Abstract
The existence of historical pigments databases is important to speed up cultural heritage research. Knowledge of their chemical composition and their manufacture contributes to the study of art history and helps develop accurate conservation-restoration strategies. In this study, a total of nineteen pigments, among which we find silicates (Egyptian blue, natural and synthetic blue ultramarine, green earth and chrysocolla), oxides (natural and synthetic hematite, red and yellow natural ochres, and chromium green), carbonates (natural and synthetic azurite, natural and synthetic malachite, and white lead), sulphides (natural and synthetic cinnabar, and orpiment) and acetates, (verdigris) have been characterized by Fourier Transform Infrared-Spectroscopy in Attenuated Total Reflection (ATR-FTIR) and Diffuse Reflectance (DRIFT) modalities. Considering the latter, there is still a great deal of uncertainty in the interpretation of the different IR vibrational bands. Therefore, a comparative study between these two techniques has been carried out to highlight the potential of DRIFT spectroscopy as a portable and non-destructive technique that allows the differentiation and characterization of historical pigments in the field of cultural heritage. Before performing FTIR analysis, pigments were analysed using X-ray diffraction (XRD) to detect impurities and/or additives in the pigments. Differentiation between natural and synthetic pigments was possible due to the identification of impurities in natural pigments, and manufacture-related compounds or additives in synthetic pigments. Results obtained in this study have proven DRIFT to be a very useful analytical technique for in situ characterization of heritage materials. This study serves as an initial step in clarifying the challenges and uncertainties associated with interpreting spectra obtained through the DRIFT modality. However, the use of other complementary analytical techniques is required.
1. Introduction
Identifying pigments in art is one of the most important goals in the study of cultural heritage for numerous reasons, ranging from historical research and archaeometry to adequate conservation strategies [1]. From a historical and artistic point of view, the use of specific pigments is linked to specific time periods, thus helping to date artworks, providing useful information for experts, e.g., to confirm or refute the authenticity of paintings [2]. Moreover, by studying the pigments and techniques an artist used, historians can gain insights into the artistic process and the context in which the artwork was produced. On the other hand, from a conservation and restoration standpoint, pigment identification is essential for developing effective preservation strategies. The identification of pigments enables conservators to understand how to preserve painted artworks, acknowledging their reactions to environmental factors such as light, moisture, temperature fluctuations, etc., thereby mitigating deterioration and ensuring the long-term stability of the artwork [2]. In short, the scientific study of pigments not only enhances our comprehension of art history and artistic practices but also plays a crucial role in the safeguarding of cultural heritage for future generations.
There are several analytical techniques that can be used for this purpose (e.g., X-ray diffraction, X-ray fluorescence, Fourier transform infrared spectroscopy, optical and scanning electronic microscopy modalities, chromatographical techniques, etc.) [3]. However, most of them are sample-invasive, and therefore not universally appreciated [3]. Nowadays, non-destructive instruments are broadly used for the in situ characterization of materials which are often more acceptable and convenient since they respect the integrity of the artwork [3,4]. The quality and performance of the equipment available have drastically improved, leading to the possibility of obtaining more information from the objects without the need for sampling [4].
Among the different analytical approaches, Fourier transform infrared spectroscopy (FTIR) can be used to characterize both organic (e.g., binders) and inorganic materials (e.g., pigments). FTIR spectroscopy utilises infrared radiation to measure the fraction of incident light absorbed at specific wavelengths. This process generates a spectrum that represents the vibrational modes of molecular bonds, enabling the structural analysis of various materials. Each spectrum serves as a chemical fingerprint for identifying minerals and provides unique insights into their molecular structure. In addition, FTIR devices have improved in the last two decades in terms of spectral resolution and detection limit, granting their consolidation as an effective and versatile technique for the study of cultural heritage materials [5,6,7,8]. FTIR can be used in transmission, Attenuated Total Reflection (ATR-FTIR) and in reflectance modality, obtaining an IR spectrum that yields valuable information [7,8]. This is because the energy of most molecular vibrations is found within the IR region, particularly the mid-infrared (MIR, from 4000 to 450 cm−1) [6]. The kind of sample available will generally determine the modality applied as follows [8]:
- i.
- When the IR light can pass through the sample, transmission, which usually provides high spectral resolution, is the most suitable modality. However, it is considered destructive as a pelleting procedure is usually required; the sample must be grinded and mixed with another solid, typically potassium bromide (KBr). Samples can also be examined in a diamond anvil cell by previously compressing the sample (ergo, also destructive).
- ii.
- When the IR light cannot pass completely through the sample, ATR-FTIR is the most suitable since the light only interacts with the first few microns (µm) of the sample. However, in order to apply ATR (with benchtop or portable equipment), it is necessary to exert pressure with the equipment plunger against the FTIR crystal (made up of diamond, germanium or zinc selenide), and is therefore destructive.
- iii.
- Lastly, reflectance modality can also be used and is recommended when samples cannot be taken from the artwork and in situ measurements are needed. Here, measurements can be performed in three different manners as follows: (1) reflection-absorption where the IR light passes through a very thin sample and reflects on a reflective substrate, which is useful for analysing thin tissues or coatings; (2) specular reflection, where the IR light is bounced off a reflective surface, which is useful for examining samples like polymers, gemstones, metals, thin films, glass, etc.; and (3) diffuse reflection, where the light is scattered off a sample surface (Diffuse Reflectance Infrared Fourier Transform spectroscopy, DRIFT), which is commonly applied to characterise rough and matte surfaces such as stones and paintings.
Regarding results, ATR-FTIR spectra are like those acquired in transmission modality, as stated by Silva et al. [9]. However, Navas et al. [10] compared the transmission and DRIFT spectra of several blue pigments and observed significant differences. It was concluded from their findings that DRIFT modality was more effective in the identification of pigments whether they were pure or mixed with rabbit glue. Moreover, Arrizabalaga et al. [11] also observed great differences when comparing the spectra of different materials (nitrates, sulphates, carbonates, oxides and oxalates) obtained with a FTIR benchtop equipment (in transmission and ATR modalities) and with a handheld device in DRIFT modality. Overall, handheld DRIFT devices can yield interesting results, though this technique has been scarcely used in cultural heritage and is vaguely reported in scientific literature (e.g., [9,10,12,13,14,15]) as opposed to the other FTIR modalities (transmission and ATR).
Beyond the evident advantages of portability and non-invasiveness in handheld DRIFT devices, this IR spectroscopy technique is particularly beneficial due to its ability to simultaneously identify both organic and inorganic materials [16]. However, DRIFT modality may encounter challenges related to spectral interferences which can arise from low material quantities, surface roughness and distortions in the spectra [16]. In particular, spectral distortion can occur because the diffusion and specular reflection components cannot be optically separated, leading to signal distortion [17]. This occurs since the refractive index (n) and absorption index (k) of materials influence the specular reflectance [11]. Thus, when a sample contains a compound with k > 1, Reststrahlen bands appear (German word meaning ‘residual rays’ [6,8]), and this can happen with many inorganic materials (carbonates, sulphates, silicates, etc.) [18,19]. These bands basically refer to specific wavelengths of light that are strongly reflected rather than transmitted or absorbed by the material, making it difficult to interpret the spectra [6]. This is more pronounced when several compounds are present in a sample, as is the case of pigments where impurities or additives are usually present.
In this study, an ATR-FTIR and DRIFT comparative study has been carried out for the analysis of nineteen pigments. This comparison aimed to enhance the identification of characteristic functional groups, addressing the ongoing challenges and uncertainties in interpreting spectra obtained through DRIFT modality. Regarding the selection of pigments, there are various ways of classifying pigments in the literature [2,20,21,22,23,24]: origin of manufacture (natural or synthetic), composition (organic and inorganic), colour (blue, red, green, etc.), among others. This study examines the primary chemical composition of various pigments, encompassing silicates (Egyptian blue, natural and synthetic blue ultramarine, green earth and chrysocolla), oxides (natural and synthetic hematite, natural red and yellow ochres, and synthetic chromium green), carbonates (natural and synthetic azurite, natural and synthetic malachite, and white lead), sulphides (natural and synthetic cinnabar, and orpiment) and acetates (verdigris). These pigments were selected due to their historical significance as they are among the most frequently documented in pictorial heritage, regardless of the support (e.g., wall or easel painting) or the painting technique employed (e.g., fresco or mixed with egg yolk, rabbit glue, linseed oil or Arabic gum). Furthermore, by classifying pigments according to their chemical composition, this study highlights their shared spectroscopic characteristics. For instance, all silicate-based pigments exhibit similar Si–O vibrational bands. This classification facilitates the differentiation of pigments with overlapping infrared (IR) spectral features, providing valuable information for professionals. A prior mineralogical characterization by X-ray diffraction (XRD) was carried out to fully characterise the pigments, identifying the possible presence of other mineral phases associated to impurities additives, etc., which could provide other IR features in the spectrum.
2. Materials and Methods
The nineteen pigments studied were supplied by Kremer Pigments GmbH & Co. KG (Aichstetten, Germany): silicates (Egyptian blue, natural and synthetic blue ultramarine, green earth and chrysocolla), oxides (natural and synthetic hematite, red and yellow natural ochres, and chromium green), carbonates (natural and synthetic azurite, natural and synthetic malachite, and white lead), sulphides (natural and synthetic cinnabar, and orpiment) and acetates (verdigris). The data provided by the supplier for each pigment are included in Table 1.

Table 1.
Pigments used in the study. Data provided by the supplier and compared to the authors characterization by X-ray diffraction (XRD).
The mineralogical composition of the powdered pigments was determined using X-ray diffraction (XRD) with an XPert PRO PANalytical B.V. (Almelo, The Netherlands), according to the random-powder method. Analyses were performed with Cu-Kα radiation, Ni filter, 45 kV voltage and 40 mA intensity. The exploration range was 3° to 60° 2θ and the goniometer speed was 0.05° 2θ s−1. The oriented aggregate method was also used in green earth and natural ochre pigments since it is common for these pigments to have clay minerals in their composition. The mineral phases were identified using X’Pert HighScore software (version 4.9.0.27512). Table 1 compares the specifications provided by the supplier with those obtained from the mineralogical characterization conducted by the authors. The detection limit of this technique was ca. 3 wt.%; therefore, other mineral phases in low percentage could not be discarded.
The molecular composition of the pigments was obtained by Fourier Transform Infrared Spectroscopy (FTIR) by directly analysing the powder pigments. The pigments were analysed using a laboratory FTIR benchtop Thermo Nicolet 6700 equipment (Thermo Fisher, Waltham MA, USA) with a 2 cm−1 resolution in Attenuated Total Reflection (ATR) modality, with 100 scans (automatically averaged into a single spectrum), operating in the mid-infrared spectral region (4000–650 cm−1). The powdered pigments were individually analysed by placing less than 1 gram and pressing the plunger against a diamond crystal, exerting the maximum pressure that the equipment allows to ensure the best possible contact with the diamond crystal. A 4300 Handheld FTIR Spectrometer manufactured by Agilent Technologies S.L. (Santa-Clara, CA, USA) was also used in Diffuse Reflectance Infrared Fourier Transform spectroscopy (DRIFT) modality, operating in the same infrared spectral region, with 32 scans (automatically averaged into a single spectrum), with a spectral resolution of 4 cm−1 and a penetration depth of 2–3 µm. A Coarse Gold Reference Cap (G8180-67560) was used for background subtraction. In this procedure, approximately 2–3 grams of each powdered pigment were individually placed on glass slides and then flattened by gently pressing another glass slide on top, creating a smooth and uniform surface. Once compacted, the top glass slide was carefully removed, and the equipment was positioned to ensure full contact with the flattened surface. This setup was adequate to cover the 6 mm spot diameter effectively. A total of three measurements were obtained by direct contact, exerting as much pressure as possible without breaking the glass slide. The spectra with the highest absorbance peaks (ergo, more representative) and higher spectral resolution were selected. Regardless of the modality used, pigments were analysed under controlled laboratory conditions (50 ± 10% relative humidity and 20 ± 2 °C temperature).
3. Results
In this section, the ATR-FTIR and DRIFT results are included for each pigment, grouped according to their chemical nature, i.e., silicates, oxides, carbonates, sulphides and acetates. For each subsection, tables are included, comparing the characteristic bands identified for each modality, the vibration modes and the mineralogical assignment made for each band. Due to the lack of knowledge in the use of DRIFT modality, some of the assignments were made by the authors by considering the position of the bands identified in ATR-FTIR modality, which were already reported by other authors. Figures comparing the spectra in both modalities are also included for each pigment.
Considering the FTIR spectra obtained, it must be considered that the bands in the region between 2400 and 1900 cm−1 in the ATR-FTIR spectra were assigned to the signal from the diamond crystal from the benchtop equipment used [25]. As for the DRIFT spectra, they generally showed high intensity bands due to atmospheric CO2, around 2400–2300 cm−1 (marked in the figures with “CO2”), as well as broad bands between 4000 and 3100 cm−1, assigned to –OH [25,26]. Moreover, many of the DRIFT spectra showed bands between 3100 and 2800 cm−1, 2650 and 2500 cm−1 and 1780 and 1270 cm−1 related to organic compounds [25,26]. Their presence could be related to the extraction process of the pigment from the mineral ore, as it was the case of natural blue ultramarine (UL-N), explained below. In other cases, the organic components could be due to a levigation process, i.e., mineral particle separation. This method was developed by Michael Price (MP) for pigment commercialization in 1997 as a technique for separating powder pigment into various pigment sizes, thus obtaining different shades [27]. The MP method involved washing the pigment with different mediums: e.g., egg yolk for azurite and malachite, rabbit glue for cinnabar or casein for realgar [27]. This could justify the presence of characteristic bands generally assigned to organic compounds, such as methylene groups or amino acids, which were present in many of the pigment spectra (marked in the figures with “OM”, i.e., organic matter). However, these “OM” bands were not included in Table 2, Table 3, Table 4, Table 5 and Table 6 since their assignment to one organic component or another was not essential for pigment allocation nor within the aims of this study. In addition, in order to identify the compound nature, the application of other analytical techniques such as chromatographic techniques, such as gas chromatography (GC) and High Performance Liquid Chromatography (HPLC), would be necessary.
3.1. Silicate Pigments
Silicate pigments are mainly characterized by bands assigned to Si–O bonds found in the range 1250–650 cm−1 in both modalities (Table 2). The mineralogical characterization of Egyptian blue (EGB-S) by XRD, composed of cuprorivaite and quartz in relation to the manufacturing process of the pigment [28], was visible in the IR spectra regardless of the modality. In addition, calcite was also identified through both FTIR modalities in EGB-S: CO32− group characteristic bands were identified in the ATR-FTIR spectra at 1490 cm−1, and at 1735 and 1353 cm−1 in the DRIFT spectra. Therefore, while XRD results did not report the presence of calcite, probably because it was below the detection limit (ca. 3 wt.%) of the XRD equipment, FTIR did. Calcite is part of the raw materials used during the manufacturing process of Egyptian blue [28], therefore, its presence is not surprising. High intensity bands assigned to cuprorivaite were present in both spectra (Figure 1a,b), though at higher wavenumbers in DRIFT (Figure 1b). The bands assigned to quartz impurities showed higher intensities in DRIFT modality, additionally showing a Reststrahlen band around 684 cm−1 [29] (marked with a black arrow in Figure 1b). One aspect to be highlighted is the presence of a stairstep characteristic pattern in the DRIFT spectra assigned to cuprorivaite (marked with a black rectangle in Figure 1b) as was stated by Wiseman et al. [29].
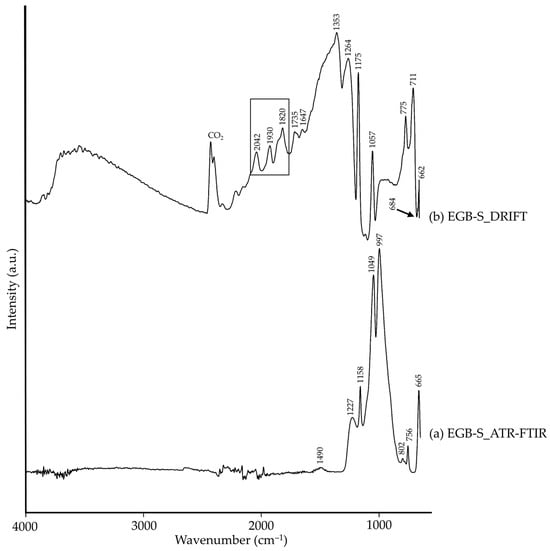
Figure 1.
Absorbance spectra of Egyptian blue (EGB-S) obtained by ATR-FTIR (a) and DRIFT (b) modalities. See Table 1 for the explanation of the pigment identification codes.

Table 2.
Silicate-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
Table 2.
Silicate-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
| Pigment | ATR-FTIR | DRIFT | ||
|---|---|---|---|---|
| Wavenumber (cm−1) | Assignment 1 | Wavenumber (cm−1) | Assignment 1 | |
| EGB-S | 2042, 1930, 1820 | ν+δ(Si–O), cuprorivaite [14] | ||
| 1735 | νs+δ(CO32−), calcite [26] | |||
| 1647 | –OH [26] | |||
| 1490 | CO32−, calcite [26] | 1353 | νas(CO32−), calcite [26] | |
| 1227, 1158, 1049, 997 | νas(Si–O), cuprorivaite [30,31] | 1264, 1175, 1057, 662 | νs(Si–O), cuprorivaite [32,33] | |
| 802 | νs(Si–O), quartz [8,34] | 775, 711, 684 (Reststrahlen) | Si–O, quartz [29,35] | |
| 756, 665 | νs(Si–O), cuprorivaite [30,31] | |||
| UL-N | 3600–3000 | –OH [26], muscovite (?) | 3800–3000 | –OH [26], muscovite (?) |
| 2911, 2859, 2639 | ν(–CH), organic matter [26] | 2607, 2172, 2093, 1850, 1731 | ν(–CH), organic matter [26] | |
| 1647 | –OH [26] | 1688 | –OH [26] | |
| 1430 | CO32− [26], calcite (?) | 1435 | CO32− [26], calcite (?) | |
| 1255 | ν(C–O–C), calcite [26] | |||
| 1157, 1054 | SiO32−, silicate [26] | 1234, 1030 (Reststrahlen) | νas(Si–O or Si,Al–O), lazurite [15,36,37] | |
| 965 | Si,Al–O, sodalite [38,39] | 970 | νas(Si–O), sodalite [10] | |
| 750 | Al–O–Si, muscovite [40] | 752, 728 | Al–O–Si, muscovite (?) | |
| 700 | νas(Si,Al–O), lazurite [9,41] | |||
| 664 | –SO42−, lazurite [41] | 667 | –SO42−, lazurite [15] | |
| UL-S | 3690, 3621 | δ(–OH), kaolinite [42] | 3805–3700 | –OH, kaolinite [35] |
| 1920 | CO32−, carbonate [26] | |||
| 1648 | –OH [26] | 1684 | –OH [26] | |
| 1240, 1160 | SiO32−, silicate [26] | 1280, 1240 | νas(Si–O) or νas(Si,Al–O), lazurite [36,37] | |
| 990 | Si,Al–O, sodalite [38,39] | 1006, 940, 800 | Al–OH, kaolinite [35] | |
| 800, 752, 692 | Al,Si–O, kaolinite [43] | 753, 735, 705 | Si–O, silicate [26] | |
| 665 | –SO42−, lazurite [41] | 675 | –SO42−, lazurite [15] | |
| GE-N | 3500–3100 | ν(–OH), aluminosilicates [44] | 3800–2900 | –OH, aluminosilicates [26] |
| 1840 | νs+δ(CO32−), calcite [14] | |||
| 1632 | –OH [26] | 1695 | –OH [26] | |
| 1175–1150 | Si–O [26], silicates | |||
| 1425, 873 | νas(CO32−), calcite [8,45] | 1493, 1430 | CO32−, calcite [11,35] | |
| 1065(Reststrahlen) | ν(Si–O), glauconite [15] | |||
| 970, 724 | Si–O, glauconite/celadonite [43,46,47] | 960, 920 | Si,Al–O, kaolinite [26] | |
| 777, 735 | Si–O [26], silicates | |||
| 671 | δ(Si–O), glauconite [48] | |||
| CHR-N | 3600, 3321 | –OH [26,49], malachite (?) | 3800–3000 | ν(–OH), malachite [50,51] |
| 3392 | δ(–OH), malachite [18] | |||
| 1630 | δ(–OH), chrysocolla [18] | 1689 | –OH [26] | |
| 1492, 1388, 1095, 823 | CO32−, malachite [18] | 1483 | CO32−, malachite (?) | |
| 998 | Si–O, chrysocolla [49] | 1214, 1152 | SiO32−, silicate [26] | |
| 752 | Si–O [26], chrysocolla (?) β(CO32−), malachite [52] | 1056 (Reststrahlen) | ν(Si–O), chrysocolla (?) | |
| 670 | Si–O, quartz [8,34] | 790–690, 660 | Si–O, quartz [26] | |
1 Vibration mode: νs, symmetric stretching; νas, asymmetric stretching; δ, bending; (?), possible assignment.
The blue colour in blue ultramarine pigments is due to sodalite group minerals, mainly lazurite and sodalite [53], as detected by XRD and is present in both variants (natural and synthetic). Additionally, natural blue ultramarine (UL-N) was also composed of calcite, diopside, pyrite, albite, muscovite and wollastonite, all mineral phases related to the mineral ore [53]. As for synthetic blue ultramarine (UL-S), nepheline (another sodalite-group mineral) and kaolinite were detected, the latter related to its manufacturing process [53]. Their characteristic IR characteristic bands of lazurite/sodalite were present in both spectra (Table 2), regardless of the modality (ATR-FTIR or DRIFT) and the pigment’s nature (natural-N or synthetic-S). These bands were positioned at similar wavenumbers (±5 cm−1) in both ATR-FTIR and DRIFT. This similarity was also reported by Navas et al. [10] in a study comparing DRIFT and transmission modalities. Still, whilst the ATR-FTIR spectra of UL-N (Figure 2a) and UL-S (Figure 2c) were similar, DRIFT spectra showed differences between them (Figure 2b,d, respectively). UL-N showed a Reststrahlen band in the DRIFT spectrum at 1030 cm−1 assigned to lazurite (marked with a black arrow in Figure 2b), not present in the UL-S DRIFT spectrum [15,36,37]. Additionally, bands in the region 2200–1600 cm−1 in the UL-N spectrum (marked with a black dashed-line rectangle in Figure 2b) suggested the presence of organic matter (OM) [26]. Indeed, as reported by the supplier, the method of obtaining this pigment is by grinding the mineral (lapis lazuli) and mixing it with melted wax, resins and oils; a complex process reported by medieval authors such as Cennino Cennini [54]. Lastly, DRIFT spectra of both UL-N and UL-S showed bands between 1350 and 1200 cm−1 (marked with a black rectangle in Figure 2b,d) related to Si–O or Si, Al–O overlapping [36,37], not present in the ATR-FTIR spectrum.
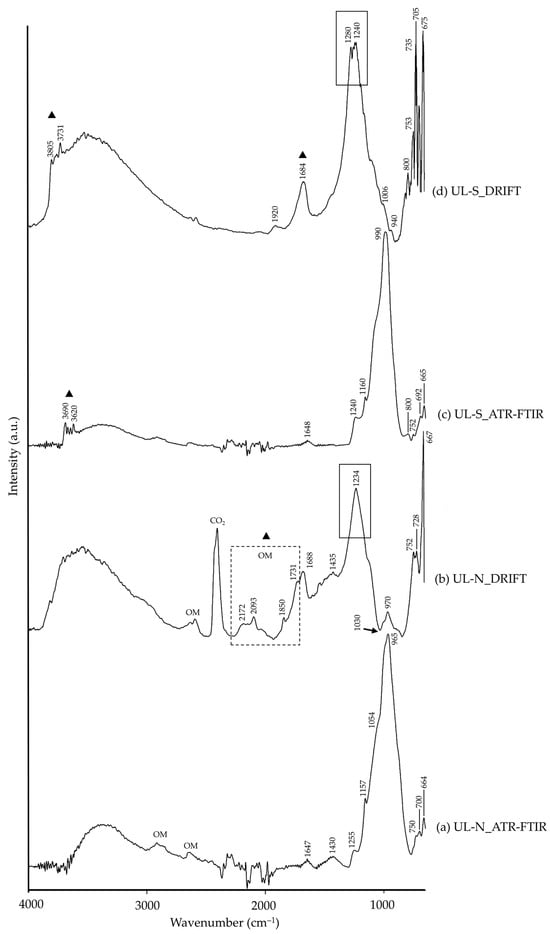
Figure 2.
Absorbance spectra of natural blue ultramarine (UL-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities and synthetic blue ultramarine (UL-S) by ATR-FTIR (c) and DRIFT (d) modalities. See Table 1 for the explanation of the pigment identification codes.
In green earth pigments, the green colour is due to mica-group phases, mainly glauconite/celadonite [55], as detected by XRD. These were observed in both modalities (Table 2). Other mica-group minerals were identified by XRD, such as muscovite, together with calcite, clinochlore, albite and anorthite. Moreover, XRD of oriented-aggregates allowed the identification of clay minerals such as montmorillonite and kaolinite. All these impurities are common among commercial green-earth pigments [47,56]. In the FTIR spectra, the main difference was observed through the presence of a Reststrahlen band at 1065 cm−1 (marked with a black arrow in Figure 3b) in the DRIFT spectrum, assigned to glauconite [15]. In addition, DRIFT modality showed a higher number of bands when compared to the ATR-FTIR spectra, all ascribable to the silicate pigment impurities (Table 2).
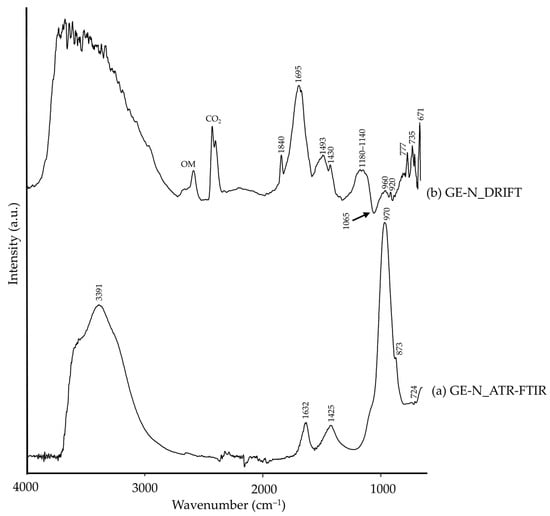
Figure 3.
Absorbance spectra of natural green earth (GE-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities. See Table 1 for the explanation of the pigment identification codes.
Lastly, CHR-N was not only composed by chrysocolla by XRD; malachite and quartz were also detected, mineral phases associated to chrysocolla [49]. These phases were also identified by FTIR analysis, as observed in Table 2. ATR-FTIR (Figure 4a) showed silicate characteristic bands assigned to chrysocolla and quartz, as well as carbonate group bands ascribable to malachite. These were also present in the DRIFT spectrum (Figure 4b), in addition to a Reststrahlen band at 1056 cm−1 (marked with a black arrow in Figure 4b).
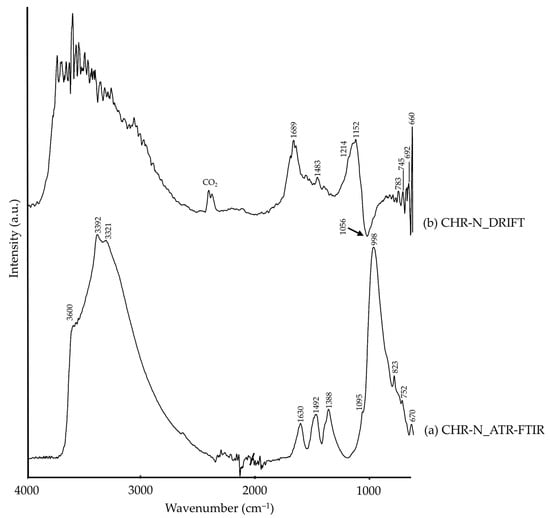
Figure 4.
Absorbance spectra of chrysocolla (CHR-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities. See Table 1 for the explanation of the pigment identification codes.
Overall, when comparing ATR-FTIR and DRIFT modalities, the silicate characteristic bands were present at higher wavenumbers in the later. In addition, Reststrahlen bands were observed by DRIFT between 1065 and 1030 cm−1 in UL-N, GE-N and CHR-N, and at 684 cm−1 for EG-S, all assigned to Si–O bonding. Moreover, while ultramarine pigments (natural and synthetic) showed similar ATR-FTIR spectra, differences were observed in DRIFT modality. These must be considered as markers since they allow the differentiation between the natural and the synthetic variant (marked with black triangles in Figure 2b,d, respectively). That is, on the one hand, the presence of organic matter (OM) in UL-N is due to the pigment extraction process; and on the other hand, the presence of kaolinite in UL-S.
3.2. Oxide Pigments
Hematite pigments, natural (HE-N) and synthetic (HE-S), owe their red hue to the presence of iron oxide, i.e., hematite, as detected by XRD. Nevertheless, the natural variety presented kaolinite, muscovite and dolomite, phases reported as common impurities associated to the mineral ore [57]. The IR Fe–O strong characteristic bands assigned to iron oxides are located in the region below 600 cm−1 in both ATR-FTIR [58,59,60] and DRIFT modalities [35,61,62]; thus, they are not visible in the spectra presented in this study. Nevertheless, the impurities in HE-N were identified by characteristic bands assigned to aluminosilicates (kaolinite or muscovite) and dolomite (CaMg(CO3)2), as shown in Table 3, in both ATR-FTIR (Figure 5a) and DRIFT spectra (Figure 5b). Lastly, in HE-S, both spectra (Figure 5c,d) analyses did not provide valuable signals, just bands assigned to Si–O, showing the presence of a small amount of quartz [35], probably present as an impurity or inert filler.
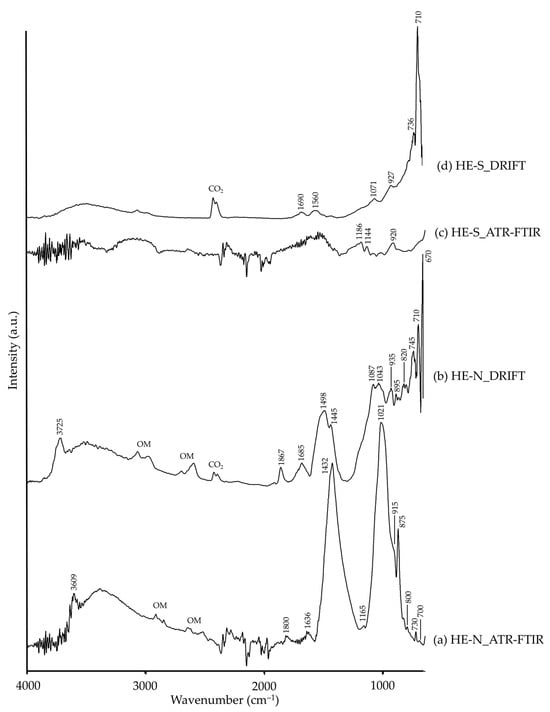
Figure 5.
Absorbance spectra of natural hematite (HE-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities and synthetic hematite (HE-S) by ATR-FTIR (c) and DRIFT (d) modalities. See Table 1 for the explanation of the pigment identification codes.

Table 3.
Oxide-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
Table 3.
Oxide-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
| Pigment | ATR-FTIR | DRIFT | ||
|---|---|---|---|---|
| Wavenumber (cm−1) | Assignment 1 | Wavenumber (cm−1) | Assignment 1 | |
| HE-N | ~3609 | ν(–OH), aluminosilicate [42] | 3725 | ν(–OH), aluminosilicate [35] |
| 3540–3045 | –OH [26], aluminosilicate (?) | 3650–3150 | –OH [26], aluminosilicate (?) | |
| 1800 | νs+δ(CO32−), dolomite (?) | 1867 | νs+δ(CO32−) [14], dolomite | |
| 1636 | –OH [26] | 1685 | –OH [26] | |
| 1432, 875, 730 | CO32−, dolomite [63] | 1498, 1445 | CO32− [26], dolomite (?) | |
| 1165 | Si–O, quartz [44] | 1087, 1043, 745, 710 | Si–O, quartz [26] | |
| 1021 | Si–O, kaolinite [44] | 935, 895, 820 | Al–OH, kaolinite [35] | |
| 915 | Al–OH, kaolinite [44] | |||
| 800 | Al,Si–O, kaolinite [43] | |||
| 700 | Al–O [26], kaolinite (?) | 670 | Si–O, quartz [35] | |
| HE-S | 1690, 1560 | –OH [26] | ||
| 1186, 1144, 920 | Si–O, quartz [26] | 1071, 927, 736, 710 | Si–O, quartz [26,35] | |
| RO-N | 3700–3000 | ν(–OH), aluminosilicate [26], illite (?) | 3800–2900 | ν(–OH), aluminosilicate [35,64], illite (?) |
| 2053 | ν+δ(Si–O) [14,35], quartz (?) | |||
| 1925, 1839 | νs+δ(CO32−), calcite [26] | |||
| 1680, 1620 | –OH [26], gypsum (?) | 1720–1660 | –OH [26], gypsum (?) | |
| 1269, 1186 | C–O [26], calcite (?) | |||
| 1080, 1023, 793 | Si–O, quartz [8,34] | 1030–1060 | Si–O, silicate [26] | |
| 828, 777, 749, 718 | Si–O, quartz [35] | |||
| 700, 670 | ν(S–O) [26], gypsum (?) | |||
| YO-N | 3650–3100 | –OH [26], goethite (?) | 3700–3150, 3072, 2960 | ν(–OH), goethite [26,65] |
| 1792 | C=O, calcite [8,45] | 1845 | δ(CO32−), calcite [26] | |
| 1640 | –OH [26], goethite (?) | 1680 | –OH [26], goethite (?) | |
| 1395 | CO32−, calcite [8,45] | 1481, 1430, 725 | CO32−, calcite [35] | |
| 1266, 1187 | C–O [26], calcite (?) | |||
| 1090, 1014, 804 | Si–O, silicate [26] | 1090, 1035, 924 | Si–O, silicate [26] | |
| 875 | νas(CO32−), calcite [8,45] | 890, 860, 815, 682 | δ(CO32−), calcite [26] | |
| 715 | νs(CO32−), calcite [8,45] | |||
| CG-S | 3570–3050 | –OH [26] | ||
| 1158, 1104 | Si–O, quartz [35] | 1170, 1070, 880, 830, 802, 775, 760 | Si–O, quartz [35] | |
1 Vibration mode: νs, symmetric stretching; νas, asymmetric stretching; δ, bending; (?), possible assignment.
As for natural ochre pigments, their colour depends on the nature of the iron oxide chromophore. As such, the reddish hue in RO-N is mainly due to anhydrous iron oxide (Fe2O3), whilst in YO-N, the yellow hue is due to hydrated iron oxides such as goethite (FeO·OH), as identified by XRD analysis. Their natural provenance implied the presence of impurities, as shown in Table 1: quartz, gypsum, diopside and illite in RO-N, and calcite in YO-N [66]. As with what occurred with the hematite pigments, RO-N and YO-N only showed IR signals assigned to the impurities (Table 3) since Fe–O characteristic bands appear at lower wavenumbers (>600 cm−1), as observed in other pigments, i.e., bands assigned to quartz or gypsum (in RO-N) or calcite (in YO-N) appeared at higher wavenumbers, and generally with higher intensity, in DRIFT modality (Figure 6b,d, respectively).
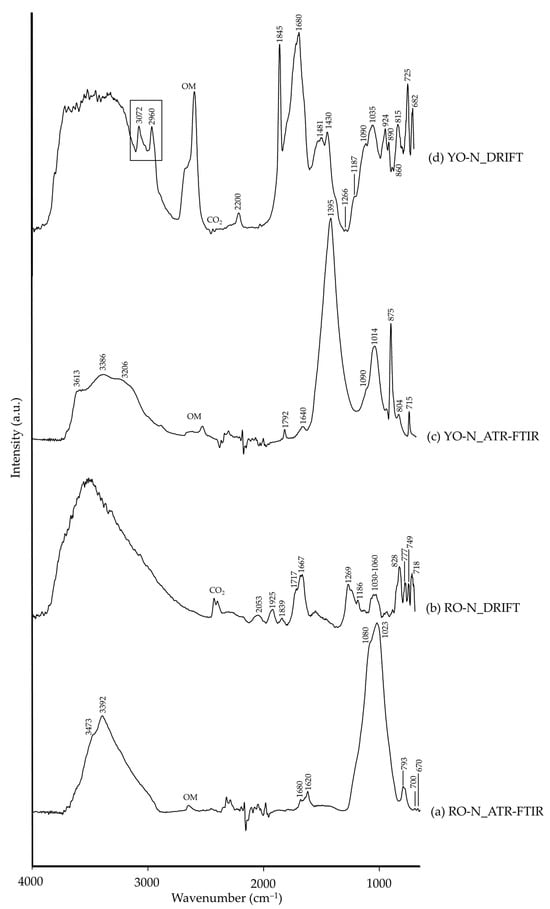
Figure 6.
Absorbance spectra of natural red ochre (RO-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities and natural yellow ochre (YO-N) by ATR-FTIR (c) and DRIFT (d) modalities. See Table 1 for the explanation of the pigment identification codes.
Lastly, regarding synthetic chromium green, the Cr–O tension modes are reported to be present in the far-IR region (≈605, 577, 497, 474, 460 and 450 cm−1 [67,68]). However, in DRIFT modality (Figure 7b) we observed high intensity bands at 1170, 1070, 880, 830, 802, 775 and 760 cm−1 ascribable to quartz [35]. This must be assigned to impurities present in low percentage as it was not identified by XRD (Table 1).
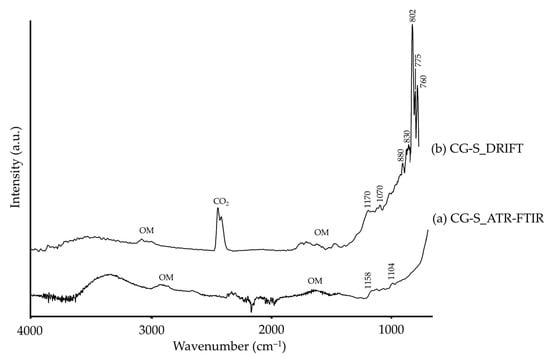
Figure 7.
Absorbance spectra of synthetic chromium green (CG-S) obtained by ATR-FTIR (a) and DRIFT (b) modalities. See Table 1 for the explanation of the pigment identification codes.
Overall, the identification of Fe–O (in HE-N, HE-S, RO-N and YO-N) or Cr–O (in CH-S) tension modes were not observed in the spectra shown in Figure 5, Figure 6 and Figure 7, therefore, these pigments would not be easily identified. Nevertheless, the presence of bands assigned to impurities (HE-N, RO-N and YO-N) could be used as IR markers to differentiate between natural and synthetic pigments. Finally, no Reststrahlen bands were identified in these oxide pigments.
3.3. Carbonate Pigments
All five carbonate-based pigments (AZ-N, AZ-S, MA-N, MA-S and WL-S) presented characteristic bands assigned to carbonate groups (CO32−), though with slight differences in both ATR-FTIR and DRIFT modalities (Table 4). Similar to what occurred with the oxide-based pigments, bands related to copper ions (Cu2+) in AZ-N, AZ-S, MA-N, MA-S, or related to lead ions (Pb2+) in white lead (WL-S) were not identified as they are generally present below 600 cm−1 [26]. Nevertheless, other characteristic IR bands, ascribable to each pigment, were observed.

Table 4.
Carbonate-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
Table 4.
Carbonate-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
| Pigment | ATR-FTIR | DRIFT | ||
|---|---|---|---|---|
| Wavenumber (cm−1) | Assignment 1 | Wavenumber (cm−1) | Assignment 1 | |
| AZ-N | 3955 | –OH [26] | ||
| 3692, 3619 | δ(–OH), kaolinite [42] | 3810, 3731 | –OH, kaolinite [35] | |
| 3425 | ν(–OH), azurite [52] | 3550, 3475 | ν(–OH), azurite [14,15] | |
| 1498, 1412 | νas(CO32−), azurite [52] | 1914, 1677, 1545 | CO32−, carbonate [26], azurite (?) | |
| 1465 | ν(C–O), azurite [69] | 1487, 1445 (Reststrahlen), 1421 | νas(CO32−), azurite [14,15] | |
| 1090, 1006 | νs(CO32−), azurite [52] | 1135, 1090 (Reststrahlen), 1007 | νs(CO32−), azurite [14] | |
| 1030, 950 | β(–OH), azurite [52] | 1040, 935 | Al–OH, kaolinite [35] | |
| 833, 813 | δ(CO32−), azurite [52] | 834 | δ(CO32−), azurite [10,14,15] | |
| 765, 695 | β(CO32−), azurite [52] | 783, 710 | β(CO32−), azurite [14] | |
| AZ-S | 3957, 3850 | –OH [26] | ||
| 3425 | ν(–OH), azurite [52] | 3750–2900 | ν(–OH), azurite [14,15] | |
| 1508, 1396 | νas(CO32−), azurite [52] | 1913, 1885, 1685, 1620, 1530 | CO32−, carbonate [26], azurite (?) | |
| 1469 | ν(C–O), azurite [69] | 1491, 1450 (Reststrahlen), 1407 | νas(CO32−), azurite [14,15] | |
| 1090 | νs(CO32−), azurite [52] | 1116, 1090 (Reststrahlen), 993 | νs(CO32−), azurite [14] | |
| 950 | β(–OH), azurite [52] | 954 | δ(–OH), azurite [15] | |
| 870 | CO32−, calcite [35] | |||
| 818 | δ(CO32−), azurite [52] | |||
| 767, 739 | β(CO32−), azurite [52] | 792, 765, 685 | β(CO32−), azurite [14] | |
| MA-N | 3395, 3308 | δ(–OH), malachite [18] | 3700–3000 | ν(–OH), malachite [9] |
| 1970 | CO32− [26], malachite (?) | |||
| 1852, 1605 | CO32− [26], malachite (?) | |||
| 1490, 1379 | νas(CO32−), malachite [18,52] | 1484, 1400 | νas(CO32−), malachite [9] | |
| 1095 | νs(CO32−), malachite [52] | 1128, 1095, 1040, 935 | νs(CO32−), malachite [9] | |
| 1042, 865 | β(–OH), malachite [52] | |||
| 815 | δ(CO32−), malachite [52] | 867 | CO32− [35], malachite (?) | |
| 770 | CO32−, malachite (?) | |||
| 776, 745, 705 | β(CO32−), malachite [52] | 680 | Si–O, quartz [35] | |
| MA-S | 3403, 3320 | δ(–OH), malachite [18] | 3700–3000 | ν(–OH), malachite [9] |
| 1850, 1594 | CO32− [26], malachite (?) | |||
| 1490, 1382 | νas(CO32−), malachite [18,52] | 1478 | CO32−, malachite (?) | |
| 1105 | νs(CO32−), malachite [52] | 1129, 1090 | νs(CO32−), malachite [9] | |
| 1047, 873 | β(–OH), malachite [52] | 940–910 | Si–O, quartz [25] | |
| 815 | δ(CO32−), malachite [52] | 845 | δ(CO32−), malachite (?) | |
| 750, 712 | β(CO32−), malachite [52] | 769, 695, 675 | Si–O, quartz [26] | |
| WL-S | 3560–3100 | –OH [26], hydrocerussite (?) | 3800–3200 | ν(–OH), hydrocerussite [14,70] |
| 1730, 1065 | νs(CO32−), cerussite [14,71] | 1780, 1135, 1080, 1020 | νs(CO32−), cerussite [14,15] | |
| 1430, 1370 | νas(CO32−), cerussite [15] | 1550 | ν(CO32−), carbonate [14,70] | |
| 1125 | SO42−, lead carbonate [72] | 1465 | νas(CO32−), cerussite [13,15] | |
| 995 | Pb–OH [72], hydrocerussite (?) | |||
| 850 | β(CO32−), cerussite [72] | 860 | β(CO32−), cerussite [13,15] | |
| 790 | β(Pb–OH) [73], hydrocerussite (?) | |||
| 690 | δ(CO32−), cerussite [72] | 713, 695 | δ(CO32−), cerussite [72] | |
1 Vibration mode: νs, symmetric stretching; νas, asymmetric stretching; δ, bending; (?), possible assignment.
Regarding copper carbonates, azurite is a basic blue copper carbonate (Cu3(CO3)2(OH)2). The natural version (AZ-N) was additionally composed of quartz, kaolinite and phlogopite by XRD, all of which are common impurities to which the mineral ore is associated [74]. As for AZ-S, calcite was also present, related to the raw materials used in its manufacturing process [74]. When comparing AZ-N and AZ-S (Figure 8), differences between them were observed. The presence of impurities in the natural pigment, recognizable through the characteristic bands of kaolinite (–OH and Al–OH bonds [35,42]) and those assigned to quartz (Si–O bonds [8,34,35]), in both ATR-FTIR and DRIFT spectra, marked a clear distinction between both pigments. Even though these bands were also present in both modalities, though present at higher wavenumbers in DRIFT spectra, other IR bands were observed. Both AZ-N (Figure 8b) and AZ-S (Figure 8d) showed bands assigned to organic matter (OM) which could be related to a levigation process. This is very remarkable since DRIFT modality was able to obtain IR signals (of considerable intensity) assigned to organic matter when ATR-FTIR spectra were unable to provide significant signals.
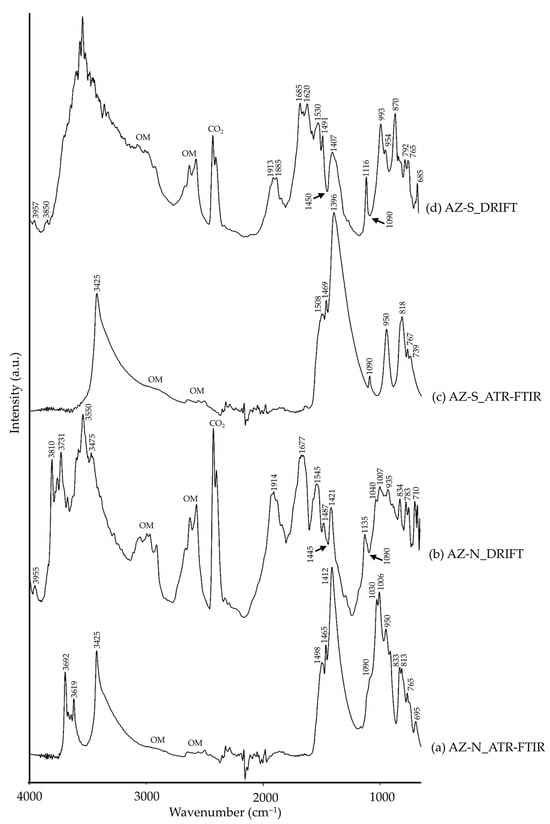
Figure 8.
Absorbance spectra of natural azurite (AZ-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities and synthetic azurite (AZ-S) by ATR-FTIR (c) and DRIFT (d) modalities. See Table 1 for the explanation of the pigment identification codes.
Malachite is also a copper carbonate (Cu2(CO3)(OH)2), similar to azurite, therefore sharing similar absorption bands regarding hydroxyl (–OH) and carbonate (CO32−) groups, as observed in Table 4. Natural malachite (MA-N) was also composed of hematite and cuprite, impurities to which the mineral ore is commonly associated [49]. However, none have IR signals in the 4000–650 cm−1 spectral region. As for synthetic malachite (MA-S), quartz was also present related to the raw materials used in the pigment manufacture [49]. The ATR-FTIR spectra of both MA-N (Figure 9a) and MA-S (Figure 9c) presented very similar –OH and CO32− group characteristic bands, though at slightly higher wavenumbers (±5 cm−1) in MA-S. As for the DRIFT spectra, results were very similar between them.
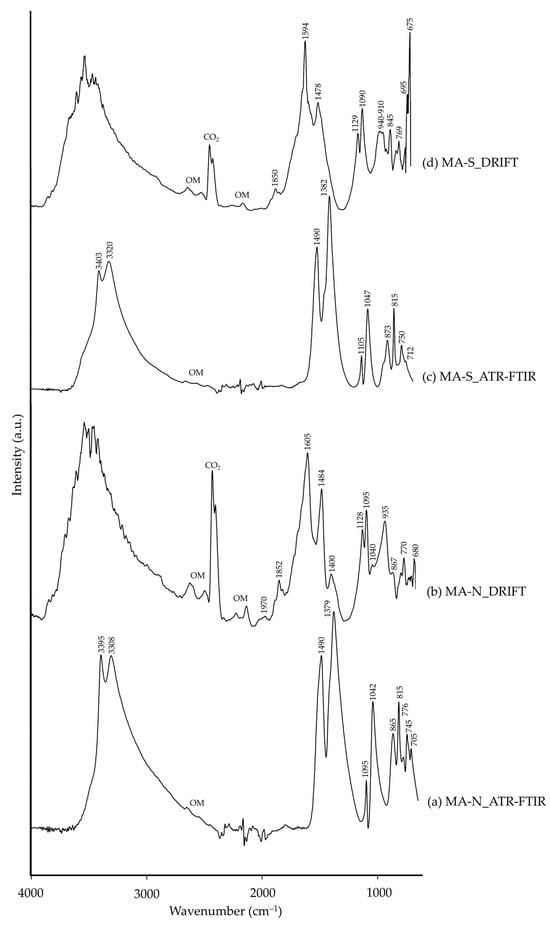
Figure 9.
Absorbance spectra of natural malachite (MA-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities and synthetic malachite (MA-S) by ATR-FTIR (c) and DRIFT (d) modalities. See Table 1 for the explanation of the pigment identification codes.
Regarding white lead (WL-S), this pigment was composed of a carbonate salt, cerussite, recognizable through its characteristic CO32− groups in both modalities (Table 4). However, in DRIFT (Figure 10b), they appeared at higher frequencies, and generally with higher intensities. Additionally, a –OH broad band was present in both modalities, more pronounced in DRIFT, which could be related to hydrocerussite, commonly present in white lead [75]. The Pb–O band, characteristic of white lead, was not observed in these spectra as it is usually present around 466 cm−1 in the ATR-FTIR spectra [73]. Lastly, organic matter (OM) was also detected, exclusively in DRIFT modality, similarly to azurite pigments (Figure 7).
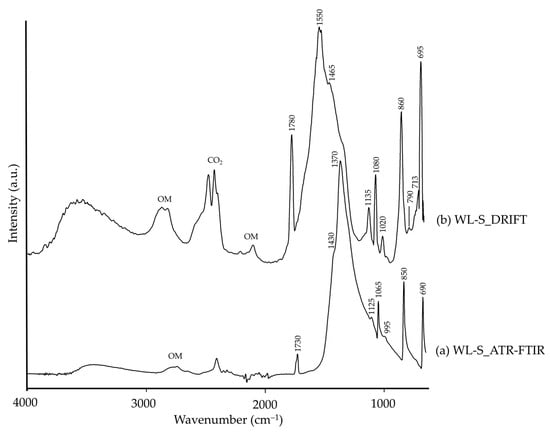
Figure 10.
Absorbance spectra of synthetic white lead (WL-S) obtained by ATR-FTIR (a) and DRIFT (b) modalities. See Table 1 for the explanation of the pigment identification codes.
In short, copper-carbonate pigments were distinguishable regardless of the modality used. In azurite pigments, –OH bands were present as a single peak (around 3425 cm−1, as observed in Figure 8a,c, respectively), whilst in malachite pigments they were present as doublets at ~3400 and ~3310 cm−1 (Figure 9a,c, respectively). Moreover, Reststrahlen bands were identified exclusively in azurite pigments in DRIFT modality, regardless of their nature (natural or synthetic), marked with a black arrow in Figure 8b,d. These must also be considered as markers to differentiate between azurite and malachite pigments, as Miliani et al. also reported [14]. Finally, the presence of organic matter was observed in all the carbonate pigments when using DRIFT modality, something that is undoubtedly of great interest to professionals in the conservation science field.
3.4. Sulphide Pigments
It is widely reported in the literature that the characterization of sulphide-based pigments, e.g., cinnabar, vermilion or orpiment, cannot be achieved by FTIR analysis [76]. For instance, mercury sulphide pigments, i.e., cinnabar and vermilion, show Hg–S tension bands in the far-IR spectral region (345 and 283 cm−1 on both pigments [77]). Nevertheless, Figure 11 showed that ATR-FTIR and DRIFT spectra of both CI-N and VE-S had IR signals. On the one hand, this is related to SO2 stretching (marked with black arrows in Figure 11), characteristic of sulphide pigments, and which showed better resolution in the synthetic pigment (VE-S). On the other hand, even though CI-N and VE-S were only composed of cinnabar (HgS) by XRD analysis, the presence of IR bands assigned to impurities such as calcite and quartz were detected by FTIR analysis (see Table 5). Calcite and quartz are among the characteristic impurities found to be associated with the natural ore, as well as used to synthesize the synthetic version [78]. Also, the DRIFT spectra showed higher resolution and more information when compared to the ATR-FTIR spectra, regardless of the pigment (CI-N or VE-S, Figure 11b,d, respectively). Interestingly, VE-S showed –OH broad bands in both ATR-FTIR and DRIFT, though the authors could not ascribe it to a mineral phase. Lastly, organic matter (OM) was present in both pigments which should be related to a levigation process, as with what was observed in carbonate pigments.
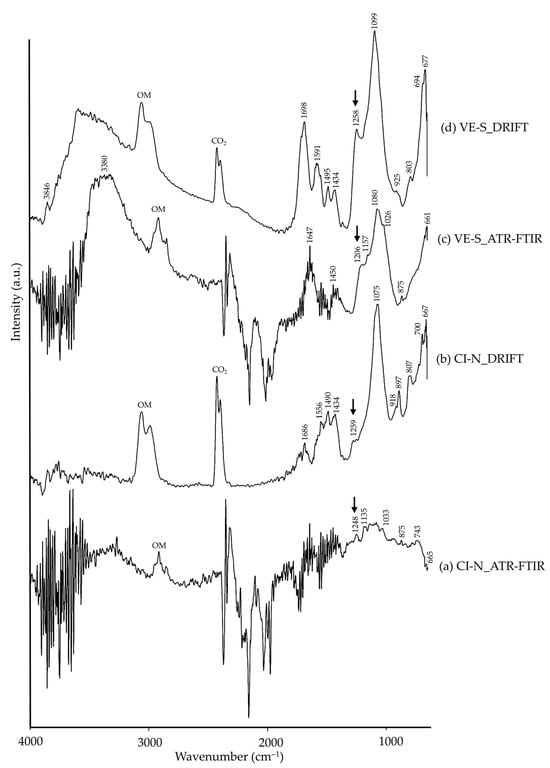
Figure 11.
Absorbance spectra of natural cinnabar (CI-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities and synthetic cinnabar (CI-S) by ATR-FTIR (c) and DRIFT (d) modalities. See Table 1 for the explanation of the pigment identification codes.

Table 5.
Sulphide-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
Table 5.
Sulphide-based pigments analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included where possible. See Table 1 for the explanation of the pigment identification codes.
| Pigment | ATR-FTIR | DRIFT | ||
|---|---|---|---|---|
| Wavenumber (cm−1) | Assignment 1 | Wavenumber (cm−1) | Assignment 1 | |
| CI-N | 1686, 1556, 1490, 1434 | CO32−, carbonate [26], calcite (?) | ||
| 1248 | νas(SO2) [26], cinnabar (?) | 1259 | νas(SO2) [26], cinnabar (?) | |
| 1135, 1033 | ν(Si–O), quartz [8,34] | 1075, 918, 897, 807, 700 | ν(Si–O), quartz [35] | |
| 875 | νas(CO32−), calcite [8,45] | |||
| 743, 665 | ν(Si–O), quartz [8,34] | 667 | Si–O, quartz [35] | |
| VE-S | 3500–3000 | –OH [26] | 3846, 3700–3200 | –OH [26] |
| ~1647, ~1450 | CO32−, carbonate [26], calcite (?) | 1698, 1591, 1495, 1434 | CO32−, carbonate [26], calcite (?) | |
| 1258 | νas(SO2) [26], vermilion (?) | |||
| 1206 | νas(SO2) [26], vermilion (?) | |||
| 1157, 1080, 1026, 661 | ν(Si–O), quartz [8,34] | 1099, 925, 803, 694, 677 | ν(Si–O), quartz [35] | |
| 875 | νas(CO32−), calcite [8,45] | |||
| OR-N | ~1420 | CO32−, carbonate [26], calcite (?) | 1690, 1491 | CO32−, carbonate [26], calcite (?) |
| 1244 | νas(SO2) [26], orpiment/realgar (?) | 1285, 1260 | νas(SO2) [26], orpiment/realgar (?) | |
| 1145, 1035, 845, 798, 710 | ν(Si–O), quartz [8,34] | 1180, 1140, 1070, 893, 860, 815, 700, 675, 660 | ν(Si–O), quartz [35] | |
| 870 | νas(CO32−), calcite [8,45] | |||
1 Vibration mode: νs, symmetric stretching; νas, asymmetric stretching; δ, bending; (?), possible assignment.
Regarding orpiment (OR-N), a very similar situation to that mentioned in CI-N and VE-S was observed. The raw pigment was composed of orpiment and realgar (by XRD), both arsenic sulphides. However, even though As–S characteristic bands were not identified as they are generally present around 480 cm−1 [26], the presence of SO2 stretching vibration was present (marked with black arrows in Figure 12). In Figure 12 it can be seen that the ATR-FTIR spectra showed higher noise than DRIFT spectra. Also, DRIFT showed higher number of bands, all of which could be related to calcite or quartz (see Table 5), likely to be present as impurities (even though they were not identified by XRD, Table 1). Moreover, organic matter (OM) was again present, though more pronounced in DRIFT modality.
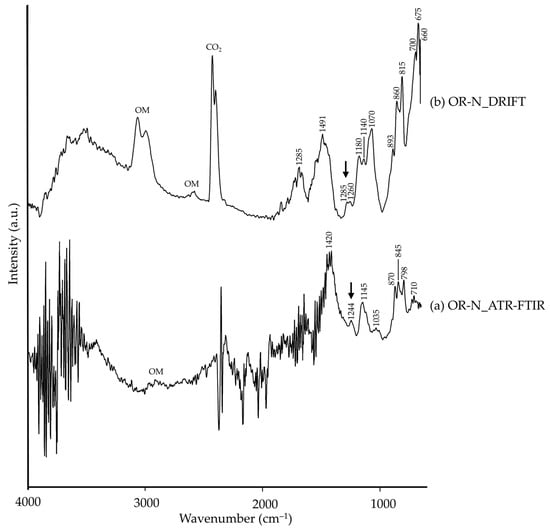
Figure 12.
Absorbance spectra of natural orpiment (OR-N) obtained by ATR-FTIR (a) and DRIFT (b) modalities. See Table 1 for the explanation of the pigment identification codes.
Overall, it was observed that the identification of sulphide pigments is not an easy task by IR spectroscopy, regardless of the modality. Though their impurities appeared in the spectra, the three pigments showed the same mineral phases (calcite and quartz). Therefore, the use of other portable techniques such as Raman spectroscopy should be considered for their identification. Nevertheless, it was observed that DRIFT modality provided more information than ATR-FTIR. Also, all three showed bands assigned to organic matter (OM) which could be related to a levigation process, where rabbit glue was used for CI-N and VE-S, and casein in OR-N, as explained in [27].
3.5. Acetate Pigment
Neutral copper acetate, i.e., verdigris, corresponds chemically and structurally with the mineral hoganite (Cu(CH3COO)2·H2O) [79], as detected by XRD. Based on the literature, the main ATR-FTIR characteristic bands that can be associated to this pigment were detected (Table 6, Figure 13a). However, the study of the VER-S by DRIFT modality showed differences, specially related to hydroxyl groups (–OH bonds) of the acetate ion. Whilst in ATR-FTIR we observed three characteristic bands at 3447, 3362 and 3268 cm−1, the DRIFT spectrum showed a broad band between 3700 and 3200 cm−1 likely due to the interaction with atmospheric moisture (Figure 13b). The acetate group was also detected by DRIFT modality, though at higher wavenumbers and with lower intensity (marked with a black rectangle in Figure 13).
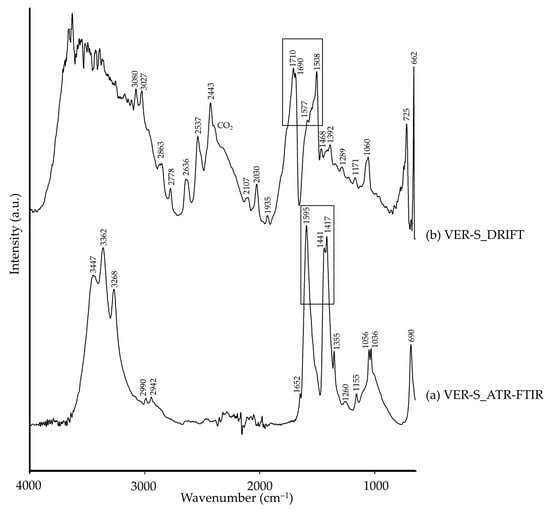
Figure 13.
Absorbance spectra of synthetic verdigris (VER-S) obtained by ATR-FTIR (a) and DRIFT (b) modalities. See Table 1 for the explanation of the pigment identification codes.

Table 6.
Acetate-based pigment analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included when possible. See Table 1 for the explanation of the pigment identification codes.
Table 6.
Acetate-based pigment analysed by ATR-FTIR and DRIFT modalities. Bands (cm−1) and assignment (vibration mode and mineral associated) are included when possible. See Table 1 for the explanation of the pigment identification codes.
| Pigment | ATR-FTIR | DRIFT | ||
|---|---|---|---|---|
| Wavenumber (cm−1) | Assignment 1 | Wavenumber (cm−1) | Assignment 1 | |
| VER-S | 3447, 3362, 3268 | ν(–OH), acetate ion [80,81,82] | 3800–3200 | ν(–OH) [26], acetate ion (?) |
| 2990, 2942 | ν(C–H), acetate [83] | 3080, 3027 | ν(C–H) [26], acetate (?) | |
| 2863 | νs(CH3) [26], acetate (?) | |||
| 2778–1935 | Not assigned | |||
| 1652 | C=O [26] | |||
| 1595 | νs(COO−), acetate [80,81,82] | 1710, 1690 | νs(COO−) [26], acetate (?) | |
| 1441, 1417 | νas(COO−), acetate [80,81,82] | 1577,1508 | νas(COO−) [26], acetate (?) | |
| 1468 | (COO−) [26], acetate (?) | |||
| 1260 | νs(C=O) [80,81,82] | 1289 | νs(C=O) [26] | |
| 1155 | -CH3 [80,81,82] | 1171 | -CH3 (?) | |
| 1355, 1056, 1036 | C–H from the CH3 in the acetate group [80,81,82] | 1392, 1060 | C–H from the CH3 in the acetate group (?) | |
| 690 | δ(O–C–O), acetate ion [80,81,82,83] | 725, 662 | O–C–O [26] | |
1 Vibration mode: νs, symmetric stretching; νas, asymmetric stretching; δ, bending; (?), possible assignment.
4. Conclusions
This study presents the results obtained from the analysis of nineteen pigments by infrared spectroscopy comparing Fourier Transform Infrared-Spectroscopy in Attenuated Total Reflection (ATR-FTIR) and Diffuse Reflectance (DRIFT) modalities. Prior characterization using X-ray diffraction (XRD) played a crucial role in identifying characteristic bands assigned to impurities and/or additives, highlighting discrepancies between the actual composition of the pigments and the information provided by manufacturer. Furthermore, additional mineral phases were detected through their infrared characteristic bands in both ATR-FTIR and DRIFT modalities, which were not identified by XRD—likely due to their low concentration—falling below the detection threshold (ca. 3 wt.%) of the XRD equipment.
With regards to FTIR modalities, the DRIFT modality proved to be highly effective at differentiating pigments with similar chemical natures such as silicates, oxides, carbonates, sulphides and acetates. Also, DRIFT enabled the distinction between natural and synthetic analogues by detecting impurities in natural pigments, as well as manufacturing-related compounds or additives, mainly quartz and calcite, in synthetic ones. These features generally appeared at higher wavenumbers and with higher intensity compared to the spectra obtained by ATR-FTIR modality.
DRIFT modality offers notable advantages, particularly its enhanced sensitivity in detecting organic compounds. Its non-destructive nature allowed for the identification of spectral bands associated with organic substances, which may be linked to the levigation process. These organic-related bands were observed in all analysed pigments except for Egyptian blue, synthetic blue ultramarine, chrysocolla, synthetic hematite and verdigris. However, DRIFT also presents certain limitations, including the presence of spectral interferences from atmospheric moisture (broad bands between 4000 and 3100 cm⁻¹) and carbon dioxide (doublet band between 2400 and 2300 cm⁻¹). These external contributions can complicate spectral interpretation and affect analytical precision.
In all, this study sets the foundation for addressing the uncertainty related to the interpretation of the spectra obtained by DRIFT modality. The results presented are considered a starting point for the identification of pigments in pictorial artworks (whatever their nature—mural or easel—or the painting technique) for in situ characterization, without the need for sampling, thus respecting the integrity of the original work. However, given the complexity of heritage materials, it is common practice in heritage science to integrate multiple analytical techniques. Therefore, combining DRIFT spectroscopy with complementary methods such as Raman spectroscopy enhances the depth and accuracy of pigment characterization, providing more comprehensive information.
Finally, future studies involving the analysis of real pictorial works should consider the results obtained here in order to provide veracity and establish differences that will help the scientific community.
Author Contributions
Conceptualization, D.J.-D. and J.S.P.-A.; methodology, D.J.-D. and J.S.P.-A.; software, D.J.-D. and J.S.P.-A.; validation, D.J.-D. and J.S.P.-A.; formal analysis, D.J.-D. and J.S.P.-A.; investigation, D.J.-D. and J.S.P.-A.; resources, J.S.P.-A.; data curation, D.J.-D.; writing—original draft preparation, D.J.-D.; writing—review and editing, J.S.P.-A.; visualization, D.J.-D. and J.S.P.-A.; supervision, J.S.P.-A.; project administration, J.S.P.-A.; funding acquisition, J.S.P.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the PID2021-123395OA-I00 research project funded by MICIU/AEI/10.13039/501100011033, by “ERDF A way of making Europe” and, by the Xunta de Galicia ED431F 2022/07 project. For more information: https://laseringph.webs.uvigo.es/ (accessed on 28 March 2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding author.
Acknowledgments
Analyses were performed in the CACTI (Centro de Apoyo a la Investigación) Research Support Centre and at the CINTECX (Centro de Investigación en Tecnologías, Energías y Procesos Industriales) Research Centre at the University of Vigo. D. Jiménez-Desmond was supported by the ED481A-2023/086 predoctoral contract through “Programa de axudas á etapa predoutoral da Xunta de Galicia” cofinanced by the European Union within the framework of the FSE+ Galicia 2021–2027 programme. J.S. Pozo-Antonio was supported by the RYC2020-028902-I project funded by MICIU/AEI/10.13039/501100011033 and, by “ESF Investing in your future”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Harth, A. The Study of Pigments in Cultural Heritage: A Review Using Machine Learning. Heritage 2024, 7, 3664–3695. [Google Scholar] [CrossRef]
- Mayer, R. Materiales y Técnicas del Arte; Hermann Blume Ediciones: Madrid, Spain, 2005. [Google Scholar]
- Artioli, G. Scientific Methods and Cultural Heritage: An Introduction to the Application of Materials Science to Archaeometry and Conservation Science; Oxford University Press Inc.: New York, NY, USA, 2010. [Google Scholar]
- Aucouturier, M.; Darque-Ceretti, E. Materials Surface Science Applied to the Investigation of Cultural Heritage Artefacts. Procedia Mater. Sci. 2015, 9, 31–47. [Google Scholar] [CrossRef]
- Low, M.J.D.; Baer, N.S. Application of Infrared Fourier Transform Spectroscopy to problems in Conservation. Stud. Conserv. 1977, 22, 116–128. [Google Scholar] [CrossRef]
- Prati, S.; Sciutto, G.; Bonacini, I.; Mazzeo, R. New Frontiers in Application of FTIR Microscopy for Characterization of Cultural Heritage Materials. In Analytical Chemistry for Cultural Heritage. Topics in Current Chemistry Collections; Mazzeo, R., Ed.; Springer: Cham, Switzerland, 2017; Volume 374. [Google Scholar] [CrossRef]
- Poliszuk, A.; Ybarra, G. Analysis of Cultural Heritage materials by infrared spectroscopy. In Infrared Spectroscopy: Theory, Developments and Applications; Cozzolino, D., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 519–536. [Google Scholar]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Scientific Tools for Conservation. Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Silva, C.E.; Silva, L.P.; Edwards, H.G.M.; De Oliveira, L.F.C. Diffuse reflection FTIR spectral database of dyes and pigments. Anal. Bioanal. Chem. 2006, 386, 2183–2191. [Google Scholar] [CrossRef]
- Navas, N.; Romero-Pastor, J.; Manzano, E.; Cardell, C. Benefits of applying combined diffuse reflectance FTIR spectroscopy and principal component analysis for the study of blue tempera historical painting. Anal. Chim. Acta 2008, 630, 141–149. [Google Scholar] [CrossRef]
- Arrizabalaga, I.; Gómez-Laserna, O.; Carrero, J.A.; Bustamante, J.; Rodríguez, A.; Arana, G.; Madariaga, J.M. Diffuse reflectance FTIR database for the interpretation of the spectra obtained with a handheld device on built heritage materials. Anal. Methods 2015, 7, 1061–1070. [Google Scholar] [CrossRef]
- Arrizabalaga, I.; Gómez-Laserna, O.; Aramendia, J.; Arana, G.; Madariaga, J.M. Applicability of a Diffuse Reflectance Infrared Fourier Transform handheld spectrometer to perform in situ analyses on Cultural Heritage materials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 259–267. [Google Scholar] [CrossRef]
- Steger, S.; Stege, H.; Bretz, S.; Hahn, O. Capabilities and limitations of handheld Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) for the analysis of colourants and binders in 20th-century reverse paintings on glass. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 195, 103–112. [Google Scholar] [CrossRef]
- Miliani, C.; Rosi, F.; Daveri, A.; Brunetti, B.G. Reflection infrared spectroscopy for the non-invasive in situ study of artists’ pigments. Appl. Phys. A Mater. Sci. Process. 2012, 106, 295–307. [Google Scholar] [CrossRef]
- Zaffino, C.; Guglielmi, V.; Faraone, S.; Vinaccia, A.; Bruni, S. Exploiting external reflection FTIR spectroscopy for the in-situ identification of pigments and binders in illuminated manuscripts. Brochantite and posnjakite as a case study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1076–1085. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, X.; Li, L. Origins of baseline drift and distortion in Fourier transform spectra. Molecules 2022, 27, 4287. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, T.; Bécue, T.; Gautier, S. Diffuse Reflection Infrared Spectroscopy (DRIFTS): Application to the in situ Analysis of Catalysts. Oil Gas Sci. Tech. 2004, 59, 215–237. [Google Scholar] [CrossRef]
- Buti, D.; Rosi, F.; Brunetti, B.G.; Miliani, C. In-situ identification of copper-based green pigments on paintings and manuscripts by reflection FTIR. Anal. Bioanal. Chem. 2013, 405, 2699–2711. [Google Scholar] [CrossRef]
- Monico, L.; Rosi, F.; Miliani, C.; Daveri, A.; Brunetti, B.G. Non-invasive identification of metal-oxalate complexes on polychrome artwork surfaces by reflection mid-infrared spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 270–280. [Google Scholar] [CrossRef]
- Bevilacqua, N.; Borgioli, L.; Adrover Gracia, I. Pigmenti Nell’arte Dalla Preistoria Alla Rivoluzione Industriale; II Prato: Saonara, Italy, 2010. [Google Scholar]
- Borgioli, L. I Pigmenti dell’800; II Prato: Saonara, Italy, 2022. [Google Scholar]
- Pedrola, A. Materiales, Procedimientos y Técnicas Pictóricas; Editorial Ariel: Barcelona, Spain, 2019. [Google Scholar]
- Matteini, M.; Moles, A. La Química en la Restauración: Los Materiales del Arte Pictórico; Editorial Nerea: Basque Country, Spain, 2001. [Google Scholar]
- Doerner, M. The Materials of the Artist and Their Use in Painting, with Notes on the Techniques of the Old Masters, 1998th ed.; Houghton Mifflin Harcourt: Boston, MA, USA, 1921. [Google Scholar]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Temperature dependences of IR spectra of humic substances of brown coal. Agronomy 2021, 11, 1822. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: New York, NY, USA, 2004. [Google Scholar]
- Price, M. A Renaissance of Color: Particle Separation and Preparation of Azurite for Use in Oil Painting. Leonardo 2000, 33, 281–288. [Google Scholar] [CrossRef]
- Riederer, J. Egyptian Blue. In Artists Pigments. A Handbook of Their History and Characteristics; FitzHugh, E.W., Ed.; Archetype Publications: London, UK, 1997; Volume 3, pp. 23–46. [Google Scholar]
- Wiseman, G.; Barnes, S.; Helwig, K. Investigation of Egyptian Blue on a Fragmentary Egyptian Head Using ER-FTIR Spectroscopy and VIL Imaging. Heritage 2023, 6, 993–1006. [Google Scholar] [CrossRef]
- Mirtit, P.; Appolonia, L.; Casoli, A.; Ferrari, R.P.; Laurent, E.; Amisano Canesi, A.; Chiari, G. Spectrochemical and structural studies on a Roman sample of Egyptian blue. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995, 51, 437–446. [Google Scholar] [CrossRef]
- Coimbra, M.M.; Martins, I.; Bruno, S.M.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Rudić, S.; Nolasco, M.M. Shedding Light on Cuprorivaite, the Egyptian Blue Pigment: Joining Neutrons and Photons for a Computational Spectroscopy Study. Cryst. Growth Des. 2023, 23, 4961–4969. [Google Scholar] [CrossRef]
- Manfredi, M.; Barberis, E.; Aceto, M.; Marengo, E. Non-invasive characterization of colorants by portable diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy and chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 171–179. [Google Scholar] [CrossRef]
- Edreira, M.C.; Feliu, M.J.; Fernández-Lorenzo, C.; Martín, J. Spectroscopic study of Egyptian blue mixed with other pigments. Helv. Chim. Acta 2003, 86, 29–49. [Google Scholar] [CrossRef]
- Anbalagan, G.; Prabakaran, A.R.; Gunasekaran, S. Spectroscopic characterization of Indian standard sand. J. Appl. Spectrosc. 2010, 77, 86–94. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Janik, L.J.; Raupach, M. Diffuse reflectance infrared fourier transform (DRIFT) spectroscopy in soil studies. Aust. J. Soil Res. 1991, 29, 49–67. [Google Scholar] [CrossRef]
- Climent-Pascual, E.; De Paz, J.R.; Rodríguez-Carvajal, J.; Suard, E.; Sáez-Puche, R. Synthesis and characterization of the ultramarine-type analog Na 8-X[Si6Al6O24]·(S2,S3,CO3)1-2. Inorg. Chem. 2009, 48, 6526–6533. [Google Scholar] [CrossRef]
- Byrappa, K.; Devaraju, M.K.; Madhusudan, P.; Dayananda, A.S.; Kumar, B.V.S.; Girish, H.N.; Ananda, S.; Lokanatha Rai, K.M.; Javeri, P. Synthesis and characterization of calcium aluminum silicate hydroxide (CASH) mineral. J. Mater. Sci. 2006, 41, 1395–1398. [Google Scholar] [CrossRef]
- Zilio, S.C.; Bagnato, V.S. Infrared Spectra of Natural Sodalite. J. Phys. Chem. 1984, 88, 1373–1376. [Google Scholar] [CrossRef]
- Mofrad, A.M.; Peixoto, C.; Blumeyer, J.; Liu, J.; Hunt, H.K.; Hammond, K.D. Vibrational Spectroscopy of Sodalite: Theory and Experiments. J. Phys. Chem. C 2018, 122, 24765–24779. [Google Scholar] [CrossRef]
- Friedrich, F.; Heissler, S.; Faubel, W.; Nüesch, R.; Weidler, P.G. Cu(II)-intercalated muscovite: An infrared spectroscopic study. Vib. Spectrosc. 2007, 43, 427–434. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Helwig, K. The characterisation of iron earth pigments using infrared spectroscopy. In Postprints of IRUG2; Pretzel, B., Ed.; Victoria & Albert Museum (V&A): London, UK, 1998; pp. 83–91. [Google Scholar]
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; Brown, A.J. Reflectance and emission spectroscopy study of four groups of phyllosilicates: Smectites, kaolinite-serpentines, chlorites and micas. Clay Miner. 2008, 43, 35–54. [Google Scholar] [CrossRef]
- Genestar, C.; Pons, C. Earth pigments in painting: Characterisation and differentiation by means of FTIR spectroscopy and SEM-EDS microanalysis. Anal. Bioanal. Chem. 2005, 382, 269–274. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torrens-Martín, D.; Morales, L.M.; Martínez-Ramírez, S. Infrared spectroscopy in the analysis of building and construction materials. In Infrared Spectroscopy: Materials Science, Engineering and Technology; Theophanides, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 369–381. [Google Scholar]
- Moretto, L.M.; Orsega, E.F.; Mazzocchin, G.A. Spectroscopic methods for the analysis of celadonite and glauconite in Roman green wall paintings. J. Cult. Herit. 2011, 12, 384–391. [Google Scholar] [CrossRef]
- Fanost, A.; Gimat, A.; de Viguerie, L.; Martinetto, P.; Giot, A.C.; Clémancey, M.; Blondin, G.; Gaslain, F.; Glanville, H.; Walter, P.; et al. Revisiting the identification of commercial and historical green earth pigments. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124035. [Google Scholar] [CrossRef]
- Buckley, H.A.; Bevan, J.C.; Brown, K.M.; Johnson, L.R.; Farmer, V.C. Glauconite and celadonite: Two separate mineral species. Miner. Mag. 1978, 42, 373–382. [Google Scholar] [CrossRef]
- Gettens, R.J.; FitzHugh, E.W. Malachite and Green Verditer. In Artists Pigments. A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications: London, UK, 1993; Volume 2, pp. 183–202. [Google Scholar]
- Van Der Grift, C.J.G.; Geus, J.W.; Kappers, M.J.; Van Der Maas, J.H. Characterization of copper-silica catalysts by means of in situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Catal. Lett. 1989, 3, 159–168. [Google Scholar] [CrossRef]
- Van Der Grift, C.J.G.; Wielers, A.F.H.; Mulder, A.; Geus, J.W. The reduction behaviour of silica-supported copper catalysts prepared by deposition-precipitation. Thermochim. Acta 1990, 171, 95–113. [Google Scholar] [CrossRef]
- Plesters, J. Ultramarine Blue, Natural and Artificial. In Artists Pigments. A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications: London, UK, 1993; Volume 2, pp. 37–66. [Google Scholar]
- Cennini, C. Libro Dell’arte, 1998th ed.; Akal: Madrid, Spain, 1437. [Google Scholar]
- Grissom, C.A. Green Earth. In Artists Pigments. A Handbook of Their History and Characteristics; Feller, R.L., Ed.; Archetype Publications: London, UK, 1986; Volume 1, pp. 141–168. [Google Scholar]
- Rafalska-Lasocha, A.; Kaszowska, Z.; Lasocha, W.; Dziembaj, R. X-ray powder diffraction investigation of green earth pigments. Powder Diffr. 2010, 25, 38–45. [Google Scholar] [CrossRef]
- Cloutis, E.; MacKay, A.; Norman, L.; Goltz, D. Identification of Historic Artists’ Pigments Using Spectral Reflectance and X-Ray Diffraction Properties I. Iron Oxide and Oxy-Hydroxide-Rich Pigments. J. Near Infrared Spectrosc. 2016, 24, 27–45. [Google Scholar] [CrossRef]
- Wang, F.; Qin, X.F.; Meng, Y.F.; Guo, Z.L.; Yang, L.X.; Ming, Y.F. Hydrothermal synthesis and characterization of α-Fe2O3 nanoparticles. Mater. Sci. Semicond. Process 2013, 16, 802–806. [Google Scholar] [CrossRef]
- Xiao, W.; Jones, A.M.; Collins, R.N.; Bligh, M.W.; David Waite, T. Use of fourier transform infrared spectroscopy to examine the Fe(II)-Catalyzed transformation of ferrihydrite. Talanta 2017, 175, 30–37. [Google Scholar] [CrossRef]
- Salama, W.; El Aref, M.; Gaupp, R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1816–1826. [Google Scholar] [CrossRef]
- Wang, Y.; Muramatsu, A.; Sugimoto, T. FTIR analysis of well-defined α-Fe203 particles. Colloids Surf. A Physicochem. Eng. Asp. 1998, 134, 281–297. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. The behavior of hydroxyl units of synthetic goethite and its dehydroxylated product hematite. Spectrochim. Acta Part A 2001, 57, 2575–2586. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Ramasamy, V.; Ponnusamy, V.; Sabari, S.; Anishia, S.R.; Gomathi, S.S. Effect of grinding on the crystal structure of recently excavated dolomite. Indian J. Pure Appl. Phys. 2009, 47, 586–591. [Google Scholar]
- Ritz, M.; Vaculíková, L.; Plevová, E. Identification of Clay Minerals by Infrared Spectroscopy and Discriminant Analysis. Appl. Spectrosc. 2010, 64, 1379–1387. [Google Scholar]
- Rosi, F.; Miliani, C.; Clementi, C.; Kahrim, K.; Presciutti, F.; Vagnini, M.; Manuali, V.; Daveri, A.; Cartechini, L.; Brunetti, B.G.; et al. An integrated spectroscopic approach for the non-invasive study of modern art materials and techniques. Appl. Phys. A Mater. Sci. Process 2010, 100, 613–624. [Google Scholar] [CrossRef]
- Mastrotheodoros, G.P.; Beltsios, K.G. Pigments—Iron-based red, yellow, and brown ochres. Archaeol. Anthropol. Sci. 2022, 14, 35. [Google Scholar] [CrossRef]
- Wiesinger, R.; Pagnin, L.; Anghelone, M.; Moretto, L.M.; Orsega, E.F.; Schreiner, M. Pigment and Binder Concentrations in Modern Paint Samples Determined by IR and Raman Spectroscopy. Angew. Chem. 2018, 57, 7401–7407. [Google Scholar] [CrossRef]
- Bai, Y.L.; Xu, H.; Zhang, Y.; Li, Z.H. Reductive conversion of hexavalent chromium in the preparation of ultra-fine chromia powder. J. Phys. Chem. Sol. 2006, 67, 2589–2595. [Google Scholar] [CrossRef]
- Goldsmith, J.A.; Ross, S.D. The infrared spectra of azurite and malachite. Spectrochim. Acta A 1968, 24, 2131–2137. [Google Scholar] [CrossRef]
- Lluveras, A.; Boularand, S.; Andreotti, A.; Vendrell-Saz, M. Degradation of azurite in mural paintings: Distribution of copper carbonate, chlorides and oxalates by SRFTIR. Appl. Phys. A 2010, 99, 363–375. [Google Scholar] [CrossRef]
- Farmer, V.C. The infrared spectra of mineral. Mineral. Soc. Monogr. 1974, 4, 331–363. [Google Scholar]
- Rosi, F.; Daveri, A.; Miliani, C.; Verri, G.; Benedetti, P.; Piqué, F.; Brunetti, B.G.; Sgamellotti, A. Non-invasive identification of organic materials in wall paintings by fiber optic reflectance infrared spectroscopy: A statistical multivariate approach. Anal. Bioanal. Chem. 2009, 395, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Siidra, O.; Nekrasova, D.; Depmeier, W.; Chukanov, N.; Zaitsev, A.; Turner, R. Hydrocerussite-related minerals and materials: Structural principles, chemical variations and infrared spectroscopy. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2018, 74, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Flores, A.; Villalobos, M.; Pi-Puig, T.; Martínez-Villegas, N.V. Revised aqueous solubility product constants and a simple laboratory synthesis of the Pb(II) hydroxycarbonates: Plumbonacrite and hydrocerussite. Geochem. J. 2017, 51, 315–328. [Google Scholar] [CrossRef]
- Gettens, R.J.; FitzHugh, E.W. Azurite and Blue Verditer. In Artists Pigments. A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications: London, UK, 1993; Volume 2, pp. 23–36. [Google Scholar]
- Gettens, R.; Kühn, H.W.T.C. Lead White. In Artists Pigments. A Handbook of Their History and Characteristics; FitzHugh, E.W., Ed.; Archetype Publications: London, UK, 1997; Volume 3, pp. 67–82. [Google Scholar]
- Franquelo, M.L.; Duran, A.; Herrera, L.K.; Jimenez de Haro, M.C.; Perez-Rodriguez, J.L. Comparison between micro-Raman and micro-FTIR spectroscopy techniques for the characterization of pigments from Southern Spain Cultural Heritage. J. Mol. Struct. 2009, 924–926, 404–412. [Google Scholar] [CrossRef]
- Vahur, S.; Knuutinen, U.; Leito, I. ATR-FT-IR spectroscopy in the region of 500–230 cm−1 for identification of inorganic red pigments. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 764–771. [Google Scholar] [CrossRef]
- Gettens, R.J.; Feller, R.L.; Chase, W.T. Vermilion and Cinnabar. In Artists Pigments. A Handbook of Their History and Characteristics; Roy, A., Ed.; Archetype Publications: London, UK, 1993; Volume 2, pp. 159–182. [Google Scholar]
- Scott, D.A. Copper and Bronze in Art. Corrosion, Colorants, Conservation; Getty Publications: Los Angeles, CA, UAS, 2002. [Google Scholar]
- Salvadó, N.; Butí, S.; Cotte, M.; Cinque, G.; Pradell, T. Shades of green in 15th century paintings: Combined microanalysis of the materials using synchrotron radiation XRD, FTIR and XRF. Appl. Phys. A Mater. Sci. Process. 2013, 111, 47–57. [Google Scholar] [CrossRef]
- Prati, S.; Bonacini, I.; Sciutto, G.; Genty-Vincent, A.; Cotte, M.; Eveno, M.; Menu, M.; Mazzeo, R. ATR-FTIR microscopy in mapping mode for the study of verdigris and its secondary products. Appl. Phys. A Mater. Sci. Process. 2016, 122, 10. [Google Scholar] [CrossRef]
- San Andrés, M.; De La Roja, J.M.; Baonza, V.G.; Sancho, N. Verdigris pigment: A mixture of compounds. Input from Raman spectroscopy. J. Raman Spectros. 2010, 41, 1468–1476. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, NY, USA, 1963. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).