Abstract
This paper presents a novel small-scale system for drinking water treatment from surface waters, designed to rely on gravity as the only source of energy driving the treatment process. The pilot-scale setup, designed for a flow rate of 0.5 L/s, was tested at the Cornell University Water Filtration Plant (CWFP) for a total period of 5 months of operation. The experiments evaluated the influence of selected process parameters on system performance. The identified best operation practices were used to complete a comparative study against CWFP’s full-scale treatment process and to conduct a performance assessment in the context of various legislative landscapes. The objective of the work was to determine both the advantages and disadvantages of the proposed technology over established solutions. Over the study period, the average turbidity of the produced water was equal to 0.54 NTU. The pilot complied with the United States Environmental Protection Agency (US EPA) turbidity standard of <0.3 NTU 47.1% of the time and <1 NTU for 89.9% of the time, thus falling short of the standard of <0.3 NTU 95% of the time and <1 NTU 100% of the time. For 99.5% of the time, it complied with the World Health Organization turbidity guideline of <5 NTU for chlorination treatment. The benchmark conventional system outperformed the tested prototype, complying with the US EPA standards for the entire duration of the study. The tested process also generated a waste stream, which accounted on average for more than 10% of the total raw water volume.
1. Introduction
1.1. Background
Municipal water treatment technologies encompass a wide range of techniques, which can be classified into “plus approaches,” which involve chemical additives, and “minus approaches,” which rely on physical or biological processes without added chemicals. Reid et al. argued in favor of minus approaches with respect to chlorine addition due to their lower risk of forming disinfection byproducts (DBPs), a growing health concern in drinking water [1]. While chlorination is effective against acute waterborne infections, it can generate halogenated organic compounds linked to chronic health issues, including bladder cancer and miscarriages [2]. Reducing DBP formation while ensuring microbial safety often involves eliminating particulate and dissolved organic carbon from water. Strategies include biodegradation, oxidative treatment, adsorption, and membrane separation—all aimed at minimizing both chlorine and organic matter, the main DBP precursors. The Netherlands has long employed this approach, achieving microbiologically safe water without chlorine by eliminating heterotrophic nutrients. Although the US regulations require chlorine residuals in treated water, focusing on organic precursor removal remains a viable strategy for DBP mitigation [3,4,5,6,7,8].
Drinking water treatment for human consumption must address a wide range of contaminants that pose risks to human health. These include biological contaminants such as bacteria, viruses, and protozoa; chemical contaminants like arsenic, nitrates, and specific organic contaminants; and physical impurities such as turbidity and suspended solids. Contamination can arise from both natural sources—including geogenic arsenic in groundwater or volcanic activity—and anthropogenic activities, such as agricultural runoff, industrial discharge, and insufficient sanitation infrastructure [9,10]. In rural and underserved communities globally, these challenges are often compounded by limited infrastructure, intermittent electricity, and low technical capacity. Consequently, treatment solutions in such contexts often rely on decentralized, low-cost, and easy-to-maintain technologies—such as biosand filtration or point-of-use chemical disinfection—to provide safe drinking water [11,12].
A widely used method to ensure microbiological safety in drinking water is chlorination, valued for its cost-effectiveness and the ability to maintain a disinfectant residual throughout distribution systems. However, its effectiveness is heavily influenced by the presence of other contaminants in the water, which can interfere with microbial inactivation and react with chlorine to form harmful DBPs. To address this, the World Health Organization (WHO) only recommends chlorination at a maximum turbidity of 5 nephelometric turbidity units (NTUs), with an operational target of ≤1 NTU to ensure reliable disinfection performance [13]. Similarly, the US Environmental Protection Agency (EPA) imposes specific turbidity and chlorine residual requirements under the Surface Water Treatment Rules. For systems using conventional or direct filtration, the EPA mandates that turbidity must never exceed 1 NTU, and that 95% of samples each month must be below 0.3 NTU, and E. coli needs to be <1 MPN/100 mL [4]. For chlorination, the EPA requires a minimum free chlorine residual of 0.2 mg/L at the entry point to the distribution system and a detectable residual throughout the system to maintain microbial protection [14]. These standards reflect the critical importance of clarity and chemical stability for effective disinfection and public health protection. In low-resource settings, consistently achieving these targets remains a technical challenge, emphasizing the need for context-appropriate designs that integrate effective pretreatment (e.g., coagulation, sedimentation, and filtration) with disinfection.
In industrial contexts, water treatment approaches differ widely depending on the specific application. Industries such as semiconductors, pharmaceuticals, and food and beverage production often require ultra-pure water to avoid contamination of products or processes. To achieve their quality targets, these sectors commonly use membrane technologies, UV disinfection, ion exchange, and ozonation. Disinfection in such cases not only targets pathogens but also prevents biofouling and microbial growth in sensitive equipment. In contrast, industries relying on water for cooling, boiler feed, or cleaning often focus on scaling and corrosion control rather than pathogen inactivation. Chlorination is frequently avoided in these systems because of its high reactivity, which can lead to equipment degradation and the formation of unwanted halogenated byproducts. For example, chlorine can react with organic matter or metals, forming corrosive or toxic compounds that may be detrimental to the factory equipment or products [15]. Therefore, while both municipal and industrial systems aim to improve water quality, their treatment priorities and disinfection strategies are shaped by distinct technical, economic, and regulatory considerations.
Rural communities in Latin America and globally often face disproportionately high levels of contaminants due to inadequate treatment systems [16,17,18,19]. Various attempts have been made to create a method for equitable, sustainable water treatment, which could withstand the specific challenges of these remote locations, often experiencing limited access to resources and workforce. Tested treatment methods relied on adapting both well-established and novel treatment technologies to the specific challenges of the remote communities. One example of such a treatment method is the Multi-Stage Filtration method (in Spanish “Filtración en Múltiples Etapas”, FiME) developed at the Inter-regional Center for Water Supply and Removal (Centro Inter-regional de Abastecimiento y Remoción de Agua, CINARA) operating within the University of Valle, Colombia [20]. FiME systems rely on the slow sand filtration principle and are commonly implemented throughout rural Latin America, due to their robustness and ease of operation. They only require minimum maintenance, as slow sand filtration relies on the microbial action within the schmutzdecke—German for “dirt layer”—which is a shallow hypogeal biological layer formed just beneath the surface of the sand. In this type of biological filtration, sand serves as a support matrix for the microbial communities, which are the active component in the treatment process [21].
FiME systems, which would be considered a minus approach method according to Reid’s classification, pose challenges related to their large size and maintenance problems. To address this, the Pan-American Center for Sanitary Engineering and Environmental Sciences (Centro Panamericano de Ingeniería Sanitaria y Ciencias del Ambiente, CEPIS), located in Lima, Peru, created another set of guidelines for water treatment plant design [22,23]. Established in 1968, CEPIS was an environmental technology center established under the Pan-American Health Organization. The mission of this institution was to cooperate with the countries of the Americas in the evaluation and control of environmental risk factors that affect the health of their populations. One of the main contributions developed by the scientists and engineers affiliated with the Center was the creation of a set of designs and recommendations for resilient water treatment systems, based on the conventional coagulation/sedimentation/filtration process [24]. These solutions, known as “CEPIS plants”, contributed significantly to the efforts made by the countries of Latin America to address their health and environmental challenges. CEPIS technology relied on the conventional water treatment process but was heavily oriented towards passive components, which rely on the flow channel geometry and a creative use of fluid dynamics instead of using mechanical components. Notably, water treatment systems based on the CEPIS specifications became widespread and popular beyond small-scale applications.
Coagulation-based systems like those from CEPIS have reliably delivered safe drinking water for over a century. While largely unchanged, modern enhancements include ballasted flocculation (e.g., Actiflo) and sludge blanket clarifiers (e.g., Superpulsator) [25,26,27,28]. Ongoing research explores “enhanced coagulation” via optimized dosing, auxiliary chemicals, and improved tank designs [29,30,31]. Coagulation is also being combined with filtration, membranes, adsorbents, oxidants, and biological processes to boost performance [32,33]. Despite these innovations, basic coagulation-based systems remain globally prevalent.
1.2. AguaClara Technology
The AguaClara (AC) group, launched in 2005 at Cornell University’s School of Civil and Environmental Engineering, aims to reinvent the conventional coagulation process and develop context-appropriate water treatment solutions for underserved communities in the Global South. Working in partnership with Agua Para el Pueblo, a Honduran NGO, AC focuses on improving the condition of the Water, Sanitation, and Hygiene (WASH) sector in rural Central America through the deployment of off-grid, gravity-powered treatment plants [34,35].
AC builds on CEPIS designs, following a user-centric, low-maintenance approach to design. The group developed an off-grid, gravity-powered water treatment method that relies on passive components and novel flow channel geometries to accomplish the same process goals conventionally achieved by mechanical components and external power sources, such as mixing, flow monitoring, maintaining the sludge blanket, or performing the filter backwash. This type of implementation eliminates the need for electricity and therefore reduces operational costs and possible failure modes typically associated with conventional water treatment plants.
AC made four main improvements to the original CEPIS technology:
- Creating a novel off-grid system for flow monitoring and reliable dosing of the chemical agents [36].
- Redesigning the baffled flocculator to generate a more uniform collision potential and allow for shorter hydraulic retention times [37].
- Adapting the sludge blanket process, which conventionally requires vacuum pumps and large valves, for passive operation only driven by gravity [38].
- Designing a stacked rapid sand filter, which reduces the head loss over the filtration media and minimizes the need for backwash water [39,40].
These improvements aim to deliver reliable, efficient water treatment with minimal maintenance, enabling off-grid operation. Deployed in various locations in Central America, AC plants have improved access to safe water, fostered community engagement, and built local capacity through community empowerment and training operators. This section is only meant as an introduction to the design approach, presenting the main improvements to the conventional treatment process developed by the AC group. The exact information about the designs, such as flow rates, dimensions, and other parameters necessary to recreate the system, can be found in the documents published by AC, as well as in the other materials available in the public domain [41].
1.3. Research Gap
Passive water treatment systems like the ones proposed by AC can be a sustainable and energy-independent solution for delivering safe drinking water, especially in rural and off-grid communities. Operation without pumps, by relying on gravitational force to move water through treatment stages, such as sedimentation, filtration, and disinfection, contributes to their simplicity and low maintenance requirements, making them particularly well-suited for remote and resource-constrained environments. Reported performance evaluations of full-scale passive plant, such as those implemented in rural Honduras based on the AC designs, indicate consistent removal of turbidity to levels below 1 NTU even during variable weather conditions, meeting WHO recommendations for safe drinking water in the distribution system [42]. Similar results were reported for a pilot-scale household rainwater system in Australia, where turbidity was reduced to 0.3–0.4 NTU using a gravity-driven membrane and granular activated carbon filter [43]. In another study, a gravity-fed membrane system using silver nanoparticle-coated microfilters successfully treated high-turbidity water (40–700 NTU), achieving a final turbidity <1 NTU, while also eliminating microbial contaminants [44]. These findings indicate that the passive systems, when well-designed and maintained, can achieve high levels of physical and microbiological water quality. However, their long-term success depends on factors such as source water variability, system operation, and protection against recontamination during storage or distribution.
However, in the context of the AC technology, a key gap in the literature is the lack of controlled, long-term performance data for the operating systems. This study addresses that gap through the evaluation of PF200—a miniature, prefabricated water treatment plant based on AC design principles. Intended to serve a small community of approximately 200 people, PF200 offers a portable, low-cost platform for testing and optimization in a controlled setting. Figure 1 compares this pilot unit with a 10 L/s full-scale AC plant built in 2018. Figure 1 shows the comparison of a full-scale AC design of a 10 L/s plant created in 2018 and the tested pilot. For this study, PF200 was used as a representative pilot test platform. The goal of the experiments was to optimize the operation and evaluate the performance of a water treatment process in an AC plant. As a test rig, it has the advantages of being relatively low cost and portable, enabling its easy deployment in a controlled environment, which allows for better experiment monitoring than the full-scale implementations of the AC technology in Central America.
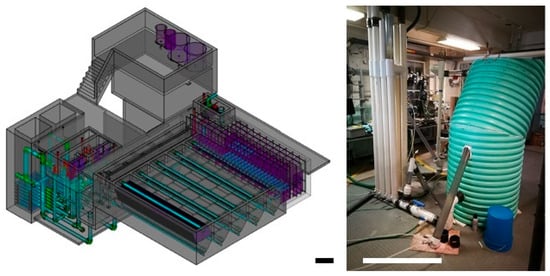
Figure 1.
Comparison between the full-scale AC plant with a 10 L/s flow rate (left) and the PF200 (right) with a 0.5 L/s flow rate. A scale bar of 1 m scale provided for reference.
The long-term success of the water treatment system depends not only on design but also on factors like source water variability, operational reliability in coagulant dosing, and post-treatment contamination risks. Furthermore, performance evaluations conducted by non-technical users may be prone to subjective biases and operational inconsistencies, limiting the accuracy of field assessments. The goal of this article is to conduct tests of PF200 in a controlled environment and to evaluate the performance of this setup against a full-scale conventional water treatment system built according to the United States Environmental Protection Agency (US EPA) design recommendations.
2. Materials and Methods
2.1. Pilot System
The pilot system replicates the core components of AC technology and was designed for a nominal flow rate of 0.5 L/s, with operational flexibility between 0.2 and 0.7 L/s. It includes the following unit processes:
- Inlet and Coagulant Dosing: Raw water mixed with coagulant is sourced from the rapid mix chamber of the benchmark facility, ensuring identical physical–chemical characteristics and water characteristics shared between the two systems. Flow is regulated by a PID loop using a 1.5″ motorized control valve and a SITRANS FM MAG 3100 flow meter from Siemens (Plano, TX, USA). The coagulant used is PCH-180 aluminum chloride hydroxide (PACl), with an active aluminum concentration of 5.6% by mass, sourced from the Holland Company (Adams, MA, USA).
- Flocculation: Post-coagulation, water enters a flocculation unit comprising eight vertical 3″ PVC pipes with internal baffles to ensure uniform head loss. The segments are open at the top and include pipe stubs to accommodate the required height of the water column.
- Sedimentation: Flocculated water flows into a clarifier made from 3′ corrugated PVC pipe. This unit mimics the full-scale AC sludge blanket clarifier with ~1 m sludge layer, a sludge compaction zone, and narrow-spaced settling plates. Water enters through a 3″ PVC manifold with nozzles for uniform distribution. Waste sludge is continuously drained from the sludge compaction zone via a 3/4″ motorized valve, with flow monitored by an SM6001 flow meter from IFM (Malvern, PA, USA).
- Filtration: Clarified water is directed into a stacked rapid sand filter built from 14″ PVC pipe. The enclosed design enables backwashing via hydraulic siphon, while following the same inlet/outlet tank arrangement as in larger AC filters. The filter is 170 cm tall and includes four inlet and three outlet manifolds spaced 20 cm apart, creating six filtration layers with a total of 130 cm of anthracite media. Head loss is monitored using a liquid level sensor in the inlet tank, calibrated for 0–80 cm.
Figure 2 shows a schematic drawing of the tested prototype. Raw water flow rate is measured by the flow gauge q 1 and adjusted by the control valve CV 1. It is then analyzed by the turbidimeter NTU 1, before passing through the rapid mix unit. Water is then fed into a baffled flocculator and subsequently into the bottom of the sludge blanket clarifier. Sludge drain rate is measured by the flow gauge q 2 and controlled by the control valve CV 2. Clarified water is collected by the manifold at the top of the clarifier tank, and the turbidity is measured by the NTU 2. Water is then fed into the filter inlet tank, where the water level is constantly monitored by the pressure gauge p 1. When the head loss reaches a tank capacity, the pressure gauge signals to open the control valve CV 3, which activates the backwash sequence. Filtered water is collected in the filter outlet manifold, and the turbidity is monitored by the NTU 3. A detailed manual, describing the materials, dimensions, and the fabrication process of the presented pilot unit prepared by the AC group, is provided in the Supplementary Materials S1.
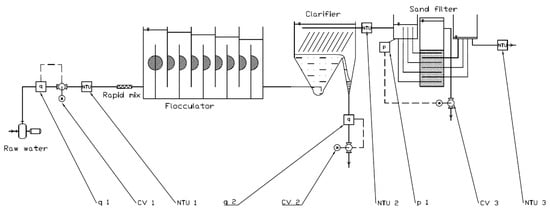
Figure 2.
Schematic drawing of the experimental setup. Key unit operations are labeled (Raw water source, Rapid mix, Flocculator, Clarifier, and Sand filter), and auxiliary elements are denoted by NTU (turbidimeters), CV (control valves), q (flow gauges), and p (pressure gauge/level sensor). Solid lines indicate the hydraulic connections, and the dashed lines indicate signal loops.
The pilot system incorporates a Supervisory Control and Data Acquisition (SCADA) setup for automated monitoring and process control. Unlike large-scale AC plants, which avoid electrical components to enhance resilience against power outages, in this study, automation enabled continuous experiments and real-time adjustment of system parameters without constant oversight of the system. Process control was managed using ProCoDa (version 3.0.0.12), an open-source software developed by the AC group, built on the National Instruments LabVIEW platform (LabVIEW 2019 SP1, 64-bit). It supports signal acquisition, basic computation, and actuator communication. Data acquisition was performed using a National Instruments NI 6008 card (National Instruments, Austin, TX, USA), modified in-house to produce 4–20 mA output signals on two analog channels and collect voltage inputs on eight analog channels. Turbidity was continuously monitored at three stages—raw, clarified, and filtered water—using HF Scientific MTOL + online turbidimeters. Sampling was driven by a Cole Parmer 7523-70 Masterflex L/S peristaltic pump (Cole Parmer, Chicago, IL, USA) with three stacked Easy-Load 900-1315 pump heads, ensuring constant and uniform flow through all turbidimeters. Each channel was sampled at 1-s intervals.
2.2. Test Site
The studied PF200 unit was commissioned on the premises of a full-scale water treatment facility, Cornell University Water Filtration Plant (CWFP). The plant is located in Ithaca (NY) and is responsible for providing water to Cornell University’s campus and the local community. Owned and operated by Cornell University, CWFP uses Fall Creek as the primary water source and relies on a conventional coagulation/filtration/sedimentation process for water treatment. It operates in compliance with US EPA requirements, exceeding the water standards enforced by the National Primary Drinking Water Regulations [45]. During the period of the tests, CWFP was operating below its design capacity of 3.6 Mgal/day, treating water at an average flow rate of 1.7 Mgal/day. As a result, the facility was only running one or two shifts per day, with stopovers every night. The previously described PCH-180 sPACl coagulant that was used in the pilot plant is also used for the treatment process at CWFP and was sourced from the facility for the pilot tests.
Besides the regular quality monitoring conducted by CWFP, Fall Creek water quality is also monitored by the Community Science Institute (CSI). At their website, CSI maintains a public archive that contains water quality data from Central New York stream and lake samples collected by local volunteer partners [46]. Table 1 provides a compilation of the most important water quality parameters sourced from the CSI database. The provided data were based on the measurements collected from 2010 to 2024 in the lower section of Fall Creek, downstream from the hamlet of McLean. Relevant information regarding the materials and methods used by CSI is provided on their website.

Table 1.
Raw water quality parameters for Fall Creek, the raw water source for the PF200 drinking water system study; P5 and P95 indicate the 5th and the 95th percentile.
2.3. Experiment Design, Monitoring, and Control
PF200 was run daily during the periods of CWFP operation, except for maintenance stopovers. The study was divided into two phases.
In the first phase, operational parameters were optimized—specifically, the sludge drainage rate and filter backwash duration. A series of randomized experiments was conducted, with systematic adjustments to these parameters. Each setting was applied for a fixed duration: either a full clarifier operation day or a complete filter cycle. Randomization minimized bias due to the order of experiments, and performance was continuously monitored to identify optimal operating conditions.
In the second phase, performance data were collected and compared to the benchmark full-scale treatment system. Turbidity served as the primary performance metric, offering a general indication of process efficiency. As coagulation works by destabilizing and aggregating dispersed particles, turbidity removal is widely accepted as a reliable proxy for overall treatment effectiveness [4,47].
Data collection occurred from April to December, with the goal of capturing seasonal variation in raw water quality. Maintenance and unexpected shutdowns limited data availability, resulting in approximately five months of usable data. The dataset was post-processed in Python (version 3.7.6), beginning with the removal of downtime and malfunction periods. The remaining data were smoothed using a Savitzky–Golay filter (20-point window, 3rd-degree polynomial, equal weights) to reduce instrument noise. The final results are presented using percentile plots to illustrate performance trends over time.
3. Results
3.1. Good Operation Practice: Minimizing Wastestreams
3.1.1. Clarifier Sludge Drain
For reliable system performance, sludge in the clarifier was continuously drained via a control valve. To optimize drainage, 54 experiments were conducted, testing six valve openings ranging from 13% to 23%. Each valve setting was randomly assigned per day, and the corresponding sludge flow rate was recorded. The objective was to minimize sludge volume while avoiding increases in clarified water turbidity and preventing drain clogging.
Figure 3 presents the average, minimum, and maximum flow rates observed across experiments. Clogging emerged as a key issue, driven by sludge compaction over time, which reduced flow and eventually obstructed the drain, leading to critical clarifier failure. The 19% opening of the sludge drain valve, corresponding to experiments 30–39, was the lowest setting that allowed for consistent and reliable flow, equal to approximately 45 mL/s. The drawback of this setting is that it equals approximately 9% of the design throughput of the entire system, meaning that the sludge is highly diluted, and the pilot generates a large volume of wastewater compared to the drinking water produced.
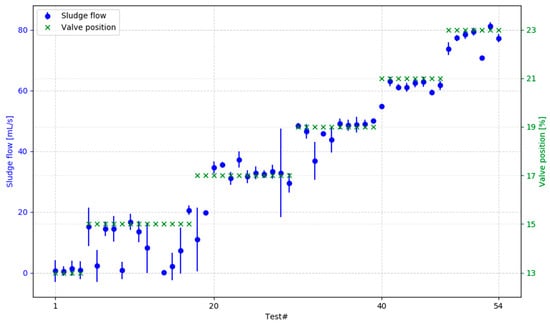
Figure 3.
Multiple experiments testing the sludge drain clogging in varying operation conditions; flow was recorded over the entire shift and is represented as bars indicating minimum, maximum, and average flow rate.
3.1.2. Filter Backwash Duration
Filter backwash time was the second operational parameter optimized. At the end of each filter run, backwash duration was randomly varied between 90 and 900 s to assess its impact on filtration performance. A total of 47 experiments were conducted.
Two metrics were used to evaluate performance:
- Post-backwash pressure drop across the filter media.
- Clean bed filtration efficiency, calculated as the negative logarithm of the effluent-to-influent turbidity ratio pC*.
The pC* value, representing turbidity removal [48], is defined by Equation (1):
Negative value indicates more turbidity in the filter effluent than in the influent, therefore suggesting that there are still particles in the filter bed after the backwash cycle is completed. Consequently, it is recommended to run the backwash until the clean bed log removal of turbidity equals 0.
The results showed that the pressure drop remained unaffected by backwash duration. However, filtration efficiency improved with longer backwash times, up to an optimal duration of 300 s (5 min). Beyond this point, additional backwash time had no measurable benefit. Conversely, shorter durations increased flow but compromised filtration performance and reduced filter run time. Thus, a 5 min backwash was identified as optimal, balancing filtration efficiency and operational throughput. Figure 4 illustrates the relationship between backwash duration and filter performance.
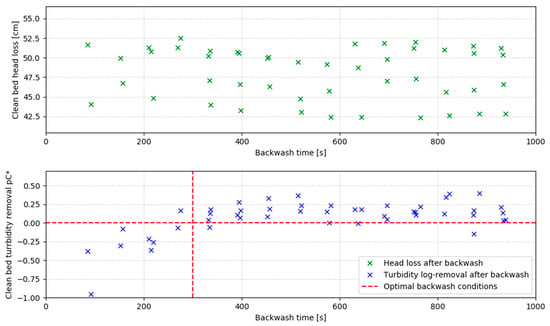
Figure 4.
Clean bed head loss (top) and clean bed log turbidity removal (bottom) are used as potential indicators for the filter bed cleanliness; no correlation between the backwash time and the head loss was observed, and optimal backwash time was set as the backwash time needed for clean bed particle concentration to be equivalent to influent turbidity (here called the clean bed turbidity removal).
The duration of the filter runs was dependent on the clarified water turbidity and usually stayed within the range of 6 to 8 h per filter run. With a 5 min backwash, this results in an additional 1.2% of the water lost due to the backwash operation and a total loss of more than 10% of the raw water fed into the system over the entire treatment process.
3.2. PF200 Operation and Comparative Tests
The total duration of the pilot’s operation was equal to 5 months. Turbidity was measured at three points—at the inlet (raw water taken from the adjacent river), after the clarifier, and after the filter. The extensive duration of the tests (1100 h total operation time) combined with a high sampling frequency of once per second resulted in a significant amount of data. A convenient way to represent this information is as a frequency distribution on a percentile plot. This removes the influence of the periodic variations of the analyzed process parameters, allowing for a comparison with the data collected by the SCADA system at the CWFP. Data downloaded from SCADA for the same period are also converted to the percentile plot and directly compared with the results for PF200.
Over the period of the tests, the average dosing of PACl coagulant was equal to 18.15 ppm, equivalent to 1.02 mgAl/L, with the minimum and maximum dose over that period ranging from 13 to 50 ppm. Raw water turbidity in that period averaged 12.2 NTU, ranging from 0.24 to 500 NTU. Sludge blanket clarification resulted in up to a 10-fold decrease in turbidity (1-log removal efficiency according to the definition established by the EPA). During the experiments, on average, it resulted in a turbidity of 0.2 NTU, and it ranged from the detection limit of the system at 0.02 NTU to 2.1 NTU. Stacked rapid sand filters provide up to another 1-log turbidity removal. Consequently, the overall turbidity decrease over the entire system approaches 99%. However, these values could only be achieved in conditions of high raw water turbidity. Over the 5 months of operation, the filter produced water meeting a requirement of less than 0.3 NTU only 75% of the time, while the US EPA legislation requires turbidity below 0.3 NTU for 95% of the time and below 1 NTU for the remaining part of the operation.
For the evaluation against CWFP, the turbidity removal efficiency of both systems is used to assess their performance. In comparison, the performance of the full-scale CWFP system is superior to the tested pilot at each stage of the process. Over the period of experiments, the clarifier reliably allowed for more than a 1-log decrease of turbidity, while the filter served as a polishing step, bringing the final treated water turbidity to the range from the detection limit of 0.02 NTU to 0.12 NTU, with an average value of 0.05 NTU. This performance corresponded to CWFP meeting the EPA turbidity standards 100% of the time. Figure 5 shows the performance comparison between the two systems, revealing that the water quality at each stage of the pilot process is inferior to the water quality of the benchmark conventional system. It is important to keep in mind that CWFP operates below its design capacity and has a longer flocculator residence time, a long track record of good performance, and well-established good operation practice compared to the PF200.
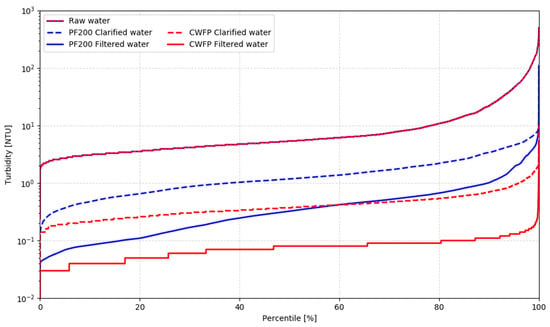
Figure 5.
PF200 comparison against a full-scale conventional plant at the Cornell University campus.
4. Discussion
The tested pilot showed various advantages. Firstly, its maintenance requirements were notably low, requiring only 2 h of operation and maintenance (O&M) work per week. This time was spent mostly on cleaning the analytical equipment and maintaining the sampling pumps, which proves the claim of low maintenance requirements of the treatment process itself. The tested system operated in a stable manner, without the need for adjustments to the process parameters or other modifications. In a full-scale implementation, this aspect could translate to reduced operational costs and downtime when compared to conventional water treatment systems that demand more O&M effort. It might be a good solution for specific needs of entities such as small utilities, which often operate understaffed and prefer systems that are simple and reliable.
Energy efficiency was another advantage of the tested system. The low need for energy results directly from the off-grid design philosophy of the AC group, which obviously contributes to a sustainable operation. The main water pump was the primary power consumer, with a nameplate power demand of 750 W. The remaining components of the system required only a 200 W power supply necessary to maintain the automation, quality control, and data logging. According to the Smart Energy Design Assistance Center, an applied research program at the University of Illinois at Urbana-Champaign, a typical energy consumption required of a large surface water treatment system is equal to 2.019 Wh/gal [49]. Based on the nameplate power demand of the equipment, the peak energy consumption for the presented technology is equal to approximately 2 Wh/gal, at the flow rate of just 500 mL/s. This result positions the prototype at a similar energy efficiency to much larger facilities. Since the power demand does not scale linearly with the capacity, it leaves plenty of room for future optimization in the field of energy use.
Despite the obvious advantages, the first major concern observed during testing was the excessive production of wastewater. Overall, the pilot wasted more than 10% of the total drawn raw water as combined clarifier sludge and the filter backwash stream. Depending on the conditions, technologies relying on coagulation are expected to produce less than 3% total water losses in the treatment process [50], which shows a substantial deficit in PF200 environmental metrics. Elevated environmental impact is a critical factor to address, as it raises questions about the system’s overall sustainability and its potential adverse effects on the ecosystem.
Pilot tests also revealed some crucial drawbacks associated with the performance of the tested system. The quality of treated water was significantly inferior when compared to the benchmark water treatment plant, as well as to the reported industry standards [51]. This outcome poses a clear competitive disadvantage, indicating that further refinement is necessary before considering it as a viable alternative to existing technologies. Figure 6 shows that when compared to the drinking water turbidity standards of different countries, the tested technology fell short of most countries’ standards. CWFP was continuously meeting the US water quality standards, which are the most stringent among the analyzed sets of regulations and require turbidity below 0.3 NTU 95% of the time, and below 1 NTU for the rest of the time [4]. PF200 was not complying with the US, Polish [52], and Colombian standards [53]. For 99.5% of the time, it was meeting the least stringent, Honduran turbidity standard of 5 NTU. This value is considered by the WHO to be a bare minimum, allowing for effective subsequent disinfection with chlorine [13]. In conclusion, PF200 operating under the conditions presented in this paper, such as raw water physicochemical properties and the concentration of the coagulant, only met the 0.3 NTU US limit 45% of the time, rather than 95% of the time.
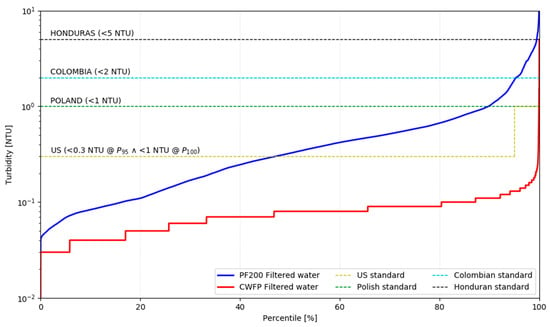
Figure 6.
Performance of both systems vs. water quality standards from different countries.
The final consideration around performance is related to the full-scale AC systems and how well they are represented by the PF200. Several water treatment plants designed by the AC group are currently operating in Honduras and Nicaragua. Water quality produced by one of them—a 16 L/s water treatment plant in Moroceli (Honduras)—was monitored for one year between 2016 and 2017 and is provided in the Supplemental Materials S2. Figure 7 and Table 2 compare the PF200 performance presented in this paper with the far superior performance data provided for Moroceli. A significant discrepancy between the water quality delivered by both units should raise questions about both the degree to which PF200 represents the full-scale AC plant and indicates the need for a technology performance assessment conducted in a controlled environment.
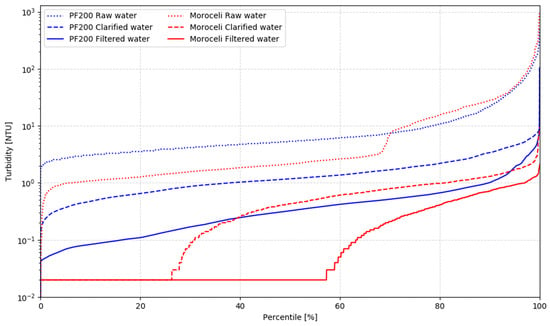
Figure 7.
PF200 vs. Moroceli; comparison with a 16 L/s AC plant in Moroceli (Honduras) reveals that the reported performance of the full-scale plant is much better than the registered performance of the pilot system.

Table 2.
PF200 vs. Moroceli: main turbidity statistics for both systems.
The quality of raw water plays a foundational role in determining the efficiency and reliability of drinking water treatment systems. Variability in raw water—caused by seasonal changes, pollution, or climate events—can significantly affect operational processes such as coagulation, filtration, and disinfection. High levels of turbidity, organic matter, or microbial contaminants in untreated water may increase chemical dosing requirements, reduce filtration efficiency, and compromise disinfection, potentially leading to health risks and regulatory non-compliance. Studies have shown that raw water quality parameters such as turbidity and UV254 absorbance are strong predictors of the final treated water quality, particularly in small systems with limited infrastructure [54]. Advanced control systems that monitor real-time raw water quality can help adjust chemical dosing and stabilize output water quality, as demonstrated by successful implementations in China and elsewhere [55]. Furthermore, large-scale risk assessments have shown that addressing raw water quality at the catchment level—by identifying key land use and climate pressures—enhances the resilience and sustainability of water supply systems over time [56]. As such, comprehensive monitoring and management of raw water quality are essential for both short-term treatment efficiency and long-term water security.
This leads to questions about the viability of performance assessment in an environment where a wide range of external factors affect the tested installation, which also corresponds to the double inflection of the raw water turbidity curve on the frequency distribution plot, with an additional inflection point around the 70% rank. This, combined with a high value of standard deviation, implies a significant influence of an unknown external factor over the data. Various possible factors could have contributed to that behavior, such as changes in the watershed, weather conditions, human error, or malfunction of the analytical equipment. Independent of the nature of the disturbances influencing the results, it confirms the need for performance tests conducted in a controlled environment.
The need for further performance assessment in a controlled environment is fortified by a paper from 2018, which analyzes the operation of six gravity-fed water treatment systems built in rural Honduras, including the Moroceli plant, according to AC design recommendations [42]. The authors focused on two metrics characterizing the water quality—turbidity and the E. coli counts—and measured them at different stages of the treatment process. Performance evaluations show consistent removal of turbidity to levels below 1 NTU, meeting WHO recommendations for safe drinking water even during variable weather conditions. Additionally, all the systems studied show a decrease in the E. coli counts below the detection limit, independently of the raw water quality. Future work with the PF200 will need further testing to improve turbidity removal, including experimenting with coagulant dosing. Additionally, testing should be performed with the PF200 for other key parameters dictated by legislators, like biological contamination (e.g., E. coli) and emerging contaminants. The use of a sludge blanket can potentially provide a nutrient-rich growth environment for the opportunistic pathogens characteristic of the water treatment and distribution systems, such as Legionella. Microorganisms grown at the stage of the process would not be removed by the clarifier and the filter, posing a threat of directly contaminating the distribution network. In such cases, the entire process would have to be redesigned to incorporate other means of protection against microbiological contamination. A similar set of factors should be taken into account for other aspects of the design as well. The performance of the tested technology in terms of nutrient removal, capturing microplastics, per- and polyfluoroalkyl substances, as well as other parameters regulated by the drinking water legislation, should be evaluated, as no similarities between the technologies should be assumed without evidence.
These aspects are highly important, given the fact that at the time of the tests, the studied technology was not validated by the EPA as an approved method of water treatment for human consumption. Despite the similarities between the conventional process and PF200, it is unclear to what degree it replicates performance on the established treatment methods. Further research is necessary to answer those questions.
5. Conclusions
The evaluation of the PF200 water treatment system reveals both potential advantages and significant challenges. The system’s low energy consumption and minimal maintenance demands are attractive, while the technical and operational issues limit its current applicability. Over the study period, the average turbidity of the treated water was 0.54 NTU, resulting in only 47.1% compliance with the US EPA standard of <0.3 NTU. Additionally, the process generated a waste stream averaging over 10% of the raw water volume, reducing overall efficiency. Future work should emphasize improved coagulant dosing strategies, enhanced design to minimize waste, and comprehensive testing against a broader range of contaminants, including bacterial and viral contaminants, nutrients, and emerging contaminants like microplastics and PFAS.
Robust performance validation and life cycle assessments are required to confirm PF200’s viability as a portable, small-scale water treatment alternative. The tested water treatment system shows promise due to its low maintenance requirements and energy efficiency, qualities particularly relevant in regions facing workforce shortages and infrastructure challenges [57,58]. However, both the poor performance and the significant wastewater production of the pilot pose significant obstacles to its widespread adoption. Contingent on meeting the quality standards and other legal requirements, clear advantages in the field of O&M might lead to positioning PF200 as an attractive alternative for the users in need of a robust, portable, small-scale water treatment system.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15126668/s1, S1—Manual: construction manual for the tested PF200; S2—Moroceli: performance data of the AguaClara plant in Moroceli, Honduras.
Author Contributions
Conceptualization, M.S. and R.E.R.; methodology, M.S. and R.E.R.; software, M.S.; validation, M.S., P.K. and R.E.R.; formal analysis, M.S. and P.K.; investigation, M.S.; resources, R.E.R.; data curation, M.S. and P.K.; writing—original draft preparation, M.S. and P.K.; writing—review and editing, M.S., P.K. and R.E.R.; visualization, M.S.; supervision, R.E.R.; project administration, R.E.R.; funding acquisition, R.E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States National Science Foundation, Grant #s 1919084 and 1704472.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available online; information about the technology released to the public domain and available online at https://github.com/aguaclara (accessed on 10 June 2025).
Acknowledgments
The authors would like to acknowledge Monroe Weber-Shirk, founder of AguaClara, for advising on the design and construction of the PF200 setup, as well as Christopher Bordlemay Padilla and the rest of the Cornell University Water Filtration Plant personnel, for their ongoing support and for hosting the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reid, E.; Igou, T.; Zhao, Y.; Crittenden, J.; Huang, C.-H.; Westerhoff, P.; Rittmann, B.; Drewes, J.E.; Chen, Y. The Minus Approach Can Redefine the Standard of Practice of Drinking Water Treatment. Environ. Sci. Technol. 2023, 57, 7150–7161. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, D.L.; Von Gunten, U. The Chlorine Dilemma. Science 2011, 331, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Smeets, P.W.M.H.; Medema, G.J.; Van Dijk, J.C. The Dutch Secret: How to Provide Safe Drinking Water without Chlorine in the Netherlands. Drink. Water Eng. Sci. 2009, 2, 1–14. [Google Scholar] [CrossRef]
- US EPA, O. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 31 August 2021).
- Rittmann, B.E.; Snoeyink, V.L. Achieving Biologically Stable Drinking Water. J. Am. Water Work. Assoc. 1984, 76, 106–114. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Huck, P.M.; Bouwer, E.J. Biological Treatment of Public Water Supplies. Crit. Rev. Environ. Control. 1989, 19, 119–184. [Google Scholar] [CrossRef]
- Van Der Wielen, P.W.J.J.; Brouwer-Hanzens, A.; Italiaander, R.; Hijnen, W.A.M. Initiating Guidance Values for Novel Biological Stability Parameters in Drinking Water to Control Regrowth in the Distribution System. Sci. Total Environ. 2023, 871, 161930. [Google Scholar] [CrossRef]
- Prest, E.I.; Hammes, F.; Van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef]
- Arcentales-Ríos, R.; Carrión-Méndez, A.; Cipriani-Ávila, I.; Acosta, S.; Capparelli, M.; Moulatlet, G.M.; Pinos-Vélez, V. Assessment of Metals, Emerging Contaminants, and Physicochemical Characteristics in the Drinking Water and Wastewater of Cuenca, Ecuador. J. Trace Elem. Miner. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U.; Trakal, L.; Mohan, D. Emerging Technologies for Arsenic Removal from Drinking Water in Rural and Peri-Urban Areas: Methods, Experience from, and Options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef]
- Delgado, C.D. Abastecimiento de Agua Potable Para Pequeñas Comunidades Rurales Por Medio de un Sistema de Colección de Lluvia-Planta Potabilizadora; Universidad Autónoma del Estado de México: Toluca, México, 2000; Volume 7. [Google Scholar]
- Rodríguez, C.; García, B.; Pinto, C.; Sánchez, R.; Serrano, J.; Leiva, E. Water Context in Latin America and the Caribbean: Distribution, Regulations and Prospects for Water Reuse and Reclamation. Water 2022, 14, 3589. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed. + 1st add; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- US EPA. Surface Water Treatment Rules. Available online: https://www.epa.gov/dwreginfo/surface-water-treatment-rules (accessed on 31 August 2021).
- Rajagopal, S.; Jenner, H.A.; Venugopalan, V.P. (Eds.) Operational and Environmental Consequences of Large Industrial Cooling Water Systems; Springer: Boston, MA, USA, 2012; ISBN 978-1-4614-1697-5. [Google Scholar]
- Jansz, S. A Study into Rural Water Supply Sustainability in Niassa Province, Mozambique; A WaterAid Report March; WaterAid: London, UK, 2011. [Google Scholar]
- Access to Drinking Water. Available online: https://data.unicef.org/topic/water-and-sanitation/drinking-water/ (accessed on 9 May 2024).
- Water, Sanitation and Hygiene (WASH) Situation and Issues for Urban Poor People and Vulnerable Groups, Cambodia; WaterAid Cambodia: Phnom Penh, Cambodia, 2015.
- de Moraes, S.; Freitas, D. Los Retos de La Adopción Tecnologíca En El Sector Hídrico de Latinoamérica; Instituto Mexicano de Tecnología del Agua: Jiutepec, México, 2014; ISBN 978-607-9368-01-2. [Google Scholar]
- Sánchez, L.D.; Sánchez, A.; Galvis, G.; Latorre, J. Filtración en Múltiples Etapas; IRC Centro Internacional en Agua y Saneamiento/CINARA: The Hague, The Netherlands, 2007; p. 68. [Google Scholar]
- Clarke, B.A.; Jones, C.J.; Evans, H.L.; Crompton, J.L.; Dorea, C.C.; Bertrand, S. Multi-Stage Filtration for Developing World Surface Water Treatment. Proc. Inst. Civ. Eng. Water Manag. 2004, 157, 143–149. [Google Scholar] [CrossRef]
- Arboleda-Valencia, J. A New Approach to Treatment Plant Design and Construction in Latin America. J. AWWA 1986, 78, 92–105. [Google Scholar] [CrossRef]
- García-Ávila, F.; Zhindón-Arévalo, C.; Álvarez- Ochoa, R.; Donoso-Moscoso, S.; Tonon-Ordoñez, M.D.; Flores Del Pino, L. Optimization of Water Use in a Rapid Filtration System: A Case Study. Water-Energy Nexus 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Arboleda Valencia, J.; Buitrago León, I.A.; Jaramillo Gómez, L.A. Teoría y Práctica de la Purificación del Agua Potable. Tomo 1, 4th ed.; Ecoe Ediciones: Bogotá, Colombia, 2023; ISBN 978-958-50-3647-5. [Google Scholar]
- Home, G.P.; Stockley, M.; Shaw, G. The Sirofloc Process at Redmires Water-Treatment Works. Water Environ. J. 1992, 6, 10–18. [Google Scholar] [CrossRef]
- Srivastava, S.; Brighu, U.; Gupta, A.B. Performance Assessment of Pulsating Floc Blanket Clarifiers and Conventional Clariflocculators in Pilot-scale Models. Water Environ. Res. 2021, 93, 887–895. [Google Scholar] [CrossRef]
- Su, Z.Y.; Li, X.; Yang, Y.L.; Zhou, Z.W.; Wu, Y. Ballasted Flocculation of Micro-Sand/Magnetic Powder for Landscape Water Treatment. Adv. Mater. Res. 2013, 777, 56–59. [Google Scholar] [CrossRef]
- Plum, V.; Dahl, C.P.; Bentsen, L.; Petersen, C.R.; Napstjert, L.; Thomsen, N.B. The Actiflo Method. Water Sci. Technol. 1998, 37, 269–275. [Google Scholar] [CrossRef]
- Cui, H.; Huang, X.; Yu, Z.; Chen, P.; Cao, X. Application Progress of Enhanced Coagulation in Water Treatment. RSC Adv. 2020, 10, 20231–20244. [Google Scholar] [CrossRef]
- Mirbagheri, S.A.; Malekmohamadi, S.; Nasrabadi, S.S. A Comparison of Different Pilot Constructed Clarifiers with the Purpose of Achieving the Optimum Condition in Turbidity Removal at Water Treatment Plants in Tehran. Water Pract. Technol. 2017, 12, 576–588. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Chiang, P.-C.; Shah, K.J. Evaluation and Optimization of Enhanced Coagulation Process: Water and Energy Nexus. Water-Energy Nexus 2019, 2, 25–36. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Suja, F. A Comparative Study of Coagulation, Granular- and Powdered-Activated Carbon for the Removal of Perfluorooctane Sulfonate and Perfluorooctanoate in Drinking Water Treatment. Environ. Technol. 2015, 36, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, N.; Sanz, J.; Ocen, C.; Lobo, A.; Temprano, J.; Tejero, I. Reverse Osmosis Pretreatment Alternatives: Demonstration Plant in the Seawater Desalination Plant in Carboneras, Spain. Desalination 2011, 265, 229–236. [Google Scholar] [CrossRef]
- González Rivas, M.; Beers, K.; Warner, M.E.; Weber-Shirk, M. Analyzing the Potential of Community Water Systems: The Case of AguaClara. Water Policy 2014, 16, 557–577. [Google Scholar] [CrossRef]
- The AguaClara Drinking Water Treatment Program Delivered in the Republic of Honduras; AguaClara Reach Inc.: Ithaca, NY, USA, 2019.
- Swetland, K.A.; Weber-Shirk, M.L.; Lion, L.W. Gravity-Powered Chemical Dose Controller for Sustainable, Municipal-Scale Drinking Water Treatment. J. Environ. Eng. 2013, 139, 1023–1034. [Google Scholar] [CrossRef]
- Pennock, W.H.; Lion, L.W.; Weber-Shirk, M.L. Design Algorithm for Vertically-Baffled Flocculators. Environ. Eng. Sci. 2021, 38, 592–606. [Google Scholar] [CrossRef]
- Garland, C.; Weber-Shirk, M.; Lion, L.W. Revisiting Hydraulic Flocculator Design for Use in Water Treatment Systems with Fluidized Floc Beds. Environ. Eng. Sci. 2017, 34, 122–129. [Google Scholar] [CrossRef]
- Adelman, M.J.; Weber-Shirk, M.L.; Cordero, A.N.; Coffey, S.L.; Maher, W.J.; Guelig, D.; Will, J.C.; Stodter, S.C.; Hurst, M.W.; Lion, L.W. Stacked Filters: Novel Approach to Rapid Sand Filtration. J. Environ. Eng. 2012, 138, 999–1008. [Google Scholar] [CrossRef]
- Adelman, M.J.; Weber-Shirk, M.L.; Will, J.C.; Cordero, A.N.; Maher, W.J.; Lion, L.W. Novel Fluidic Control System for Stacked Rapid Sand Filters. J. Environ. Eng. 2013, 139, 939–946. [Google Scholar] [CrossRef]
- The Physics of Water Treatment Design—AguaClara v1.4.37 Documentation. Available online: https://aguaclara.github.io/Textbook/ (accessed on 9 May 2024).
- Brooks, Y.M.; Tenorio-Moncada, E.A.; Gohil, N.; Yu, Y.; Estrada-Mendez, M.R.; Bardales, G.; Richardson, R.E. Performance Evaluation of Gravity-Fed Water Treatment Systems in Rural Honduras: Verifying Robust Reduction of Turbidity and Escherichia Coli during Wet and Dry Weather. Am. J. Trop. Med. Hyg. 2018, 99, 881–888. [Google Scholar] [CrossRef]
- Kus, B.; Kandasamy, J.; Vigneswaran, S.; Shon, H.K.; Moody, G. Household Rainwater Harvesting System—Pilot Scale Gravity Driven Membrane-Based Filtration System. Water Supply 2013, 13, 790–797. [Google Scholar] [CrossRef]
- Mecha, C.A.; Pillay, V.L. Development and Evaluation of Woven Fabric Microfiltration Membranes Impregnated with Silver Nanoparticles for Potable Water Treatment. J. Membr. Sci. 2014, 458, 149–156. [Google Scholar] [CrossRef]
- Drinking Water System Updates and Water Quality Reports|Facilities and Campus Services. Available online: https://fcs.cornell.edu/departments/energy-sustainability/utilities-production-distribution/water/drinking-water-system-updates-water-quality-reports (accessed on 9 May 2024).
- Community Science Institute Database. Available online: https://database.communityscience.org/ (accessed on 11 April 2025).
- Gregory, J. Flocculation Fundamentals. In Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 459–491. ISBN 978-3-642-20664-1. [Google Scholar]
- Pennock, W.H.; Weber-Shirk, M.L.; Lion, L.W. A Hydrodynamic and Surface Coverage Model Capable of Predicting Settled Effluent Turbidity Subsequent to Hydraulic Flocculation. Environ. Eng. Sci. 2018, 35, 1273–1285. [Google Scholar] [CrossRef]
- Benchmarking for Water and Wastewater Treatment Plants; SEDAC Smart Energy Design Assistance Center at The University of Illinois: Urbana-Champaign, IL, USA, 2022.
- Cornwell, D.A.; Lee, R.G. Waste Stream Recycling: Its Effect on Water Quality. J. AWWA 1994, 86, 50–63. [Google Scholar] [CrossRef]
- Alver, A. Evaluation of Conventional Drinking Water Treatment Plant Efficiency According to Water Quality Index and Health Risk Assessment. Environ. Sci. Pollut. Res. 2019, 26, 27225–27238. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Zdrowia z Dnia 7 Grudnia 2017 r. w Sprawie Jakości Wody Przeznaczonej Do Spożycia Przez Ludzi. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 17 May 2024).
- Resolución 2115–2007|Minvivienda. Available online: https://minvivienda.gov.co/normativa/resolucion-2115-2007 (accessed on 17 May 2024).
- Scheili, A.; Delpla, I.; Sadiq, R.; Rodriguez, M.J. Impact of Raw Water Quality and Climate Factors on the Variability of Drinking Water Quality in Small Systems. Water Resour. Manag. 2016, 30, 2703–2718. [Google Scholar] [CrossRef]
- Wang, D. Raw Water Quality Assessment for the Treatment of Drinking Water. Environ. Earth Sci. 2016, 75, 1327. [Google Scholar] [CrossRef]
- Vorstius, C.; Rowan, J.S.; Brown, I.; Frogbrook, Z.; Palarea-Albaladejo, J. Large-Scale Risk Screening of Raw Water Quality in the Context of Drinking Water Catchments and Integrated Response Strategies. Environ. Sci. Policy 2019, 100, 84–93. [Google Scholar] [CrossRef]
- The Cost of Aging Water Infrastructure and Environmental Racism: The Jackson, Mississippi Water Crisis and Access to Funding. Available online: https://www.liebertpub.com/doi/epdf/10.1089/env.2022.0117 (accessed on 10 June 2024).
- Grigg, N.S. Aging Water Distribution Systems: What Is Needed? Public Work. Manag. Policy 2017, 22, 18–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).