Recovery of Bioactive Components from Strawberry Seeds Residues Post Oil Extraction and Their Cosmetic Potential
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Chromatographic Analysis
2.4. Cell Line Assay
2.4.1. Cell Viability
2.4.2. Cytoskeleton Organization
2.4.3. Intracellular Levels of Reactive Oxygen Species (ROS)
2.5. Statistic
3. Results and Discussion
3.1. Phenolic Composition of the Plant Material
3.2. Optimization of Extraction Procedure to Obtain Phenolic Rich Fraction
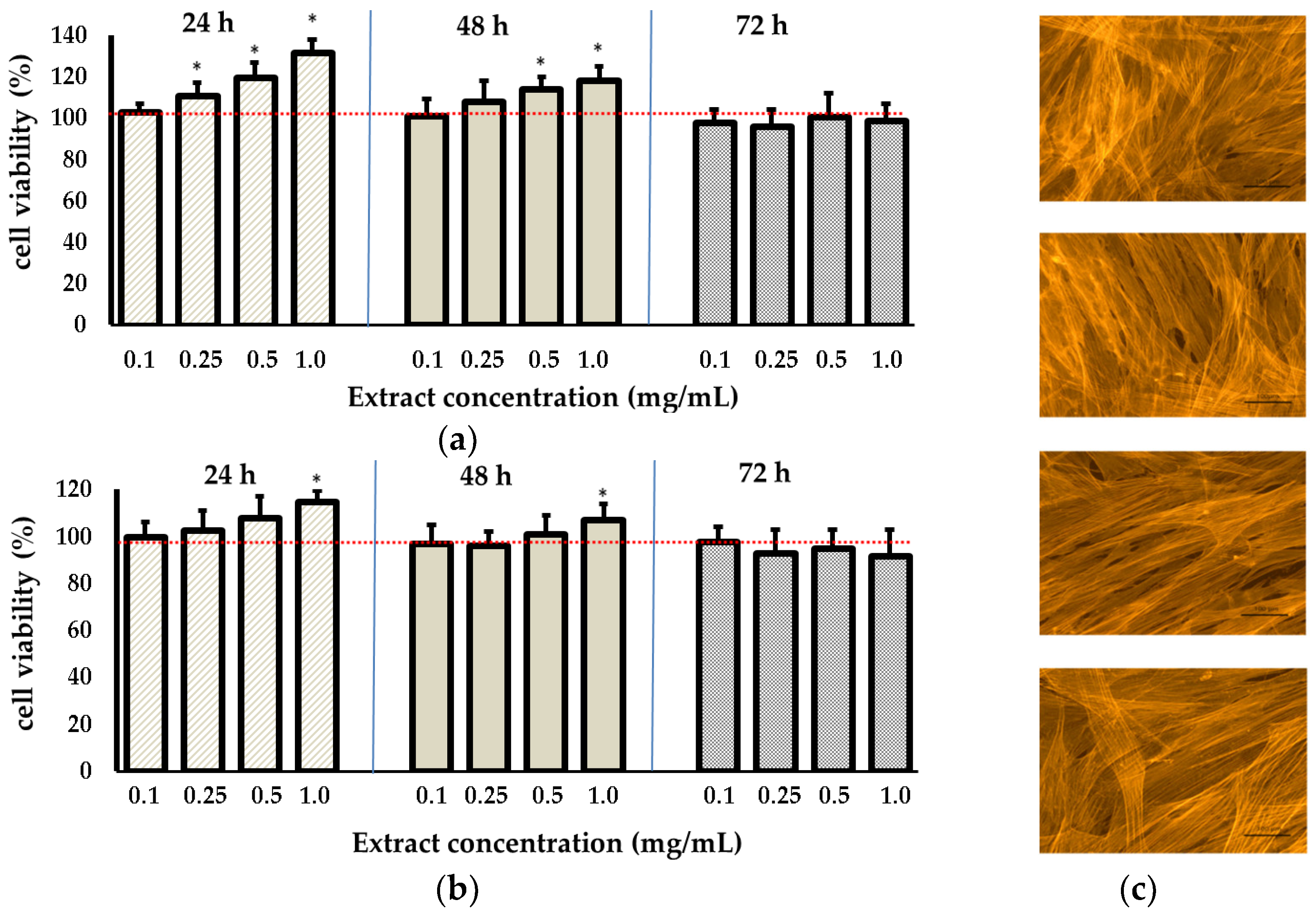
3.3. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaim, U. Methods for the Valorisation of Agri-Food Waste According to the Circular Bioeconomy Concept. Eng. Sci. Technol. 2021, 2021, 94–112. [Google Scholar] [CrossRef]
- Facchini, F.; Silvestri, B.; Digiesi, S.; Lucchese, A. Agri-Food Loss and Waste Management: Win-Win Strategies for Edible Discarded Fruits and Vegetables Sustainable Reuse. Innov. Food Sci. Emerg. Technol. 2023, 83, 103235. [Google Scholar] [CrossRef]
- Sławińska, N.; Prochoń, K.; Olas, B. A Review on Berry Seeds—A Special Emphasis on Their Chemical Content and Health-Promoting Properties. Nutrients 2023, 15, 1422. [Google Scholar] [CrossRef]
- Qaderi, R.; Mezzetti, B.; Capocasa, F.; Mazzoni, L. Stability of Strawberry Fruit (Fragaria × Ananassa Duch.) Nutritional Quality at Different Storage Conditions. Appl. Sci. 2022, 13, 313. [Google Scholar] [CrossRef]
- Salas-Arias, K.; Irías-Mata, A.; Sánchez-Kopper, A.; Hernández-Moncada, R.; Salas-Morgan, B.; Villalta-Romero, F.; Calvo-Castro, L.A. Strawberry Fragaria × Ananassa Cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules 2023, 28, 1865. [Google Scholar] [CrossRef] [PubMed]
- Newerli-Guz, J.; Śmiechowska, M.; Drzewiecka, A.; Tylingo, R. Bioactive Ingredients with Health-Promoting Properties of Strawberry Fruit (Fragaria × Ananassa Duchesne). Molecules 2023, 28, 2711. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.D.S.; Sebastià, N.; Montoro, A.; García-Martínez, E. Strawberry (Fragaria × Ananassa) and Kiwifruit (Actinidia Deliciosa) Extracts as Potential Radioprotective Agents: Relation to Their Phytochemical Composition and Antioxidant Capacity. Appl. Sci. 2023, 13, 8996. [Google Scholar] [CrossRef]
- Pukalskienė, M.; Pukalskas, A.; Dienaitė, L.; Revinytė, S.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Recovery of Bioactive Compounds from Strawberry (Fragaria × Ananassa) Pomace by Conventional and Pressurized Liquid Extraction and Assessment Their Bioactivity in Human Cell Cultures. Foods 2021, 10, 1780. [Google Scholar] [CrossRef]
- Davidson, M.; Louvet, F.; Meudec, E.; Landolt, C.; Grenier, K.; Périno, S.; Ouk, T.-S.; Saad, N. Optimized Single-Step Recovery of Lipophilic and Hydrophilic Compounds from Raspberry, Strawberry and Blackberry Pomaces Using a Simultaneous Ultrasound-Enzyme-Assisted Extraction (UEAE). Antioxidants 2023, 12, 1793. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Moccia, F.; Tedesco, I.; Adabbo, E.; Panzella, L.; Russo, G.L.; Napolitano, A. Stillage Waste from Strawberry Spirit Production as a Source of Bioactive Compounds with Antioxidant and Antiproliferative Potential. Antioxidants 2023, 12, 421. [Google Scholar] [CrossRef]
- Raczyk, M.; Bryś, J.; Brzezińska, R.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Górska, A. Quality Assessment of Cold-Pressed Strawberry, Raspberry and Blackberry Seed Oils Intended for Cosmetic Purposes. Acta Sci. Pol. Technol. Aliment. 2021, 20, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Milala, J.; Grzelak-Błaszczyk, K.; Sójka, M.; Kosmala, M.; Dobrzyńska-Inger, A.; Rój, E. Changes of Bioactive Components in Berry Seed Oils during Supercritical CO 2 Extraction. J. Food Process. Preserv. 2018, 42, e13368. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Gastélum-Estrada, A.; Chuck-Hernandez, C.; Antunes-Ricardo, M.; Reza-Zaldivar, E.E.; Piagentini, A.; Jacobo-Velázquez, D.A. Kinetic Ultrasound-Assisted Extraction as a Sustainable Approach for the Recovery of Phenolics Accumulated through UVA Treatment in Strawberry By-Products. Foods 2023, 12, 2989. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Zduńczyk, Z.; Juśkiewicz, J.; Jurgoński, A.; Karlińska, E.; Macierzyński, J.; Jańczak, R.; Rój, E. Chemical Composition of Defatted Strawberry and Raspberry Seeds and the Effect of These Dietary Ingredients on Polyphenol Metabolites, Intestinal Function, and Selected Serum Parameters in Rats. J. Agric. Food Chem. 2015, 63, 2989–2996. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Król, B.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Żary-Sikorska, E. Physiological Properties of Dietary Ellagitannin-Rich Preparations Obtained from Strawberry Pomace Using Different Extraction Methods. Pol. J. Food Nutr. Sci. 2015, 65, 199–209. [Google Scholar] [CrossRef]
- Grzelak-Błaszczyk, K.; Karlińska, E.; Grzęda, K.; Rój, E.; Kołodziejczyk, K. Defatted Strawberry Seeds as a Source of Phenolics, Dietary Fiber and Minerals. LWT 2017, 84, 18–22. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Duda, A.; Poliszko, N.; Różańska, M.B.; Jeżowski, P.; Tomkowiak, A.; Mildner-Szkudlarz, S.; Baranowska, H.M. Wheat Bread Enriched with Raspberry and Strawberry Oilcakes: Effects on Proximate Composition, Texture and Water Properties. Eur. Food Res. Technol. 2019, 245, 2591–2600. [Google Scholar] [CrossRef]
- Wójciak, M.; Mazurek, B.; Tyśkiewicz, K.; Kondracka, M.; Wójcicka, G.; Blicharski, T.; Sowa, I. Blackcurrant (Ribes nigrum L.) Seeds—A Valuable Byproduct for Further Processing. Molecules 2022, 27, 8679. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Kocjan, R.; Nowak, R. Effect of Different Extraction Techniques on Quantification of Oleanolic and Ursolic Acid in Lamii Albi Flos. Ind. Crops Prod. 2013, 44, 373–377. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Podgórski, R. Influence of Different Extraction Methods on the Quantification of Selected Flavonoids and Phenolic Acids from Tilia Cordata Inflorescence. Ind. Crops Prod. 2015, 76, 509–514. [Google Scholar] [CrossRef]
- Skalska-Kamińska, A.; Wójciak, W.; Żuk, M.; Paduch, R.; Wójciak, M. Protective Effect of Urtica Dioica Extract against Oxidative Stress in Human Skin Fibroblasts. Life 2023, 13, 2182. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant Potential of Diosmin and Diosmetin against Oxidative Stress in Endothelial Cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef]
- Sowa, I.; Paduch, R.; Strzemski, M.; Zielińska, S.; Rydzik-Strzemska, E.; Sawicki, J.; Kocjan, R.; Polkowski, J.; Matkowski, A.; Latalski, M.; et al. Proliferative and Antioxidant Activity of Symphytum officinale Root Extract. Nat. Prod. Res. 2018, 32, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef]
- Ratz-Łyko, A.; Arct, J.; Majewski, S.; Pytkowska, K. Influence of Polyphenols on the Physiological Processes in the Skin. Phytother. Res. 2015, 29, 509–517. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Locatelli, M.; Granica, S.; Cacciagrano, F.; Tomczyk, M. A Review on the Dietary Flavonoid Tiliroside. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1395–1421. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Shimoda, H.; Takarada, T.; Imokawa, G. Strawberry Seed Extract and Its Major Component, Tiliroside, Promote Ceramide Synthesis in the Stratum Corneum of Human Epidermal Equivalents. PLoS ONE 2018, 13, e0205061. [Google Scholar] [CrossRef]
- Tomczyk, M.; Bazylko, A.; Staszewska, A. Determination of Polyphenolics in Extracts of Potentilla Species by High-performance Thin-layer Chromatography Photodensitometry Method. Phytochem. Anal. 2010, 21, 174–179. [Google Scholar] [CrossRef]
- Nowak, R. Separation and Quantification of Tiliroside from Plant Extracts by SPE/RP-HPLC. Pharm. Biol. 2003, 41, 627–630. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Nowak, R.; Pielczyk, J.; Łamacz, K. Optimization of Extraction Conditions for Determination of Tiliroside in Tilia L. Flowers Using an LC-ESI-MS/MS Method. J. Anal. Methods Chem. 2019, 2019, 9052425. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Šamec, D.; Šalić, A. Modern Techniques for Flavonoid Extraction—To Optimize or Not to Optimize? Appl. Sci. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Babich, H.; Borenfreund, E. Applications of the Neutral Red Cytotoxicity Assay to In Vitro Toxicology. Altern. Lab. Anim. 1990, 18, 129–144. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.-L. Assessment of the Anti-Inflammatory Activity and Free Radical Scavenger Activity of Tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Wang, T.; Lin, Q.; Feng, X.; Jiang, Q.; Liu, Y.; Chen, D. Role of the P-Coumaroyl Moiety in the Antioxidant and Cytoprotective Effects of Flavonoid Glycosides: Comparison of Astragalin and Tiliroside. Molecules 2017, 22, 1165. [Google Scholar] [CrossRef]
- Baek, B.; Lee, S.H.; Kim, K.; Lim, H.-W.; Lim, C.-J. Ellagic Acid Plays a Protective Role against UV-B-Induced Oxidative Stress by up-Regulating Antioxidant Components in Human Dermal Fibroblasts. Korean J. Physiol. Pharmacol. 2016, 20, 269. [Google Scholar] [CrossRef]
- Park, S.N.; Kim, S.Y.; Lim, G.N.; Jo, N.R.; Lee, M.H. In Vitro Skin Permeation and Cellular Protective Effects of Flavonoids Isolated from Suaeda Asparagoides Extracts. J. Ind. Eng. Chem. 2012, 18, 680–683. [Google Scholar] [CrossRef]
- Taiwo, F.O.; Oyedeji, O.; Osundahunsi, M.T. Antimicrobial and Antioxidant Properties of Kaempferol-3-O-Glucoside and 1-(4-Hydroxyphenyl)-3-Phenylpropan-1-One Isolated from the Leaves of Annona Muricata (Linn.). J. Pharm. Res. Int. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Carola, C.; Pfluecker, F.; Graf, R.; Epstein, H.; Wirth, C. Tiliroside and Dihydroxy Methylchromone: From Nature to Cosmetic Applications. J. Appl. Cosmetol. 2010, 28, 109–123. [Google Scholar]
- Bae, J.; Choi, J.; Kang, S.; Lee, Y.; Park, J.; Kang, Y. Dietary Compound Ellagic Acid Alleviates Skin Wrinkle and Inflammation Induced by UV-B Irradiation. Exp. Dermatol. 2010, 19, e182–e190. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J.; Wei, D.; Wang, Z.; Tao, X. Tyrosinase Inhibitory Effect and Inhibitory Mechanism of Tiliroside from Raspberry. J. Enzym. Inhib. Med. Chem. 2009, 24, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Tavares, W.D.S.; Martin Pastor, M.; Pérez, L.; Morán, M.D.C.; Sousa, F.F.O.D. Skin Repairing Potential of Ellagic Acid-Loaded Zein Nanoparticles: Chemical and Biopharmaceutical Characterization, Enzymatic Inhibition and Cytotoxicity over Keratinocytes. J. Mol. Liq. 2023, 384, 122198. [Google Scholar] [CrossRef]
- Shimogaki; Tanaka; Tamai; Masuda. In Vitro and in Vivo Evaluation of Ellagic Acid on Melanogenesis Inhibition. Int. J. Cosmet. Sci. 2000, 22, 291–303. [Google Scholar] [CrossRef] [PubMed]



| RT (min) | [M − H]− | Formula | Name | Seeds Defatted at 230 PSI (mg/100 g) | Seeds Defatted at 330 PSI (mg/100 g) |
|---|---|---|---|---|---|
| 7.73 | 451.12448 | C21H24O11 | catechin hexoside 2 | 8.7 ± 1.1 a | 9.1 ± 1.2 a |
| 10.07 | 577.13615 | C30H26O12 | Procyanidin 2 | + | + |
| 10.41 | 289.07211 | C15H14O6 | Catechin 1 | 5.6 ± 0.6 a | 6.1 ± 0.7 a |
| 13.68 | 517.09892 | C24H22O13 | pelargonidin-3-(malonyl)-glucoside 2 | 4.4 ± 0.5 a | 4.1 ± 0.5 a |
| 17.70 | 447.05881 | C20H16O12 | ellagic acid rhamnoside 1 | 3.6 ± 0.3 a | 4.4 ± 0.4 b |
| 18.00 | 300.99905 | C14H6O8 | ellagic acid 1 | 65.8 ± 5.9 a | 77.2 ± 6.8 b |
| 18.44 | 463.08843 | C21H20O12 | quercetin glucoside 1 | 8.1 ± 0.8 a | 10.3 ± 0.6 a |
| 20.07 | 447.09445 | C21H20O11 | kaempferol glucoside 1 | 23.5 ± 2.2 a | 24.9 ± 2.4 a |
| 20.55 | 461.07357 | C21H18O12 | methylellagic acid hexoside 2 | 3.8 ± 0.4 a | 4.9 ± 0.5 b |
| 26.32 | 593.13207 | C30H26O13 | tiliroside 1 | 131.7 ± 11.6 a | 136.4 ± 12.1 a |
| Condition | Kaempferol-G | Ellagic Acid | Tiliroside | Yield (%) |
|---|---|---|---|---|
| HRE (60 °C) 80% | 90.8 ± 8.7 d | 355.5 ± 34.9 b,c | 874.9 ± 84.5 d | 6.91 ± 0.7 a |
| HRE (60 °C) 90% | 88.1 ± 8.4 d | 360.1 ± 35.8 a,b | 900.8 ± 90.1 c,d | 6.34 ± 0.6 a |
| HRE (60 °C) 100% | 87.4 ± 8.8 d | 365.1 ± 36.8 a,b | 898.7 ± 90.7 c,d | 5.71 ± 0.6 b |
| ME (40 °C) 80% | 111.8 ± 10.4 a,b | 350.3 ± 34.4 b,c | 898.7 ± 87.6 c,d | 4.51 ± 0.5 c |
| ME (40 °C) 90% | 101.5 ± 10.1 b,c | 357.9 ± 35.8 a–c | 910.4 ± 90.4 b | 4.66 ± 0.4 c |
| ME (40 °C) 100% | 99.1 ± 9.8 b,c | 366.1 ± 36.4 a,b | 905.5 ± 88.7 b,c | 4.84 ± 0.5 c |
| UAE 80% | 122.3 ± 11.1 a | 345.4 ± 23.5 c | 984.1 ± 90.7 a | 4.49 ± 0.5 c |
| UAE 90% | 119.3 ± 10.4 a | 371.3 ± 33.3 a | 973.2 ± 91.4 a | 4.61 ± 0.5 c |
| UAE 100% | 102.3 ± 9.9 b,c | 370.3 ± 34.6 a | 986.5 ± 98.3 a | 4.75 ± 0.5 c |
| MAE 80% | 97.9 ± 9.8 c | 357.2 ± 33.9 a–c | 940.5 ± 88.8 a,b | 5.98 ± 0.6 b |
| MAE 90% | 96.7 ± 8.9 c,d | 370.5 ± 35.4 a | 911.8 ± 90.9 b | 5.81 ± 0.5 b |
| MAE 100% | 91.9 ± 9.4 d | 376.3 ± 36.7 a | 898.7 ± 86.5 c,d | 5.65 ± 0.5 b |
| Component | Extraction without Pretreatment | Pretreatment with Water | ||
|---|---|---|---|---|
| 1 Step UAE 1 × 15 min | 2 Steps UAE 2 × 15 min | 1 Step UAE 30 min | ||
| Kaempferol glucoside | 2.59 ± 0.21 a | 3.44 ± 0.31 b | 4.05 ± 0.44 b | 3.84 ± 0.31 b |
| Ellagic acid | 8.05 ± 0.84 a | 10.71 ± 1.02 b | 13.5 ± 1.11 c | 11.4 ± 0.91 b |
| Tiliroside | 21.11 ± 1.82 a | 28.08 ± 2.28 b | 36.5 ± 3.24 c | 31.8 ± 1.99 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójciak, W.; Żuk, M.; Sowa, I.; Mazurek, B.; Tyśkiewicz, K.; Wójciak, M. Recovery of Bioactive Components from Strawberry Seeds Residues Post Oil Extraction and Their Cosmetic Potential. Appl. Sci. 2024, 14, 783. https://doi.org/10.3390/app14020783
Wójciak W, Żuk M, Sowa I, Mazurek B, Tyśkiewicz K, Wójciak M. Recovery of Bioactive Components from Strawberry Seeds Residues Post Oil Extraction and Their Cosmetic Potential. Applied Sciences. 2024; 14(2):783. https://doi.org/10.3390/app14020783
Chicago/Turabian StyleWójciak, Weronika, Magdalena Żuk, Ireneusz Sowa, Barbara Mazurek, Katarzyna Tyśkiewicz, and Magdalena Wójciak. 2024. "Recovery of Bioactive Components from Strawberry Seeds Residues Post Oil Extraction and Their Cosmetic Potential" Applied Sciences 14, no. 2: 783. https://doi.org/10.3390/app14020783
APA StyleWójciak, W., Żuk, M., Sowa, I., Mazurek, B., Tyśkiewicz, K., & Wójciak, M. (2024). Recovery of Bioactive Components from Strawberry Seeds Residues Post Oil Extraction and Their Cosmetic Potential. Applied Sciences, 14(2), 783. https://doi.org/10.3390/app14020783






