The Effect of Heat- and Salt Treatment on the Stability and Rheological Properties of Chickpea Protein-Stabilized Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CPC-Stabilized Emulsions
2.3. Stability Tests
2.4. Rheological Tests
2.5. Particle Size Distribution (PSD)
2.6. Confocal Laser Scanning Microscopy (CLSM)
2.7. Statistical Methods
3. Results and Discussion
3.1. The Influence of Pre-Treatment of the Protein Dispersions on the Properties of the CPC-Stabilized Emulsions
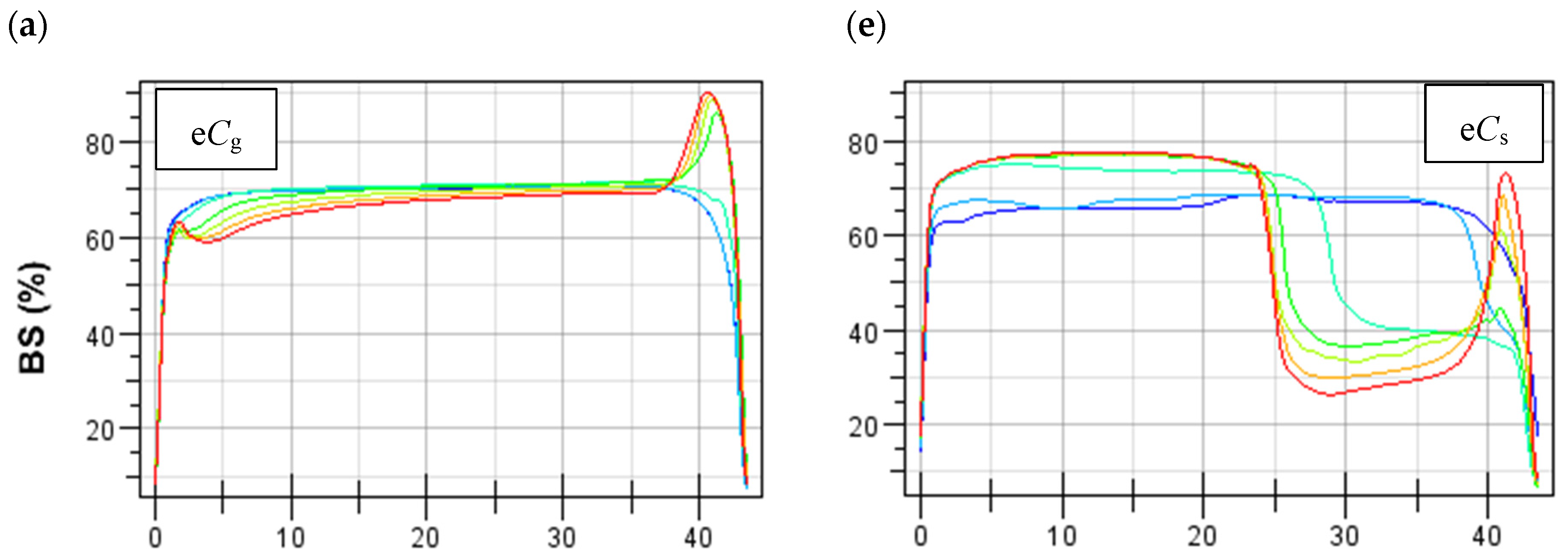
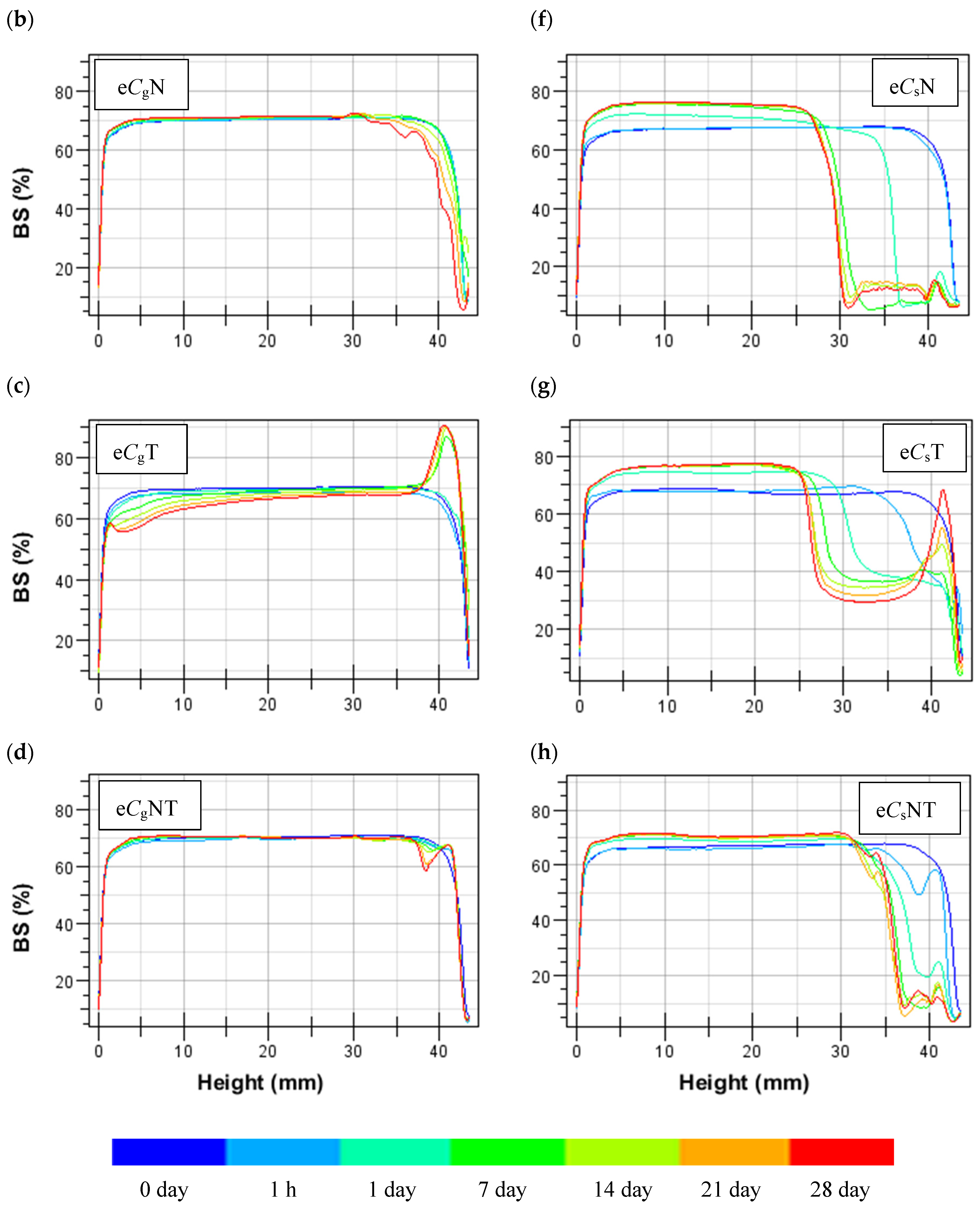
3.2. Emulsion Stability
3.2.1. Untreated CPC-Stabilized Emulsions
3.2.2. Heat- and Salt-Treated CPC-Stabilized Oil-in-Water Emulsions
Emulsions Stabilized by Gelling CPC
Emulsions Stabilized by Standard CPC
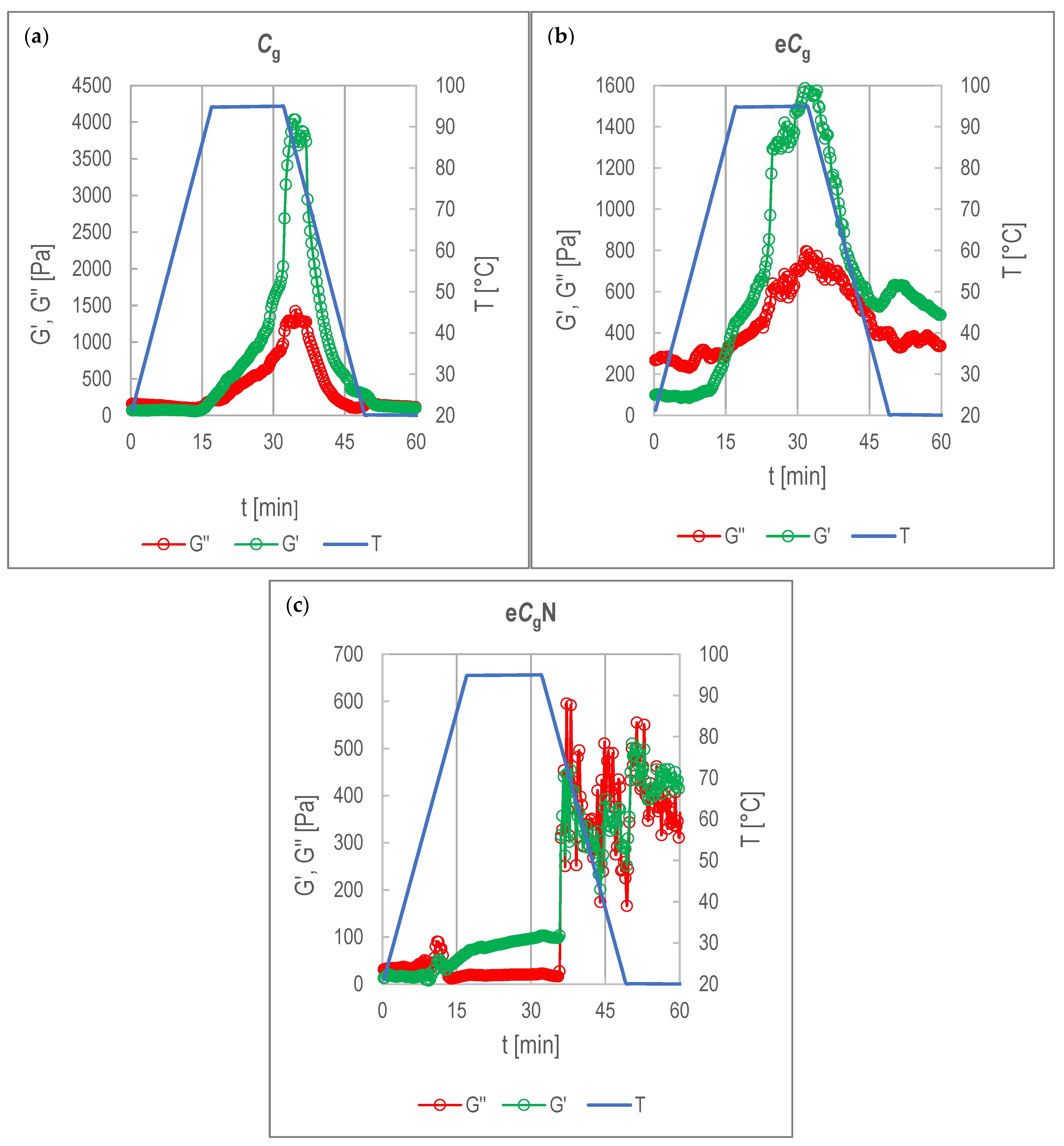
3.3. Viscoelastic Behavior of CPC-Stabilized Emulsions
3.3.1. Emulsions Stabilized by Gelling CPC
3.3.2. Emulsions Stabilized by Standard CPC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glusac, J.; Isaschar-Ovdat, S.; Fishman, A. Transglutaminase modifies the physical stability and digestibility of chickpea protein-stabilized oil-in-water emulsions. Food Chem. 2020, 315, 126301. [Google Scholar] [CrossRef]
- Goldstein, N.; Reifen, R. The potential of legume-derived proteins in the food industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, M.; Ettelaie, R.; Sarkar, A. Pea protein microgel particles as Pickering stabilisers of oil-in-water emulsions: Responsiveness to pH and ionic strength. Food Hydrocoll. 2020, 102, 105583. [Google Scholar] [CrossRef]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Glob. Food Secur. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Gundogan, R.; Tomar, G.S.; Karaca, A.C.; Capanoglu, E.; Tulbek, M.C. Chapter 9—Chickpea Protein: Sustainable Production, Functionality, Modification, and Applications. In Sustainable Protein Sources, 2nd ed.; Nadathur, S., Janitha, P.D., Wanasundara, L.S., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 185–199. [Google Scholar] [CrossRef]
- He, Y.; Meda, V.; Reaney, M.J.; Mustafa, R. Aquafaba, a new plant-based rheological additive for food applications. Trends Food Sci. Technol. 2021, 111, 27–42. [Google Scholar] [CrossRef]
- Onyango, E. Legume Protein: Properties and Extraction for Food Applications. In Legumes Research—Volume 2; Jimenez-Lopez, J.C., Clemente, A., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Boukid, F. Chickpea (Cicer arietinum L.) protein as a prospective plant-based ingredient: A review. Int. J. Food Sci. Technol. 2021, 56, 5435–5444. [Google Scholar] [CrossRef]
- Shakoor, I.F.; Pamunuwa, G.K.; Karunaratne, D.N. Chickpea and soybean protein delivery systems for oral ingestion of hydroxycitric acid. Food Chem. Adv. 2023, 2, 100207. [Google Scholar] [CrossRef]
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional composition, health benefits and bio-active compounds of chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef]
- Patil, N. Chickpea protein: A comprehensive review on nutritional properties, processing, functionality, applications, and sustainable impact. Pharm. Innov. J. 2023, 12, 3424–3434. [Google Scholar]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.-B.; Chen, B.; Rao, J. Plant proteins from green pea and chickpea: Extraction, fractionation, structural characterization and functional properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea protein ingredients: A review of composition, functionality, and applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef]
- Papalamprou, E.; Doxastakis, G.; Biliaderis, C.; Kiosseoglou, V. Influence of preparation methods on physicochemical and gelation properties of chickpea protein isolates. Food Hydrocoll. 2009, 23, 337–343. [Google Scholar] [CrossRef]
- Ramani, A.; Kushwaha, R.; Malaviya, R.; Kumar, R.; Yadav, N. Molecular, functional and nutritional properties of chickpea (Cicer arietinum L.) protein isolates prepared by modified solubilization methods. J. Food Meas. Charact. 2021, 15, 2352–2368. [Google Scholar] [CrossRef]
- Felix, M.; Cermeño, M.; Romero, A.; FitzGerald, R.J. Characterisation of the bioactive properties and microstructure of chickpea protein-based oil in water emulsions. Food Res. Int. 2019, 121, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.P.; Nickerson, M.T.; Low, N.H. The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. J. Food Sci. Technol. 2015, 52, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Karaca, A.C.; Nickerson, M.T.; Low, N.H. Lentil and Chickpea Protein-Stabilized Emulsions: Optimization of Emulsion Formulation. J. Agric. Food Chem. 2011, 59, 13203–13211. [Google Scholar] [CrossRef] [PubMed]
- Bogahawaththa, D.; Chau, N.H.B.; Trivedi, J.; Dissanayake, M.; Vasiljevic, T. Impact of selected process parameters on solubility and heat stability of pea protein isolate. LWT 2019, 102, 246–253. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current insights into protein solubility: A review of its importance for alternative proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Keuleyan, E.; Gélébart, P.; Beaumal, V.; Kermarrec, A.; Ribourg-Birault, L.; Le Gall, S.; Meynier, A.; Riaublanc, A.; Berton-Carabin, C. Pea and lupin protein ingredients: New insights into endogenous lipids and the key effect of high-pressure homogenization on their aqueous suspensions. Food Hydrocoll. 2023, 141, 108671. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N. Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chem. 2007, 102, 366–374. [Google Scholar] [CrossRef]
- Novák, P.; Havlíček, V. 4—Protein Extraction and Precipitation. In Proteomic Profiling and Analytical Chemistry; Ciborowski, P., Silberring, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 51–62. [Google Scholar] [CrossRef]
- Yousefi, N.; Abbasi, S. Food proteins: Solubility & thermal stability improvement techniques. Food Chem. Adv. 2022, 1, 100090. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Panouillé, M.; Huc-Mathis, D.; Moulin, G.; Saint-Eve, A.; Irlinger, F.; Bonnarme, P.; Michon, C.; Souchon, I. The rheological and microstructural properties of pea, milk, mixed pea/milk gels and gelled emulsions designed by thermal, acid, and enzyme treatments. Food Hydrocoll. 2018, 77, 75–84. [Google Scholar] [CrossRef]
- Gomes, A.; Sobral, P.J.D.A. Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules 2022, 27, 60. [Google Scholar] [CrossRef]
- Bußler, S.; Steins, V.; Ehlbeck, J.; Schlüter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca’. J. Food Eng. 2015, 167, 166–174. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Liu, Q.; Chen, Q.; Kong, B. Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrason. Sonochemistry 2020, 67, 105160. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crop. Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Saricaoglu, F.T. Application of high-pressure homogenization (HPH) to modify functional, structural and rheological properties of lentil (Lens culinaris) proteins. Int. J. Biol. Macromol. 2020, 144, 760–769. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Zeng, H.; Chen, L. Effects of high pressure homogenization on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocoll. 2018, 83, 275–286. [Google Scholar] [CrossRef]
- Domian, E.; Mańko-Jurkowska, D. The effect of homogenization and heat treatment on gelation of whey proteins in emulsions. J. Food Eng. 2022, 319, 110915. [Google Scholar] [CrossRef]
- Domian, E.; Mańko-Jurkowska, D.; Górska, A. Heat-induced gelation, rheology and stability of oil-in-water emulsions prepared with patatin-rich potato protein. Food Bioprod. Process. 2023, 139, 144–156. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, Y.; Zhu, L.; Liu, X.; Zhao, G.; Wang, S.; Yang, L.; Ma, T.; Liu, H. Stability and rheological properties of water-in-oil (W/O) emulsions prepared with a soyasaponin-PGPR system. Futur. Foods 2021, 4, 100096. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Håkansson, A. Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annu. Rev. Food Sci. Technol. 2019, 10, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Z.; Subramanian, P.; Dar, B. Functionalization of legume proteins using high pressure processing: Effect on technofunctional properties and digestibility of legume proteins. LWT 2022, 158, 113106. [Google Scholar] [CrossRef]
- Juttulapa, M.; Piriyaprasarth, S.; Takeuchi, H.; Sriamornsak, P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J. Pharm. Sci. 2017, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.; Cunha, R. Flaxseed oil—Whey protein isolate emulsions: Effect of high pressure homogenization. J. Food Eng. 2012, 111, 449–457. [Google Scholar] [CrossRef]
- Melchior, S.; Moretton, M.; Calligaris, S.; Manzocco, L.; Nicoli, M.C. High pressure homogenization shapes the techno-functionalities and digestibility of pea proteins. Food Bioprod. Process. 2022, 131, 77–85. [Google Scholar] [CrossRef]
- Subasi, B.G.; Yildirim-Elikoğlu, S.; Altay, I.; Jafarpour, A.; Casanova, F.; Mohammadifar, M.A.; Capanoglu, E. Influence of non-thermal microwave radiation on emulsifying properties of sunflower protein. Food Chem. 2021, 372, 131275. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Krztoń-Maziopa, A.; Babut, M.; Mitrosz, P. Rheological and physical analysis of oil-water emulsion based on enzymatic structured fat. Rheol. Acta 2020, 59, 717–726. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. Factors Affecting the Stability of Emulsions Stabilised by Biopolymers. In Science and Technology behind Nanoemulsions; Karakus, S., Ed.; IntechOpen: London, UK, 2018; pp. 65–81. [Google Scholar]
- Erçelebi, E.A.; Ibanoğlu, E. Rheological properties of whey protein isolate stabilized emulsions with pectin and guar gum. Eur. Food Res. Technol. 2009, 229, 281–286. [Google Scholar] [CrossRef]
- Félix, M.; Carrera, C.; Romero, A.; Bengoechea, C.; Guerrero, A. Rheological approaches as a tool for the development and stability behaviour of protein-stabilized emulsions. Food Hydrocoll. 2020, 104, 105719. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Emulsion Formation, Stability, and Rheology. In Emulsion Formation and Stability; Tadros, T.F., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 1–75. [Google Scholar] [CrossRef]
- Niu, H.; Wang, W.; Dou, Z.; Chen, X.; Chen, X.; Chen, H.; Fu, X. Multiscale combined techniques for evaluating emulsion stability: A critical review. Adv. Colloid Interface Sci. 2023, 311, 102813. [Google Scholar] [CrossRef] [PubMed]
- Zamani, Z.; Razavi, S.M.A. Steady shear rheological properties, microstructure and stability of water in water emulsions made with basil seed gum and waxy corn starch or high pressure-treated waxy corn starch. LWT 2023, 174, 114453. [Google Scholar] [CrossRef]
- Pathak, M. Chapter 5—Nanoemulsions and Their Stability for Enhancing Functional Properties of Food Ingredients. In Nanotechnology Applications in Food; Opera, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 87–106. [Google Scholar] [CrossRef]
- Martínez, I.; Riscardo, M.A.; Franco, J.M. Effect of salt content on the rheological properties of salad dressing-type emulsions stabilized by emulsifier blends. J. Food Eng. 2007, 80, 1272–1281. [Google Scholar] [CrossRef]
- Tan, Y.; Lee, P.W.; Martens, T.D.; McClements, D.J. Comparison of Emulsifying Properties of Plant and Animal Proteins in Oil-in-Water Emulsions: Whey, Soy, and RuBisCo Proteins. Food Biophys. 2022, 17, 409–421. [Google Scholar] [CrossRef]
- Ettoumi, Y.L.; Chibane, M.; Romero, A. Emulsifying properties of legume proteins at acidic conditions: Effect of protein concentration and ionic strength. LWT 2016, 66, 260–266. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.; Kasapis, S.; Barrow, C. Interfacial and emulsifying properties of lentil protein isolate. Food Chem. 2012, 134, 1343–1353. [Google Scholar] [CrossRef]
- Jo, M.; Chang, M.J.; Goh, K.K.T.; Ban, C.; Choi, Y.J. Rheology, Microstructure, and Storage Stability of Emulsion-Filled Gels Stabilized Solely by Maize Starch Modified with Octenyl Succinylation and Pregelatinization. Foods 2021, 10, 837. [Google Scholar] [CrossRef]
- Totosaus, A.; Montejano, J.G.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. Int. J. Food Sci. Technol. 2002, 37, 589–601. [Google Scholar] [CrossRef]
- Dent, T.; Campanella, O.; Maleky, F. Enzymatic hydrolysis of soy and chickpea protein with Alcalase and Flavourzyme and formation of hydrogen bond mediated insoluble aggregates. Curr. Res. Food Sci. 2023, 6, 100487. [Google Scholar] [CrossRef] [PubMed]
- Badii, F.; Atri, H.; Dunstan, D.E. The effect of shear on the rheology and structure of heat-induced whey protein gels. Int. J. Food Sci. Technol. 2016, 51, 1570–1577. [Google Scholar] [CrossRef]
- Phillips, L.G.; Whitehead, D.M.; Kinsella, J. Structure–Function Properties of Food Proteins; Academic Press: New York, NY, USA, 1994. [Google Scholar]
- Foegeding, E.A. Food Biophysics of Protein Gels: A Challenge of Nano and Macroscopic Proportions. Food Biophys. 2006, 1, 41–50. [Google Scholar] [CrossRef]
- Ramos, L.; Pereira, J.O.; Silva, S.I.; Amorim, M.M.; Fernandes, J.C.; Lopes-Da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Effect of composition of commercial whey protein preparations upon gelation at various pH values. Food Res. Int. 2012, 48, 681–689. [Google Scholar] [CrossRef]
- Al-Ali, H.A.; Shah, U.; Hackett, M.J.; Gulzar, M.; Karakyriakos, E.; Johnson, S.K. Technological strategies to improve gelation properties of legume proteins with the focus on lupin. Innov. Food Sci. Emerg. Technol. 2021, 68, 102634. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]

| Sample 1 | PSD | Turbiscan Stability 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| 1. Day | 7. Day | 28. Day | ||||||
| d43 [μm] | span [-] | TSI [-] | Category | TSI [-] | Category | TSI [-] | Category | |
| eCg | 0.70 ± 0.02 a | 3.19 ± 0.08 c | 0.4 ± 0.0 a | A+ | 3.0 ± 0.1 a | B | 5.8 ± 0.2 a | C |
| eCgN | 1.10 ± 0.03 a | 4.64 ± 0.16 e | 0.1 ± 0.0 a | A+ | 0.7 ± 0.0 a | A | 2.8 ± 0.2 a,b | C |
| eCgT | 0.80 ± 0.00 a | 3.68 ± 0.07 d | 0.4 ± 0.2 a | A+ | 2.7 ± 1.0 a | B | 6.4 ± 0.3 a | C |
| eCgNT | 51.25 ± 0.68 c | 1.48 ± 0.02 a | 0.2 ± 0.1 a | A+ | 1.1 ± 0.3 a | B | 1.7 ± 0.3 b | B |
| eCs | 40.62 ± 0.23 b | 1.87 ± 0.01 a,b | 12.8 ± 0.2 c | D | 17.0 ± 0.4 b | D | 22.1 ± 0.7 d | D |
| eCsN | 36.83 ± 0.81 b | 2.04 ± 0.05 b | 6.1 ± 0.7 a,b | C | 29.1 ± 2.8 c | D | 36.5 ± 1.8 e | D |
| eCsT | 37.77 ± 1.67 b | 1.99 ± 0.08 b | 6.8 ± 1.8 b,c | C | 11.5 ± 1.1 b | D | 17.2 ± 0.0 c | D |
| eCsNT | 38.05 ± 0.22 b | 1.97 ± 0.01 b | 5.0 ± 2.4 a,b | C | 11.4 ± 0.8 b | D | 14.5 ± 0.0 c | D |
| Sample 1 | μ100 § [mPa·s] | VRI † [-] | Model ‡ (r ≥ 0.999) | μ [mPa·s] | k [mPa·sn] | N [-] | [mPa] |
|---|---|---|---|---|---|---|---|
| eCg | 3.42 ± 0.22 c | 0 ± 0.000 a | Newton | 3.42 ± 0.22 | - | 1 | - |
| eCgN | 11.26 ± 0.05 e | 0.341 ± 0.001 b | Ostwald–de Waele | - | 25.89 ± 0.06 | 0.819 ± 0.000 | - |
| eCgT | 3.08 ± 0.00 b,c | 0 ± 0.000 a | Newton | 3.08 ± 0.00 | - | 1 | - |
| eCgNT | 60.13 ± 0.30 f | 0.775 ± 0.002 e | Herschel–Bulkley | - | 326 ± 24 | 0.573 ± 0.013 | 1454 ± 83 |
| eCs | 8.73 ± 0.41 d | 0.531 ± 0.051 c | Herschel–Bulkley | - | 19.7 ± 4.5 | 0.810 ± 0.030 | 60.0 ± 31.7 |
| eCsN | 7.89 ± 0.05 d | 0.481 ± 0.002 c | Herschel–Bulkley | - | 16.5 ± 0.7 | 0.828 ± 0.010 | 40.9 ± 2.6 |
| eCsT | 8.93 ± 0.65 d | 0.529 ± 0.001 c | Herschel–Bulkley | - | 21.7 ± 3.2 | 0.794 ± 0.015 | 54.5 ± 1.9 |
| eCsNT | 11.03 ± 0.53 e | 0.636 ± 0.007 d | Herschel–Bulkley | - | 36.7 ± 8.1 | 0.718 ± 0.035 | 113.2 ± 6.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mańko-Jurkowska, D.; Domian, E. The Effect of Heat- and Salt Treatment on the Stability and Rheological Properties of Chickpea Protein-Stabilized Emulsions. Appl. Sci. 2024, 14, 2698. https://doi.org/10.3390/app14072698
Mańko-Jurkowska D, Domian E. The Effect of Heat- and Salt Treatment on the Stability and Rheological Properties of Chickpea Protein-Stabilized Emulsions. Applied Sciences. 2024; 14(7):2698. https://doi.org/10.3390/app14072698
Chicago/Turabian StyleMańko-Jurkowska, Diana, and Ewa Domian. 2024. "The Effect of Heat- and Salt Treatment on the Stability and Rheological Properties of Chickpea Protein-Stabilized Emulsions" Applied Sciences 14, no. 7: 2698. https://doi.org/10.3390/app14072698
APA StyleMańko-Jurkowska, D., & Domian, E. (2024). The Effect of Heat- and Salt Treatment on the Stability and Rheological Properties of Chickpea Protein-Stabilized Emulsions. Applied Sciences, 14(7), 2698. https://doi.org/10.3390/app14072698







