Effects of Packaging Material Type, Storage Time and Lipid Content on Phthalate Migration in Smoked Fish Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Chemicals and Standards
2.3. Sample Preparation
2.4. Instrumentation, Analysis and Method Validation
2.5. Lipid Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Holadová, K.; Prokupková, G.; Hajšlová, J.; Poustka, J. Headspace solid-phase microextraction of phthalic acid esters from vegetable oil employing solvent-based matrix modification. Anal. Chim. Acta 2007, 582, 24–33. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Available online: https://www.fao.org/3/ca9229en/ca9229en.pdf (accessed on 12 December 2023).
- Daviglus, M.; Sheeshka, J.; Murkin, E. Health benefits from eating fish. Comment Toxico. 2002, 8, 345–347. [Google Scholar] [CrossRef]
- Yerlikaya, P. Fitalat esterleri ve su ürünleri tüketimindeki yeri. J. Food Health Sci. 2017, 3, 59–66. [Google Scholar] [CrossRef]
- Sioen, I.; Fierens, T.; Van Holderbeke, M.; Geerts, L.; Bellemans, M.; De Maeyer, M.; Servaes, K.; Vanermen, G.; Boon, P.E.; De Henauw, S. Phthalates dietary exposure and food sources for Belgian preschool children and adults. Enviro. Inter. 2012, 48, 102–108. [Google Scholar] [CrossRef]
- Kiralan, S. Phthalate and polycyclic aromatic hydrocarbon levels in liquid ingredients of packaged fish sold in Turkish markets. J. Oleo Sci. 2020, 69, 851–858. [Google Scholar] [CrossRef]
- Ustun, I.; Sungur, S.; Okur, R.; Sumbul, A.T.; Oktar, S.; Yilmaz, N.; Gokce, C. Determination of phthalates migrating from plastic containers into beverages. Food Anal. Method. 2015, 8, 222–228. [Google Scholar] [CrossRef]
- Pereira, J.; do Céu Selbourne, M.; Poças, F. Determination of phthalates in olive oil from European market. Food Cont. 2019, 98, 54–60. [Google Scholar] [CrossRef]
- US EPA. Phthalates, TEACH Chemical Summary; Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–25. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100BNSK.PDF?Dockey=P100BNSK.PDF (accessed on 18 February 2024).
- European Commission. Commission Regulation (EU) No 143/2011 of 17 February 2011 amending Annex XIV to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (‘REACH’). Off. J. Eur. Union 2011, 44, 2–6. [Google Scholar]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- Relić, R.; Marković, Z. Farmed fish welfare, with insight into the situation in Serbia. Vet. Glas. 2021, 75, 132–144. [Google Scholar] [CrossRef]
- Plastic Europe. Plastics the Facts 2017. An Analysis of European Plastics Production, de Mand and Waste Data. Available online: https://www.plasticseurope.org/application/files/5715/1717/4180/Plastics_the_facts_2017_FINAL_for_website_one_page.pdf (accessed on 10 December 2023).
- Yadav, S.; Rai, S.; Srivastava, A.K.; Panchal, S.; Patel, D.K.; Sharma, V.P.; Jain, S.; Srivastava, J.P. Determination of pesticide and phthalate residues in tea by QuEChERS method and their fate in processing. Environ. Sci. Pollut. Res. 2017, 24, 3074–3083. [Google Scholar] [CrossRef]
- Carnol, L.; Schummer, C.; Moris, G. Quantification of six phthalates and one adipate in Luxembourgish beer using HS-SPMEGC/MS. Food Anal. Methods. 2017, 10, 298–309. [Google Scholar] [CrossRef]
- Kartalović, B.; Vranešević, J.; Petrović, J.; Ðurđević, B.; Ratajac, R. Detekcija ostataka mikroplastike-Razvoj metode za ftalate u medu. Arh. Vet. Med. 2021, 14, 19–33. [Google Scholar]
- Habschied, K.; Kartalović, B.; Lazić, D.; Krstanović, V.; Mastanjević, K. Survey on phthalates in beer packaged in aluminum cans, PET and glass bottles. Fermentation 2023, 9, 125. [Google Scholar] [CrossRef]
- SANTE. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 5 October 2023).
- Spiric, A.; Trbovic, D.; Vranic, D.; Djinovic, J.; Petronijevic, R.; Matekalo-Sverak, V. Statistical evaluation of fatty acid profile and cholesterol content in fish (common carp) lipids obtained by different sample preparation procedures. Anal. Chim. Acta 2010, 672, 66–71. [Google Scholar] [CrossRef]
- Fromme, H.; Gruber, L.; Schlummer, M.; Wolz, G.; Boehmer, S.; Angerer, J.; Mayer, R.; Liebl, B.; Bolte, G. Intake of phthalates and di(2-ethylhexyl)adipate: Results of the integrated exposure assessment survey based on duplicate diet samples and biomonitoring data. Environ. Int. 2007, 33, 1012–1020. [Google Scholar] [CrossRef]
- Bopp, K.; Altkofer, W. Survey on intake of certain phthalic acid esters in South Germany: Comparative study of two boarding schools with conventional and organic nutrition. Dtsch. Lebensm. Rundsch. 2009, 105, 35–38. [Google Scholar]
- Fierens, T.; Servaes, K.; Van Holderbeke, M.; Geerts, L.; De Henauw, S.; Sioen, I.; Vanarmen, G. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol. 2012, 50, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Arp, A.C.; Yerlikaya, P. Phthalate ester migration into food: Effect of packaging material and time. Eur. Food Res. Technol. 2020, 246, 425–435. [Google Scholar]
- Baranenko, D.; Boulkrane, M.S.; Borisova, I.; Astafyeva, B.; Lu, W.; Abd El-Aty, A.M. Translocation of phthalates from food packaging materials into minced beef. Front. Nutr. 2022, 8, 813553. [Google Scholar] [CrossRef]
- Wittassek, M.; Koch, H.; Angerer, J.; Brüning, T. Assessing exposure to phthalates-the human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Horák, T.; Olšovská, J. Phthalates in beverages—A review. Kvas Prum. 2020, 66, 264–269. [Google Scholar] [CrossRef]
- Jarošová, A.; Bogdanovičová, S. Phthalate migration from packaging materials into food. Potravinarstvo 2015, 9, 275–279. [Google Scholar]
- Bogdanović, S.; Anđelković, H.; Kostić, S.; Kocić, M.; Anđelković, D. The effects of temperature and ultrasound on the migration of di-(2-ethylhexyl) phthalate from plastic packaging into dairy products. Bulg. Chem. Commun. 2019, 51, 242–248. [Google Scholar] [CrossRef]
- Guerreiro, T.M.; Oliviera, D.N.; Melo, C.F.O.R.; Lima, E.O.; Catharino, R.R. Migration from plastic packaging into meat. Food Res. Int. 2018, 109, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Kida, M.; Koszelnik, P. Investigations of the presence and possible migration form microplastics of phthalic acid esters and polycylic aromatic hydrocarbons. J. Polym. Environ. 2021, 29, 599–611. [Google Scholar] [CrossRef]
- McGinnis, P.; Martinez, J.; Gadagbui, B.; Willis, A.; Lee, D.; Parker, A.; Vincent, M.; Thiel, S. Exposure Assessment: Potential for the Presence of Phthalates in Selected Plastics. CPSC Staff Statement on the Toxicology Excellence for Risk Assessment Report, pp. 1–101. 2015. Available online: https://www.cpsc.gov/s3fs-public/pdfs/ReportonPhthalatesinFourPlastics.pdf (accessed on 13 December 2023).
- Kim, S.; Kim, S.; Park, G.; Yoo, Y.J.; Jeong, J.S.; Nam, K.T.; Jee, S.; Lim, K.; Lee, Y. Di-2-ethylhexylphthalate promotes thyroid cell proliferation and DNA damage through activating thyrotropin-receptor-mediated pathways in vitro and in vivo. Food Chem. Toxicol. 2019, 124, 265–272. [Google Scholar] [CrossRef]


| Phthalate | Retention Time (min) X ± SD | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|---|
| Dimethyl phthalate | 6.08 ± 0.04 | 1.41 | 4.69 |
| Diethyl phthalate | 7.19 ± 0.01 | 0.30 | 1.01 |
| Diisobutyl phthalate | 9.17 ± 0.02 | 0.30 | 1.01 |
| Dibutyl phthalate | 9.83 ± 0.05 | 0.30 | 1.01 |
| Bis (2-ethylhexyl) phthalate | 13.19 ± 0.05 | 0.32 | 1.14 |
| Di-n-octyl phthalate | 14.21 ± 0.05 | 0.31 | 1.10 |
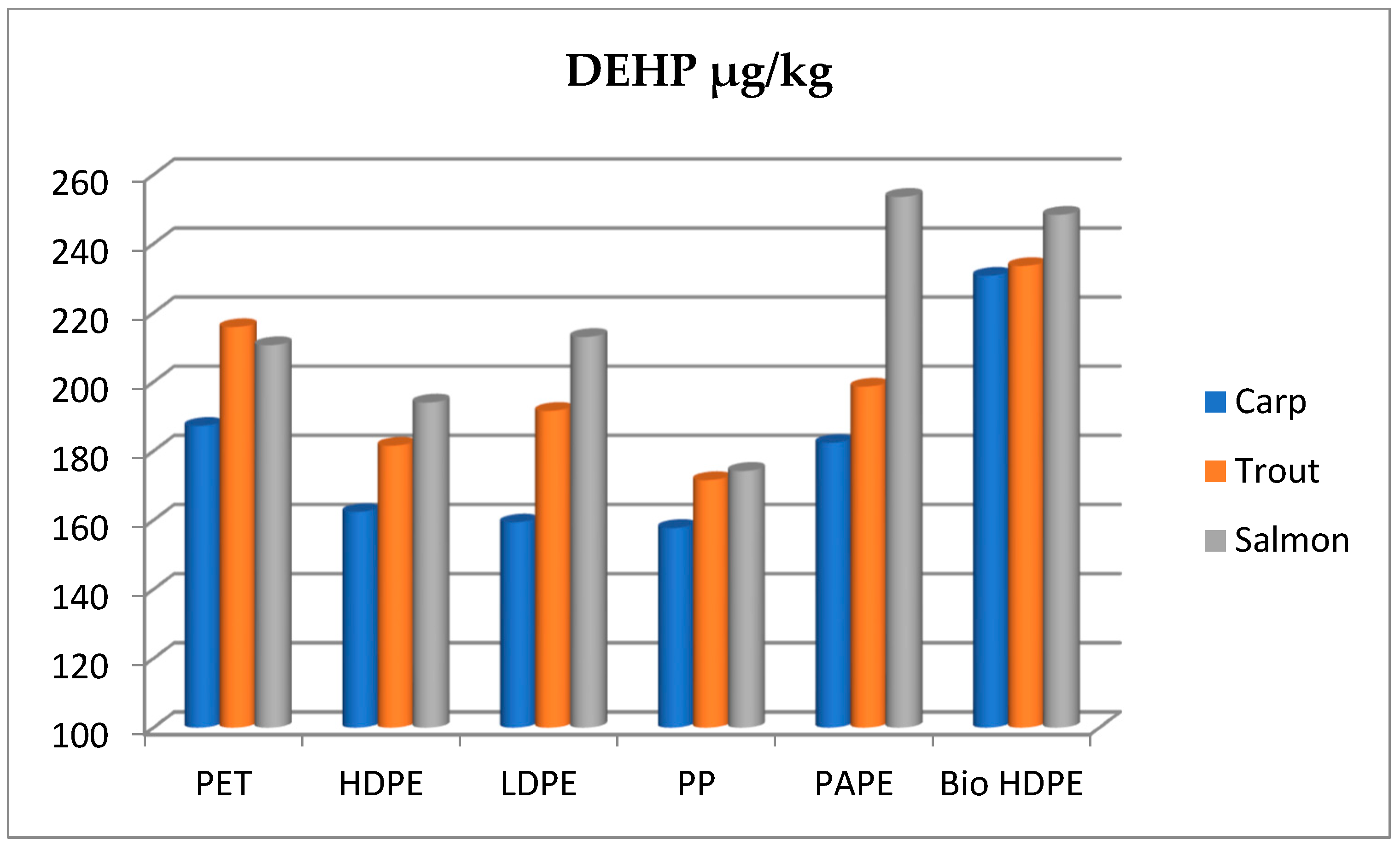
| Smoked Fish Species | Packaging Matherial | Time of Storage | Phtalathes (µg/kg) | |||||
|---|---|---|---|---|---|---|---|---|
| DMP | DEP | DIBP X ± SD | DBP X ± SD | DEHP X ± SD | DnOP | |||
| Carp | Control | - | ND * | ND | 23.25 a ± 1.02 | 31.60 a ± 0.38 | 157.69 a ± 0.91 | ND |
| Carp | PET | 3 month | ND | ND | 25.31 a ± 0.37 | 29.89 a ± 0.21 | 158.51 a ± 0.42 | ND |
| Carp | PET | 6 month | ND | ND | 30.85 b ± 0.19 | 33.22 a,c ± 0.57 | 187.25 b ± 0.39 | ND |
| Carp | HDPE | 3 month | ND | ND | 25.49 a ± 1.17 | 29.30 a ± 0.18 | 158.67 a ± 0.36 | ND |
| Carp | HDPE | 6 month | ND | ND | 39.16 c ± 0.23 | 43.34 b ± 0.26 | 162.36 a ± 1.1 | ND |
| Carp | LDPE | 3 month | ND | ND | 24.30 a ± 0.44 | 30.68 a ± 0.40 | 156.50 a ± 0.46 | ND |
| Carp | LDPE | 6 month | ND | ND | 31.84 b ± 0.35 | 32.26 a ± 0.37 | 159.34 a ± 0.88 | ND |
| Carp | PP | 3 month | ND | ND | 23.89 a ± 0.24 | 33.64 a,c ± 0.25 | 155.61 a ± 0.71 | ND |
| Carp | PP | 6 month | ND | ND | 33.18 b ± 0.58 | 37.82 c ± 0.44 | 157.72 a ± 0.75 | ND |
| Carp | PAPE | 3 month | ND | ND | 23.51 a ± 0.54 | 31.54 a ± 0.36 | 154.72 a ± 0.60 | ND |
| Carp | PAPE | 6 month | ND | ND | 38.57 c ± 0.34 | 46.68 b ± 0.39 | 182.36 b ± 1.01 | ND |
| Carp | Bio HDPE | 3 month | ND | ND | 30.49 b ± 0.27 | 37.69 c ± 0.39 | 221.74 c ± 1.13 | ND |
| Carp | Bio HDPE | 6 month | ND | ND | 40.97 c ± 0.35 | 45.82 b ± 0.27 | 230.73 c ± 1.05 | ND |
| Trout | Control | - | ND | ND | 33.44 b ± 0.27 | 32.78 a,c ± 0.23 | 172.89 a,b ± 0.54 | ND |
| Trout | PET | 3 month | ND | ND | 34.93 b ± 0.64 | 32.08 a ± 0.45 | 208.68 c,d ± 0.65 | ND |
| Trout | PET | 6 month | ND | ND | 39.87 c ± 0.65 | 45.21 b ± 0.43 | 215.85 c ± 1.05 | ND |
| Trout | HDPE | 3 month | ND | ND | 32.80 b ± 0.89 | 32.01 a ± 0.42 | 180.63 b ± 0.47 | ND |
| Trout | HDPE | 6 month | ND | ND | 38.86 c ± 0.76 | 33.35 a ± 0.58 | 181.51 b ± 0.38 | ND |
| Trout | LDPE | 3 month | ND | ND | 31.87 b ± 0.25 | 32.28 a ± 0.44 | 180.90 b ± 0.70 | ND |
| Trout | LDPE | 6 month | ND | ND | 34.72 b ± 0.3 | 42.62 b ± 0.71 | 191.59 b,d ± 0.77 | ND |
| Trout | PP | 3 month | ND | ND | 34.56 b ± 0.38 | 33.65 a,c ± 0.54 | 169.75 a,b ± 0.42 | ND |
| Trout | PP | 6 month | ND | ND | 34.69 b ± 0.58 | 37.66 c ± 0.23 | 171.57 a,b ± 0.77 | ND |
| Trout | PAPE | 3 month | ND | ND | 34.19 b ± 0.61 | 37.68 c ± 0.55 | 183.64 b ± 0.66 | ND |
| Trout | PAPE | 6 month | ND | ND | 42.21 c ± 0.38 | 42.94 b ± 0.89 | 198.62 d ± 0.57 | ND |
| Trout | Bio HDPE | 3 month | ND | ND | 31.96 b ± 0.73 | 35.53 a,c ± 0.76 | 192.13 b,d ± 0.69 | ND |
| Trout | Bio HDPE | 6 month | ND | ND | 35.19 b,c ± 0.53 | 45.96 b ± 0.71 | 233.54 c ± 0.90 | ND |
| Salmon | Control | - | ND | ND | 29.76 b ± 0.43 | 42.26 b ± 0.57 | 167.06 a,b ± 0.63 | ND |
| Salmon | PET | 3 month | ND | ND | 29.26 b ± 0.55 | 48.65 b,d ± 1.08 | 172.48 a,b ± 1.23 | ND |
| Salmon | PET | 6 month | ND | ND | 57.26 e ± 0.92 | 50.16 d ± 1.01 | 210.61 c ± 1.11 | ND |
| Salmon | HDPE | 3 month | ND | ND | 27.14 a,b± 0.63 | 39.25 c,b ± 0.57 | 172.10 a,b ± 0.87 | ND |
| Salmon | HDPE | 6 month | ND | ND | 37.49 b,c ± 1.26 | 47.16 b,d ±0.54 | 194.0 b,d ± 1.02 | ND |
| Salmon | LDPE | 3 month | ND | ND | 28.16 a,b ± 0.54 | 41.57 b ± 1.11 a | 166.27 a,b± 0.59 | ND |
| Salmon | LDPE | 6 month | ND | ND | 50.95 d ± 0.60 | 44.84 b ± 0.83 | 213.01 c± 1.06 | ND |
| Salmon | PP | 3 month | ND | ND | 28.32 a,b ± 0.28 | 44.03 b ± 0.34 a | 167.38 a,b ± 0.54 | ND |
| Salmon | PP | 6 month | ND | ND | 30.57 b ± 0.87 | 44.81 b ± 0.77 a | 174.22 a,b ± 0.56 | ND |
| Salmon | PAPE | 3 month | ND | ND | 45.49 d ± 0.62 | 41.15 b ± 0.79 a | 185.48 b ± 1.33 | ND |
| Salmon | PAPE | 6 month | ND | ND | 73.77 f ±1.28 | 78.45 e ± 1.18 | 253.56 e ± 1.23 | ND |
| Salmon | Bio HDPE | 3 month | ND | ND | 36.88 b,c ± 0.67 | 40.92 c,b ± 0.93 | 200.9 d ± 1.19 | ND |
| Salmon | Bio HDPE | 6 month | ND | ND | 45.86 d ± 0.87 | 47.40 b,d ± 1.27 | 248.37 e ± 0.44 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurić, B.; Kartalović, B.; Habschied, K.; Novakov, N.; Vranešević, J.; Brkić, B.; Mastanjević, K. Effects of Packaging Material Type, Storage Time and Lipid Content on Phthalate Migration in Smoked Fish Meat. Appl. Sci. 2024, 14, 1660. https://doi.org/10.3390/app14041660
Đurić B, Kartalović B, Habschied K, Novakov N, Vranešević J, Brkić B, Mastanjević K. Effects of Packaging Material Type, Storage Time and Lipid Content on Phthalate Migration in Smoked Fish Meat. Applied Sciences. 2024; 14(4):1660. https://doi.org/10.3390/app14041660
Chicago/Turabian StyleĐurić, Boban, Brankica Kartalović, Kristina Habschied, Nikolina Novakov, Jelena Vranešević, Boris Brkić, and Krešimir Mastanjević. 2024. "Effects of Packaging Material Type, Storage Time and Lipid Content on Phthalate Migration in Smoked Fish Meat" Applied Sciences 14, no. 4: 1660. https://doi.org/10.3390/app14041660
APA StyleĐurić, B., Kartalović, B., Habschied, K., Novakov, N., Vranešević, J., Brkić, B., & Mastanjević, K. (2024). Effects of Packaging Material Type, Storage Time and Lipid Content on Phthalate Migration in Smoked Fish Meat. Applied Sciences, 14(4), 1660. https://doi.org/10.3390/app14041660







