Featured Application
An encapsulation strategy with the use of a spray-drying technique and whey protein concentrate with maltodextrin as wall material is recommended to obtain oxidatively stable powders with linseed oil and ethyl esters of this oil. These powders can be easily applied to various food matrices, increasing the share of valuable α-linolenic acid.
Abstract
The aim of the study was to compare the encapsulation of linseed oil and its ethyl esters using two coating materials (maltodextrin with whey protein concentrate (WPC) vs. maltodextrin with gum arabic) and two drying methods (spray-drying vs. freeze-drying) to obtain powders with the highest oxidative stability. A comparison was made based on the properties of emulsions (morphology, particle size distribution, and stability) and powders (morphology, physicochemical properties, fatty acid composition, and oxidative stability). The powder’s oxidative stability was determined based on the Rancimat protocol. The most uniform distribution of oil droplets in prepared emulsions was stated for ethyl esters in a mixture of maltodextrin and gum arabic. Emulsions with WPC had a bimodal character, while those with gum arabic had a monomodal character. Gum arabic promoted emulsion stability, while in samples containing WPC, sedimentation and creaming processes were more visible. Powders obtained using spray-drying had a spherical shape, while those obtained by freeze-drying were similar to flakes. Although encapsulation efficiency was the highest for freeze-dried powders made of linseed ethyl esters with gum arabic, the highest oxidative stability was stated for powders made by spray-drying with WPC as wall material (independently of linseed sample form). These powders can be easily applied to various food matrices, increasing the share of valuable α-linolenic acid.
1. Introduction
Linseed (Linum usitatissimum) is one of the most commonly used raw materials in the oil industry. Annual oil production was close to 3.3 million tons in 2021 [1]. The consumption of linseed oil exerts a beneficial effect in the prevention and treatment of various diseases. For example, this oil contains many phytochemicals with documented activities, e.g., the lowering of plasma cholesterol and blood pressure in dyslipidemia patients [2]. Similarly, pharmacological studies have shown that α-linolenic fatty acid (n-3, ALA), as the main component of this oil, demonstrates numerous health benefits, such as reducing metabolic syndrome, anti-cancer, anti-inflammatory, antioxidant, anti-obesity, neuroprotective, and intestinal microflora-regulating effects [3]. Additionally, a recent systematic review and dose–response meta-analysis of cohort studies with a total of 1,197,564 participants confirmed that dietary ALA intake is also related with a lowered risk of mortality due to various diseases [4]. Also, non-saponifiable components of linseed oil (tocopherols, carotenoids, phytosterols, and lipophilic polyphenols) have additional pro-healthy effects [2].
The consumption of ALA is crucial for human body health balance for at least two main reasons. Firstly, it may help to achieve the recommended n-6/n-3 fatty acid ratio below 4/1 [5,6]. Secondly, ALA can be converted into longer n-3 fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are typical of marine food. They are crucial for cardiovascular health and have benefits in many other conditions, including metabolic, inflammatory, and neuropsychiatric disorders, as well as in regulating gene expression [7]. The conversion rates of ALA to n-3 fatty acids with longer chains (DHA or EPA) in the human body are generally low (up to 8% conversion to EPA in men and up to 21% in women [7]), but plants seem to be valuable n-3 fatty acid sources in the diet of many consumers, especially those who avoid marine food due to an undesirable smell/odor of these products [8] or due to a vegan/vegetarian diet choice [9]. Additionally, it is worth knowing that the conversion of ALA to DHA and EPA can be disrupted when the of n-6 to n-3 ratio in the diet is high [5]. On the other hand, when the levels of linoleic acid and estrogen (17β-oestradiol-3-benzoate) in the diet are low, the conversion of ALA into DHA is increased by upregulating the fatty acids elongases and desaturases through regulating the expression of transcription factors. Additionally, the low-linoleic acid diet and estrogen had a synergistic effect on levels of DHA in liver and serum, through increasing the expression of peroxisome proliferators-activated receptor alpha (PPAR-α) [10]. The presented data show that linseed oil, or generally all sources of ALA (such as chia and hemp oils), may constitute an important component of the daily diet of vegans, vegetarians, or consumers avoiding seafood.
ALA can be easily oxidized via autoxidation, photooxidation, or enzyme-catalyzed reactions. Microencapsulation is a common method of preventing this process. Encapsulation can protect the unsaturated fatty acids against oxidation by shielding them against oxygen and also hiding any unwanted flavors from the raw material. This technology consists of two main stages: preparing a stable emulsion and then drying it. An important element is determining the formulation of the emulsion, e.g., coating composition and the amount of oil that can be encapsulated. The most commonly used coating materials include chitosan, alginate, starch, maltodextrin, gums (arabic or guar), and whey protein (WPC) [11]. Gum arabic has been proven to be the most effective coating material for encapsulating volatiles via the spray-drying process [12]. Unfortunately, the high cost and limited availability of gum arabic limits its use as a single coating material for encapsulation. Therefore, it is mixed with other materials such as mesquite gum and maltodextrin to lower the encapsulation cost [12].
The most popular encapsulation techniques include spray-drying and freeze-drying [13]. These methods differ significantly from each other. In the spray-drying process, the oil is dispersed in a mixture of compounds creating a coating solution. Once small droplets are formed, they are then quickly dried to obtain microcapsules [14]. In turn, freeze-drying is recommended for encapsulation of thermally sensitive compounds. The action of low pressure causes ice to sublimate very quickly from the frozen emulsion. [15]. It is considered an expensive drying technology due to the use of a pump, which can provide vacuum conditions. Thus, the complete drying process (24–48 h) concerns the high energy consumption procedure [13].
Recently, several reports describing the encapsulation of linseed oil have been published. Elik et al. [16] studied the encapsulation of carotenoid-enriched linseed oil and linseed oil by spray-drying and spray freeze-drying techniques. This study showed that the spray freeze-drying technique had a lower encapsulation efficiency but better flow properties. Naz et al. [17] analyzed the effect of polymer blends (gum arabic, maltodextrin, and inulin) on the encapsulation efficiency of spray-dried microencapsulated linseed oil. Their results showed that microcapsules containing gum arabic and maltodextrin at a 1:1 ratio had the best encapsulation efficiency, close to 92%. In turn, Avci et al. [18] investigated the production of freeze-dried linseed oil powders using rocket seed gum as an alternative encapsulation material. More information on the types of materials used to encapsulate bioactive compounds is summarized in a recent review by Diaz-Montes [11].
ALA sources in diets can be real oils (linseed, chia, hemp, etc.) or their preparations like ethyl esters [19,20,21]. Data on the encapsulation of linseed oil is relatively easily accessible. In contrast, information showing the results of the encapsulation of such ethyl esters to protect them against oxidation is scarce. In our previous study, the possibility of efficient encapsulation of linseed oil ethyl esters using spray-drying with the use of pregelatinized potato starch was presented [22].
In this context, the aim of the current study is to compare the encapsulation of linseed oil and its ethyl esters using the most common coating materials (maltodextrin with WPC vs. maltodextrin with gum arabic) and methods (spray-drying vs. freeze-drying) to obtain linseed oil or its ethyl esters powders with the highest oxidative stability.
2. Materials and Methods
2.1. Materials and Reagents
Cold-pressed linseed oil (L, free fatty acid content = 0.5%, and water content = 0.06%) was purchased from the company “Olejarnia Świecie” (Świecie nad Osą, Poland), maltodextrin (DE = 17.5, moisture 57.0%, pH 4.8) from Pepees S.A. Company (Łomża, Poland), whey protein concentrate (WPC 50) from Ostrowia Company (Ostrów Mazowiecka, Poland), and gum arabic from Kremer Pigmente GmbH&Co.KG (Aichstetten, Germany). Additionally, 96% ethyl alcohol and potassium hydroxide (KOH) were purchased from Chempur (Piekary Śląskie, Poland). Chloroform, n-hexane, methanol, and zinc were purchased from Sigma-Aldrich (Poznań, Poland).
2.2. Linseed Oil Ethyl Esters Production
Linseed oil ethyl esters (E) were produced in a one-step transesterification process at elevated temperatures. Firstly, a solution of alkaline catalyst in ethyl alcohol (3 g of KOH in 400 mL of 96% ethyl alcohol) was prepared [23]. Then, the catalyst–alcohol solution was added to 200 g of linseed oil in a round flask, and the mixture was refluxed at 76 °C for 2 h in a water bath. After the transesterification process, residual ethanol was removed from the reaction mixture by distillation in a R-210 rotary vacuum evaporator (Büchi, Flawil, Switzerland), and the mixture was washed three times with distilled water (100 mL) using a laboratory separatory funnel. Finally, the ethyl esters were centrifuged at 7130× g for 15 min using the MPV-350R laboratory centrifuge (MPW Med Instruments, Warsaw, Poland) to remove any impurities (solids, glycerol, and water). The purity of the linseed oil ethyl esters was confirmed by thin-layer chromatography.
2.3. Emulsion Preparation
The samples were prepared by mixing wall materials, water, and lipid fraction using a Thermomix (Vorwerk, Germany) at 9000 rpm for 120 s (compositions of prepared emulsions are presented in Table 1). L or E were added (200 g per 1800 g of wall materials and water mixture) and mixed (at 9000 rpm for 120 s) to obtain an emulsion. The resulting emulsion was additionally homogenized (first at 24 MPa and then at 4 MPa) using a Panda 2K laboratory homogenizer (GEA Niro Soavi, Parma, Italy). The compositions of the prepared emulsions were optimized in preliminary studies.

Table 1.
Compositions of prepared emulsions (%).
2.4. Emulsion Properties Analysis
The emulsion microstructure was analyzed at a magnification of 40× using a Motic BA210E apparatus with a Motic Camera 1080p optical microscope (Motic, Kowloon, Hong Kong). The droplet size of the emulsion was measured using a Mastersizer 2000 (Malvern Instruments Ltd., Worcestershire, UK). The emulsion’s stability was determined using the turbidimetric method by measuring the backscattering of light (ΔRW) using a Turbiscan Classic 2 every 5 min for 24 h. The profile of changes in the backscattering coefficient (RW) over time was analyzed, and the slope coefficient of the curve was calculated.
2.5. Encapsulation Process
Two techniques were employed to prepare encapsulated linseed oil and its ethyl esters. To obtain spry-dried samples (SD), a pilot-plant spray-dryer (A/S Niro Atomizer, Copenhagen, Denmark) was used. The drying parameters were controlled to maintain an inlet temperature of 130 °C and an outlet temperature of 90 °C, and the feed flow rate was 77 mL/min. The freeze-dried samples (FD) were obtained with a Cryolizer Freeze Dryer type B-64 (New Brunswick Scientific Co., Inc., Edison, NJ, USA). The emulsions were included in aluminum pans and frozen at −20 °C for 24 h. Then, the samples were freeze-dried at a pressure of 0.12 mbar with a drying time of 72 h. During the freeze-drying process, the product temperature increased from an initial value of −50 °C to a final temperature of 18 °C. After the freeze-drying process, the samples were ground by using a glass rod to transform them into powder.
2.6. Encapsulation Efficiency Analysis
Firstly, the surface oil content (SOC) was determined by the extraction of 2 g of powder with 15 mL of n-hexane for 2 min at room temperature. Then, the solvent was filtered, and the residue was rinsed three times with 25 mL of n-hexane. The solvent was evaporated using a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland). The extracted oil was weighed, and the SOC was expressed as a percentage of the powder. The encapsulation efficiency (EE) was calculated based on Equation (1), where the total oil content (TOC) is the amount of oil in the anhydrous fraction of prepared emulsion (40%) calculated based on the emulsion formulation (Table 1).
2.7. Powder Basic Properties Analysis
The moisture content of the powders was determined at 105 °C according to the AOAC official method 925.10 [24]. Samples were dried until a constant weight was obtained in two consecutive readings.
The morphology of the powders was characterized using scanning electron microscopy (SEM Quanta 200; FEI Company, Hillsboro, OR, USA) according to Ogrodowska et al. [22].
Powder particle sizes were analyzed using the Mastersizer 2000. Powders were also characterized by Sauter mean diameter D3,2 (the surface weighted mean diameter), De Brouckere mean diameter D4,3 (the volume weighted mean diameter), and specific surface area (SSA). Additionally, the width of the distribution (Span) was calculated based on the distribution of diameters of the droplets at which 90%, 50% or 10% of the sample was smaller than the size measured (d0.9, d0.5, and d0.1, respectively) according to Equation (2) [25].
2.8. Fatty Acid Composition
The GC–MS technique was applied to the analysis of the fatty acids composition according to the method described previously [26]. The analysis was performed on a BPX70 (25 m × 0.22 mm × 0.25 μm) capillary column (SGE Analytical Science, Victoria, Australia) with the use of a GC–MS QP2010 PLUS system (Shimadzu, Kyoto, Japan). Helium at a flow rate of 1.3 mL/min as a carrier gas was used. The temperature of injection was set at 230 °C, and the column temperature was subsequently increased from 150 °C to 180 °C (10 °C/min), to 185 °C (1.5 °C/min), to 250 °C (30 °C/min), and then a 10 min hold. The GC–MS interface and ion source temperatures were set at 240 °C, and the ionization energy was set at 70 eV. The total ion current (TIC) mode was used in the 50–500 m/z range. Identification was based on mass spectra compared to mass spectral libraries (NIST08 library, Shimadzu, Kyoto, Japan).
2.9. Oxidative Stability Analysis
The oxidative stability index (OSI) of linseed oil, ethyl esters, and powders was tested on a Rancimat apparatus 743 (Metrohm, Herisau, Switzerland), according to Ogrodowska et al. [22]. Briefly, 2.5 g of sample was weighed in a reaction vessel and placed in a thermostated electric heating block at a temperature of 110 °C. A stream of air was forced through the vessel with a flow rate of 20 L/h. The detection of volatile oxidation products was based on the conductometric technique, and the time that elapsed until these oxidation products appeared was saved as the induction period describing the stability of samples.
2.10. Statistical Analysis
Data were analyzed statistically using Statistica 13.3 (StatSoft, Kraków, Poland), and the one-way ANOVA with Duncan’s test was applied. All stated differences were considered statistically significant if the p-value was below 0.05.
3. Results
3.1. Emulsion Properties
3.1.1. Morphology
The microstructure/morphology of emulsions could not be observed by unaided eyes since their particles are smaller than 100 μm [27]. Therefore, various types of microscopes are used to observe the microstructure of emulsions.
The images of emulsion samples taken with an optical microscope reveal their complex microstructure (Figure 1). In three samples (LMW, EMW, and LMG), the presence of agglomerates consisting of several droplets of the oil phase was observed, with these clusters being the largest in the LMG sample. In the EMG sample, no occurrence of such agglomerates was observed. Campelo et al. [28] reported that the formation of larger droplets is related with a higher viscosity of proteins and longer-chain maltodextrins of low emulsifying capacity. Additionally, lower emulsion viscosity hinders the formation of smaller droplets, which is related to the aggregation and increase of these droplets. In their study, microscopy images showed larger droplets for samples with higher amounts of WPC and maltodextrin with a low dextrose equivalent. In contrast, according to Premi and Sharma [29], the emulsion prepared from maltodextrin with WPC had a lower viscosity than maltodextrin with gum arabic and simultaneously showed larger droplets than the maltodextrin with gum arabic. The authors explained this phenomenon as an effect of the low stability of emulsion droplets and lower resistance in droplet movement in maltodextrin with WPC, which resulted in coalescence and thus, in large-diameter droplets. The results of the current study led to the adoption of this latter explanation of the stated phenomenon.
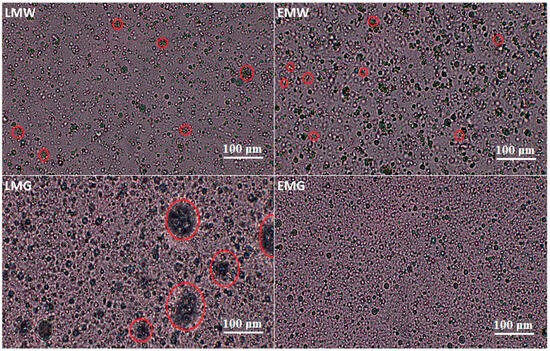
Figure 1.
Optical microscope images of emulsions (red loop—agglomerates). LMW, LMG, EMW, EMG—emulsion variants, which compositions are presented in Table 1.
3.1.2. Particle Size Distribution
The particle size distribution in the emulsion affects the physical stability of the emulsion. It is a well-known fact that the smaller the dispersed particles, the more stable the emulsion is created [30].
Samples of emulsions containing WPC (EMW and LMW) exhibited a bimodal particle size distribution (Figure 2). In both emulsions, the first fraction ranged from 0.04 to 0.95 μm and was mainly composed of protein-coated particles. The particle size of the second fraction ranged from 1.10 to 15.14 μm, representing dispersed oil phase droplets and their agglomerates. Larger agglomerates (in the range up to 100 μm) that were observed in the microscopic images of LMG likely underwent disintegration, indicating relatively weak interaction forces between dispersed particles. Similar observations of the bimodal size distribution of emulsions based on regenerated whey proteins and maltodextrin were reported by Hebishy et al. [31] and Dybowska [32]. In general, bimodal emulsions have special rheological properties, e.g., relatively low bulk viscosity for semi-solid products, high flow adaptability, and a tendency to reduce the viscosity of a particulate suspension [33].
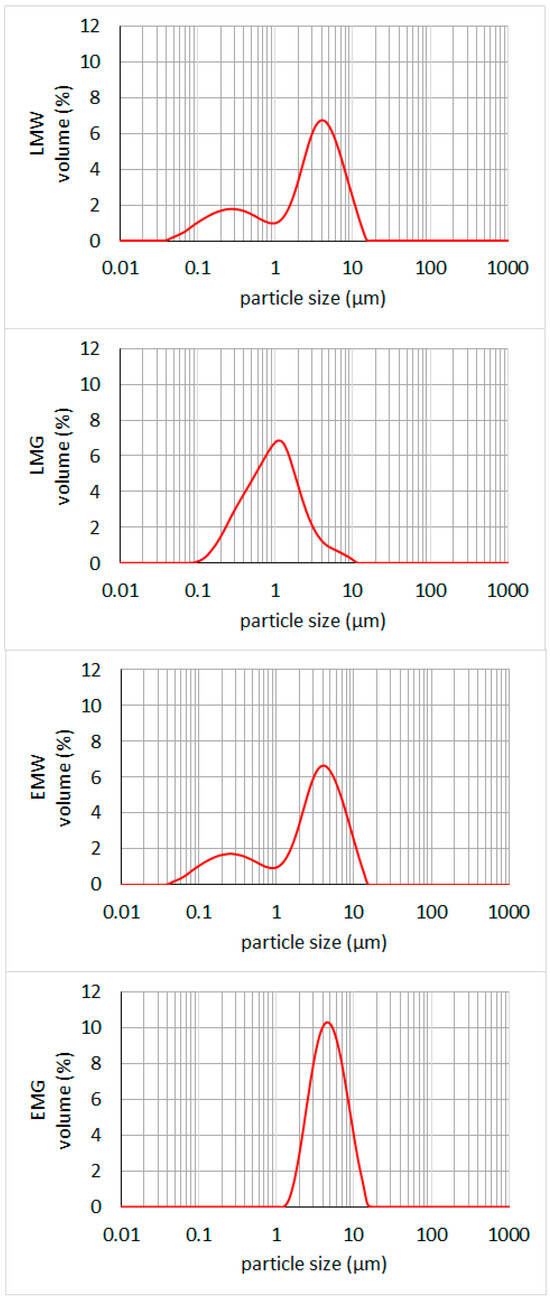
Figure 2.
Particle size distribution of emulsions. LMW, LMG, EMW, EMG—emulsion variants, the compositions of which are presented in Table 1.
The size distribution of emulsions containing arabic gum (EMG and LMG) was close to monomodal. In the case of monomodal emulsions formulation, Stokes’ law is fulfilled. This indicates that the higher the emulsion viscosity and the smaller the droplet diameter, the smaller the sedimentation is observed [34]. Monomodal distribution was particularly evident in the sample containing ethyl esters (EMG), where there was a single main particle fraction ranging from 1.44 to 17.38 μm, representing dispersed oil phase droplets. The particle size distribution in the emulsion containing oil (LMG) resembled a monomodal distribution in the particle size range of 0.08 to 10.00 μm. A clear break in the curve was visible in the diameter range of 2.19 to 5.02 μm, indicating the presence of a distinct particle fraction. Comparing the particle sizes in LMG and EMG samples, it can be observed that emulsions based on ethyl esters had larger oil droplets. Furthermore, this phenomenon did not occur in emulsions, in which WPC was used as an emulsifier.
3.1.3. Stability
The stability of an emulsion correlates with the physicochemical properties of the compounds in the interfacial layer. The competition between different surface-active ingredients is significant for this emulsion feature [35].
Turbidimetry analyses revealed that emulsions produced with the use of WPC exhibited poorer stability compared to emulsions with gum arabic (Figure 3). This is reflected in the values of the slope coefficient of the RW line, with values for the LMW sample being −1.11 and for the EMW sample being −0.53. Samples containing WPC underwent sedimentation and creaming. The slope coefficients of the RW line for the LMG and EMG emulsions were −0.20 and −0.01, respectively, with no observable tendency for creaming in the EMG emulsion. The reasons for such disparities in sample stability can be traced to the type of emulsifier used. The WPC was regenerated from spray-dried powder, and some of it consists of insoluble particles [36,37] that tend to sediment, negatively impacting the maintenance of the oil phase droplets. Gum arabic covered interfacial space better, resulting in more stable emulsion systems [38,39].
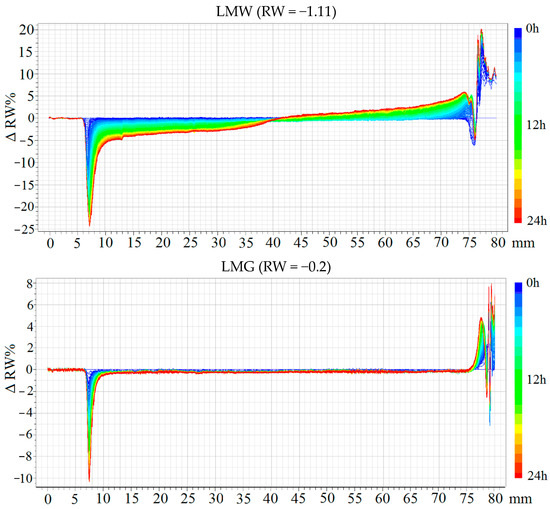
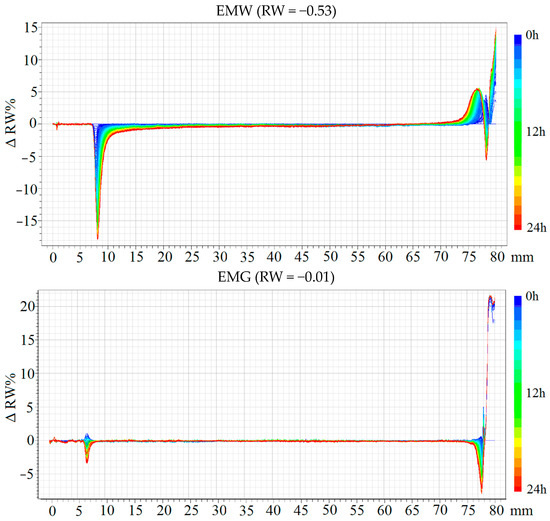
Figure 3.
Changes in the backscattering of light (ΔRW), and backscattering coefficient (RW) of emulsions. LMW, LMG, EMW, EMG—emulsion variants, which compositions are presented in Table 1.
3.2. Powders—Morphology, Particle Size Distribution and other Physicochemical Properties
Morphology, particle size distribution, and physicochemical properties of encapsulated powders are related to processing parameters, including feeding rate, initial concentration of the solid material, inlet and outlet temperatures, surface tension, and other intrinsic properties of the drying material [40].
The SEM images of SD and FD powders are shown in Figure 4. Powders obtained by the SD technique were characterized by a spherical shape, while the particles of powders obtained by FD had broken glasslike surfaces, similar to flakes. The images showed that regardless of the used core and wall ingredients, the powders were similar to each other for drying technique. Similarly, no differences were observed between microcapsules containing linseed oil and its esters. All SD samples had particles that varied in size, some being quite small and others being large. Most of the particles did not have a smooth surface, and dents were visible. Additionally, agglomeration of the same particles was observed. Similar observations were presented by Teo et al. [41] in powders containing high concentrations of maltodextrin. The authors reported that it can be related to a slow rate of crust formation and less elasticity of the wall matrix to resist structural collapse of the droplets during the spray-drying process [41]. Our results are in agreement with the findings of Tatar et al. [42]. In their study, powders containing maltodextrin and gum arabic had many dents and were remarkably shrunk. In turn, FD powders had a large, rather smooth surface. The image of LMG powder also showed the cross-section of powder particles (circled with a blue loop). Generally, FD powders had many pores on the surface as a result of the water sublimation during freeze-drying [16,43], but in the present study, it was shown that the particles did not have a porous structure. Elik et al. [16] also observed a smooth surface of FD powders containing, depending on the variant, different proportions of maltodextrin, low-methoxylated pectin, or sunflower wax. Nevertheless, by analyzing the structure of the products obtained as a result of freeze-drying, it should be taken into account that particle morphology reflects the result of grinding.
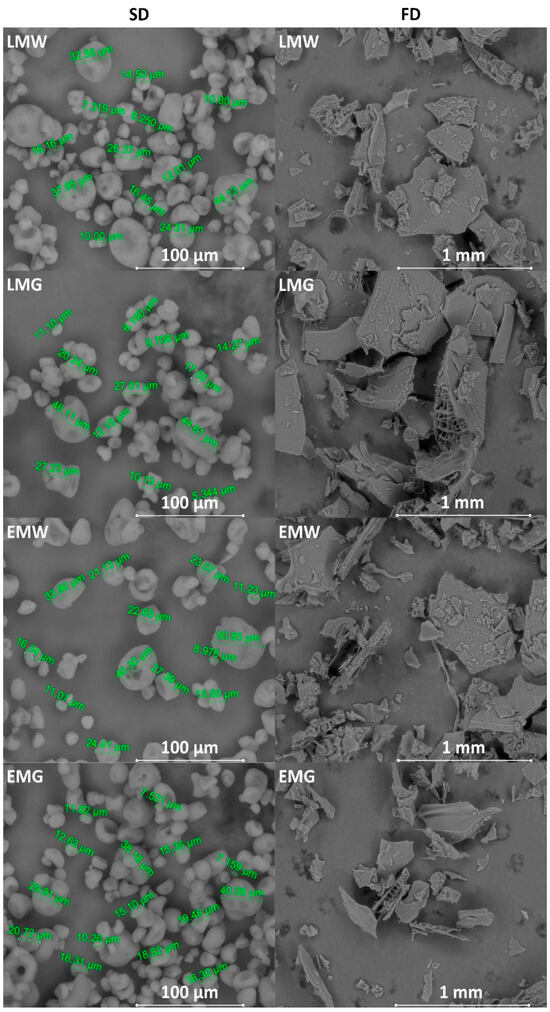
Figure 4.
Scanning electron microscope images of powders prepared by spry- (SD) and freeze-drying (FD) (SD 400×, FD 50×). LMW, LMG, EMW, EMG—powder variants obtained from emulsions, which compositions are presented in Table 1.
Table 2 presents the selected physicochemical properties of powders. Powders produced by FD were characterized by a slightly lower moisture content (1.23–1.57%) compared to powders produced by SD (2.09–2.84%). Similar moisture values of the obtained microcapsules with both drying techniques were previously stated by Quispe-Condori et al. [44] and Misra et al. [45]. The influence of coating ingredients on the moisture of powders was also observed, which was lower in the case of samples containing gum arabic. The determined variation of a powder’s moisture made with WPC and gum arabic seems to be a general rule, since in a study conducted by Silva et al. [46] powders made of a mixture of gum arabic and maltodextrin had a moisture content in the range of 1.6–2.0%, while in a study conducted by Campelo et al. [28], the moisture content was in the range of 3.83–5.35% for the powders made with WPC and maltodextrin. Overall, the drying process parameters used in the current study resulted in powders with the desired moisture level, which should not exceed the 4% allowed by the food industry [16,44].

Table 2.
Physicochemical properties of powders prepared by spry- (SD) and freeze-drying (FD).
Encapsulation efficiency is one of the most important parameters in powder production, as well as surface oil presence. The type of wall material and the drying method simultaneously showed a significant impact on the lipid content on the surface. Regardless of whether linseed oil or its ethyl esters were encapsulated, powders in which the coating material was gum arabic and maltodextrin that were dried by the FD technique were characterized by the lowest surface oil content of 4.42 and 5.52%, respectively. In contrast, powders composed of maltodextrin and WPC obtained by the same technique had the highest surface oil content: 11.48% (LMW) and 10.95% (EMW). In other variants of powders, surface oil ranged from 6.78% (EMG) to 8.78% (LMG). Ozdemir et al. [47] also obtained the lowest surface oil values for powders containing gum arabic.
The surface oil content determines encapsulation efficiency (the lower the surface oil content, the higher the encapsulation efficiency). In the current study, the opposite values were found for LMG (88.94%) and LMW (71.31%) samples, both dried by the FD technique. In the case of powders obtained by the SD technique, the encapsulation efficiency was in a narrower range, from 78.06% (LMG) to 83.06% (EMG). In a study conducted by Carneiro et al. [48], the encapsulation efficiency of linseed oil varied from 62.3 to 95.7%, with the lowest value obtained for powders with maltodextrin and WPC. In turn, Akram et al. [49] confirmed the high ability of the gum arabic and maltodextrin mixture for the encapsulation efficiency of fish oil (83.4%) and flaxseed oil (84.5%). This confirms that the encapsulation efficiency depends on the ingredients used as coating materials. Carneiro et al. [48] reported that the differences in encapsulation efficiency are related to the differences between the polymer matrices, which have different retention properties and film-forming capabilities. WPC has worse coating properties compared to gum arabic [50]. Additionally, it can also be a result of the penetration of ice crystals into oil droplets and the distribution of the interfacial membrane, therefore leaking the oil from the core to the surface, leading to a higher amount of surface oil in the powders [18]. In turn, Linke et al. [51] reported that encapsulation efficiency is correlated with particle sizes. In their study, the lowest encapsulation efficiency was achieved for the smallest particle fraction, and it was assumed that due to a large surface-to-volume ratio of small powder particles, the probability of oil droplets being in contact with the particle surface increases, which leads to more surface oil. This is in accordance with our study, where particle mean diameter D3,2 was in the range of 22.62 µm (LMG) to 24.20 µm (LMW) in SD powders. The largest average diameter of D4,3 particles was 47.35 µm for powder EMG obtained by the SD technique. This value was really high when compared to EMW (28.33 µm) and powders that contain linseed oil: 34.46 µm (LMW) and 37.35 µm (LMG). This is in agreement with the results published by Carneiro et al. [48] for linseed oil encapsulated by mixing maltodextrin with gum arabic (23.03 µm) and WPC (17.98 µm), which the authors explained by the high emulsion viscosity. Even smaller diameters D4,3 for powders that were WPC coated in the range of 10.04 to 10.91 µm were reported by Takeungwongtrakul et al. [52]. Similarly, Akram et al. [49] reported particle diameters in the range from 14.20 to 25.30 µm for powders with maltodextrin and gum arabic and attributed these results to higher emulsion viscosity, which is a crucial factor resulting in the larger microcapsule size of powders. Powders containing gum arabic also had the highest span values of 1.90 and 1.99 for samples containing oil and esters, respectively. However, the SSA value in all powders was at the same significant level (0.25–0.27 m2/g). Elsewhere, Najaf Najafi et al. [53] reported that the type and concentration of wall material significantly influence the size (D3,2) and SSA of spray-dried powders. In their study, modified starch (Hi-Cap 100, refined from waxy maize) resulted in larger particles with smaller SSA compared to skim milk powder.
Surface oil content differed between prepared powders from 4.42% (LMG) to 11.48% (LMW), both obtained by freeze-drying (Table 3). Apart from surface oil content variation, the composition of fatty acids was relatively stable and typical for high-ALA linseed oils [54,55]. ALA ranged from 50.43% (LMG) to 53.43% (EMW), in both powders prepared through freeze-drying. It points out that part of ALA can be easily oxidizable, even in the form of powder.

Table 3.
Fatty acid composition of surface oil extracted from powders prepared by spry- (SD) and freeze-drying (FD).
The induction period of used linseed oil was 2.40 h, while that of prepared ethyl esters was only 1.23 h (Figure 5). A similar phenomenon was determined using tridocosahexaenoin (more stable) and ethyl docosahexaenoate [56]. The lower oxidative stability of ethyl esters may be the result of the degradation of natural antioxidants such as tocopherols, phenols, and flavonoids present in linseed oil [57] during their preparation.
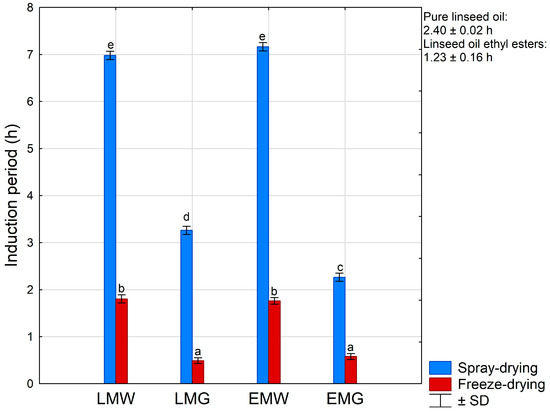
Figure 5.
Induction period (IP) (average value ± standard deviation (SD)) of linseed oil, linseed oil ethyl esters and powders prepared by spry- (SD) and freeze-drying (FD). LMW, LMG, EMW, EMG—powder variants obtained from emulsions, which compositions are presented in Table 1. Means within lines with different letters are significantly different (p < 0.05).
The induction periods of the prepared powders are presented in Figure 5. It is clearly visible that the type of drying technique was decisive for the oxidative stability of the obtained powders. Spray-drying resulted in approximately 4-fold more stable powders in relation to freeze-drying. Among spray-dried powders, the highest oxidative stability exhibited samples were made with WPC (7.17 h vs. 6.98 h for powders of ethyl esters and linseed oil, respectively). Powders with gum arabic exhibited 2–3-fold lower stability than those corresponding with WPC. As in the case of spray-dried samples and also in the case of freeze-dried powders, better oxidative stability was found for samples prepared using WPC. The form of the n-3 fatty acid source (oil or ethyl esters) had no effect on the oxidative stability of the powder.
The greater ability of the spray-drying technique in the protection of unsaturated fatty acids in selected plant oils microcapsules was recently presented by Abdel-Razek et al. [58]. Similarly, the better ability of WPC vs. gum arabic mixtures with maltodextrin as components of wall material for linseed oil protection was found by Carneiro et al. [48]. It was expected, since antioxidant properties had previously been observed for different commercial whey products (more details in the review by Corrochano et al. [59]). Whey hydrolysates and the free amino acids present in them, such as tryptophan, phenylalanine, tyrosine, cysteine, and histidine, contribute mostly to the antioxidant properties of these products [59].
4. Conclusions
Encapsulation is a common technique used in the food industry to protect various bioactive compounds. The first step in this process is to create a stable emulsion. This study showed that emulsions of linseed oil and linseed oil ethyl esters with a mixture of gum arabic had better oil droplet distribution and stability than with WPC. In contrast, these ALA preparations can be effectively encapsulated with a mixture of maltodextrin and WPC in a ratio of 1:1 to protect ALA against oxidation. Although the oxidative stability of pure linseed oil is approximately 2-fold greater than that of its ethyl esters, spray-dried powders containing both forms of ALA and WPC had the same oxidative stability. The results of the current study showed that freeze-drying should not be recommended for the preparation of oxidatively stable ALA powders. Oxidatively stable powders can be successfully used for the production of functional foods, e.g., fruit juices enriched with ALA, but such use requires further research in terms of the assessment of acceptability in sensory properties.
Author Contributions
Conceptualization, D.O., M.T., P.B. and I.Z.K.; methodology, D.O., M.T. and P.B.; formal analysis, D.O., P.B., M.W., G.D. and S.C.; investigation, M.T. and I.Z.K.; data curation, D.O., P.B. and G.D.; writing—original draft preparation, D.O., I.Z.K., M.T. and P.B.; writing—review and editing, D.O., I.Z.K., M.T., G.D. and S.C.; visualization, P.B. and G.D.; supervision, I.Z.K.; project administration, I.Z.K.; funding acquisition, D.O. and I.Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Warmia and Mazury “Rector’s Scientific Grant” project (no. NGR-180-2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Waldemar Brandt for technical assistance with part of the spray-drying process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Knoema. Linseed Production in the World. Available online: https://knoema.com/data/agriculture-indicators-production+linseed (accessed on 22 January 2024).
- Al-Madhagy, S.; Ashmawy, N.S.; Mamdouh, A.; Eldahshan, O.A.; Farag, M.A. A Comprehensive Review of the Health Benefits of Flaxseed Oil in Relation to Its Chemical Composition and Comparison with Other Omega-3-Rich Oils. Eur. J. Med. Res. 2023, 28, 240. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The Review of Alpha-linolenic Acid: Sources, Metabolism, and Pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Aune, D.; Beyene, J.; Mobarak, S.; Asadi, M.; Sadeghi, O. Dietary Intake and Biomarkers of Alpha Linolenic Acid and Risk of All Cause, Cardiovascular, and Cancer Mortality: Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. BMJ 2021, 375, n2213. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.H.; Reifen, R.; Crawford, M.A. Past and Present Insights on Alpha-Linolenic Acid and the Omega-3 Fatty Acid Family. Crit. Rev. Food Sci. Nutr. 2016, 56, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, N.R.; Loh, T.C.; Foo, H.L.; Alshelmani, M.I.; Akit, H. Influence of Dietary Ratios of n-6: N-3 Fatty Acid on Gene Expression, Fatty Acid Profile in Liver and Breast Muscle Tissues, Serum Lipid Profile, and Immunoglobulin in Broiler Chickens. J. Appl. Poult. Res. 2019, 28, 454–469. [Google Scholar] [CrossRef]
- Elgar, K. EPA/DHA: A Review of Clinical Use and Efficacy. Nutr. Med. J. 2022, 2, 97–132. [Google Scholar]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent Advances in Fishy Odour in Aquatic Fish Products, from Formation to Control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- IPSOS. An Exploration into Diets around the World. Available online: https://www.ipsos.com/sites/default/files/ct/news/documents/2018-09/an_exploration_into_diets_around_the_world.pdf (accessed on 29 November 2023).
- Kim, D.; Choi, J.-E.; Park, Y. Low-Linoleic Acid Diet and Oestrogen Enhance the Conversion of α-Linolenic Acid into DHA through Modification of Conversion Enzymes and Transcription Factors. Br. J. Nutr. 2019, 121, 137–145. [Google Scholar] [CrossRef]
- Díaz-Montes, E. Wall Materials for Encapsulating Bioactive Compounds via Spray-Drying: A Review. Polymers 2023, 15, 2659. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Damerau, A.; Ogrodowska, D.; Banaszczyk, P.; Dajnowiec, F.; Tańska, M.; Linderborg, K.M. Baltic Herring (Clupea harengus membras) Oil Encapsulation by Spray Drying Using a Rice and Whey Protein Blend as a Coating Material. J. Food Eng. 2022, 314, 110769. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef] [PubMed]
- Elik, A.; Koçak Yanık, D.; Göğüş, F. A Comparative Study of Encapsulation of Carotenoid Enriched-Flaxseed Oil and Flaxseed Oil by Spray Freeze-Drying and Spray Drying Techniques. LWT 2021, 143, 111153. [Google Scholar] [CrossRef]
- Naz, S.; Shabbir, M.A.; Aadil, R.M.; Khan, M.R.; Ciftci, O.N.; Sameen, A.; Yasmin, I.; Hayee, A.; Maqsood, M. Effect of Polymer and Polymer Blends on Encapsulation Efficiency of Spray-Dried Microencapsulated Flaxseed Oil. Int. Food Res. J. 2020, 27, 78–87. [Google Scholar]
- Avci, E.; Karadag, A.; Karasu, S. Production of Freeze-Dried Flaxseed Oil Powders by Using Rocket Seed Gum as an Alternative Novel Encapsulation Agent to Improve Oxidative Stability. Sains Malays. 2021, 51, 3647–3662. [Google Scholar] [CrossRef]
- Aguilera-Oviedo, J.; Yara-Varón, E.; Torres, M.; Canela-Garayoa, R.; Balcells, M. Sustainable Synthesis of Omega-3 Fatty Acid Ethyl Esters from Monkfish Liver Oil. Catalysts 2021, 11, 100. [Google Scholar] [CrossRef]
- Dempsey, M.; Rockwell, M.S.; Wentz, L.M. The Influence of Dietary and Supplemental Omega-3 Fatty Acids on the Omega-3 Index: A Scoping Review. Front. Nutr. 2023, 10, 1072653. [Google Scholar] [CrossRef]
- Chevalier, L.; Plourde, M. Comparison of Pharmacokinetics of Omega-3 Fatty Acid Supplements in Monoacylglycerol or Ethyl Ester in Humans: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2021, 75, 680–688. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Damerau, A.; Banaszczyk, P.; Tańska, M.; Konopka, I.Z.; Piłat, B.; Dajnowiec, F.; Linderborg, K.M. Native and Pregelatinized Potato and Rice Starches and Maltodextrin as Encapsulating Agents for Linseed Oil Ethyl Esters—Comparison of Emulsion and Powder Properties. J. Food Eng. 2024, 364, 111799. [Google Scholar] [CrossRef]
- Armenta, R.E.; Vinatoru, M.; Burja, A.M.; Kralovec, J.A.; Barrow, C.J. Transesterification of Fish Oil to Produce Fatty Acid Ethyl Esters Using Ultrasonic Energy. J. Am. Oil Chem. Soc. 2007, 84, 1045–1052. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Malvern Instruments Ltd. User Manual. Mastersizer 2000, MANO347; Malvern Instruments Ltd.: Worcestershire, UK, 2007. [Google Scholar]
- Dąbrowski, G.; Czaplicki, S.; Szustak, M.; Cichońska, E.; Gendaszewska-Darmach, E.; Konopka, I. Composition of Flesh Lipids and Oleosome Yield Optimization of Selected Sea Buckthorn (Hippophae rhamnoides L.) Cultivars Grown in Poland. Food Chem. 2022, 369, 130921. [Google Scholar] [CrossRef]
- Hu, Y.-T.; Ting, Y.; Hu, J.-Y.; Hsieh, S.-C. Techniques and Methods to Study Functional Characteristics of Emulsion Systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef]
- Campelo, P.H.; do Carmo, E.L.; Zacarias, R.D.; Yoshida, M.I.; Ferraz, V.P.; de Barros Fernandes, R.V.; Botrel, D.A.; Borges, S.V. Effect of Dextrose Equivalent on Physical and Chemical Properties of Lime Essential Oil Microparticles. Ind. Crop. Prod. 2017, 102, 105–114. [Google Scholar] [CrossRef]
- Premi, M.; Sharma, H.K. Effect of Different Combinations of Maltodextrin, Gum Arabic and Whey Protein Concentrate on the Encapsulation Behavior and Oxidative Stability of Spray Dried Drumstick (Moringa oleifera) Oil. Int. J. Biol. Macromol. 2017, 105, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Ursica, L.; Tita, D.; Palici, I.; Tita, B.; Vlaia, V. Particle Size Analysis of Some Water/Oil/Water Multiple Emulsions. J. Pharm. Biomed. Anal. 2005, 37, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Hebishy, E.; Zamora, A.; Buffa, M.; Blasco-Moreno, A.; Trujillo, A.-J. Characterization of Whey Protein Oil-in-Water Emulsions with Different Oil Concentrations Stabilized by Ultra-High Pressure Homogenization. Processes 2017, 5, 6. [Google Scholar] [CrossRef]
- Dybowska, B.E. Whey Protein-Stabilized Emulsion Properties in Relation to Thermal Modification of the Continuous Phase. J. Food Eng. 2011, 104, 81–88. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent. Polymers 2020, 12, 2207. [Google Scholar] [CrossRef] [PubMed]
- Querol, N.; Barreneche, C.; Cabeza, L. Storage Stability of Bimodal Emulsions vs. Monomodal Emulsions. Appl. Sci. 2017, 7, 1267. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving Emulsion Formation, Stability and Performance Using Mixed Emulsifiers: A Review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Písecký, J. Chapt. 10 Achieving Product Properties. In Handbook of Milk Powder Manufacture; Niro A/S: Copenhagen, Denmark, 1997. [Google Scholar]
- McKenna, A.B.; Lloyd, R.J.; Munro, P.A.; Singh, H. Microstructure of Whole Milk Powder and of Insolubles Detected by Powder Functional Testing. Scanning 1999, 21, 305–315. [Google Scholar] [CrossRef]
- Charoen, R.; Jangchud, A.; Jangchud, K.; Harnsilawat, T.; Naivikul, O.; McClements, D.J. Influence of Biopolymer Emulsifier Type on Formation and Stability of Rice Bran Oil-in-water Emulsions: Whey Protein, Gum Arabic, and Modified Starch. J. Food Sci. 2011, 76, E165–E172. [Google Scholar] [CrossRef] [PubMed]
- Chanamai, R.; McClements, D.J. Comparison of Gum Arabic, Modified Starch, and Whey Protein Isolate as Emulsifiers: Influence of PH, CaCl2 and Temperature. J. Food Sci. 2002, 67, 120–125. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Teo, A.; Lam, Y.; Lee, S.J.; Goh, K.K.T. Spray Drying of Whey Protein Stabilized Nanoemulsions Containing Different Wall Materials—Maltodextrin or Trehalose. LWT 2021, 136, 110344. [Google Scholar] [CrossRef]
- Tatar, F.; Tunç, M.T.; Dervisoglu, M.; Cekmecelioglu, D.; Kahyaoglu, T. Evaluation of Hemicellulose as a Coating Material with Gum Arabic for Food Microencapsulation. Food Res. Int. 2014, 57, 168–175. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Tańska, M.; Brandt, W. The Influence of Drying Process Conditions on the Physical Properties, Bioactive Compounds and Stability of Encapsulated Pumpkin Seed Oil. Food Bioprocess Technol. 2017, 10, 1265–1280. [Google Scholar] [CrossRef]
- Quispe-Condori, S.; Saldaña, M.D.A.; Temelli, F. Microencapsulation of Flax Oil with Zein Using Spray and Freeze Drying. LWT 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Panigrahi, C.; Mishra, H.N. A Comparative Approach on the Spray and Freeze Drying of Probiotic and Gamma-Aminobutyric Acid as a Single Entity: Characterization and Evaluation of Stability in Simulated Gastrointestinal Conditions. Food Chem. Adv. 2023, 3, 100385. [Google Scholar] [CrossRef]
- Silva, V.M.; Vieira, G.S.; Hubinger, M.D. Influence of Different Combinations of Wall Materials and Homogenisation Pressure on the Microencapsulation of Green Coffee Oil by Spray Drying. Food Res. Int. 2014, 61, 132–143. [Google Scholar] [CrossRef]
- Ozdemir, N.; Bayrak, A.; Tat, T.; Altay, F.; Kiralan, M.; Kurt, A. Microencapsulation of Basil Essential Oil: Utilization of Gum Arabic/Whey Protein Isolate/Maltodextrin Combinations for Encapsulation Efficiency and in Vitro Release. J. Food Meas. Charact. 2021, 15, 1865–1876. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Akram, S.; Bao, Y.; Butt, M.S.; Shukat, R.; Afzal, A.; Huang, J. Fabrication and Characterization of Gum Arabic- and Maltodextrin-based Microcapsules Containing Polyunsaturated Oils. J. Sci. Food Agric. 2021, 101, 6384–6394. [Google Scholar] [CrossRef]
- Vahidmoghadam, F.; Pourahmad, R.; Mortazavi, A.; Davoodi, D.; Azizinezhad, R. Characteristics of Freeze-Dried Nanoencapsulated Fish Oil with Whey Protein Concentrate and Gum Arabic as Wall Materials. Food Sci. Technol. 2019, 39, 475–481. [Google Scholar] [CrossRef]
- Linke, A.; Weiss, J.; Kohlus, R. Factors Determining the Surface Oil Concentration of Encapsulated Lipid Particles: Impact of the Emulsion Oil Droplet Size. Eur. Food Res. Technol. 2020, 246, 1933–1943. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Wall Materials and the Presence of Antioxidants Influence Encapsulation Efficiency and Oxidative Stability of Micro-encapsulated Shrimp Oil. Eur. J. Lipid Sci. Technol. 2015, 117, 450–459. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Kadkhodaee, R.; Mortazavi, S.A. Effect of Drying Process and Wall Material on the Properties of Encapsulated Cardamom Oil. Food Biophys. 2011, 6, 68–76. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Değerli, Z.; Özcan, M.M.; Babiker, E.E. Effect of Different Oil Extraction Methods on Bioactive Compounds, Antioxidant Capacity and Phytochemical Profiles of Raw Flaxseeds (Linum Usitatissimum) and after Roasting at Different Temperatures. J. Sci. Food Agric. 2023, 103, 7117–7126. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Tańska, M.; Konopka, I. Impact of the Addition of 4-Vinyl-Derivatives of Ferulic and Sinapic Acids on Retention of Fatty Acids and Terpenoids in Cold-Pressed Rapeseed and Flaxseed Oils during the Induction Period of Oxidation. Food Chem. 2019, 278, 119–126. [Google Scholar] [CrossRef]
- Ahonen, E.; Damerau, A.; Suomela, J.-P.; Kortesniemi, M.; Linderborg, K.M. Oxidative Stability, Oxidation Pattern and α-Tocopherol Response of Docosahexaenoic Acid (DHA, 22:6n–3)-Containing Triacylglycerols and Ethyl Esters. Food Chem. 2022, 387, 132882. [Google Scholar] [CrossRef] [PubMed]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Hassanein, M.M.; Ozçelik, B.; Baranenko, D.A.; El-Messery, T.M. Omega Fatty Acid-Balanced Oil Formula and Enhancing Its Oxidative Stability by Encapsulation with Whey Protein Concentrate. Food Biosci. 2022, 50, 101975. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited Review: Whey Proteins as Antioxidants and Promoters of Cellular Antioxidant Pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).