Effects of Surface Water Treatment for Drinking Water Production, Distribution and Heating on Biological Stability
Abstract
1. Introduction
2. Methods
2.1. Preparation of AOC-Free Glassware
2.2. Sampling Sites and Sample Collection
2.3. Flow Cytometry
2.4. Assessment of Regrowth Potential and Ratio of Fixed to Dissolved Nutrients
3. Results
3.1. Effect of Surface Water Treatment on Cell Numbers and Bacterial Regrowth Potentials
3.1.1. River Water Treatment
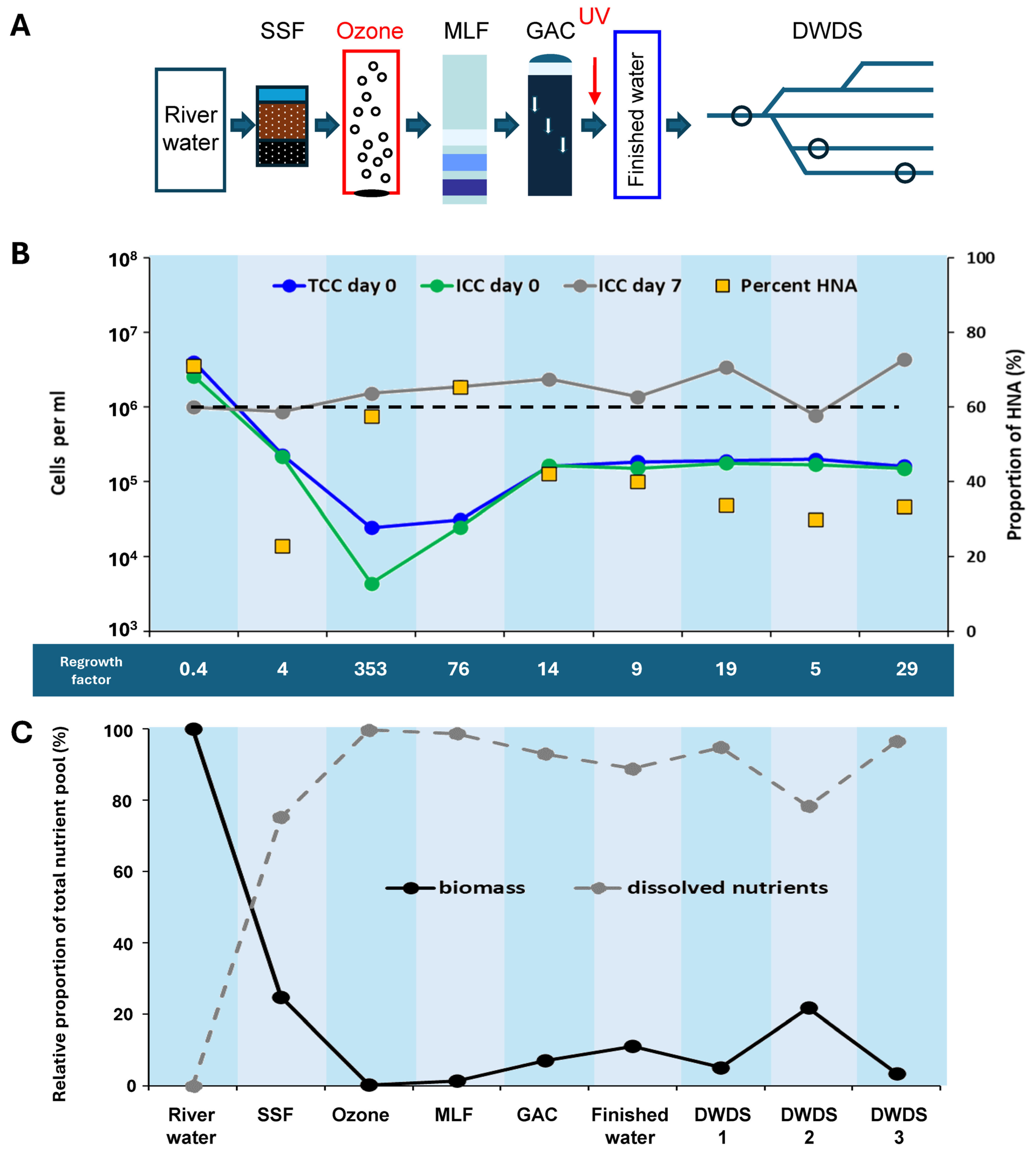
3.1.2. Reservoir Water Treatment
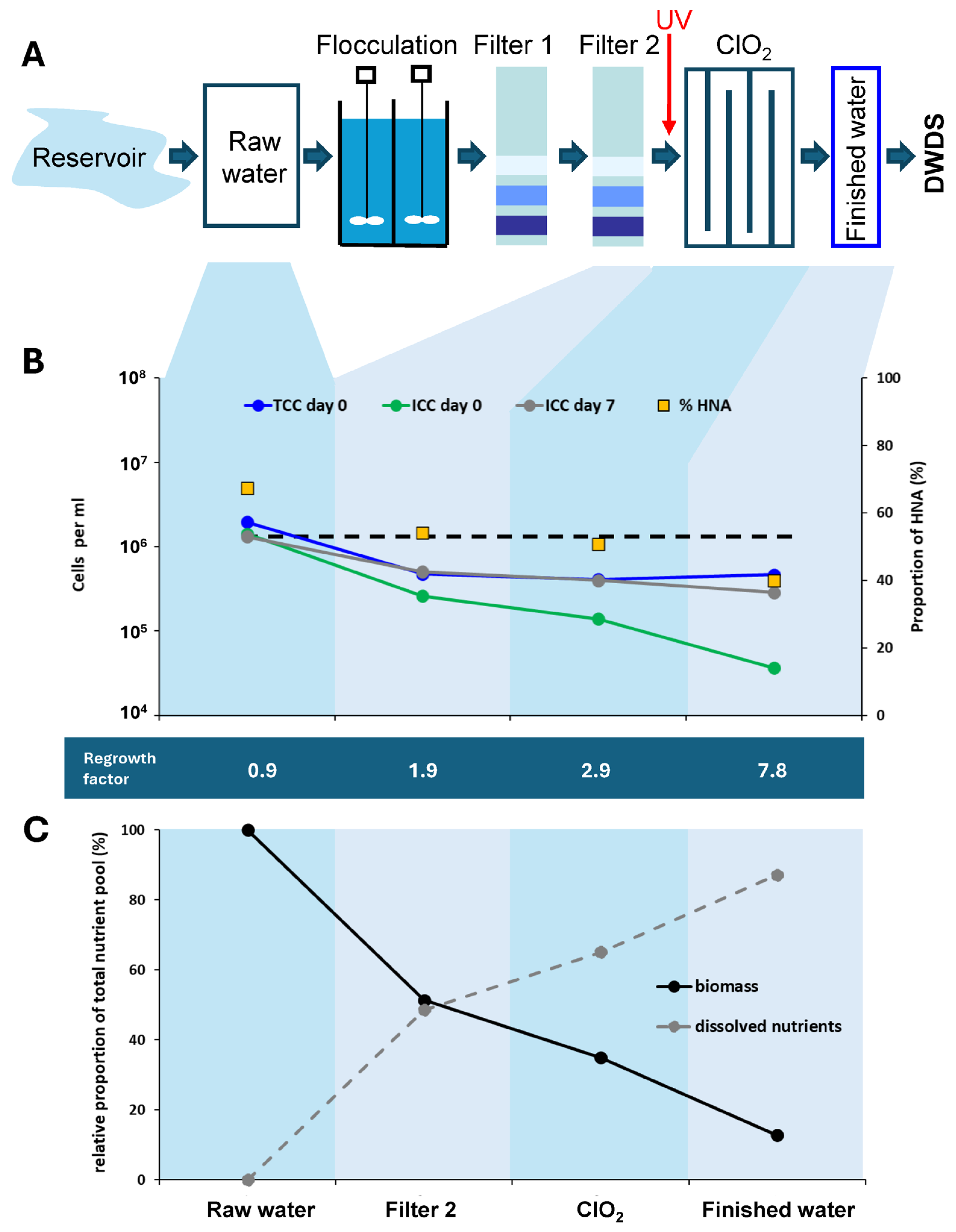
3.1.3. Canal Water Treatment
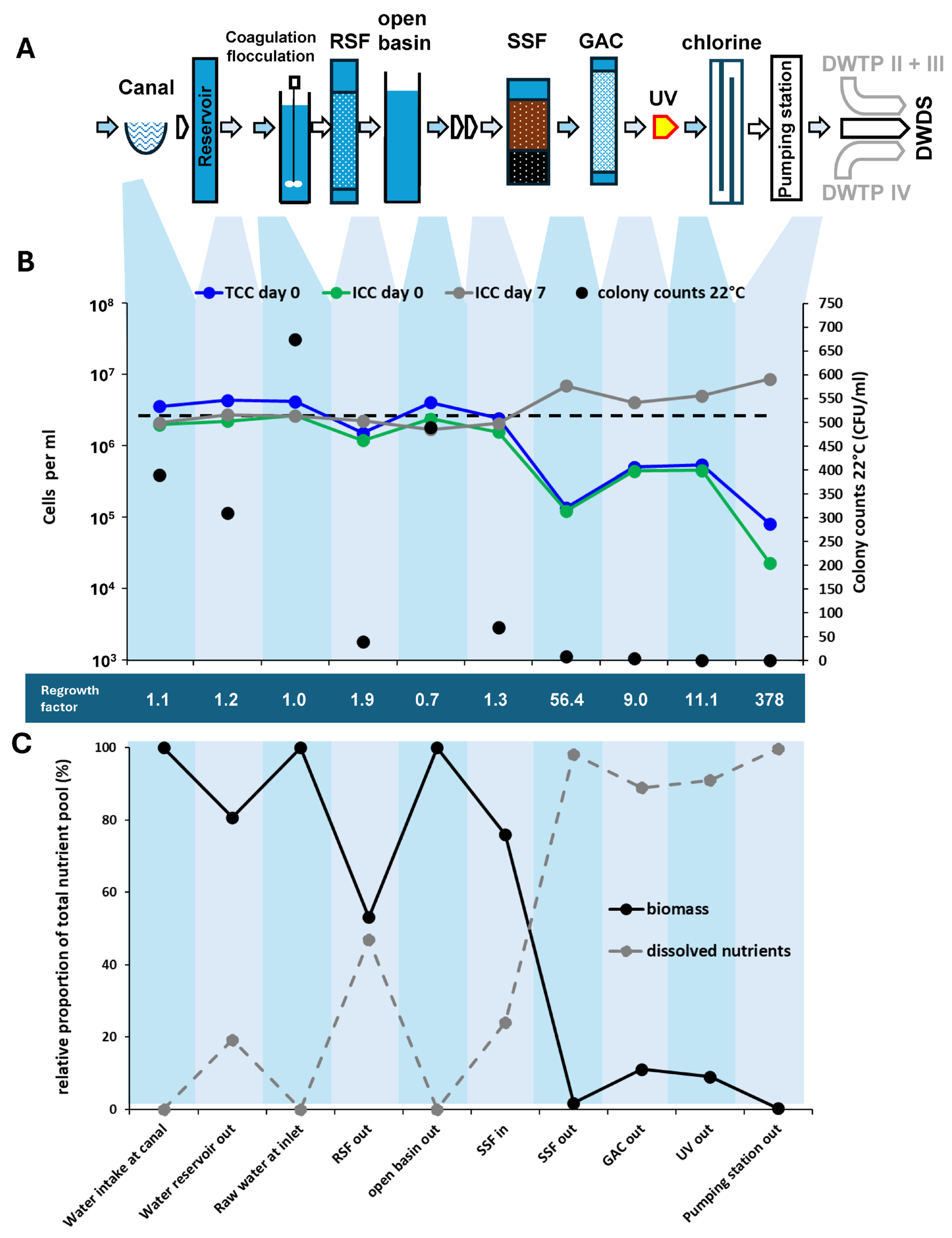
3.2. Microbiological Changes During Distribution of Chlorinated Water

3.3. Regrowth Potential at the Entry Point of Buildings
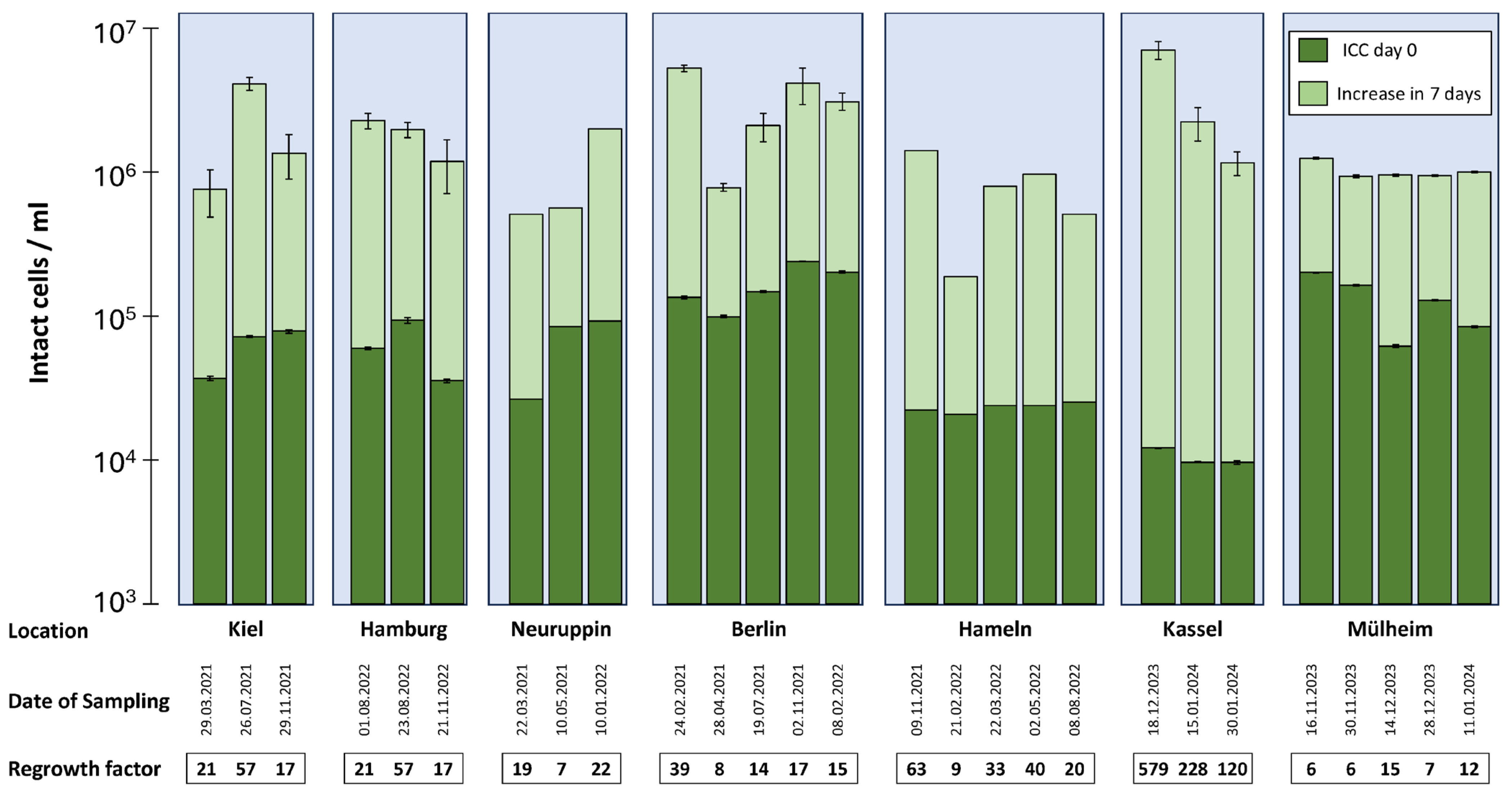
3.4. Effect of Heating Water on Production of PWH

4. Discussion
4.1. Effect of Water Treatment
4.2. Regrowth in Distribution Systems
4.3. Regrowth Potential in Drinking Water Installations and Effect of Water Heating
4.4. Biological Stability of Drinking Water and Its Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acronym | Definition |
| AOC | assimilable organic carbon |
| ATP | adenosine triphosphate |
| ClO2 | chlorine dioxide |
| DOC | dissolved organic carbon |
| DWDS | drinking water distribution system |
| DWTP | drinking water treatment plant |
| DWI | drinking water installation |
| EPDM | ethylene–propylene–diene monomer |
| FCM | flow cytometry |
| FL | fluorescence |
| GAC | granular activated carbon |
| GP | ground passage |
| HNA | high nucleic acid |
| ICC | intact cell count |
| LNA | low nucleic acid |
| MLF | multilayer filter |
| PI | propidium iodide |
| POE | point of entry |
| PWC | potable water cold |
| PWH | potable water hot |
| RSF | rapid sand filter |
| SG-I | SYBR Green I |
| SSF | slow sand filter |
| TCC | total cell count |
| TOC | total organic carbon |
References
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Rittman, B.; Snoeyinck, V.L. Achieving biologically stable drinking water. J. Am. Water Work. Assoc. 1984, 76, 106–110. [Google Scholar] [CrossRef]
- van der Kooij, D. Significance and assessment of the biological stability of drinking water. In Water Pollution. The Handbook of Environmental Chemistry; Hrubec, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 5/5B. [Google Scholar]
- Falkinham, J.O., III; Hilborn, E.D.; Arduino, M.J.; Pruden, A.; Edwards, M.A. Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 2015, 123, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Percival, S.L.; Walker, J.T. Contamination potential of biofilms in water distribution systems. Water Sci. Technol. Water Supply 2002, 2, 271–280. [Google Scholar] [CrossRef]
- Sun, H.; Shi, B.; Lytle, D.A.; Bai, Y.; Wang, D. Formation and release behavior of iron corrosion products under the influence of bacterial communities in a simulated water distribution system. Environ. Sci. Process. Impacts 2014, 16, 576–585. [Google Scholar] [CrossRef]
- Clark, R.M. Drinking water treatment and distribution systems: Their Role in Reducing Risks and Protecting Public Health. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–172. [Google Scholar]
- Uhl, W.; Schaule, G. Establishment of HPC(R2A) for regrowth control in non-chlorinated distribution systems. Int. J. Food Microbiol. 2004, 92, 317–325. [Google Scholar] [CrossRef]
- Campostrini, L.; Proksch, P.; Jakwerth, S.; Farnleitner, A.H.; Kirschner, A.K.T. Introducing bacterial community turnover times to elucidate temporal and spatial hotspots of biological instability in a large Austrian drinking water distribution network. Water Res. 2024, 252, 121188. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Hwang, C.; Liu, W.T.; Boon, N.; Köster, O.; Vrouwenvelder, H.; Egli, T.; Hammes, F. Microbiology-based multi-parametric approach towards assessing biological stability in drinking water distribution networks. Water Res. 2013, 47, 3015–3025. [Google Scholar] [CrossRef]
- Prest, E.I.; Weissbrodt, D.G.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Long-Term bacterial dynamics in a full-scale drinking water distribution system. PLoS ONE 2016, 11, e0164445. [Google Scholar] [CrossRef]
- Schleich, C.; Chan, S.; Pullerits, K.; Besmer, M.D.; Paul, C.J.; Rådström, P.; Keucken, A. Mapping Dynamics of bacterial communities in a full-scale drinking water distribution system using flow cytometry. Water 2019, 11, 2137. [Google Scholar] [CrossRef]
- Prest, E.I.; El-Chakhtoura, J.; Hammes, F.; Saikaly, P.E.; van Loosdrecht, M.C.; Vrouwenvelder, J.S. Combining flow cytometry and 16S rRNA gene pyrosequencing: A promising approach for drinking water monitoring and characterization. Water Res. 2014, 63, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Nocker, A.; Shah, M.; Dannenmann, B.; Schulze-Osthoff, K.; Wingender, J.; Probst, A.J. Assessment of UV-C-induced water disinfection by differential PCR-based quantification of bacterial DNA damage. J. Microbiol. Methods 2018, 149, 89–95. [Google Scholar] [CrossRef]
- Flemming, H.C.; Bendinger, B.; Exner, M.; Gebel, J.; Kistemann, T.; Schaule, G.; Szewzyk, U.; Wingender, J. The last meters before the tap: Where drinking water quality is at risk. In Microbial Growth in Drinking Water Supplies—Problems, Causes, Control and Research Needs; Van der Kooij, D., van der Wielen, P.W.J., Eds.; IWA Publishing: London, UK, 2014; pp. 207–238. [Google Scholar]
- Völker, S.; Schreiber, C.; Kistemann, T. Drinking water quality in household supply infrastructure—A survey of the current situation in Germany. Int. J. Hyg. Environm. Health 2010, 213, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Bucheli-Witschel, M.; Kötzsch, S.; Darr, S.; Widler, R.; Egli, T. A new method to assess the influence of migration from polymeric materials on the biostability of drinking water. Water Res. 2012, 46, 4246–4260. [Google Scholar] [CrossRef]
- Proctor, C.R.; Hammes, F. Drinking water microbiology—From measurement to management. Curr. Opin. Biotechnol. 2015, 33, 87–94. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Boon, N.; Wang, Y.; Egli, T.; Hammes, F. Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Res. 2010, 44, 4868–4877. [Google Scholar] [CrossRef]
- Lipphaus, P.; Hammes, F.; Kötzsch, S.; Green, J.; Gillespie, S.; Nocker, A. Microbiological tap water profile of a medium-sized building and effect of water stagnation. Environ. Technol. 2013, 35, 620–628. [Google Scholar] [CrossRef]
- Ross, P.S.; Hammes, F.; Dignum, M.; Magic-Knezev, A.; Hambsch, B.; Rietveld, L.D. A comparative study of three different assimilable organic carbon (AOC) methods: Results of a round-robin test. Water Sci. Technol. Water Supply 2013, 13, 1024–1033. [Google Scholar] [CrossRef]
- Egli, T.; Campostrini, L.; Leifels, M.; Füchslin, H.P.; Kolm, C.; Dan, C.; Zimmermann, S.; Hauss, V.; Guiller, A.; Grasso, L.; et al. Domestic hot-water boilers harbour active thermophilic bacterial communities distinctly different from those in the cold-water supply. Water Res. 2024, 253, 121109. [Google Scholar] [CrossRef]
- Prest, E.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef]
- van der Wielen, P.W.J.J.; Brouwer-Hanzens, A.; Italiaander, R.; Hijnen, W.A.M. Initiating guidance values for novel biological stability parameters in drinking water to control regrowth in the distribution system. Sci. Total Environ. 2023, 871, 161930. [Google Scholar] [CrossRef]
- ISO 6222:1999; Water quality—Enumeration of culturable microorganisms—Colony count by inoculation in a nutrient agar culture medium. ISO: Geneva, Switzerland, 1999.
- Gatza, E.; Hammes, F.; Prest, E. Assessing Water Quality With the BD Accuri™ C6 Flow Cytometer; White Paper; BD Biosciences: Franklin Lakes, NJ, USA, 2013. [Google Scholar]
- Hammes, F.; Broger, T.; Weilenmann, H.-U.; Vital, M.; Helbing, J.; Bosshart, U.; Huber, P.; Odermatt, R.P.; Sonnleitner, B. Development and laboratory-scale testing of a fully automated online flow cytometer for drinking water analysis. Cytom. Part A 2012, 81, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.; Lipphaus, P.; Green, J.; Parsons, S.; Weir, P.; Juskowiak, K.; Jefferson, B.; Jarvis, P.; Nocker, A. Assessing microbiological water quality in drinking water distribution systems with disinfectant residual using flow cytometry. Water Res. 2014, 65, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, I.; Nakamura, H.; Kurisu, F.; Furumari, H. Characterization of microbial regrowth potential shaped by advanced drinking water treatment. H2Open J. 2021, 4, 157–166. [Google Scholar] [CrossRef]
- Rahmatika, I.; Kurisu, F.; Furumai, H.; Kasuga, I. Dynamics of the microbial community and opportunistic pathogens after water stagnation in the premise plumbing of a building. Microbes Environ. 2022, 37, ME21065. [Google Scholar] [CrossRef]
- Hammes, F.; Berger, C.; Köster, O.; Egli, T. Assessing biological stability of drinking water without disinfectant residuals in a full-scale water supply system. J. Water Supply Res. Technol. 2010, 59, 31–40. [Google Scholar] [CrossRef]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Koster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef]
- Vital, M.; Dignum, M.; Magic-Knezev, A.; Ross, P.; Rietveld, L.; Hammes, F. Flow cytometry and adenosine tri-phosphate analysis: Alternative possibilities to evaluate major bacteriological changes in drinking water treatment and distribution systems. Water Res. 2012, 46, 4665–4676. [Google Scholar] [CrossRef]
- Ramseier, M.K.; Peter, A.; Traber, J.; von Gunten, U. Formation of assimilable organic carbon during oxidation of natural waters with ozone, chlorine dioxide, chlorine, permanganate, and ferrate. Water Res. 2011, 45, 2002–2010. [Google Scholar] [CrossRef]
- Hammes, F.; Salhi, E.; Koster, O.; Kaiser, H.P.; Egli, T.; von Gunten, U. Mechanistic and kinetic evaluation of organic disinfection by-product and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Res. 2006, 40, 2275–2286. [Google Scholar] [CrossRef]
- Hammes, F.; Meylan, S.; Salhi, E.; Köster, O.; Egli, T.; von Gunten, U. Formation of assimilable organic carbon (AOC) and specific natural organic matter (NOM) fractions during ozonation of phytoplankton. Water Res. 2007, 41, 1447–1454. [Google Scholar] [CrossRef]
- Rosińska, A.; Rakocz, K. Ozonation and changes in biodegradable organic substances in drinking water treatment: The future of green technology. Energies 2024, 17, 530. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Hwang, C.; Ling, F.; Liu, W.-T.; Boon, N.; Köster, O.; Egli, E.; Hammes, H. Abundance and composition of indigenous bacterial communities in a multi-step biofiltration-based drinking water treatment plant. Water Res. 2014, 62, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.J.; Xi, C.; Raskin, L. Bacterial community structure in the drinking water microbiome is governed by filtration processes. Environ. Sci. Technol. 2012, 46, 8851–8859. [Google Scholar] [CrossRef]
- van de Aa, L.T.J.; Rietveld, L.C.; van Dijk, J.C. Effects of ozonation and temperature on the biodegradation of natural organic matter in biological granular activated carbon filters. Drink. Water Eng. Sci. 2011, 4, 25–35. [Google Scholar] [CrossRef]
- Sallanko, J.; Iivari, P.; Heiska, E. Effect of ozone and granular activated coal (GAC) on the bioactivity of drinking water. J. Environ. Sci. Health Part A 2009, 44, 230–234. [Google Scholar] [CrossRef]
- Ohkouchi, Y.; Ly, B.T.; Ishikawa, S.; Aoki, Y.; Echigo, S.; Itoh, S. A survey on levels and seasonal changes of assimilable organic carbon (AOC) and its precursors in drinking water. Environ. Technol. 2011, 32, 1605–1613. [Google Scholar] [CrossRef]
- Guchi, E. Review on slow sand filtration in removing microbial contamination and particles from drinking water. Am. J. Food Nutr. 2015, 3, 47–55. [Google Scholar]
- van der Kooij, D.; Veenendaal, H.R.; van der Mark, E.J.; Dignum, M. Assessment of the microbial growth potential of slow sand filtrate with the biomass production potential test in comparison with the assimilable organic carbon method. Water Res. 2017, 125, 270–279. [Google Scholar] [CrossRef]
- Trikannad, S.A.; Attiani, V.; van der Wielen, P.W.J.J.; Smidt, H.; van der Hoek, J.P.; van Halem, D. Recovery of microbial biomass and purification performance after scraping of full-scale slow sand filters. J. Water Process Eng. 2024, 60, 105101. [Google Scholar] [CrossRef]
- Kirschner, A.K.T.; Lindner, G.; Jakwerth, S.; Vierheilig, J.; van Driezum, I.C.H.; Derx, J.; Blaschke, A.P.; Savio, D.; Farnleitner, A.H. Assessing biological stability in a porous groundwater aquifer of a riverbank filtration system: Combining traditional cultivation-based and emerging cultivation-independent in situ and predictive methods. Osterr. Wasser Und Abfallwirtsch 2021, 73, 490–500. [Google Scholar] [CrossRef]
- Weiss, W.J.; Bouwer, E.J.; Ball, W.P.; O’Melia, C.R.; Aboytes, R.; Speth, T.F. Riverbank filtration: Effect of ground passage on NOM. J. Water Supply: Res. Technol.–AQUA 2004, 53, 61–83. [Google Scholar] [CrossRef]
- Kondor, A.C.; Vancsik, A.V.; Bauer, L.; Szabó, L.; Szalai, Z.; Jakab, G.; Maász, G.; Pedrosa, M.; Sampaio, M.J.; Lado Ribeiro, A.R. Efficiency of the bank filtration for removing organic priority substances and contaminants of emerging concern: A critical review. Environ. Pollut. 2023, 340, 122795. [Google Scholar] [CrossRef] [PubMed]
- Storck, F.R.; Schmidt, C.K.; Lange, F.; Henson, J.W.; Hahn, K. Factors controlling micropollutant removal during riverbank filtration. J. Am. Water Work. Assoc. 2012, 104, 643–652. [Google Scholar] [CrossRef]
- Chen, W.T.; Chien, C.C.; Ho, W.S.; Ou, J.H.; Chen, S.C.; Kao, C.M. Effects of treatment processes on AOC removal and changes of bacterial diversity in a water treatment plant. J. Environ. Manag. 2022, 31, 114853. [Google Scholar] [CrossRef]
- Maul, A.; El-Shaarawi, A.H.; Block, J.C. Heterotrophic bacteria in water distribution systems. I. Spatial and temporal variation. Sci. Total Environ. 1985, 44, 201–214. [Google Scholar] [CrossRef]
- Nescerecka, A.; Rubulis, J.; Vital, M.; Juhna, T.; Hammes, F. Biological instability in a chlorinated drinking water distribution network. PLoS ONE 2014, 9, e96354. [Google Scholar] [CrossRef]
- Nescerecka, A.; Juhna, T.; Hammes, F. Identifying the underlying causes of biological instability in a full-scale drinking water supply system. Water Res. 2018, 135, 11–21. [Google Scholar] [CrossRef]
- Fransolet, G.; Villers, G.; Masschelein, W.J. Influence of temperature on bacterial development in waters. Ozone Sci. Eng. 2008, 7, 205–227. [Google Scholar] [CrossRef]
- Choi, Y.; Park, H.; Lee, M.; Lee, G.-S.; Choi, Y.-J. Seasonal variation of assimilable organic carbon and its impact to the biostability of drinking water. Environ. Eng. Res. 2019, 24, 501–512. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Wang, F.; Qian, L.; Zhou, Y.; Qi, W.; Chen, J. Effect of disinfectant residual on the interaction between bacterial growth and assimilable organic carbon in a drinking water distribution system. Chemosphere 2018, 202, 586–597. [Google Scholar] [CrossRef]
- Grimard-Conea, M.; Bédard, E.; Prévost, M. Can free chlorine residuals from the utility really be maintained in building plumbing systems to prevent microbial growth? Sci. Total Environ. 2024, 939, 173651. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Elfland, C.; Edwards, M. Impact of advanced water conservation features and new copper pipe on rapid chloramines decay and microbial regrowth. Water Res. 2012, 46, 611–621. [Google Scholar] [CrossRef]
- Ji, P.; Parks, J.; Edwards, M.A.; Pruden, A. Impact of water chemistry, pipe material and stagnation on the building plumbing microbiome. PLoS ONE 2015, 10, e0141087. [Google Scholar] [CrossRef]
- Ling, F.; Whitaker, R.; LeChevallier, M.W.; Liu, W.T. Drinking water microbiome assembly induced by water stagnation. ISME J. 2018, 12, 1520–1531. [Google Scholar] [CrossRef]
- Learbuch, K.L.G.; Smidt, H.; van der Wielen, P.W.J.J. Influence of pipe materials on the microbial community in unchlorinated drinking water and biofilm. Water Res. 2021, 194, 116922. [Google Scholar] [CrossRef]
- Spinks, A.T.; Dunstan, R.H.; Harrison, T.; Coombes, P.; Kuczera, G. Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Res. 2006, 40, 1326–1332. [Google Scholar] [CrossRef]
- Nocker, A.; Lindfeld, E.; Wingender, J.; Schulte, S.; Dumm, M.; Bendinger, B. Thermal and chemical disinfection of water and biofilms: Only a temporary effect in regard to the autochthonous bacteria. J. Water Health 2021, 19, 808–822. [Google Scholar] [CrossRef]
- Meyer, B.; Pannekens, M.; Soares, A.; Timmermann, L.; Probst, A.; Hippelein, M.; Bendinger, B.; Nocker, A. Bacterial populations in different parts of domestic drinking water systems are distinct and adapted to the given ambient temperatures. Front. Water 2023, 5, 1119951. [Google Scholar] [CrossRef]
- van der Kooij, D.; Veenendaal, H.R.; Italiaander, R.; van der Mark, E.J.; Dignum, M. Primary colonizing Betaproteobacteriales play a key role in the growth of Legionella pneumophila in biofilms on surfaces exposed to drinking water treated by slow sand iltration. Appl. Environ. Microbiol. 2018, 84, e01732-18. [Google Scholar] [CrossRef]
- Favere, J.; Waegenaar, F.; Jia, M.; Folens, K.; Verhoeven, M.; Balliu, E.; Rajkovic, A.; De Gusseme, B.; Boon, N. Production of biostable drinking water using a lab-scale biological trickling filter enriched with hydrogen-oxidizing bacteria. Npj Clean Water 2024, 7, 101. [Google Scholar] [CrossRef]
- Favere, J.; Barbosa, R.G.; Sleutels, T.; Verstraete, W.; De Gusseme, B.; Boon, N. Safeguarding the microbial water quality from source to tap. Npj Clean Water 2021, 4, 28. [Google Scholar] [CrossRef]
- van der Kooij, D. Assimilable Organic Carbon (AOC) in Treated Water: Determination and Significance. In Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; Wileys & Sons: New York, NY, USA, 2002; pp. 312–327. [Google Scholar]
- van der Kooij, D. Assimilable organic carbon as an indicator of bacterial regrowth. J. Am. Water Work. Assoc. 1992, 84, 57–65. [Google Scholar] [CrossRef]
- van der Kooij, D.; Visser, A.; Hijnen, W. Determining the concentration of easily assimilable organic carbon in drinking water. J. Am. Water Work. Assoc. 1982, 74, 540–545. [Google Scholar] [CrossRef]
- Elhadidy, A.M.; van Dyke, M.I.; Peldszus, S.; Huck, P.M. Application of flow cytometry to monitor assimilable organic carbon (AOC) and microbial community changes in water. J. Microbiol. Methods 2016, 130, 154–163. [Google Scholar] [CrossRef]
- Hammes, F.A.; Egli, T. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 2005, 39, 3289–3294. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocker, A.; Meyer, B.; Bendinger, B. Effects of Surface Water Treatment for Drinking Water Production, Distribution and Heating on Biological Stability. Appl. Sci. 2025, 15, 5843. https://doi.org/10.3390/app15115843
Nocker A, Meyer B, Bendinger B. Effects of Surface Water Treatment for Drinking Water Production, Distribution and Heating on Biological Stability. Applied Sciences. 2025; 15(11):5843. https://doi.org/10.3390/app15115843
Chicago/Turabian StyleNocker, Andreas, Benjamin Meyer, and Bernd Bendinger. 2025. "Effects of Surface Water Treatment for Drinking Water Production, Distribution and Heating on Biological Stability" Applied Sciences 15, no. 11: 5843. https://doi.org/10.3390/app15115843
APA StyleNocker, A., Meyer, B., & Bendinger, B. (2025). Effects of Surface Water Treatment for Drinking Water Production, Distribution and Heating on Biological Stability. Applied Sciences, 15(11), 5843. https://doi.org/10.3390/app15115843






