian Legislation D.Lgs. 101/20. Available online: https://www.gazzettaufficiale.it/eli/gu/2020/08/12/201/so/29/sg/pdf (accessed on 18 March 2025).
- Righi, S.; Bruzzi, L. Natural radioactivity and radon exhalation in building materials used in Italian dwellings. J. Environ. Radioact. 2006, 88, 158–170. [Google Scholar] [CrossRef]
- Nazaroff, W.W. Radon and Its Decay Products in Indoor Air; John Wiley and Sons, Incorporated: Hoboken, NJ, USA, 1988; ISBN 0-471-62810-7. [Google Scholar]
- Kotrappa, P.; Stieff, L. Application of NIST 222Rn Emanation Standards for Calibrating 222Rn Monitors. Radiat. Prot. Dosimetry 1994, 55, 211–218. [Google Scholar] [CrossRef][Green Version]
- Rabiee, S.; Sohrabi, M.; Afarideh, H. Electret ion chamber parametric studies for high sensitivity radon monitoring. Radiat. Phys. Chem. 2025, 229, 112498. [Google Scholar] [CrossRef]
- Verrecchia, E.P. Litho-diagenetic implications of the calcium oxalate-carbonate biogeochemical cycle in semiarid calcretes, nazareth, israel. Geomicrobiol. J. 1990, 8, 87–99. [Google Scholar] [CrossRef]
- Bradbury, H.J.; Halloran, K.H.; Lin, C.Y.; Turchyn, A.V. Calcium isotope fractionation during microbially induced carbonate mineral precipitation. Geochim. Cosmochim. Acta 2020, 277, 37–51. [Google Scholar] [CrossRef]
- Gomez-Laserna, O.; Irto, A.; Irizar, P.; Lando, G.; Bretti, C.; Martinez-Arkarazo, I.; Campagna, L.; Cardiano, P. Non-Invasive Approach to Investigate the Mineralogy and Production Technology of the Mosaic Tesserae from the Roman Domus of Villa San Pancrazio (Taormina, Italy). Crystals 2021, 11, 1423. [Google Scholar] [CrossRef]
- Meinhold, G. Rutile and its applications in earth sciences. Earth-Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Crupi, V.; Fazio, B.; Gessini, A.; Kis, Z.; La Russa, M.F.; Majolino, D.; Masciovecchio, C.; Ricca, M.; Rossi, B.; Ruffolo, S.A.; et al. TiO2–SiO2–PDMS nanocomposite coating with self-cleaning effect for stone material: Finding the optimal amount of TiO2. Constr. Build. Mater. 2018, 166, 464–471. [Google Scholar] [CrossRef]
- Bergamo, M.; de Ferri, L.; Cianciosi, A.; Gelichi, S.; Pojana, G. Spectroscopic investigation of early medieval tiles and bedding mortars from Nonantola (Modena, Italy) excavations. Eur. Phys. J. Plus 2019, 134, 209. [Google Scholar] [CrossRef]
- Kelloway, S.J.; Kononenko, N.; Torrence, R.; Carter, E.A. Assessing the viability of portable Raman spectroscopy for determining the geological source of obsidian. Vib. Spectrosc. 2010, 53, 88–96. [Google Scholar] [CrossRef]
- Gómez-Laserna, O.; Cardiano, P.; Diez-Garcia, M.; Prieto-Taboada, N.; Kortazar, L.; Olazabal, M.Á.; Madariaga, J.M. Multi-analytical methodology to diagnose the environmental impact suffered by building materials in coastal areas. Environ. Sci. Pollut. Res. 2018, 25, 4371–4386. [Google Scholar] [CrossRef] [PubMed]
- Pintér, F. The Combined Use of Ion Chromatography and Scanning Electron Microscopy to Assess Salt-affected Mineral Materials in Cultural Heritage. J. Am. Inst. Conserv. 2022, 61, 85–99. [Google Scholar] [CrossRef]
- Alves, C.; Figueiredo, C.A.M.; Sanjurjo-Sánchez, J.; Hernández, A.C. Salt Weathering of Natural Stone: A Review of Comparative Laboratory Studies. Heritage 2021, 4, 1554–1565. [Google Scholar] [CrossRef]
- Andreotti, S.; Franzoni, E.; Ruiz-Agudo, E.; Scherer, G.W.; Fabbri, P.; Sassoni, E.; Rodriguez-Navarro, C. New polymer-based treatments for the prevention of damage by salt crystallization in stone. Mater. Struct. 2019, 52, 17. [Google Scholar] [CrossRef]
- Nasraoui, M.; Nowik, W.; Lubelli, B. A comparative study of hygroscopic moisture content, electrical conductivity and ion chromatography for salt assessment in plasters of historical buildings. Constr. Build. Mater. 2009, 23, 1731–1735. [Google Scholar] [CrossRef]
- Munz, I.A.; Brandvoll, Ø.; Haug, T.A.; Iden, K.; Smeets, R.; Kihle, J.; Johansen, H. Mechanisms and rates of plagioclase carbonation reactions. Geochim. Cosmochim. Acta 2012, 77, 27–51. [Google Scholar] [CrossRef]
- Nuccetelli, C.; Risica, S.; D’Alessandro, M.; Trevisi, R. Natural radioactivity in building material in the European Union: Robustness of the activity concentration index I and comparison with a room model. J. Radiol. Prot. 2012, 32, 349. [Google Scholar] [CrossRef]
- Ravisankar, R.; Vanasundari, K.; Chandrasekaran, A.; Rajalakshmi, A.; Suganya, M.; Vijayagopal, P.; Meenakshisundaram, V. Measurement of natural radioactivity in building materials of Namakkal, Tamil Nadu, India using gamma-ray spectrometry. Appl. Radiat. Isot. 2012, 70, 699–704. [Google Scholar] [CrossRef]
- Guerrero, J.L.; Barba-Lobo, A.; Romero-Forte, C.; Bolívar, J.P. Assessment of metal(loid) and natural radionuclide pollution in surface sediments of an estuary affected by mining and phosphogypsum releases. Environ. Sci. Pollut. Res. 2024, 31, 51489–51503. [Google Scholar] [CrossRef]
- Shahrokhi, A.; Adelikhah, M.; Chalupnik, S.; Kovács, T. Multivariate statistical approach on distribution of natural and anthropogenic radionuclides and associated radiation indices along the north-western coastline of Aegean Sea, Greece. Mar. Pollut. Bull. 2021, 163, 112009. [Google Scholar] [CrossRef] [PubMed]
- Gruber, V.; Bossew, P.; De Cort, M.; Tollefsen, T. The European map of the geogenic radon potential. J. Radiol. Prot. 2013, 33, 51. [Google Scholar] [CrossRef] [PubMed]
- Montana, G.; Randazzo, L.; Ventura-Bordenca, C.; Giarrusso, R.; Baldassari, R.; Polito, A.M. The production cycle of lime-based plasters in the Late Roman settlement of Scauri, on the island of Pantelleria, Italy. Geoarchaeology 2019, 34, 631–647. [Google Scholar] [CrossRef]
- Belfiore, C.M.; La Russa, M.F.; Mazzoleni, P.; Pezzino, A.; Viccaro, M. Technological study of “ghiara” mortars from the historical city centre of Catania (Eastern Sicily, Italy) and petro-chemical characterisation of raw materials. Environ. Earth Sci. 2010, 61, 995–1003. [Google Scholar] [CrossRef]
- Janotka, I.; Martauz, P.; Bačuvčík, M. Design of Concrete Made with Recycled Brick Waste and Its Environmental Performance. Minerals 2021, 11, 463. [Google Scholar] [CrossRef]
- Akuo-ko, E.O.; Otoo, F.; Glover, E.T.; Amponsem, E.; Shahrokhi, A.; Csordás, A.; Kovács, T. Statistical assessment of natural radioactivity, radon activity, and associated radiological exposure due to artisanal mining in Atiwa West district of the Eastern region, Ghana. Heliyon 2024, 10, e34705. [Google Scholar] [CrossRef]
- Bulut, H.A.; Şahin, R. Radon, Concrete, Buildings and Human Health—A Review Study. Buildings 2024, 14, 510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).