Abstract
In Romania, groundwater is an important source of drinking water, especially in rural areas. This study investigated the concentrations of organophosphorus, carbamate, and triazine pesticides (OPs) along with organochlorine pesticides (OCPs) in groundwater samples collected from the Titu-Sarata Plain. Sensitive analytical techniques were employed, including Ultrahigh-Performance Liquid Chromatography coupled with Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ Mass Spectrometry (UHPLC-Orbitrap-MS/MS) and Gas Chromatography coupled with an electron capture detector (GC-ECD). Environmental and human health risks were assessed in the case of pesticides that exceeded the maximum allowed concentration. The environmental risk assessment (ERA) revealed significant risks associated with Phosdrin, Phorate, and pp’DDE. Additionally, particular concerns arose from the presence of Aldrin and Dieldrin, which pose a high carcinogenic risk, especially through groundwater consumption in agricultural areas. The results of this research highlight the need for the implementation of a continuous quality monitoring program for groundwater in the agricultural regions that were studied.
1. Introduction
Pesticides are chemicals that are widely used in agriculture in order to manage pests, weeds, plant diseases or pathogens. Their use helps to reduce and even prevent crop losses and maintain a high quality of agricultural products [1,2,3]. Pesticides are also used in homes, offices, shops, and public spaces to control infestations of insects and disease carriers such as mosquitoes, ticks, and rodents [3,4].
Due to their complex chemical structures and the effects on different species, pesticides can be grouped in several ways. One common method of categorization is based on the type of pest they target. Based on this criterion, pesticides include herbicides, insecticides, fungicides, nematicides, rodenticides or raticides, acaricides, and others [5,6]. Secondly, based on their origin and chemical composition, most pesticides are either natural or synthetic organic compounds [7].
The toxicity of pesticides depends on several factors, such as their chemical structure and the quantity that is used.
The excessive use of pesticides in the past, the lack of appropriate equipment, and the lack of specialized understanding from farmers has led to the contamination of soil, groundwater, and surface water. According to EUROSTAT data, starting from 2011, the consumption of pesticides has declined by 17.36% in the European Union and by 20% in Romania [7]. The harmful environmental effects of pesticide use vary according to their toxicity level, selectivity, and persistence. An important factor that contributes to environmental pollution is the dispersion of pesticides over very long distances and their accumulation in biomass, water, or soil [8].
Pesticides can also affect human health, especially in cases of prolonged or uncontrolled exposure. Long-term exposure to pesticides can disrupt the functionality of the nervous, endocrine, renal, cardiovascular, and respiratory systems, etc. These disruptions have been associated with an increasing incidence of chronic diseases, including cancer, Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, diabetes, chronic cardiovascular, renal diseases, and others [9].
Since 1991, the use of pesticides has been strictly regulated by EU legislation [7] to protect human health and the environment. Even pesticides with low toxicity can have a negative impact on water quality, soil health, biodiversity, and ecosystems [7]. Although the use of pesticides is strictly regulated to minimize their impact on human health and the environment, there are serious concerns about the health risks associated with the consumption of food and drinking water that has been contaminated with pesticide residues [3,4,10,11].
Pesticides might be present in the environment even if the concentrations are shown to be below the limit of quantification (LOQ) or limit of detection (LOD), but the presence of several pesticides can generate cumulative effects with a significant ecological impact [12].
Pesticide residues can reach the air, water, and soil through various transfer processes, including adsorption, leaching, and volatilization [13].
While most pesticides listed under the Stockholm Convention on Persistent Organic Pollutants [14] eventually degrade in the environment, some are highly persistent and capable of long-range dispersion. In certain cases, water contamination can persist for many years—even decades—after the pesticide has been eliminated from use. Groundwater, which serves as a vital source of drinking water in rural areas and is also used to irrigate agricultural land, is particularly vulnerable. As a result, monitoring groundwater quality is essential for effective environmental risk assessment (ERA) [15].
The International Agency for Research on Cancer (IARC) has classified several pesticides as carcinogenic agents [16]. Risk assessment is described as the probability of any adverse effect on human health occurring within a certain time frame [17,18]. By estimating the level of risk, a health risk assessment is performed for each chemical compound, leading to their classification as either carcinogenic or non-carcinogenic [19,20]. Any assessment of human health risk must take into account the fact that effects vary from one person to another. For this reason, uncertainty factors are part of the risk assessment process.
Assessing the exposure to some contaminants is a key step in evaluating risks to human health and varies according to the following main factors: the exposed populations (public or selected vulnerable groups); type of contaminants; single contaminant or a mixture of contaminants; the type and duration of the exposure, and the exposure pathways such as ingestion, inhalation, or dermal exposure [21].
Typically, people are exposed to contaminants through multiple exposure pathways. In such cases, the total exposure is calculated as the sum of exposures across all pathways [22,23].
Some studies on the contamination of surface water and sediments with pesticides have been conducted in Romania [24,25]. No scientific results were found on the contamination of groundwater with pesticides in Romania and no scientific results on the potential risk to public health due to the ingestion of the groundwater by the population were found. The present study is the first study conducted on the contamination of groundwater with pesticides and the assessment of the risk to human health due to the ingestion of groundwater by the population in the Titu-Sarata Plain areas, Romania. The findings highlight the importance of continuous monitoring in the researched areas, considering the use of groundwater as a drinking water source.
2. Materials and Methods
2.1. Study Area
Located in the central–eastern part of the Romanian Plain, the Titu-Sarata Plain is a typical subsidence plain (with specific morpho-hydrographic characteristics), with altitudes varying between 50 m (in the southwest) and 145 m (in the north). The physico-geographical particularities reflect the characteristics of a low plain: extensive floodplain terrain, with relatively pronounced dynamics of the riverbeds (islands) and river banks, with extremely low slopes (1–4‰); the average annual temperature is 10–11 °C (varying from −3 °C during January to 21.5 °C during July), and the average annual precipitation amounts is 550–600 mm; the hydrographic network runs in a dominant northwest–southeast direction (with several main rivers—Arges, Dambovita, Ialomita, and Cricovul Sarat—and a series of temporary streams); there are also several ponds and the water table is located at shallow depths (5–10 m).
The landscape is represented by agricultural crops and secondary steppe grasslands, along with small stands of oak forests and meadows (Figure 1). Consequently, the dominant soils are differentiated between Cernisols and halomorphic soils (in the eastern part) and Luvisols and Cambic soils (in the west).
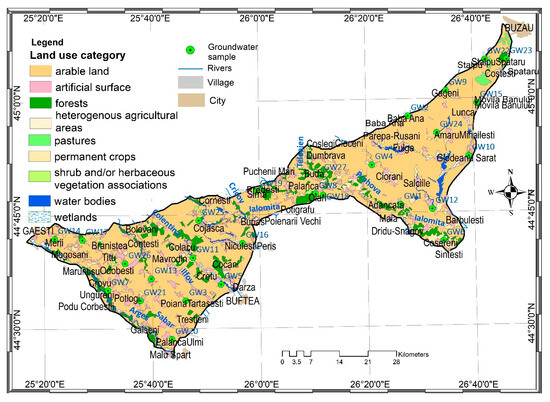
Figure 1.
Location of groundwater samples collected from Titu-Sarata Plain and surrounding land [26].
Against this general background, the population density (130 inhabitants/km2) exceeds the national average, with higher values in the southwestern sector and lower values in the eastern sector.
With the exception of three small towns (Titu, Bolintin-Vale, and Racari), the population is concentrated in villages with predominantly agricultural profiles (wheat and corn cultivation; there are also specific potato and vegetable crops in areas including Brezoaia, Lunguletu, Potlogi, and Poiana). Social and economic activity is closely linked to the existence of large cities nearby (the country’s capital, Bucharest, but also Ploiesti and Targoviste).
2.2. Sample Collection
The water samples were collected on the same day in October 2023, from 27 public dug wells with the water table level between 2 and 20 m. The sampling was performed according to the EPA Groundwater Sampling—Operating Procedure [27]. The selection of the sampling locations aimed to achieve a uniform distribution of the sampling locations for the study area. The polyethylene sampling bottles were rinsed 2–3 times with the target groundwater before samples were collected. After sampling, the groundwater samples were prelabeled, refrigerated, and transported within a 24 h timeframe at 4 °C to the laboratory for hydro-chemical analysis.
2.3. UHPLC-Orbitrap-MS/MS Analysis of OPs
Sample preparation: 250 mL water sample was filtered using glass fiber filter to remove solid components before sample preparation, followed by the pH adjustment to 3 using concentrated acetic acid. Solid phase extraction (SPE) using StrataX (500 mg/6 mL) cartridges (Phenomenex, Aschaffenburg, Germany) was used to extract the pesticides from the water samples. The acidified water samples were passed through SPE cartridges preconditioned with 10 mL acetonitrile (ACN), followed by 10 mL water before the extraction, at a flow rate of 3–5 mL/min. Afterwards, 10 mL of ultrapure water was added to remove water-soluble interferences and the residual water in the column was dried under low-pressure vacuum for 10 min. The SPE cartridges were eluted with 10 mL methanol–methylene chloride mixture (1:1, v/v) at a flow rate of 1 mL/min, after which the solvent was removed under a stream of nitrogen at 40 °C, and the extract was reconstituted with acetonitrile–water (1:9, v/v), followed by UHPLC-MS/MS analysis.
Quantitative UHPLC-Orbitrap-MS/MS analysis of pesticides: All samples were analyzed with Ultrahigh-Performance Liquid Chromatography coupled to a Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ mass spectrometer (UHPLC-Orbitrap-MS/MS, Thermo Fisher Scientific, San Jose, CA, USA) using electrospray ionization (ESI). The samples were injected once each under ESI positive mode for organophosphorus and organophosphate pesticides, triazine pesticides, and carbamates and ESI negative mode for acid herbicides. For both ESI positive and negative modes, a reversed-phase Syncronis C18 column (50 mm × 2.1 mm, 1.7 μm) (Thermo Fisher Scientic, San Jose, CA, USA) was used with a binary mobile phase gradient consisting of (A) water and (B) methanol, both containing 0.1% formic acid and 5 mM ammonium formiate. The injection volume was 10 µL throughout the whole separation, the flow rate was 0.4 mL/min, the sampler compartment temperature was 15 °C, and the column temperature was 25 °C. The Orbitrap-MS/MS was operated in the data-dependent acquisition mode, with full scans acquired in the range of m/z 100–1000, at resolution of 70,000 FWHM and m/z 200, while the fragmentation was performed at a resolution of 35,000 FWHM. The MS detector was run under the following conditions: spray voltage 3.0 kV for negative mode and 3.5 kV for positive mode, capillary temperature 320 °C, auxiliary gas heater temperature 413 °C, sheath and auxiliary gas flow (N2) 48 and 11 (arbitrary units). The target pesticides were identified and quantified according to the spectral characteristics: mass spectra, accurate mass, and specific retention time, against external standard solutions covering a linear range between 0.1 and 100 µg/L. Mass accuracy calibration of the high-resolution Orbitrap MS/MS was performed before the analysis, in both positive and negative ionization modes. Data processing was performed using Xcalibur 3.1 software package (Version 4.1) (Thermo Fisher Scientific, San Jose, CA, USA).
The solvents (methanol, acetonitrile, and water) and reagents (formic acid and ammonium formate) used for sample preparation and mass spectrometric analysis were of LC-MS-grade purity, while methylene chloride was of HPLC gradient-grade purity, all reagents being purchased from Merck (Darmstadt, Germany). Reference-grade standard solutions were purchased from LGC Dr. Ehrenstorfer (Gibraltar, UK).
2.4. GC-ECD Analysis of OCPs
Analyses were performed on a Varian 450 GC system coupled with an electron capture detector (ECD). The capillary column used to separate the compounds was an Agilent CP-Sil-5 CB 100% dimethylpolysiloxane (30 m length, 0.25 mm internal diameter, and 0.25 µm film thickness), with a stationary phase consisting of 5% diphenyl and 95% dimethyl siloxane. The oven temperature program of the GC-ECD was as follows: initial temperature of 130 °C (1 min isotherm); increase at 5 °C min−1 to 170 °C (0 min); increase at 3 °C min−1 to 180 °C (0 min); increases at 5 °C min−1 to 210 °C (2 min); and, finally, increase at 0.5 °C min−1 to 218 °C (5 min isotherm).
Extraction method
A volume of 500 mL of water sample and 100 mL of solvent petroleum ether purchased from Sigma Aldrich (Darmstadt, Germany) were put in a 1000 mL Erlenmayer vessel and stirred for 15 min on a magnetic stirrer at 1100 rpm, after which the solution was transferred to a separation funnel and left to rest for the separation of the phases. The obtained extract was collected in a conical flask. The aqueous layer was extracted by adding 50 mL of petroleum ether using a similar procedure. The obtained extracts were passed through a glass column filled with anhydrous sodium sulfate purchased from Sigma Aldrich (Darmstadt, Germany) and subsequently moistened with petroleum ether, after which the obtained extract was concentrated to 1 mL in a TurboVap500 concentrator and analyzed by GC-ECD.
Reference-grade standards were purchased from LGC Dr. Ehrenstorfer (Gibraltar, UK) with a purity range 98.2–99.9%.
The method was validated according to correlation coefficient (r2), recovery, and sensitivity.
The correlation coefficient (r2) was assessed using a five-point calibration curve over the range of 2.5–25 µg/L, running in duplicate with each set of samples.
The sensitivity of the method was determined by the limit of quantification (LOQ) based on the lowest measurable signal corresponding to a signal–noise ratio of 10:1 and by the limit of detection (LOD) based on the lowest detectable signal corresponding to a signal–noise ratio of 3:1.
The validation parameters and their acceptance criteria are summarized in Table 1.

Table 1.
Tests and acceptance criteria in determining the OCP concentrations.
2.5. Environmental Risk Assessment
The environmental risk was assessed using risk quotients (s). According to the approach proposed by Barbieri, s were defined as the ratio between the exposure concentration () and the predicted no-effect concentration () (Equation (1)) [15,28,29]:
Experts recommend using the lowest values as quality targets for environmental risk assessment (ERA) in freshwater systems. These values should preferably be derived from experimental ecotoxicity data. However, in cases where the experimental data are insufficient or are lacking, Quantitative Structure–Activity Relationship (QSAR) models can be employed for a preliminary screening and to estimate provisional values [15,28,30].
A resulting < 1 indicates that the chemical exposure is lower than the benchmark threshold (), suggesting that adverse environmental effects are unlikely.
The assessment of the degree of water pollution can be made based on the total risk coefficients (RQtot), calculated as the sum of the individual values (RQi) of all compounds present in a specific sample (Equation (2)) [28]:
RQtot = Σni=1 RQi,
Having its limitations resulting from not taking into account the unpredictable synergistic or antagonistic effects of different compounds in the water, RQtot allows for the assessment of the ecotoxicological risk that may result from the presence of all pesticides present in a given location.
2.6. Human Health Risk Assessment
The assessment of the potential non-carcinogenic and/or carcinogenic health risks associated with the ingestion of pesticide-contaminated water across different age groups (adults, children, and infants) can be evaluated using through hazard quotients (HQs), hazard indices (HIs) and incremental lifetime cancer risks (ILCRs).
2.6.1. Non-Carcinogenic Risk Assessment
To determine the chronic daily intake (CDI) of pesticides via oral exposure, we used Equation (3), derived from the methodology established by United States Environmental Protection Agency (USEPA) [17,31,32,33,34,35,36,37]:
where
CDI is the chronic daily intake (mg/kg/day);
Cw is the concentration of pesticides in water samples (mg/L);
DI is the daily average intake (L/day);
EF is the exposure frequency (days/year);
ED is the exposure duration (years);
AT is the average contact time (days);
BW is the average body weight (kg) for each age group of consumers.
Data on water consumption and body weight used in the risk assessment are presented in Supplementary Data (Table S1). For estimating the body weight of children aged 1–5 years, the formula [Weight (kg) = 2 × (age in years + 5)] was applied. Based on this, the body weight for a 2-year-old child was estimated to be 14 kg. For children under 2 years of age, an average body weight of 7 kg was considered [37].
According to the USEPA guidelines, the health risk associated with each pesticide for non-carcinogenic effects is estimated by calculating the hazard quotient (), as defined by Equation (4) [38]:
where is the oral reference dose via oral ingestion (mg/kg/day).
The assessment of the total potential for non-carcinogenic health effects resulting from exposure to a pesticide mixture in water can be performed based on the hazard index (). The is calculated based on the EPA guidelines for health risk assessment [38,39,40,41,42] as the sum of the resulting HQs for each pesticide, according to Equation (5):
or values less than 1 indicate that there are no significant non-carcinogenic risks. or values greater than or equal to 1 indicate that there are significant non-carcinogenic risks that increase as or increases [38,41,42].
2.6.2. Carcinogenic Risk Assessment
ILCR is a measure used to assess the likelihood that a person will develop cancer as a result of exposure to a particular pollutant or chemical over their lifetime. It is a key concept in public health risk assessment and is calculated based on the following factors:
1. Exposure dose: The amount of substance a person is exposed to daily, based on their body weight.
2. Cancer slope factor (CSF): A measure of the cancer risk associated with a unit dose of the substance.
3. Duration of exposure: The length of time a person is exposed to the substance, usually expressed in years.
ILCR resulting from exposure to a certain given dose of pesticide present in drinking water is calculated using Equation (6) [11,43]:
where
ILCR = ADI × CSF,
CSF is the cancer slope factor and it is defined as the risk resulting from an average lifetime dose of 1 mg per kg body weight per day of a carcinogenic contaminant and is compound-specific.
ADI is the average daily intake of OCPs.
3. Results
3.1. Occurrence and Distribution of Pesticides in the Groundwater Sample from Titu-Sarata Plain, Romania
In the collected groundwater samples, OPs as well as OCPs were identified and quantified. The results show that, out of all of the groundwater samples collected from 27 locations, only 9 groundwater samples exceeded the maximum permissible concentration for individual OPs in drinking water (0.1 μg/L per pesticide), as specified by current regulations [44]. Furthermore, in five of the groundwater samples, the total pesticide concentration also surpassed the cumulative limit of 0.5 μg/L.
Using Geostatistical Analyst extension from ArcGIS 10.8, Inverse Distance Weighting (IDW) interpolation method was used to predict the spatial distribution of total pesticide concentration in Titu-Sarata Plain (Equation (7)):
where is the estimated value of pesticide concentration in the water sample from the ith dug well, xi is the measured value of the pesticide concentration in the ith well, n is the number of dug wells located around the predicted location; d is the distance between dug wells, and i and 0 and k are the weights assigned to each measured pesticide concentration, which is usually considered equal to two.
The spatial distribution of the total pesticide concentration in the groundwater from the studied areas is presented in Figure 2.
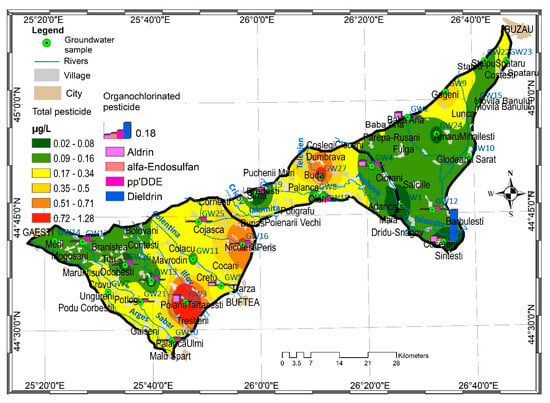
Figure 2.
Spatial distribution of the total pesticide concentration in the Titu-Sarata Plain, Romania.
The OP and OCP concentrations determined in the collected groundwater samples are presented in Supplementary Data (Table S2 and Table S3, respectively).
For data analysis, Microsoft Excel 2021 and Python 3.10.9 were used. Descriptive statistics (Supplementary Table S4, Figure 3) analysis was used to interpret the results.
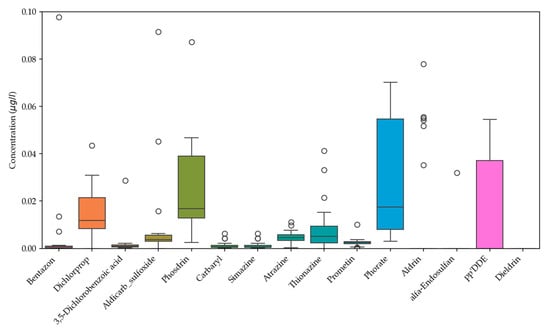
Figure 3.
Box-plot diagram of pesticide concentrations (µg/L) in groundwater samples.
The order of the median concentrations of OPs in groundwater samples was Carbaryl; Simazine < Bentazon < 3,5-Dichlorobenzoic acid < Prometin < Aldicarb sulfoxide < Thionazine < Dichlorprop < Phosdrin < Phorate.
With the exception of Aldrin and pp’DDE, respectively, very strong positive skewness values and high or very high kurtosis values were observed for the detected pesticides, which showed a lot of very low values and few high concentration values. This is linked to the effects of the factors mainly originating from agricultural anthropogenic sources and geogenic conditions of the sampling sites.
To assess the relationships between pesticides present in the groundwater samples, a Pearson correlation analysis was performed and the obtained correlation matrix is presented in Figure 4.
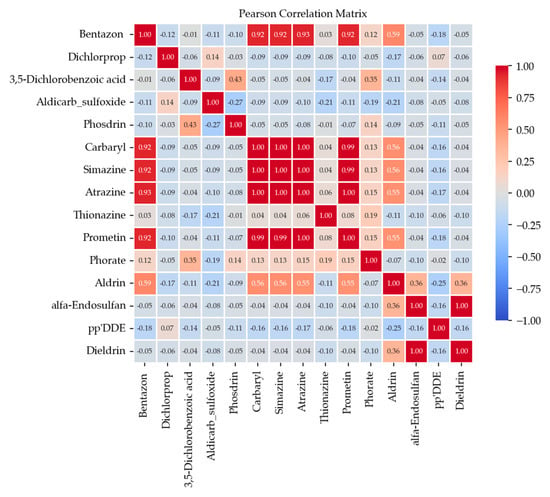
Figure 4.
Pearson’s correlation matrix of pesticides in groundwater, p < 0.05.
Strong positive correlations were identified between Bentazon, Carbaryl, Simazine, Atrazine, and Prometin, and between Dieldrin and alfa endosulfan (r > 0.9), respectively, indicating potential common contamination sources. A moderate correlation (r = 0.5–0.6) was observed between Aldrin and Bentazon, Carbaryl, Simazine, Atrazine, and Prometin, respectively, while weak or non-significant correlations (r < 0.40) indicate distinct contamination sources.
There is no significant relationship between the water depth and total pesticide concentration (Figure 5), as indicated by the very low R2 value (0.0292). Most data points, clustered between 2 and 10 m in depth, exhibit relatively low pesticide concentrations, generally below 0.4 µg/L. A few outliers, such as the point near 1.4 µg/L, are observed but appear to be isolated incidents. These may suggest localized contamination, potentially linked to intensive pesticide use in nearby agricultural areas.
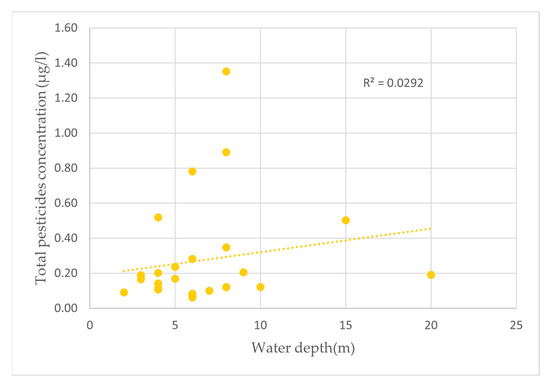
Figure 5.
The relationship between total pesticide concentrations (µg/L) and water depth (m).
3.2. Environmental Risk Assessment
The lowest s were obtained from the NORMAN Ecotoxicology Database [30] and are presented in Table 2.

Table 2.
The lowest here is no significant relationship between the water.
The individual environmental risk quotient values (RQis) calculated for each pesticide (OPs and OCPs) are presented in Table 3. Since the s for aldrin and dieldrin are zero, s cannot be determined for them. To assess the degree of water pollution and potential ecological risks, the total risk coefficients (RQtot) for each location were calculated (Table 3).

Table 3.
The values of individual environmental risk quotients (s) and total environmental risk coefficients (RQtot).
The presence of alfa endosulfan was identified only in the commune of Cosereni, where it induces a moderate risk for the environment ( = 6.38).
3.3. Human Health Risk Assessment
To assess the potential non-carcinogenic and/or carcinogenic health risks with respect to different age groups (adults, adolescents, children, and infants) posed by the ingestion of pesticide-contaminated groundwater from the Titu-Sarata Plain, Romania, HQ, HI, and ILCR values were calculated.
3.3.1. Non-Carcinogenic Risk Assessment
For the health risk assessment, the and CSF of the pesticide values used are presented in Table 4:

Table 4.
The and CSF of pesticide values used for human health risk assessments [45].
The values were calculated for aldicarb sulfoxide, carbaryl, simazine, atrazine, and phorate for the three age groups that were considered (infants, children, and adults). These values are presented in Supplementary Data (Table S5). The values for each location were determined as the sum of the individual values of the previously mentioned pesticides and are presented in Table 5.

Table 5.
The values obtained and used to estimate non-carcinogenic risk in locations where OPs are present.
To assess the potential non-carcinogenic risks caused by the presence of OCPs in the groundwater samples studied, the values were calculated. The obtained values for aldrin and pp’DDE are presented in the Supplementary Data (Table S6).
Dieldrin and alfa endosulfan were detected in only one groundwater sample, collected from the Cosereni location. The obtained values for dieldrin are as follows: 7.6 × 10−4 for infants; 3.5 × 10−4 for children; and 2 × 10−4 for adults.
By summing the values of each detected OCP, the values were calculated for each location where the OCPs were detected (Table 6).

Table 6.
The values obtained and used to estimate non-carcinogenic risk in locations where OCPs are present.
3.3.2. Carcinogenic Risk Assessment
Using the CSF values presented in Table 4, the ILCR values for aldrin and pp’DDE were determined and are presented in Supplementary Data (Table S7). In the case of dieldrin, the ILCR values are as follows: 6.1 × 10−4 for infants; 2.8 × 10−4 for children; and 1.6 × 10−4 for adults. Also, the obtained ILCR values for atrazine and simazine are of the order of 10−10–10−13.
The total ILCR values for each location where OCPs were present detected in the groundwater are shown in Table 7.

Table 7.
The total ILCR values for OCPs for each location where OCPs were detected in the groundwater.
4. Discussion
Addressing human health issues related to pesticide exposure is essential. Knowledge of potential health risks can empower individuals to take measures to protect themselves from pesticide residues in drinking water.
Several OPs were identified in the groundwater (used as drinking water) samples collected from the Titu-Sarata Plain, Romania. The results obtained showed that nine samples (from Brezoaia, Crovu, Gageni, Niculesti, Palanca, Romanesti, Spataru, Suseni Bilciuresti, and Sicrita) did not meet the minimum requirements regarding pesticide values for drinking water quality (0.1 μg/L/pesticide) [44].
Exceedances of the maximum permitted concentration in the analyzed groundwater samples were observed for the following pesticides: bentazon, dichlorprop, aldicarb sulfoxide, phosdrin, carbaryl, simazine, atrazine, thionazine, and phorate.
Concerning the total values of OP concentrations in the water samples, it was observed that the permissible limit (0.5 μg/L total pesticides) [44] was exceeded in the groundwater samples collected from five locations—Brezoaia, Gageni, Niculesti, Suseni Bilciuresti, and Sicrita—which shows that even low concentrations of some pesticides can be harmful.
In Romania, few studies have been conducted concerning the presence of pesticides in groundwater and few public datasets are available. In two regions of Transylvania, Turda and Sighișoara, a study was conducted, and the results revealed the presence of atrazine and simazine in the groundwater samples. The concentrations of atrazine ranged from 3.07 µg/L to 8.07 µg/L, values which were higher than those found in the present study. Also, the concentration of simazine ranged from 0.1 µg/L to 0.15 µg/L, values which were comparable to those obtained in the present study [46].
A particularly serious problem is that, especially in rural areas and in small family farms, the population still uses organochlorine pesticides, most likely due to a lack of knowledge on crop protection and pest control methods.
Although these compounds have been banned in the European Union due to their toxicity and high amounts of residues in manufacturing, marketing, and use, they still pollute the waters of Romania. Although they have been banned in Romania, counterfeit pesticides continue to be illegally imported into the country [47].
The obtained results show the exceedance of the maximum permitted concentration of OCPs (0.030 μg/L for drinking water) for over 55% of the groundwater samples collected from the Titu-Sarata Plain, Romania. Thus, for aldrin, there were exceedances in the groundwaters collected from 6 locations: Baba Ana, Brezoaia, Baraitaru, Ciocanesti, Cosereni, and Fanari; for pp’DDE, there were exceedances in groundwaters collected from 11 locations: Baraitaru, Ciocanesti, Jilavele, Lunguletu, Niculesti, Odaia Turcului, Olarii Vechi, Predesti, Palanca, Suseni Bilciuresti, and Salcuta; and for alfa endosulfan and dieldrin, there were exceedances only in the groundwater collected from one location: Cosereni.
The values obtained for Phosdrin in all of the water samples were higher than 10, indicating a high risk for the environment. In the case of phorate, the values were observed to be between 1 and 10 for the Palanca and Spataru locations (moderate risk) and higher than 10 for the other locations (high risk). Although the individual environmental risk factor () values in the cases of dichloroprop, aldicarb sulfoxide, carbaryl, simazine, and atrazine were lower than 1, indicating that they do not pose a risk for the environment, they do contribute to the increase in the total risk (RQtot). These results demonstrate that even low concentrations of some pesticides can be harmful if we consider all the contaminants present in a water sample.
Concerning the values for OCPs, it has been observed that pp’DDE ( ranging from 27 to 136) poses a high risk for the environment in the areas of Baraitaru, Ciocanesti, Jilavele, Lunguletu, Niculesti, Odaia Turcului, Olarii Vechi, Predesti, Palanca, Romanesti, Suseni Bilciuresti, and Salcuta, and alfa endosulfan induces a moderate risk for the environment ( = 6.38) only in the Cosereni area.
In order to capture the real situation of the degree of water pollution and environmental risk, we must take into account all of the pesticides present in the water sample from each location. Great attention must be given in the future to the areas where both OPs and OCPs were found in the groundwater, like Brezoaia, Crovu, Gageni, Spataru, and Salcuta ( ranging from 61.4 to 1036.3).
The values obtained in the cases of the ingestion of contaminated groundwater with OPs like aldicarb sulfoxide, carbaryl, simazine, atrazine, and phorate, for the three age groups considered (infants, children, and adults), are much lower than 1 (on the order of 10−4–10−10). Also, the values (7.5 × 10−6–3.7 × 10−4 for infants; 8.5 × 10−6 to 1.7 × 10−4 for children; and 6 × 10−6 to 10 × 10−5 for adults) show that the ingestion of the sampled groundwater causes low or negligible non-carcinogenic risks for the population in the studied areas.
The values obtained in the cases of the ingestion of contaminated groundwater from the studied areas, with OCPs like aldrin ( ranging from 1.2 × 10−4 to 2.7 × 10−4 for infants; from 5.8 × 10−5 to 1.2 × 10−4 for children; and from 3.3 × 10−5 to 7.4 × 10−5 for adults) and pp’DDE ( ranging from 9.6 × 10−6 to 1.1 × 10−5 for infants; from 5.4 × 10−6 to 1 × 10−6 for children; and from 1.5 × 10−6 to 3.1 × 10−6 for adults) indicates that the ingestion of groundwater from the researched areas have low or negligible non-carcinogenic risks for the population. In addition, for the Cosereni area, where the groundwater was found to be contaminated with dieldrin, in the case of groundwater ingestion, the obtained values (7.6 × 10−4 for infants; 3.5 × 10−4 for children; and 2 × 10−4 for adults) showed low or negligible non-carcinogenic risks for the population.
The very low ILCR values obtained in the case of groundwater contamination with atrazine and simazine (ILCR values ranging from 10−10 to 10−13) demonstrate that the carcinogenic risks that could be induced by ingestion of the groundwater contaminated with the mentioned pesticides are negligible.
Regarding the population exposure to pp’DDE, the ILCR values showed an acceptable (ILCR ranging from 10−6 to 1 × 10−4) or negligible (ILCR ranging from 10−7 to 10−8) carcinogenic risk for all the age groups of the populations from Baraitaru, Ciocanesti, Jilavele, Lunguletu, Niculesti, Odaia Turcului, Olarii Vechi, Predesti, Palanca, Suseni Bilciuresti, and Salcuta areas.
Also, regarding the population exposure to aldrin due to the ingestion of contaminated groundwater with this OCP from the Baba Ana, Baraitaru, Ciocanesti and Fanari areas, the ILCR values, ranging from 10−6 to 1 × 10−4, indicate an acceptable carcinogenic risk for all age groups of the population.
In contrast, the ILCR value greater than 10−4 obtained for the Brezoaia area indicates an unsafe carcinogenic risk caused by the ingestion of groundwater contaminated with aldrin. Also, particular attention should be given to population exposure to dieldrin. The ILCR values for all of the age groups of the population from the Cosereni area (ILCR > 10−4) show an unsafe carcinogenic risk.
5. Conclusions
The present study is the first study conducted on the contamination of groundwater with pesticides and the assessment of the risk to human health due to the ingestion of groundwater by the population in the Titu-Sarata Plain areas, Romania.
The obtained results highlight the importance of continuous monitoring in the studied areas, considering the use of groundwater as a drinking water source.
Rural communities, particularly those in the Brezoaia and Cosereni areas, where the groundwater is a drinking water source, could be exposed to certain health risks over a long period of time. Therefore, modernizing drinking water sources is a necessary and mandatory measure.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15115880/s1: Table S1: The parameter values used for the health risk assessment for each age group; Table S2: The OP concentration (µg/L) values in collected water samples; Table S3: OCP concentration (µg/L) values in collected water samples; Table S4: Descriptive statistics of the measured concentrations (µg/L) of pesticides in groundwaters samples; Table S5: The HQ values obtained and used to estimate non-carcinogenic risk for OPs; Table S6: The HQ values obtained and used to estimate non-carcinogenic risks for aldrin and pp’DDE; Table S7: The obtained ILCR values for aldrin and pp’DDE in the analyzed groundwater samples.
Author Contributions
Conceptualization: C.D. and C.S.; data curation, C.D. and C.S.; formal analysis, E.I.G., C.T.C. and D.I.P.; investigation, C.D., C.S., D.C., D.T. and P.B.; methodology, C.D., C.S., D.C., E.I.G., C.T.C., D.I.P., D.T. and P.B.; software, C.S. and P.B.; validation, C.D., C.S. and D.C.; writing—original draft, C.D., C.S., D.T. and P.B.; writing—review and editing, C.D., C.S. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EPA | Environmental Protection Agency |
| ERA | Environmental risk assessment |
| USEPA | United States Environmental Protection Agency |
| GC-ECD | Gas Chromatography coupled with an electron capture detector |
| UHPLC-Orbitrap-MS/MS | Ultrahigh-Performance Liquid Chromatography coupled with Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ Mass Spectrometry |
| EUROSTAT | Statistical Office of the European Union |
| OPs | Organophosphorus, carbamate, and triazine pesticides |
| OCPs | Organochlorine pesticides |
| ACN | Acetonitrile |
| HQ | Hazard quotient |
| HI | Hazard index |
| IARC | International Agency for Research on Cancer |
| PNEC | Predicted no-effect concentration |
| RfD | Oral reference dose via oral ingestion |
| RQ | Risk quotient |
| IARC | International Agency for Research on Cancer |
| ILCR | Incremental lifetime cancer risk |
References
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Damalas, C.A. Understanding benefits and risks of pesticide use. Sci. Res. Essays 2009, 4, 945–949. [Google Scholar]
- Maroni, M.; Fanetti, A.C.; Metruccio, F. Risk assessment and management of occupational exposure to pesticides in agriculture. La Med. Del Lav. 2006, 97, 430–437. [Google Scholar]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, S.; Esrafili, A.; Kalantary, R.R.; Gholami, M.; Sobhi, H.R. Synthesis and evaluation of the performance of g-C3N4/Fe3O4/Ag photocatalyst for the efficient removal of diazinon: Kinetic studies. J. Photochem. Photobiol. Chem. 2020, 389, 112279. [Google Scholar] [CrossRef]
- Li, Z. Spatiotemporal pattern models for bioaccumulation of pesticides in common herbaceous and woody plants. J. Environ. Manag. 2020, 276, 111334. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2021/1165. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_consumption_of_pesticides (accessed on 10 April 2025).
- Dumitrescu, C. Poluanti Organici; Bibliotheca: Targoviste, Romania, 2008; pp. 87–93. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating. pp. 329–490. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 19 May 2025).
- Yue, N.; Wu, J.; Qi, W.; Su, R. Algae-derived biochar nanozyme array for discrimination and detection of multiple pesticides in soil, water and food. Food Chem. 2024, 438, 137946. [Google Scholar] [CrossRef] [PubMed]
- Kalantary, R.R.; Barzegar, G.; Jorfi, S. Monitoring of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers using Monte Carlo simulation in Behbahan City, Iran. Chemosphere 2022, 286, 131667. [Google Scholar] [CrossRef]
- Längin, A.; Schuster, A.; Kümmerer, K. Chemicals in the environment—The need for a clear nomenclature: Parent compounds, metabolites, transformation products and their elimination. CLEAN—Soil Air Water 2008, 36, 349–350. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Stockholm Convention on Persistent Organic Pollutants. Available online: https://www.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx (accessed on 19 May 2025).
- Khezami, F.; Gómez-Navarro, O.; Barbieri, M.V.; Khiari, N.; Chkirbene, A.; Chiron, S.; Khadhar, S.; Pérez, S. Occurrence of contaminants of emerging concern and pesticides and relative risk assessment in Tunisian groundwater. Sci. Total Environ. 2024, 906, 167319. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Kimura, S.Y. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2019, 92, 473–505. [Google Scholar] [CrossRef]
- Rastkari, N.; Ahmadkhaniha, R.; Soleymani, F.; Ravanipour, M. Pesticide residues in drinking water treatment plants and human health risk assessment: A case study from Northern Iran. Environ. Geochem. Health 2024, 46, 68. [Google Scholar] [CrossRef] [PubMed]
- Bempah, C.K.; Ewusi, A. Heavy metals contamination and human health risk assessment around Obuasi gold mine in Ghana. Environ. Monit. Assess. 2016, 188, 261. [Google Scholar] [CrossRef]
- Wongsasuluk, P.; Chotpantarat, S.; Siriwong, W.; Robson, M. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ. Geochem. Health 2014, 36, 169–182. [Google Scholar] [CrossRef]
- Zhong, T.; Xue, D.; Zhao, L.; Zhang, X. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health 2018, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Risk Assessment. Training for the Health Sector. Available online: https://ec.europa.eu/health/ph_projects/2003/action3/docs/2003_3_09_a23_en.pdf (accessed on 23 April 2025).
- Ahmadi, M.; Akhbarizadeh, R.; Jaafarzadeh, N.; Barzegar, G.; Jorfi, S. Geochemical determination and pollution assessment of heavy metals in agricultural soils of south western of Iran. J. Environ. Health Sci. Eng. 2019, 17, 657–669. [Google Scholar] [CrossRef]
- Husain Khan, A.; Abdul Aziz, H.; Khan, N.A.; Dhingra, A.; Ahmed, S.; Naushad, M. Effect of seasonal variation on the occurrences of high-risk pharmaceutical in drain-laden surface water: A risk analysis of Yamuna River. Sci. Total Environ. 2021, 794, 148484. [Google Scholar] [CrossRef]
- Ferencs, L.; Balog, A. A Pesticide survey in soil, water and foodstuffs from Central Romania. Carpathian J. Earth Environ. Sci. 2010, 5, 111–118. [Google Scholar]
- Ciucure, C.T.; Geana, E.I.; Arseni, M.; Ionete, R.E. Status of different anthropogenic organic pollutants accumulated in sediments from Olt River Basin, Romania: From distribution and sources to risk assessment. Sci. Total Environ. 2023, 886, 163967. [Google Scholar] [CrossRef]
- CORINE Land Cover 2018 (Vestor/Raster 100m), Europe, 6-Yearly. Available online: https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 (accessed on 10 March 2025).
- EPA Groundwater Sampling-Operating Procedure-U.S. Environmental Protection Agency. Science and Ecosystem Support Division, Operating Procedure—Groundwater Sampling. 2017. Available online: https://www.epa.gov/sites/production/files/2017-07/documents/groundwater_sampling301_af.r4.pdf (accessed on 10 March 2025).
- Barbieri, M.V.; Peris, A.; Postigo, C.; Moya-Garces, A.; Monllor-Alcaraz, L.S.; Rambla-Alegre, M.; Eljarrat, E.; de Alda, M.L. Evaluation of the occurrence and fate of pesticides in a typical Mediterranean delta ecosystem (Ebro River Delta) and risk assessment for aquatic organisms. Environ. Pollut. 2021, 274, 115813. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, Y.; Shi, L.; Song, J.; Jiang, Y. Organochlorine pesticides and polychlorinated biphenyls in sediments of the Lanzhou reach of Yellow River (China): Spatial distribution, sources and risk assessment. Mar. Pollut. Bull. 2024, 208, 116962. [Google Scholar] [CrossRef] [PubMed]
- NORMAN Ecotoxicology Database. Available online: https://www.norman-network.com/nds/ecotox/ (accessed on 10 March 2025).
- Ebrahimzadeh, G.; Alimohammadi, M.; Rezaei Kahkha, M.R.; Mahvi, A.H. Contamination level and human non-carcinogenic risk assessment of diazinon pesticide residue in drinking water resources—A case study, IRAN. Int. J. Environ. Anal. Chem. 2020, 102, 4726–4737. [Google Scholar] [CrossRef]
- Ravanipour, M.; Nabipour, I.; Yunesian, M.; Rastkari, N.; Mahvi, A.H. Exposure sources of polychlorinated biphenyls (PCBs) and health risk assessment: A systematic review in Iran. Environ. Sci. Pollut. Res. 2022, 29, 55437–55456. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A). 1989. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/rags_a.pdf (accessed on 10 March 2025).
- Wu, B.; Zhao, D.Y.; Jia, H.Y.; Zhang, Y.; Zhang, X.X.; Cheng, S.P. Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 2009, 82, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hubal, E.A.C.; de Wet, T.; Du Toit, L.; Firestone, M.P.; Ruchirawat, M.; van Engelen, J.; Vickers, C. Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: Results of a World Health Organization review. Regul. Toxicol. Pharmacol. 2014, 69, 113–124. [Google Scholar] [CrossRef]
- Mekonen, S.; Argaw, R.; Simanesew, A.; Houbraken, M.; Senaeve, D.; Ambelu, A.; Spanoghe, P. Pesticide residues in drinking water and associated risk to consumers in Ethiopia. Chemosphere 2016, 162, 252–260. [Google Scholar] [CrossRef]
- Tinning, K.; Acworth, J. Make your Best Guess: An updated method for paediatric weight estimation in emergencies. Emerg. Med. Australas. 2007, 19, 528–534. [Google Scholar] [CrossRef]
- USEPA. Chapter 7: Characterizing Risk and Hazard. U.S. EPA Region 6. 2005. Available online: https://archive.epa.gov/epawaste/hazard/tsd/td/web/pdf/05hhrap7.pdf (accessed on 10 March 2025).
- Bamuwamye, M.; Ogwok, P.; Tumuhairwe, V. Cancer and non-cancer risks associated with heavy metal exposures from street foods: Evaluation of roasted meats in an urban setting. J. Environ. Pollut. Hum. Health 2015, 3, 24–30. [Google Scholar]
- Huang, M.; Zhou, S.; Sun, B.; Zhao, Q. Heavy metals in wheat grain: Assessment of potential health risk for inhabitants in Kunshan, China. Sci. Total Environ. 2008, 405, 54–61. [Google Scholar] [CrossRef]
- Meftaul, I.M.; Venkateswarlu, K.; Annamalai, P.; Parven, A.; Megharaj, M. Degradation of four pesticides in five urban landscape soils: Human and environmental health risk assessment. Environ. Geochem. Health 2022, 45, 1599–1614. [Google Scholar]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy metals in vegetables and potential risk for human health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- Sultana, M.S.; Rana, S.; Yamazaki, S.; Aono, T.; Yoshida, S. Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ. Sci. 2017, 3, 1291107. [Google Scholar] [CrossRef]
- European Commission. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast). Official Journal of the European Union. 2020. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC201243/ (accessed on 25 April 2025).
- US EPA. Baseline Human Health Risk Assessment: Vasquez Boulevard and I-70 Superfund Site, Denver, CO; U.S. Environmental Protection Agency: Denver, CO, USA, 2001.
- Dragus, A.B. Assessment of the Incidence of Triazine Pesticides in Environmental Samples and Food Products. Ph.D. Thesis, Universitatea Babeș-Bolyai din Cluj-Napoca, Cluj-Napoca, Romania, 2015. Available online: https://teze.doctorat.ubbcluj.ro/doctorat/teza/fisier/2513 (accessed on 19 May 2025).
- OLAF Teams Up with Europol Against Illegal Pesticides. Available online: https://anti-fraud.ec.europa.eu/media-corner/news/olaf-teams-europol-against-illegal-pesticides-2021-06-17_en (accessed on 17 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).