Fermentative Liberation and Transformation of Bioactive Compounds: Ellagic Acid from Nut Press Cake Ellagitannins
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Walnut Press Cake (WPC)
2.3. Preparation of WPC for Fermentation
2.4. WPC Inoculation
2.5. Fermentation
2.6. Sampling, Measuring pH, and Water Activity (aw)
2.7. Extraction of EA
2.8. HPLC–DAD Analysis
2.9. HPLC–MS Analysis
2.10. Satistical Analysis
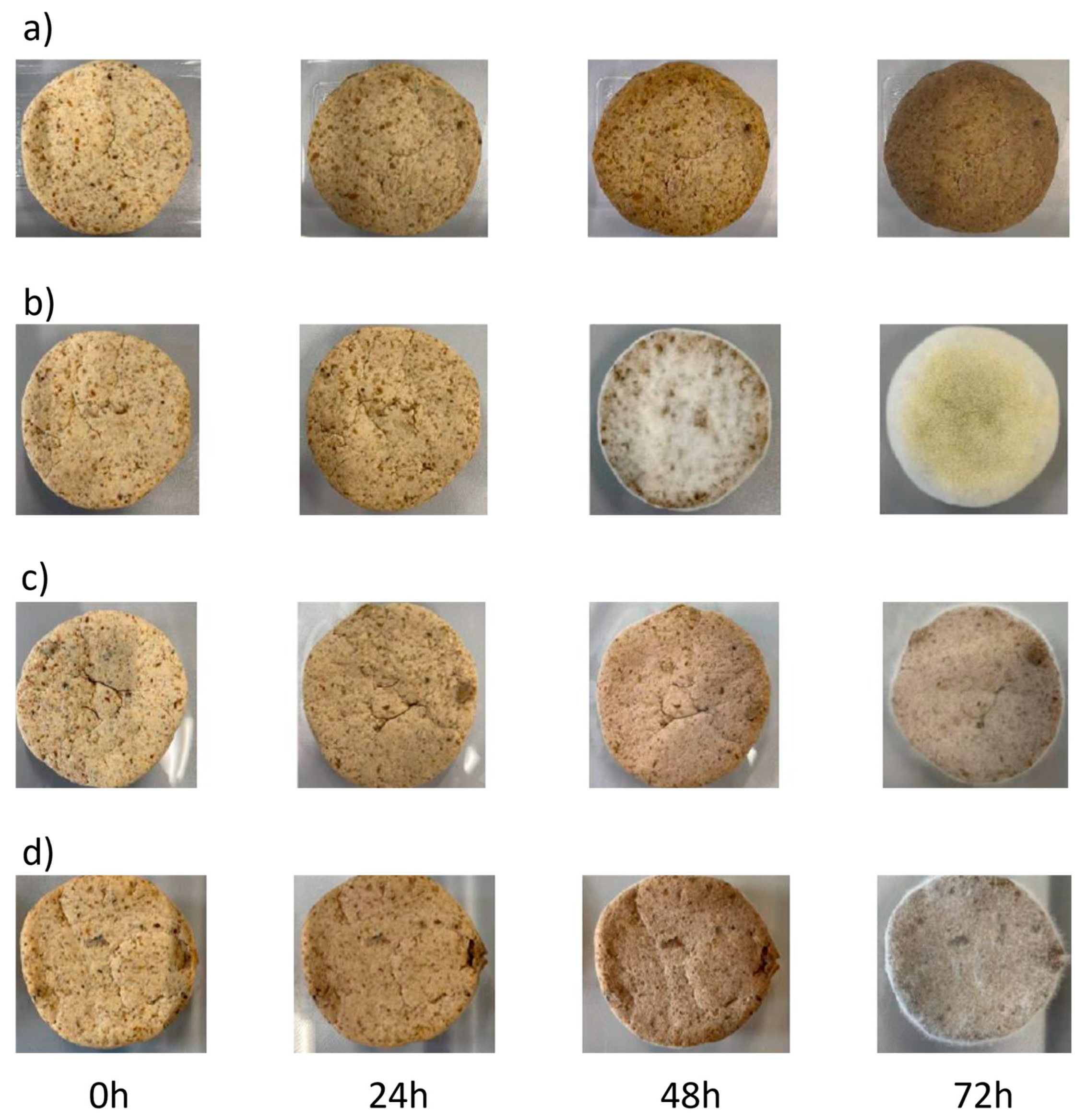
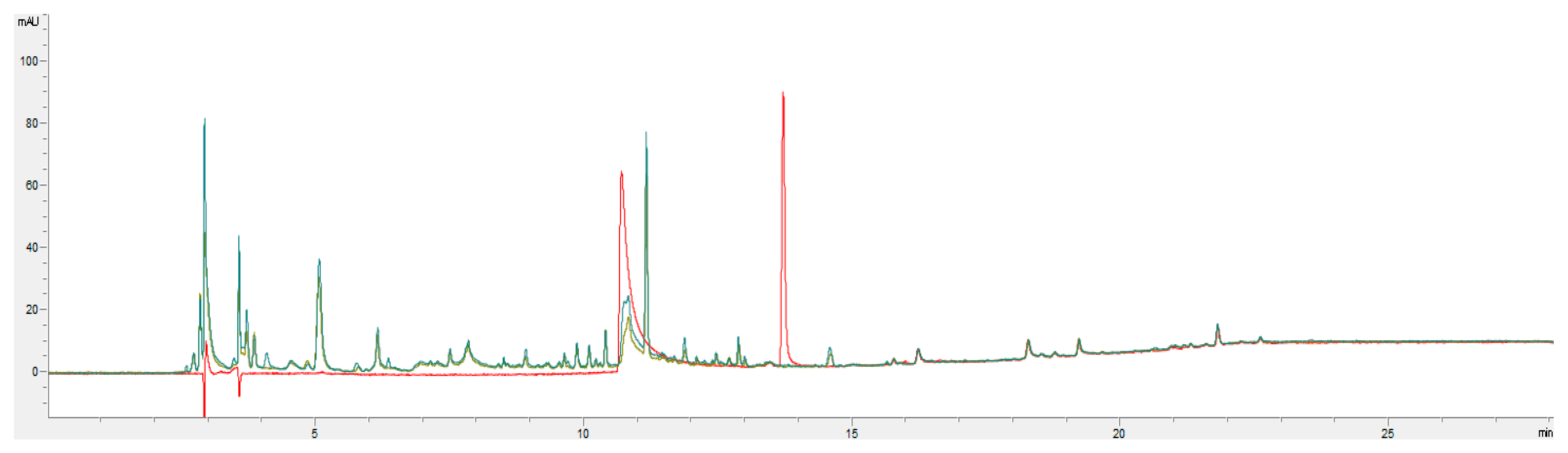
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakkalbasi, E.; Meral, R.; Dogan, I.S. Bioactive Compounds, Physical and Sensory Properties of Cake Made with Walnut Press-Cake. J. Food Qual. 2015, 38, 422–430. [Google Scholar] [CrossRef]
- Bakkalbaşı, E. Oxidative stability of enriched walnut oil with phenolic extracts from walnut press-cake under accelerated oxidation conditions and the effect of ultrasound treatment. J. Food Meas. Charact. 2019, 13, 43–50. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Pop, A.; Păucean, A.; Socaci, S.A.; Alexa, E.; Man, S.M.; Mureșan, V.; Chiş, M.S.; Salanță, L.; Popescu, I.; Berbecea, A.; et al. Quality Characteristics and Volatile Profile of Macarons Modified with Walnut Oilcake By-Product. Molecules 2020, 25, 2214. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mendoza, M.d.P.; Espinosa-Pardo, F.A.; Savoire, R.; Etchegoyen, C.; Harscoat-Schiavo, C.; Subra-Paternault, P. Recovery and antioxidant activity of phenolic compounds extracted from walnut press-cake using various methods and conditions. Ind. Crops Prod. 2021, 167, 113546. [Google Scholar] [CrossRef]
- Ran, J.; Su, Y.; Wang, P.; Yang, W.; Li, R.; Jiao, L.; Zhao, R. Effect of Lactobacillus acidophilus fermentation on bioaccessibility: The relationship between biotransformation and antioxidant activity of apple polyphenols based on metabolomics. LWT 2023, 190, 115360. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining Antioxidants and Natural Preservatives from Food By-Products through Fermentation: A Review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Doriya, K.; Jose, N.; Gowda, M.; Kumar, D.S. Chapter Six—Solid-State Fermentation vs Submerged Fermentation for the Production of l-Asparaginase. In Advances in Food and Nutrition Research; Kim, S.-K., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 78, pp. 115–135. [Google Scholar]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Chapter 23—Solid-State Fermentation Strategy for Microbial Metabolites Production: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–354. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Anwar, F.; Qadir, R.; Abbas, A. Chapter 44—Cold pressed walnut (Juglans regia L.) oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 491–495. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Gutierrez-Sánchez, G.; Prado-Barragán, L.A.; Rodríguez-Herrera, R.; Aguilar-Zárate, P.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Tafolla-Arellano, J.C.; Aguilar, C.N. Influence of culture conditions on ellagitannase expression and fungal ellagitannin degradation. Bioresour. Technol. 2021, 337, 125462. [Google Scholar] [CrossRef]
- Nevara, G.A.; Giwa Ibrahim, S.; Syed Muhammad, S.K.; Zawawi, N.; Mustapha, N.A.; Karim, R. Oilseed meals into foods: An approach for the valorization of oilseed by-products. Crit. Rev. Food Sci. Nutr. 2023, 63, 6330–6343. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Nasser, H.A.; Mahmoud, M.; Tolba, M.M.; Radwan, R.A.; Gabr, N.M.; ElShamy, A.A.; Yehya, M.S.; Ziemke, A.; Hashem, M.Y. Pros and cons of using green biotechnology to solve food insecurity and achieve sustainable development goals. Euro-Mediterr. J. Environ. Integr. 2021, 6, 29. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, P.-K.; Wong, H.-C.; Zhao, D. Oral supplementation of Gordonibacter urolithinfaciens promotes ellagic acid metabolism and urolithin bioavailability in mice. Food Chem. 2024, 437, 137953. [Google Scholar] [CrossRef]
- Huang, W.; Niu, H.; Gong, G.H.; Lu, Y.R.; Li, Z.S.; Li, H. Individual and combined effects of physicochemical parameters on ellagitannin acyl hydrolase and ellagic acid production from ellagitannin by Aspergillus oryzae. Bioprocess Biosyst. Eng. 2007, 30, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro Figueroa, J.; Mireles, M.; Ascacio-Valdés, J.; Aguilera-Carbo, A.; Sepúlveda, L.; Contreras-Esquivel, J.; Rodriguez, R.; Aguilar, C. Enzymatic Biotransformation of Pomegranate Ellagitannins: Initial Approach to Reaction Conditions. Iran. J. Biotechnol. 2020, 18, 30–36. [Google Scholar] [CrossRef]
- Brück, W.M.; Díaz Escobar, V.D.; Droz-dit-Busset, L.; Baudin, M.; Nicolet, N.; Andlauer, W. Fermentative Liberation of Ellagic Acid from Walnut Press Cake Ellagitannins. Foods 2022, 11, 3102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, S.; Ye, Q.; Hou, X.; Yang, G.; Lu, J.; Hai, Y.; Shen, J.; Fang, Y. A Novel Streptococcus thermophilus FUA329 Isolated from Human Breast Milk Capable of Producing Urolithin A from Ellagic Acid. Foods 2022, 11, 3280. [Google Scholar] [CrossRef]
- Varzakas, T. Rhizopus oligosporus mycelial penetration and enzyme diffusion in soya bean tempe. Process Biochem. 1998, 33, 741–747. [Google Scholar] [CrossRef]
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Solid-State Production of Phenolic Antioxidants from Cranberry Pomace by Rhizopus oligosporus. Food Biotechnol. 2002, 16, 189–210. [Google Scholar] [CrossRef]
- Hu, H.L.; van den Brink, J.; Gruben, B.S.; Wösten, H.A.B.; Gu, J.D.; de Vries, R.P. Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int. Biodeterior. Biodegrad. 2011, 65, 248–252. [Google Scholar] [CrossRef]
- Moccia, F.; Flores-Gallegos, A.C.; Chávez-González, M.L.; Sepúlveda, L.; Marzorati, S.; Verotta, L.; Panzella, L.; Ascacio-Valdes, J.A.; Aguilar, C.N.; Napolitano, A. Ellagic Acid Recovery by Solid State Fermentation of Pomegranate Wastes by Aspergillus niger and Saccharomyces cerevisiae: A Comparison. Molecules 2019, 24, 3689. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Liu, S.; Hai, Y.; Yang, G.; Lu, J.; He, F.; Zhao, Y.; Xia, M.; Hou, X.; Fang, Y. Lactococcus garvieae FUA009, a Novel Intestinal Bacterium Capable of Producing the Bioactive Metabolite Urolithin A from Ellagic Acid. Foods 2022, 11, 2621. [Google Scholar] [CrossRef]
- Xia, M.; Mu, S.; Fang, Y.; Zhang, X.; Yang, G.; Hou, X.; He, F.; Zhao, Y.; Huang, Y.; Zhang, W.; et al. Genetic and Probiotic Characteristics of Urolithin A Producing Enterococcus faecium FUA027. Foods 2023, 12, 1021. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, Y.; Yang, G.; Hou, X.; Hai, Y.; Xia, M.; He, F.; Zhao, Y.; Liu, S. Isolation and characterization of a novel human intestinal Enterococcus faecium FUA027 capable of producing urolithin A from ellagic acid. Front. Nutr. 2022, 9, 1039697. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Álvarez, I.; Landete, J.M. Bifidobacterium pseudocatenulatum INIA P815: The first bacterium able to produce urolithins A and B from ellagic acid. J. Funct. Foods 2018, 45, 95–99. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; García-Villalba, R.; Beltrán, D.; Frutos-Lisón, M.D.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Gut Bacteria Involved in Ellagic Acid Metabolism To Yield Human Urolithin Metabotypes Revealed. J. Agric. Food Chem. 2023, 71, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
| Temperature [°C] | CO2 [ppm] | Humidity [%] |
|---|---|---|
| 25 | 611 | 57.2 |
| 30 | 611 | 46.1 |
| 37 | 499 | 33.9 |
| Time (h) | S. thermophilus | A. oryzae | R. oligosporus | S. thermophilus and R. oligosporus Co-Culture | ||||
|---|---|---|---|---|---|---|---|---|
| pH | aw | pH | aw | pH | aw | pH | aw | |
| 0 | 5.95 ± 0.02 | 0.95 | 5.90 ± 0.02 | 0.96 | 5.88 ± 0.02 | 0.96 | 5.99 ± 0.02 | 0.96 |
| 24 | 5.76 ± 0.01 | 5.83 ± 0.01 | 5.77 ± 0.03 | 5.80 ± 0.02 | ||||
| 48 | 5.70 ± 0.03 | 5.87 ± 0.01 | 5.67 ± 0.06 | 5.81 ± 0.03 | ||||
| 72 | 5.61 ± 0.02 | 0.94 | 5.85 ± 0.03 | 0.95 | 5.61 ± 0.07 | 0.93 | 5.79 ± 0.02 | 0.96 |
| Fermentation | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| S. thermophilus | 0.14 ± 0.01 | 0.13 ± 0.00 | 0.14 ± 0.00 | 0.17 ± 0.03 |
| A. oryzae | 0.15 ± 0.04 | 0.18 ± 0.03 | 0.14 ± 0.04 | 0.13 ± 0.02 |
| R. oligosporus | 0.26 ± 0.01 | 0.21 ± 0.13 | 0.22 ± 0.14 | 0.28 ± 0.07 |
| S. thermophilus and R. oligosporus co-culture | 0.19 ± 0.03 | 0.21 ± 0.06 | 0.20 ± 0.02 | 0.16 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brück, W.M.; Erismann, Y.; Andlauer, W. Fermentative Liberation and Transformation of Bioactive Compounds: Ellagic Acid from Nut Press Cake Ellagitannins. Appl. Sci. 2024, 14, 1141. https://doi.org/10.3390/app14031141
Brück WM, Erismann Y, Andlauer W. Fermentative Liberation and Transformation of Bioactive Compounds: Ellagic Acid from Nut Press Cake Ellagitannins. Applied Sciences. 2024; 14(3):1141. https://doi.org/10.3390/app14031141
Chicago/Turabian StyleBrück, Wolfram Manuel, Yannick Erismann, and Wilfried Andlauer. 2024. "Fermentative Liberation and Transformation of Bioactive Compounds: Ellagic Acid from Nut Press Cake Ellagitannins" Applied Sciences 14, no. 3: 1141. https://doi.org/10.3390/app14031141
APA StyleBrück, W. M., Erismann, Y., & Andlauer, W. (2024). Fermentative Liberation and Transformation of Bioactive Compounds: Ellagic Acid from Nut Press Cake Ellagitannins. Applied Sciences, 14(3), 1141. https://doi.org/10.3390/app14031141







