Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara scolymus L.) and Artichoke By-Products: A Review
Abstract
1. Introduction
2. Nutritional Characteristics
2.1. Aproximate Composition of Artichoke
| Nutrient | Value | Ref. | |
|---|---|---|---|
| Energy | (kcal/100 g FW) | 79.73 ± 0.04 | [24] |
| Moisture | (g/100 g FW) | 74.54 ± 0.21 | [27] |
| Ash | (g/100 g DW) | 6.88 ± 0.14 | [27] |
| Proteins | (g/100 g DW) | 24.27 ± 0.12 | [26] |
| Carbohydrates | (g/100 g DW) | 56.62 ± 1.41 | [31] |
| Fats | (g/100 g DW) | 2.06 ± 0.05 | [27] |
| SFAs | (%) | 53.2 ± 0.5 | [24] |
| MUFAs | (%) | 2.26 ± 0.05 | [24] |
| PUFAs | (%) | 44.5 ± 0.6 | [24] |
2.2. Minerals
2.3. Vitamins
2.4. Dietary Fiber
2.5. Phenolic Compounds
2.6. Enzymes
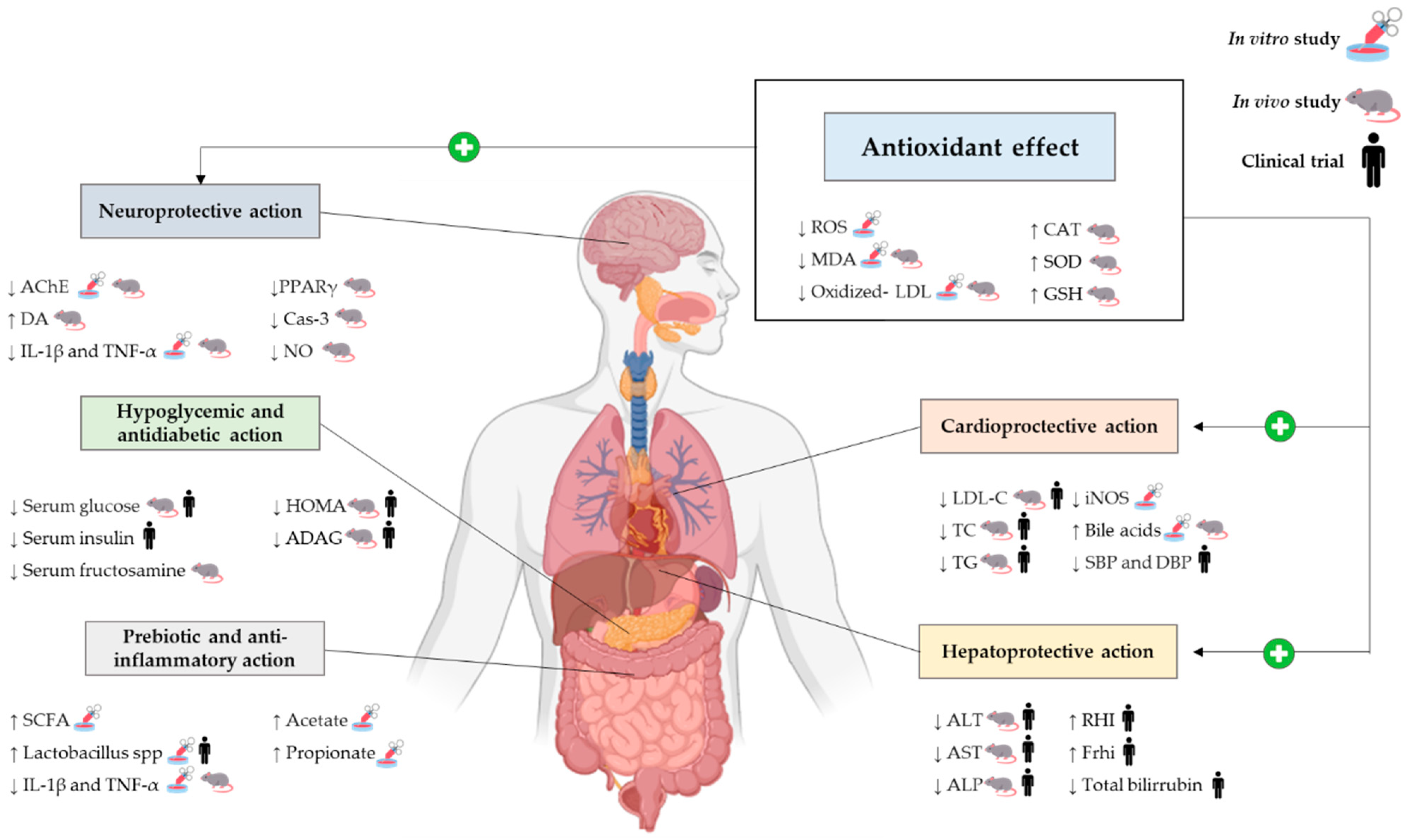
3. Health Benefits
3.1. Antioxidant Activity
3.2. Hepatoprotective Action
3.3. Prebiotic and Anti-Inflammatory Effect
3.4. Hypoglycemic Action
3.5. Cardioprotective Effect
3.6. Neuroprotective Effect
4. Food Industry Application
4.1. Use of Artichoke as a Functional Ingredient
4.2. Antimicrobial Effect
5. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Gatto, A.; De Paola, D.; Bagnoli, F.; Vendramin, G.G.; Sonnante, G. Population Structure of Cynara cardunculus Complex and the Origin of the Conspecific Crops Artichoke and Cardoon. Ann. Bot. 2013, 112, 855–865. [Google Scholar] [CrossRef]
- Meng, D.; Xiaomei, Z.; Wenzhen, K.; Xu, Z. Detecting Useful Genetic Markers and Reconstructing the Phylogeny of an Important Medicinal Resource Plant, Artemisia Selengensis, Based on Chloroplast Genomics. PLoS ONE 2019, 14, e0211340. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 7 April 2024).
- Grabowska, A.; Caruso, G.; Mehrafarin, A.; Kalisz, A.; Gruszecki, R.; Kunicki, E.; Sękara, A. Application of Modern Agronomic and Biotechnological Strategies to Valorise Worldwide Globe Artichoke (Cynara cardunculus L.) Potential—An Analytical Overview. Ital. J. Agron. 2018, 13, 279–289. [Google Scholar] [CrossRef]
- Ciancolini, A.; Alignan, M.; Pagnotta, M.A.; Miquel, J.; Vilarem, G.; Crinò, P. Morphological Characterization, Biomass and Pharmaceutical Compounds in Italian Globe Artichoke Genotypes. Ind. Crops Prod. 2013, 49, 326–333. [Google Scholar] [CrossRef]
- García-Martínez, N.; Andreo-Martínez, P.; Almela, L. Characterization of Six Artichoke Cultivars and Their Suitability for Agro-Industrial Processing. J. Food Nutr. Res. 2017, 5, 234–242. [Google Scholar] [CrossRef]
- Gostin, A.I.; Waisundara, V.Y. Edible Flowers as Functional Food: A Review on Artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Jiménez-moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-yoldi, M.J.; Ancín-azpilicueta, C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe Artichoke: A Functional Food and Source of Nutraceutical Ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Rotondo, R.; Cruz, P.S.; Masin, M.; Bürgi, M.; Girardini, J.; García, S.M.; Rodríguez, G.R.; Furlan, R.L.E.; Escalante, A.M. Artichoke Extracts with Potential Application in Chemoprevention and Inflammatory Processes. Braz. J. Pharm. Sci. 2022, 58, e19238. [Google Scholar] [CrossRef]
- Salekzamani, S.; Ebrahimi-Mameghani, M.; Rezazadeh, K. The Antioxidant Activity of Artichoke (Cynara scolymus): A Systematic Review and Meta-Analysis of Animal Studies. Phytother. Res. 2019, 33, 55–71. [Google Scholar] [CrossRef]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In Vitro Antimicrobial, Antioxidant and Anticancer Activities of Globe Artichoke (Cynara cardunculus Var. Scolymus L.) Bracts and Receptacles Ethanolic Extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar] [CrossRef]
- Hassabou, N.F.; Farag, A.F. Anticancer Effects Induced by Artichoke Extract in Oral Squamous Carcinoma Cell Lines. J. Egypt. Natl. Cancer Inst. 2020, 32, 17. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Hussein, R.; Motaal, A.A.; Fouad, M.A.; Aziz, M.A.; El-Sayed, A. Artichoke Edible Parts Are Hepatoprotective as Commercial Leaf Preparation. Rev. Bras. Farmacogn. 2018, 28, 165–178. [Google Scholar] [CrossRef]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a Sustainable Crop for Biomass and Bioactive Compounds Production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef]
- Zayed, A.; Farag, M.A. Valorization, Extraction Optimization and Technology Advancements of Artichoke Biowastes: Food and Non-Food Applications. LWT-Food Sci. Technol. 2020, 132, 109883. [Google Scholar] [CrossRef]
- Amrani, A.; Al Amrani, H.A.; khalaf Aneed, I. Artichoke and Health (Food and Medicine): A Review. J. Genet. Environ. Resour. Conserv. 2023, 11, 114–124. [Google Scholar]
- Al-Subhi, F.M.M. Artichoke as a Tool to Natural Antioxidants for Lowering Diabetics and Hypolipidemia Parameters. Alex. Sci. Exch. J. 2020, 41, 215–224. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Chemical and Morphological Characteristics of New Clones and Commercial Varieties of Globe Artichoke (Cynara cardunculus Var. Scolymus). Plant Foods Hum. Human. Nutr. 2011, 66, 291–297. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of Genotype, Harvest Time and Plant Part on Polyphenolic Composition of Globe Artichoke [Cynara cardunculus L. Var. Scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Effect of Storage Temperature and Genotype on Quality of Globe Artichoke [Cynara cardunculus L. Subsp. Scolymus (L.) Hegi] Head. Acta Hortic. 2007, 730, 449–454. [Google Scholar] [CrossRef]
- Sękara, A.; Kalisz, A.; Gruszecki, R.; Grabowska, A.; Kunicki, E. Globe Artichoke—A Vegetable Herb and Ornamental of Value in Central Europe: A Review. J. Hortic. Sci. Biotechnol. 2015, 90, 365–374. [Google Scholar] [CrossRef]
- Allahdadi, M.; Raey, Y.; Raei, Y. Growth and Chlorogenic Acid Content of Artichoke (Cynara scolymus L.) Affected by Bio and Chemical Fertilizer. J. Biodivers. Environ. Sci. 2017, 11, 63–73. [Google Scholar]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value and Chemical Composition of Greek Artichoke Genotypes. Food Chem. 2018, 267, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Alicandri, E.; Paolacci, A.R.; Catarcione, G.; Del Lungo, A.; Iacoponi, V.; Pati, F.; Scarascia Mugnozza, G.; Ciaffi, M. Morphological, Molecular, and Nutritional Characterisation of the Globe Artichoke Landrace “Carciofo Ortano”. Plants 2023, 12, 1844. [Google Scholar] [CrossRef] [PubMed]
- Claus, T.; Maruyama, S.A.; Palombini, S.V.; Montanher, P.F.; Bonafé, E.G.; de Oliveira Santos Junior, O.; Matsushita, M.; Visentainer, J.V. Chemical Characterization and Use of Artichoke Parts for Protection from Oxidative Stress in Canola Oil. LWT-Food Sci. Technol. 2015, 61, 346–351. [Google Scholar] [CrossRef]
- Magied, M.M.A.; Hussien, S.E.D.; Zaki, S.M.; Said, R.M. EL Artichoke (Cynara scolymus L.) Leaves and Heads Extracts as Hypoglycemic and Hypocholesterolemic in Rats. J. Food Nutr. Res. 2016, 4, 60–68. [Google Scholar] [CrossRef]
- Hernández-Hernández, O.; Ruiz-Aceituno, L.; Sanz, M.L.; Martínez-Castro, I. Determination of Free Inositols and Other Low Molecular Weight Carbohydrates in Vegetables. J. Agric. Food Chem. 2011, 59, 2451–2455. [Google Scholar] [CrossRef]
- El-Hadidy, G.S.; Elmeshad, W.; Abdelgaleel, M.; Ali, M. Extraction, Identification, and Quantification of Bioactive Compounds from Globe Artichoke (Cynara cardunculus Var. Scolymus). Sains Malays. 2022, 51, 2843–2855. [Google Scholar] [CrossRef]
- Hussein, L.; El-Fouly, M.M.; El-Baz, F.K.; Ghanem, S.A. Nutritional Quality and the Presence of Anti-Nutritional Factors in Leaf Protein Concentrates (LPC). Int. J. Food Sci. Nutr. 1999, 50, 333–343. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Dosi, R.; Guida, V.; Severino, V.; Maro, A. Di Nutritional and Metabolic Profiling of the Globe Artichoke (Cynara scolymus L. “Capuanella” Heads) in Province of Caserta, Italy. Aust. J. Crop Sci. 2013, 7, 1927–1934. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation: 10–14 November 2008, Geneva; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; ISBN 978-9-25-106733-8. [Google Scholar]
- Sharma, M.; Sharma, M.; Bithel, N.; Sharma, M. Ethnobotany, Phytochemistry, Pharmacology and Nutritional Potential of Medicinal Plants from Asteraceae Family. J. Mt. Res. 2022, 17, 67–83. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Mocan, A.; Atanasov, A.G. Cynaropicrin: A Comprehensive Research Review and Therapeutic Potential as an Anti-Hepatitis C Virus Agent. Front. Pharmacol. 2016, 7, 231724. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Cankar, K.; Comino, C.; Moglia, A.; Hehn, A.; Bourgaud, F.; Bouwmeester, H.; Menin, B.; Lanteri, S.; Beekwilder, J. Cytochrome P450s from Cynara cardunculus L. CYP71AV9 and CYP71BL5, Catalyze Distinct Hydroxylations in the Sesquiterpene Lactone Biosynthetic Pathway. Plant Sci. 2014, 223, 59–68. [Google Scholar] [CrossRef] [PubMed]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, Agronomical, Phytochemical, and Pharmacological Overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Corrado, G.; Colla, G.; Cardarelli, M.; de Pascale, S.; Rouphael, Y. Phytochemical Profile, Mineral Content, and Bioactive Compounds in Leaves of Seed-Propagated Artichoke Hybrid Cultivars. Molecules 2020, 25, 3795. [Google Scholar] [CrossRef] [PubMed]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.V.; Moses, J.A.; Anandharamakrishnan, C. Iron Deficiency Anemia: A Comprehensive Review on Iron Absorption, Bioavailability and Emerging Food Fortification Approaches. Trends Food Sci. Technol. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. The Important Role of Zinc in Neurological Diseases. Biomolecules 2022, 13, 28. [Google Scholar] [CrossRef]
- Wessels, I.; Fischer, H.J.; Rink, L. Dietary and Physiological Effects of Zinc on the Immune System. Annu. Rev. Nutr. 2021, 41, 133–175. [Google Scholar] [CrossRef]
- Eman, A.M.; Wafaa; Hanem, M. Evaluation of Globe Artichoke By-Products for Enhancing Functional Properties of Some Foods. J. Adv. Agric. Res. 2018, 112, 112–129. [Google Scholar]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Mineral Profile in Globe Artichoke as Affected by Genotype, Head Part and Environment. J. Sci. Food Agric. 2011, 91, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Nazeri, P.; Azizi, F. Dietary Sodium to Potassium Ratio and the Incidence of Hypertension and Cardiovascular Disease: A Population-Based Longitudinal Study. Clin. Exp. Hypertens. 2018, 40, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Biel, W.; Witkowicz, R.; Piątkowska, E.; Podsiadło, C. Proximate Composition, Minerals and Antioxidant Activity of Artichoke Leaf Extracts. Biol. Trace Elem. Res. 2020, 194, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauro, R.P.; Mauromicale, G. Mineral Profile in the Floral Stem of Some Globe Artichoke Cultivars. Acta Hortic. 2013, 983, 433–437. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Svecova, E.; Rea, E.; Lucini, L. Effects of Saline Stress on Mineral Composition, Phenolic Acids and Flavonoids in Leaves of Artichoke and Cardoon Genotypes Grown in Floating System. J. Sci. Food Agric. 2013, 93, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Grigelmo-Miguel, N.; Martmh N-Belloso, O. Comparison of Dietary Fibre from By-Products of Processing Fruits and Greens and from Cereals. LWT-Food Sci. Technol. 1999, 32, 503–508. [Google Scholar] [CrossRef]
- Quintero Ruiz, N.A.; Paolucci, M.; Siano, F.; Mamone, G.; Picariello, G.; Puppo, M.C.; Cascone, G.; Volpe, M.G. Characterization of Soluble and Insoluble Fibers in Artichoke By-Products by ATR-FTIR Spectroscopy Coupled with Chemometrics. Int. J. Food Prop. 2021, 24, 1693–1704. [Google Scholar] [CrossRef]
- Boubaker, M.; Omri, A.E.L.; Blecker, C.; Bouzouita, N. Fibre Concentrate from Artichoke (Cynara scolymus L.) Stem by-Products: Characterization and Application as a Bakery Product Ingredient. Food Sci. Technol. Int. 2016, 22, 759–768. [Google Scholar] [CrossRef]
- Fissore, E.N.; Domingo, C.S.; Pujol, C.A.; Damonte, E.B.; Rojas, A.M.; Gerschenson, L.N. Upgrading of Residues of Bracts, Stems and Hearts of Cynara cardunculus L. Var. Scolymus to Functional Fractions Enriched in Soluble Fiber. Food Funct. 2014, 5, 463–470. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Conesa, M.A.; Ferreres, F. The Effect of Storage Temperatures on Vitamin C and Phenolics Content of Artichoke (Cynara scolymus L.) Heads. Innov. Food Sci. Emerg. Technol. 2001, 2, 199–202. [Google Scholar] [CrossRef]
- Cavini, S.; Guzzetti, L.; Givoia, F.; Regonesi, M.E.; Di Gennaro, P.; Magoni, C.; Campone, L.; Labra, M.; Bruni, I. Artichoke (Cynara cardunculus Var. Scolymus L.) by-Products as a Source of Inulin: How to Valorise an Agricultural Supply Chain Extracting an Added-Value Compound. Nat. Prod. Res. 2022, 36, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Mauromicale, G. Choice of Time of Harvest Influences the Polyphenol Profile of Globe Artichoke. J. Funct. Foods 2013, 5, 1822–1828. [Google Scholar] [CrossRef]
- Schütz, K.; Persike, M.; Carle, R.; Schieber, A. Characterization and Quantification of Anthocyanins in Selected Artichoke (Cynara scolymus L.) Cultivars by HPLC-DAD-ESI-MSn. Anal. Bioanal. Chem. 2006, 384, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The Influence of Pre-Harvest Factors on the Quality of Globe Artichoke. Sci. Hortic. 2018, 233, 479–490. [Google Scholar] [CrossRef]
- Romo-Hualde, A.; Sáiz-Abajo, M.J.; Yetano-Cunchillos, A.I.; González-Ferrero, C.; Alonso-Santibanez, D.; Salvadó-Casadevall, M.; Lahoz, I.; Macua, J.I. Characterization of Bioactive Substances in Various Artichoke Varieties. Acta Hortic. 2012, 942, 395–400. [Google Scholar] [CrossRef]
- Ahmad El-Sohaimy, S. The Effect of Cooking on the Chemical Composition of Artichoke (Cynara scolymus L.). Afr. J. Food Sci. Technol. 2013, 4, 182–187. [Google Scholar] [CrossRef]
- Kalala, G.; Kambashi, B.; Everaert, N.; Beckers, Y.; Richel, A.; Pachikian, B.; Neyrinck, A.M.; Delzenne, N.M.; Bindelle, J. Characterization of Fructans and Dietary Fibre Profiles in Raw and Steamed Vegetables. Int. J. Food Sci. Nutr. 2018, 69, 682–689. [Google Scholar] [CrossRef]
- Abedo, A.; Salman, F.M.; El-Nomeary, Y.A.A.; Abedo, A.A.; Abd El-Rahman, H.H.; Mohamed, M.I.; Ahmed, S.M. Utilization of Artichoke (Cynara scolymus) By-Products in Sheep Feeding. J. Agric. Environ. Sci. 2014, 14, 624–630. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Nutritional and Functional Properties of Cynara Crops (Globe Artichoke and Cardoon) and Their Potential Applications: A Review. Int. J. Appl. Sci. Technol. 2012, 2, 64–70. [Google Scholar]
- Wan, X.; Guo, H.; Liang, Y.; Zhou, C.; Liu, Z.; Li, K.; Niu, F.; Zhai, X.; Wang, L. The Physiological Functions and Pharmaceutical Applications of Inulin: A Review. Carbohydr. Polym. 2020, 246, 116589. [Google Scholar] [CrossRef] [PubMed]
- Leroy, G.; Grongnet, J.F.; Mabeau, S.; le Corre, D.; Baty-Julien, Ć. Changes in Inulin and Soluble Sugar Concentration in Artichokes (Cynara scolymus L.) during Storage. J. Sci. Food Agric. 2010, 90, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and Functional Properties of the Different By-Products of Artichoke (Cynara scolymus L.) from Industrial Canning Processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Fissore, E.N.; Santo Domingo, C.; Gerschenson, L.N.; Giannuzzi, L. A Study of the Effect of Dietary Fiber Fractions Obtained from Artichoke (Cynara cardunculus L. Var. Scolymus) on the Growth of Intestinal Bacteria Associated with Health. Food Funct. 2015, 6, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, Y.K.; Singh, M.; Manorama, K.; Lakra, N.; Zaid, A.; Zulfiqar, F. Plant Phenolics: Neglected Secondary Metabolites in Plant Stress Tolerance. Braz. J. Bot. 2023, 1–19. [Google Scholar] [CrossRef]
- Feiden, T.; Valduga, E.; Zeni, J.; Steffens, J. Bioactive Compounds from Artichoke and Application Potential. Food Technol. Biotechnol. 2023, 61, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic Compounds from the Leaf Extract of Artichoke (Cynara scolymus L.) and Their Antimicrobial Activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Pinelli, P.; Cantini, C.; Cimato, A.; Heimler, D. Characterization of Violetto Di Toscana, a Typical Italian Variety of Artichoke (Cynara scolymus L.). Food Chem. 2006, 95, 221–225. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H.; Athmouni, K.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Chemicals Compositions, Antioxidant and Anti-Inflammatory Activity of Cynara scolymus Leaves Extracts, and Analysis of Major Bioactive Polyphenols by HPLC. Evid. Based Complement. Altern. Med. 2017, 2017, 4951937. [Google Scholar] [CrossRef]
- Xia, N.; Pautz, A.; Wollscheid, U.; Reifenberg, G.; Förstermann, U.; Li, H. Artichoke, Cynarin and Cyanidin Downregulate the Expression of Inducible Nitric Oxide Synthase in Human Coronary Smooth Muscle Cells. Molecules 2014, 19, 3654–3668. [Google Scholar] [CrossRef]
- Montesano, V.; Negro, D.; Sonnante, G.; Laghetti, G.; Urbano, M. Polyphenolic Compound Variation in Globe Artichoke Cultivars as Affected by Fertilization and Biostimulants Application. Plants 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants. In Edible Medicinal and Non-Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 1–738. [Google Scholar] [CrossRef]
- Pompili, V.; Mazzocchi, E.; Moglia, A.; Acquadro, A.; Comino, C.; Rotino, G.L.; Lanteri, S. Structural and Expression Analysis of Polyphenol Oxidases Potentially Involved in Globe Artichoke (C. Cardunculus Var. Scolymus L.) Tissue Browning. Sci. Rep. 2023, 13, 12288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef] [PubMed]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) Byproducts as a Potential Source of Health-Promoting Antioxidant Phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Sanz, S.; Olarte, C.; Ayala, F.; Echávarri, J.F. Evolution of Quality Characteristics of Minimally Processed Asparagus during Storage in Different Lighting Conditions. J. Food Sci. 2009, 74, S296–S302. [Google Scholar] [CrossRef] [PubMed]
- Todaro, A.; Peluso, O.; Catalano, A.E.; Mauromicale, G.; Spagna, G. Polyphenol Oxidase Activity from Three Sicilian Artichoke [ Cynara cardunculus L. Var. Scolymus L. (Fiori)] Cultivars: Studies and Technological Application on Minimally Processed Production. J. Agric. Food Chem. 2010, 58, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.E.; Obregón, W.D.; Avilés, F.X.; Caffini, N.O.; Vairo-Cavalli, S. Use of Artichoke (Cynara scolymus) Flower Extract as a Substitute for Bovine Rennet in the Manufacture of Gouda-Type Cheese: Characterization of Aspartic Proteases. Food Chem. 2014, 159, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Curti, E.; Anedda, R.; Pacifico, S.; Caputo, E.; Piccolella, S.; Mandrich, L. Exploring New Fruit- and Vegetable-Derived Rennet for Cheese Making. Appl. Sci. 2024, 14, 2257. [Google Scholar] [CrossRef]
- Nicosia, F.D.; Puglisi, I.; Pino, A.; Caggia, C.; Randazzo, C.L. Plant Milk-Clotting Enzymes for Cheesemaking. Foods 2022, 11, 871. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant Activity of Brazilian Vegetables and Its Relation with Phenolic Composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Dhen, N.; Gargouri, M.; Boulila, A. Chemical Composition, Antioxidant Potential and Enzymes Inhibitory Properties of Globe Artichoke By-Products. Chem. Biodivers. 2020, 17, e2000073. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Zapolski-Downar, A.; Naruszewicz, M.; Siennicka, A.; Krasnodbska, B.; Kolodziej, B. Protective Properties of Artichoke (Cynara scolymus) against Oxidative Stress Induced in Cultured Endothelial Cells and Monocytes. Life Sci. 2002, 71, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Augimeri, G.; Ceramella, J.; Vivacqua, A.; Sinicropi, M.S.; Pataro, G.; Bonofiglio, D.; Ferrari, G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Garbetta, A.; Linsalata, V.; Minervini, F.; Cardinali, A. Polyphenols from Artichoke Heads (Cynara cardunculus (L.) Subsp. Scolymus Hayek): In Vitro Bio-Accessibility, Intestinal Uptake and Bioavailability. Food Funct. 2015, 6, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, M.; Ben Abdallah Kolsi, R.; Dhouibi, R.; Ksouda, K.; Charfi, S.; Yaich, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Jamoussi, K.; et al. Protective Effects of Cynara scolymus Leaves Extract on Metabolic Disorders and Oxidative Stress in Alloxan-Diabetic Rats. BMC Complement. Altern. Med. 2017, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- El-Boshy, M.; Ashshi, A.; Gaith, M.; Qusty, N.; Bokhary, T.; AlTaweel, N.; Abdelhady, M. Studies on the Protective Effect of the Artichoke (Cynara scolymus) Leaf Extract against Cadmium Toxicity-Induced Oxidative Stress, Hepatorenal Damage, and Immunosuppressive and Hematological Disorders in Rats. Environ. Sci. Pollut. Res. 2017, 24, 12372–12383. [Google Scholar] [CrossRef]
- Tang, X.; Wei, R.; Deng, A.; Lei, T. Protective Effects of Ethanolic Extracts from Artichoke, an Edible Herbal Medicine, against Acute Alcohol-Induced Liver Injury in Mice. Nutrients 2017, 9, 1000. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, G.; Saleh, D.; El-Awdan, S. Beneficial Effect of Artichoke Leaf Extract on Ethylene Glycol-Induced Urolithiasis in Rats. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 960–967. [Google Scholar]
- Deng, A.; Wang, Y.; Huang, K.; Xie, P.; Mo, P.; Liu, F.; Chen, J.; Chen, K.; Wang, Y.; Xiao, B. Artichoke (Cynara scolymus L.) Water Extract Alleviates Palmitate-Induced Insulin Resistance in HepG2 Hepatocytes via the Activation of IRS1/PI3K/AKT/FoxO1 and GSK-3β Signaling Pathway. BMC Complement. Med. Ther. 2023, 23, 460. [Google Scholar] [CrossRef]
- Miccadei, S.; Venere, D.D.; Cardinali, A.; Romano, F.; Durazzo, A.; Foddai, M.S.; Fraioli, R.; Mobarhan, S.; Maiani, G. Antioxidative and Apoptotic Properties of Polyphenolic Extracts from Edible Part of Artichoke (Cynara scolymus L.) on Cultured Rat Hepatocytes and on Human Hepatoma Cells. Nutr. Cancer 2008, 60, 276–283. [Google Scholar] [CrossRef]
- El Morsy, E.M.; Kamel, R. Protective Effect of Artichoke Leaf Extract against Paracetamol-Induced Hepatotoxicity in Rats. Pharm. Biol. 2015, 53, 167–173. [Google Scholar] [CrossRef]
- Celepli, S.; Çolak, B.; Celepli, P.; Bigat, İ.; Batur, H.G.; Soysal, F.; Karakurt, S.; Hücümenoğlu, S.; Kısmet, K.; Şahin, M. Effects of Artichoke Leaf Extract on Hepatic Ischemia-Reperfusion Injury. Rev. Assoc. Med. Bras. 2021, 68, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Rafieian-Kopaei, M. Protective Effect of Artichoke (Cynara scolymus) Leaf Extract against Lead Toxicity in Rat. Pharm. Biol. 2013, 51, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The Synergistic Effect of Citrus Bergamia and Cynara cardunculus Extracts on Vascular Inflammation and Oxidative Stress in Non-Alcoholic Fatty Liver Disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Rangboo, V.; Noroozi, M.; Zavoshy, R.; Rezadoost, S.A.; Mohammadpoorasl, A. The Effect of Artichoke Leaf Extract on Alanine Aminotransferase and Aspartate Aminotransferase in the Patients with Nonalcoholic Steatohepatitis. Int. J. Hepatol. 2016, 2016, 4030476. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Atkin, S.L.; Butler, A.E.; Jafari, R.; Badeli, R.; Sahebkar, A. Efficacy of Artichoke Leaf Extract in Non-Alcoholic Fatty Liver Disease: A Pilot Double-Blind Randomized Controlled Trial. Phytother. Res. 2018, 32, 1382–1387. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Villar, A.; Zangara, A.; Smidt, C.R.; Risco, E. In Vitro Evaluation of Prebiotic Properties of a Commercial Artichoke Inflorescence Extract Revealed Bifidogenic Effects. Nutrients 2020, 12, 1552. [Google Scholar] [CrossRef]
- Holgado, F.; Campos-Monfort, G.; de las Heras, C.; Rupérez, P. Assessment of the Prebiotic Potential of Globe Artichoke By-Product through in Vitro Fermentation by Human Faecal Microbiota. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100328. [Google Scholar] [CrossRef]
- Vacca, M.; Pinto, D.; Annunziato, A.; Ressa, A.; Calasso, M.; Pontonio, E.; Celano, G.; De Angelis, M. Gluten-Free Bread Enriched with Artichoke Leaf Extract In Vitro Exerted Antioxidant and Anti-Inflammatory Properties. Antioxidants 2023, 12, 845. [Google Scholar] [CrossRef]
- Mateus, V.; Estarreja, J.; Silva, I.; Barracosa, P.; Teixeira-Lemos, E.; Pinto, R. Effect of Cynara cardunculus L. Var. Altilis (DC) in Inflammatory Bowel Disease. Appl. Sci. 2021, 11, 1629. [Google Scholar] [CrossRef]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal Anti-Inflammatory Effects of Artichoke Pectin and Modified Pectin Fractions in the Dextran Sulfate Sodium Model of Mice Colitis. Artificial Neural Network Modelling of Inflammatory Markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Kolida, S.; Klinder, A.; Gietl, E.; Buerlein, M.; Frohberg, C.; Landschtze, V.; Gibson, G.R. A Double-Blind, Placebo-Controlled, Cross-over Study to Establish the Bifidogenic Effect of a Very-Long-Chain Inulin Extracted from Globe Artichoke (Cynara scolymus) in Healthy Human Subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Soofiniya, Y. Hypolipidemic and Hypoglycemic Effects of Aerial Part of Cynara scolymus in Streptozotocin-Induced Diabetic Rats. J. Med. Plants Res. 2011, 5, 2717–2723. [Google Scholar]
- Alves, I.; Carvalho, B.; Terra, M.; Oliveira, C.H.; Silva, A.; Costa, M.; Rodrigues, R.; Barros, G.; Salles, B. Effects of artichoke (Cynara Scolumus L.) extract on biochemical parameters in diabetic rats. Authorea Prepr. 2020. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Bernardinelli, L.; Fazia, T.; Peroni, G.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; et al. The Metabolic Effects of Cynara Supplementation in Overweight and Obese Class I Subjects with Newly Detected Impaired Fasting Glycemia: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients 2020, 12, 3298. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Sala, P.; Perna, S.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Giacosa, A. Metabolic Management in Overweight Subjects with Naive Impaired Fasting Glycaemia by Means of a Highly Standardized Extract From Cynara scolymus: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Phytother. Res. 2014, 28, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nazni, P.; Poongodi Vijayakumar, T.; Alagianambi, P.; Amirthaveni, M. Hypoglycemic and Hypolipidemic Effect of Cynara scolymus among Selected Type 2 Diabetic Individuals. Pak. J. Nutr. 2006, 5, 147–151. [Google Scholar]
- Frigerio, J.; Tedesco, E.; Benetti, F.; Insolia, V.; Nicotra, G.; Mezzasalma, V.; Pagliari, S.; Labra, M.; Campone, L. Anticholesterolemic Activity of Three Vegetal Extracts (Artichoke, Caigua, and Fenugreek) and Their Unique Blend. Front. Pharmacol. 2021, 12, 726199. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, M.; Affes, H.; Dhouibi, R.; Charfi, S.; Turki, M.; Hammami, S.; Ayedi, F.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Effect of Artichoke (Cynara scolymus) on Cardiac Markers, Lipid Profile and Antioxidants Levels in Tissue of HFD-Induced Obesity. Arch. Physiol. Biochem. 2022, 128, 184–194. [Google Scholar] [CrossRef]
- Mocelin, R.; Marcon, M.; Santo, G.D.; Zanatta, L.; Sachett, A.; Schönell, A.P.; Bevilaqua, F.; Giachini, M.; Chitolina, R.; Wildner, S.M.; et al. Hypolipidemic and Antiatherogenic Effects of Cynara scolymus in Cholesterol-Fed Rats. Rev. Bras. Farmacogn. 2016, 26, 233–239. [Google Scholar] [CrossRef]
- Mejri, F.; Baati, T.; Martins, A.; Selmi, S.; Luisa Serralheiro, M.; Falé, P.L.; Rauter, A.; Casabianca, H.; Hosni, K. Phytochemical Analysis and in Vitro and in Vivo Evaluation of Biological Activities of Artichoke (Cynara scolymus L.) Floral Stems: Towards the Valorization of Food by-Products. Food Chem. 2020, 333, 127506. [Google Scholar] [CrossRef]
- Villanueva-Suárez, M.J.; Mateos-Aparicio, I.; Pérez-Cózar, M.L.; Yokoyama, W.; Redondo-Cuenca, A. Hypolipidemic Effects of Dietary Fibre from an Artichoke By-Product in Syrian Hamsters. J. Funct. Foods 2019, 56, 156–162. [Google Scholar] [CrossRef]
- Roghani-Dehkordi, F.; Kamkhah, A.F. Artichoke Leaf Juice Contains Antihypertensive Effect in Patients with Mild Hypertension. J. Diet. Suppl. 2009, 6, 328–341. [Google Scholar] [CrossRef]
- Rondanelli, M.; Castellazzi, A.M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Naso, M.; Nichetti, M.; Tagliacarne, C.; Valsecchi, C.; et al. Natural Killer Response and Lipo-Metabolic Profile in Adults with Low HDL-Cholesterol and Mild Hypercholesterolemia: Beneficial Effects of Artichoke Leaf Extract Supplementation. Evid.-Based Complement. Altern. Med. 2019, 2019, 2069701. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Veronesi, M.; Borghi, C. Short-Term Effects of Dry Extracts of Artichokeand Berberis in Hypercholesterolemic Patients without Cardiovascular Disease. Am. J. Cardiol. 2019, 123, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Carres, L.; Bruno, A.; D’antuono, I.; Linsalata, V.; Cardinali, A.; Neilson, A.P. In Vitro Evidences of the Globe Artichoke Antioxidant, Cardioprotective and Neuroprotective Effects. J. Funct. Foods 2023, 107, 1756–4646. [Google Scholar] [CrossRef]
- Piccinini, A.; Oliveira, M.P.; Silva, M.R.; Bett, G.S.; Becker, I.B.; Mendes, T.F.; Salla, D.H.; Silva, L.E.; Vilela, T.C.; Moraes, F.M.; et al. Effects of Ethanolic Extract of Cynara cardunculus (Artichoke) Leaves on Neuroinflammatory and Neurochemical Parameters in a Diet-Induced Mice Obesity Model. Neurochem. Res. 2022, 47, 1888–1903. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Yousef, M.I.; Ghareeb, D.A.; Augustyniak, M.; Giesy, J.P.; Aboul-Soud, M.A.M.; El Wakil, A. Artichoke Leaf Extract-Mediated Neuroprotection against Effects of Aflatoxin in Male Rats. Biomed. Res. Int. 2022, 2022, 4421828. [Google Scholar] [CrossRef] [PubMed]
- Elsayyad, A.; Reyad, Y.A.; Elshafey, B.A.; Aziz, E.K.; Metwally, M.M.M.; Abd-Elhakim, Y.M.; Abdel-Warith, A.-W.A.; Younis, E.M.; Davies, S.J.; El-Houseiny, W.; et al. Artichoke (Cynara scolymus) Leaf Extract Abates the Neurotoxic and Neurobehavioral Outcomes of Fluoride in Nile Tilapia (Oreochromis Niloticus) via Balancing Oxidative Stress, Inflammation, Apoptosis, and Acetylcholinesterase Activity. Aquaculture 2024, 584, 740684. [Google Scholar] [CrossRef]
- Mekkey, S.M.; Rahmah, A.; Raghif, A.; Abdul, H.; Alkafaji, R.; Hadi, N.R. The Anti-Parkinson Effects of Cyanara Scoluymus (Artichoke) Extract in Rat Model of Rotenone Induced Parkinsonism. Ann. Rom. Soc. Cell Biol. 2021, 25, 2318–2329. [Google Scholar]
- Cicek, B.; Genc, S.; Yeni, Y.; Kuzucu, M.; Cetin, A.; Yildirim, S.; Bolat, I.; Kantarci, M.; Hacimuftuoglu, A.; Lazopoulos, G.; et al. Artichoke (Cynara scolymus) Methanolic Leaf Extract Alleviates Diethylnitrosamine-Induced Toxicity in BALB/c Mouse Brain: Involvement of Oxidative Stress and Apoptotically Related Klotho/PPARγ Signaling. J. Pers. Med. 2022, 12, 2012. [Google Scholar] [CrossRef]
- Moradi, M.; Sohrabi, G.; Golbidi, M.; Yarmohammadi, S.; Hemati, N.; Campbell, M.S.; Moradi, S.; Kermani, M.a.H.; Farzaei, M.H. Effects of Artichoke on Blood Pressure: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2021, 57, 102668. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cicco, N.; Linsalata, V. Antioxidant Activities of Artichoke Phenolics. Acta Hortic. 2005, 681, 421–428. [Google Scholar] [CrossRef]
- Divyajanani, S.; Harithpriya, K.; Ganesan, K.; Ramkumar, K.M. Dietary Polyphenols Remodel DNA Methylation Patterns of NRF2 in Chronic Disease. Nutrients 2023, 15, 3347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef]
- Takei, K.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Cynaropicrin Attenuates UVB-Induced Oxidative Stress via the AhR-Nrf2-Nqo1 Pathway. Toxicol. Lett. 2015, 234, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.M.; Farag, M.A. Therapeutic Potential of Artichoke in the Treatment of Fatty Liver: A Systematic Review and Meta-Analysis. J. Med. Food 2022, 25, 931–942. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikhhossein, F.; Talebyan, A.; Bazshahi, E.; Djafari, F.; Hekmatdoost, A. Effects of Artichoke Supplementation on Liver Enzymes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. Res. 2022, 11, 228–239. [Google Scholar] [CrossRef]
- Moradi, S.; Shokri-Mashhadi, N.; Saraf-Bank, S.; Mohammadi, H.; Zobeiri, M.; Clark, C.C.T.; Rouhani, M.H. The Effects of Cynara scolymus L. Supplementation on Liver Enzymes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2021, 75, e14726. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Neuman, M.G.; Cohen, L.B.; Nanau, R.M. Biomarkers in Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2014, 28, 607–618. [Google Scholar] [CrossRef]
- Liu, X.; Huang, K.; Niu, Z.; Mei, D.; Zhang, B. Protective Effect of Isochlorogenic Acid B on Liver Fibrosis in Non-Alcoholic Steatohepatitis of Mice. Basic Clin. Pharmacol. Toxicol. 2019, 124, 144–153. [Google Scholar] [CrossRef]
- Al-Jameil Dr, N.; Khan, F.A.; Arjumand, S.; Khan, M.F.; Tabassum, H. Associated Liver Enzymes with Hyperlipidemic Profile in Type 2 Diabetes Patients. Int. J. Clin. Exp. Pathol. 2014, 7, 4345. [Google Scholar]
- Fritsche, J.; Beindorff, C.M.; Dachtler, M.; Zhang, H.; Lammers, J.G. Isolation, Characterization and Determination of Minor Artichoke (Cynara scolymus L.) Leaf Extract Compounds. Eur. Food Res. Technol. 2002, 215, 149–157. [Google Scholar] [CrossRef]
- Holgado, F.; Campos-Monfort, G.; de las Heras, C.; Rupérez, P. In Vitro Fermentability of Globe Artichoke By-Product by Lactobacillus acidophilus and Bifidobacterium bifidum. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100286. [Google Scholar] [CrossRef]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Marakis, G.; Booth, J.C.L. Artichoke Leaf Extract Reduces Symptoms of Irritable Bowel Syndrome and Improves Quality of Life in Otherwise Healthy Volunteers Suffering from Concomitant Dyspepsia: A Subset Analysis. J. Altern. Complement. Med. 2004, 10, 667–669. [Google Scholar] [CrossRef]
- Holtmann, G.; Adam, B.; Haag, S.; Collet, W.; Grünewald, E.; Windeck, T. Efficacy of Artichoke Leaf Extract in the Treatment of Patients with Functional Dyspepsia: A Six-Week Placebo-Controlled, Double-Blind, Multicentre Trial. Aliment. Pharmacol. Ther. 2003, 18, 1099–1105. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent Developments in Probiotics: An Emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Ueno, K.; Koga, T.; Kato, K.; Golenbock, D.T.; Gendler, S.J.; Kai, H.; Kim, K.C. MUC1 Mucin Is a Negative Regulator of Toll-like Receptor Signaling. Am. J. Respir. Cell Mol. Biol. 2008, 38, 263–268. [Google Scholar] [CrossRef]
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of Diabetes: An Overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef]
- Jalili, C.; Moradi, S.; Babaei, A.; Boozari, B.; Asbaghi, O.; Lazaridi, A.V.; Hojjati Kermani, M.A.; Miraghajani, M. Effects of Cynara scolymus L. on Glycemic Indices: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Complement. Ther. Med. 2020; 52, 102496. [Google Scholar]
- Fantini, N.; Colombo, G.; Giori, A.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Carai, M.A.M. Evidence of Glycemia-Lowering Effect by a Cynara scolymus L. Extract in Normal and Obese Rats. Phytother. Res. 2011, 25, 463–466. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evid. Based Complement. Altern. Med. 2013, 2013, 11. [Google Scholar] [CrossRef]
- Giuntini, E.B.; Sardá, F.A.H.; de Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Kim, M.; Seo, C.R.; Yoo, H.J.; Lee, S.H.; Lee, J.H. The Effects of Jerusalem Artichoke and Fermented Soybean Powder Mixture Supplementation on Blood Glucose and Oxidative Stress in Subjects with Prediabetes or Newly Diagnosed Type 2 Diabetes. Nutr. Diabetes 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary Fibre and Whole Grains in Diabetes Management: Systematic Review and Meta-Analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care Clin. Off. Pract. 2013, 40, 195–211. [Google Scholar] [CrossRef]
- Amini, M.R.; Sheikhhossein, F.; Alvani, M.; Shoura, S.M.S.; Sohrabnavi, A.; Heidarian, E.; Hekmatdoost, A. Anti-Hypertensive Effects of Artichoke Supplementation in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. Res. 2022, 11, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Bueno, A.A.; Mota, J.F. The Effect of Artichoke on Lipid Profile: A Review of Possible Mechanisms of Action. Pharmacol. Res. 2018, 137, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Maki, K.C. Effects of Dietary Inulin on Serum Lipids. J. Nutr. 1999, 129, 1474S–1477S. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Förstermann, U. Flavonoids from Artichoke (Cynara scolymus L.) Up-Regulate Endothelial-Type Nitric-Oxide Synthase Gene Expression in Human Endothelial Cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How Neuroinflammation Contributes to Neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization from M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Vaknine, S.; Soreq, H. Central and Peripheral Anti-Inflammatory Effects of Acetylcholinesterase Inhibitors. Neuropharmacology 2020, 168, 108020. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Fan, X. Role of Polyphenols as Antioxidant Supplementation in Ischemic Stroke. Oxid. Med. Cell Longev. 2021, 2021, 5471347. [Google Scholar] [CrossRef]
- Číž, M.; Dvořáková, A.; Skočková, V.; Kubala, L. The Role of Dietary Phenolic Compounds in Epigenetic Modulation Involved in Inflammatory Processes. Antioxidants 2020, 9, 691. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Tyagi, E. Diet and Cognition: Interplay between Cell Metabolism and Neuronal Plasticity. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 726–733. [Google Scholar] [CrossRef]
- Lu, C.-W.; Lin, T.-Y.; Hsieh, P.; Chiu, K.-M.; Lee, M.-Y.; Wang, S.-J. Cynarin, a Caffeoylquinic Acid Derivative in Artichoke, Inhibits Exocytotic Glutamate Release from Rat Cortical Nerve Terminals (Synaptosomes). Neurochem. Int. 2023, 167, 105537. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases—What Is the Evidence? Front. Neurosci. 2015, 9, 170294. [Google Scholar] [CrossRef] [PubMed]
- Amoriello, T.; Mellara, F.; Ruggeri, S.; Ciorba, R.; Ceccarelli, D.; Ciccoritti, R. Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project. Sustainability 2022, 14, 14778. [Google Scholar] [CrossRef]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from Artichoke Processing Industry: Reuse in Bread-Making and Evaluation of the Physico-Chemical Characteristics of the Final Product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef]
- Le, T.T.; Vo, A.P.; Thao, V.; Dang, N.; Viet, V.; Le, M. Crackers Fortified with Various Ratios of Cynara scolymus L. Leaf Extract Residue: Nutritional, Physical and Sensory Quality. Chem. Eng. Trans. 2023, 106, 859–864. [Google Scholar] [CrossRef]
- Dadalı, C. Artichoke Bracts as Fat and Wheat Flour Replacer in Cake: Optimization of Reduced Fat and Reduced Wheat Flour Cake Formulation. J. Food Meas. Charact. 2023, 17, 98–107. [Google Scholar] [CrossRef]
- San José, F.J.; Collado-Fernández, M.; López, R. Sensory Evaluation of Biscuits Enriched with Artichoke Fiber-Rich Powders (Cynara scolymus L.). Food Sci. Nutr. 2018, 6, 160–167. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.M.; Ahmed, R.A.-K. Utilization of Artichoke Processing Wastes as Fat Replacer in Beef Sausage. Egypt. J. Food Sci. 2023, 51, 13–31. [Google Scholar] [CrossRef]
- Ergezer, H.; Kaya, H.İ.; ŞiMşek, Ö. Antioxidant and Antimicrobial Potential of Artichoke (Cynara scolymus L.) Extract in Beef Patties. Czech J. Food Sci. 2018, 36, 154–162. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Licciardello, F.; Aurelio, S.; Muratore, G.; Giovanni, M.; Restuccia, C. Effect of Cynara cardunculus extract on the shelf life of aubergine burgers. Ital. J. Food Sci. 2018, 30, 19–25. [Google Scholar]
- Essid, I.; Tajine, S.; Gharbi, S.; Bellagha, S. Use of Pomegranate Peel and Artichoke Leaf Extracts to Improve the Quality of Marinated Sardine (Sardinella aurita) Fillets. J. Food Sci. Technol. 2020, 57, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, J.; Mohammad Mortazavian, A.; Khomeiri, M.; Ghasem Nejad, A. Effects of artichoke (Cynara scolymus L.) extract addition on microbiological and physico-chemical properties of probiotic yogurt. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 536–541. [Google Scholar] [CrossRef]
- Frutos, M.J.; Guilabert-Antón, L.; Tomás-Bellido, A.; Hernández-Herrero, J.A. Effect of Artichoke (Cynara scolymus L.) Fiber on Textural and Sensory Qualities of Wheat Bread. Food Sci. Technol. Int. 2008, 14, 49–55. [Google Scholar] [CrossRef]
- Valerga, L.; Quintero-Ruiz, N.A.; Concellón, A.; Puppo, M.C. Artichoke, Eggplant and Tomato Flours as Nutritional Ingredients for Wheat Dough: Hydration Properties. J. Food Sci. Technol. 2020, 57, 1954. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, B.; Çelik, B.; Kiyak, S.; Savlak, N.; Kumru, F. New Look at Waste Utilization; Use of Artichoke (Cynara scolymus L.) Leaves in the Production of Functional Crackers. Turk. Tarim. Gida Bilim. Teknol. Derg. 2020, 8, 358–364. [Google Scholar] [CrossRef]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F.; Gambacorta, G. Enrichment of Fresh Pasta with Antioxidant Extracts Obtained from Artichoke Canning By-Products by Ultrasound-Assisted Technology and Quality Characterisation of the End Product. Int. J. Food Sci. Technol. 2017, 52, 2078–2087. [Google Scholar] [CrossRef]
- Boude, E.; Elshafei, S.; Ahmed, A.; Elgrwany, L. Utilization of Artichoke And Potato Flours In Bakery Products. J. Home Econ.-Menofia Univ. 2014, 24, 57–70. [Google Scholar] [CrossRef]
- Demir, T.; Ağaoğlu, S. Antioxidant, Antimicrobial and Metmyoglobin Reducing Activity of Artichoke (Cynara scolymus) Powder Extract-Added Minced Meat during Frozen Storage. Molecules 2021, 26, 5494. [Google Scholar] [CrossRef]
- Iran, J. Chem Chemical Characteristics, and Effect of Inulin Extracted from Artichoke (Cynara scolymus L.) Root on Biochemical Properties of Synbiotic Yogurt at the End of Fermentation. Iran. J. Chem. Chem. Eng. (IJCCE) 2018, 37, 219–230. [Google Scholar]
- Llorente, B.E.; Brutti, C.B.; Caffini, N.O. Purification and Characterization of a Milk-Clotting Aspartic Proteinase from Globe Artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2004, 52, 8182–8189. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, B.A.E.Y.; Ibrahim, O.A.E.H.; El-Sayed, H.A.E.R. Purification and Characterization of Milk Clotting Enzyme from Artichoke (Cynara cardunculus l.) Flowers as Coagulant on White Soft Cheese. Int. J. Dairy Sci. 2017, 12, 254–265. [Google Scholar] [CrossRef]
- Chazarra, S.; Sidrach, L.; López-Molina, D.; Rodríguez-López, J.N. Characterization of the Milk-Clotting Properties of Extracts from Artichoke (Cynara scolymus L.) Flowers. Int. Dairy J. 2007, 17, 1393–1400. [Google Scholar] [CrossRef]
- Metwalli, A.A.A.; Al-Askalany, S.A.; Negm, M.S. Utilization of Artichoke Leaves as Healthy Drink. Available online: https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=UTILIZATION+OF+ARTICHOKE+LEAVES+AS+HEALTHY+DRINK&btnG= (accessed on 24 April 2024).
- Schuina, G.L.; Quelhas, J.O.F.; de CASTILHOS, M.B.M.; de CARVALHO, G.B.M.; Del Bianchi, V.L. Alternative Production of Craft Lager Beers Using Artichoke (Cynara scolymus L.) as a Hops Substitute. Food Sci. Technol. 2019, 40, 157–161. [Google Scholar] [CrossRef]
- Mirderikvandi, M.; Kiani, A.; Khaldari, M.; Alirezaei, M. Effects of Artichoke (Cynara scolymus L.) Extract on Antioxidant Status in Chicken Thigh Meat. Iran. J. Vet. Med. 2016, 73–81. [Google Scholar] [CrossRef]
- Al-Masari, A.I.; Al-Himdany, H.Q. EFFECT OF ADDING ARTICHOKE LEAVES EXTRACTPOWDER (Cynara scolymus L.) TO THE DIET ON THE PRODUCTIVE PERFORMANCE OF BROILERS. Iraqi J. Agric. Sci. 2022, 53, 9–15. [Google Scholar] [CrossRef]
- Abdel-Shakur Ali, M.; Marrez, D.; Ali, M.A.; Shallan, M.A.; Meshrf, W.A.; Marrez, D.A. Phenolic Constituents, Antioxidant and Antimicrobial Activities of Globe Artichoke (Cynara scolymus L.) Aqueous Extracts Tropical Journal of Natural Product Research Phenolic Constituents, Antioxidant and Antimicrobial Activities of Globe Artichoke (Cynara scolymus L.) Aqueous Extracts. Trop. J. Nat. Prod. Res. 2021, 5, 1986–1994. [Google Scholar] [CrossRef]
- Moselhy, M.A.; Abd-Elhafez, K.A.; El-Kholany, E.A.; Gohar, M.R.; Nasr, N.F. Antimicrobial, Antioxidant and Anticancer Properties of Globe Artichoke and Grape by-Products as a Source of the Bio-Active Phenolic Compounds. Egypt. J. Chem. 2023, 66, 609–624. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R.; Lu, Y. Antimicrobial Activities of Cynara scolymus L. Leaf, Head, and Stem Extracts. J. Food Sci. 2005, 70, M149–M152. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhang, H.X.; Lo, R. Antifungal Activity of Cynara scolymus L. Extracts. Fitoterapia 2005, 76, 108–111. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Alves, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Artichoke and Milk Thistle Pills and Syrups as Sources of Phenolic Compounds with Antimicrobial Activity. Food Funct. 2016, 7, 3083–3090. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Piaz, F.D. Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
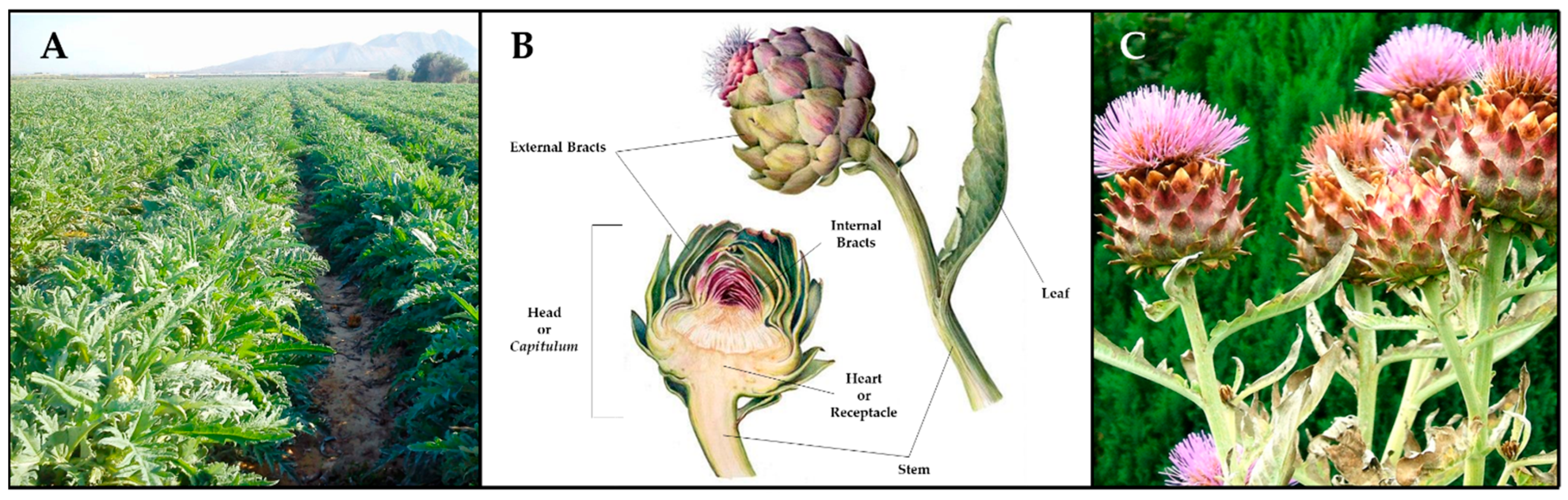
| Composition | Head | Leaves | Bracts | Stem | |
|---|---|---|---|---|---|
| Minerals | |||||
| Macroelements | |||||
| K | (mg/100 g DW) | 2160 1 | 506.3 2 | 1540 1 | 2100 3 |
| Ca | (mg/100 g DW) | 380 1 | 386.9 2 | 310 1 | 290 3 |
| Mg | (mg/100 g DW) | 2001 | 220 2 | 130 1 | 80 3 |
| Na | (mg/100 g DW) | 100 1 | 194.4 2 | 80 1 | 130 3 |
| Microelements | |||||
| Zn | (mg/100 g DW) | 3.14 1 | 2.1 2 | 2.09 1 | 4.16 4 |
| Fe | (mg/100 g DW) | 3.69 1 | 1.6 2 | 4.81 1 | 3.1 3 |
| Mn | (mg/100 g DW) | 0.97 1 | 0.8 2 | 1.46 1 | 0.46 3 |
| Cu | (mg/100 g DW) | 0.75 1 | 3.6 5 | 0.77 1 | 0.53 3 |
| Dietary Fiber | |||||
| Total dietary fiber | (g/100 g DW) | 58.8 6 | 31.47 7 | 60.35 8 | 85.28 9 |
| Insoluble dietary fiber | (g/100 g DW) | 44.5 6 | - | 38.51 8 | 67.95 9 |
| Soluble dietary fiber | (g/100 g DW) | 14.3 6 | - | 21.84 8 | 17.33 9 |
| Inulin | (g/100 g DW) | 13.9 10 | 7.5 11 | 20.2 10 | 25 10 |
| Celullose | (g/100 g DW) | 18 12 | 15.76 12 | 24.15 12 | 14.87 4 |
| Hemicellulose | (g/100 g DW) | 8.27 12 | 7.89 12 | 10.86 12 | 12.05 4 |
| Lignin | (g/100 g DW) | 14.06 12 | 10.78 12 | 15.62 12 | 6.43 4 |
| Vitamins | |||||
| Vitamin A | (mg/100 g FW) | 0.024 13 | 0.014 13 | 0.016 13 | - |
| Vitamin B1 | (mg/100 g FW) | 0.071 3 | 0.04 13 | 0.05 13 | - |
| Vitamin B2 | (mg/100 g FW) | 0.058 13 | 0.033 13 | 0.042 13 | - |
| Vitamin B6 | (mg/100 g FW) | 0.11 13 | 0.1 13 | 0.1 13 | - |
| Vitamin B9 | (mg/100 g FW) | 68 13 | 55 13 | 59 13 | - |
| Vitamin C | (mg/100 g FW) | 10 14 | 5 13 | 5.4 13 | - |
| Vitamin E | (mg/100 g FW) | 0.25 13 | 0.2 13 | 0.2 13 | - |
| Vitamin K | (μg/100 g FW) | 0.015 13 | 0.012 13 | 0.012 13 | - |
| Phenolic compounds | |||||
| Hydroxycinnamic acids | |||||
| 1-O-Caffeoylquinic acid | (mg/100 g DW) | 11 15 | nd 15 | 4 15 | 11 15 |
| 3-O-Caffeoylquinic acid | (mg/100 g DW) | 87.61 20 | nd 15 | nd 15 | Trace 15 |
| 5-O-Caffeoylquinic acid | (mg/100 g DW) | 13 15 | 126 15 | 122 15 | 478 15 |
| 4-O-Caffeoylquinic acid | (mg/100 g DW) | 13 15 | nd 15 | nd 15 | 10 15 |
| Caffeic acid | (mg/100 g DW) | 59.02 20 | 11.1 12 | nd 16 | nd 16 |
| 1,3-O-Dicaffeoylquinic acid | (mg/100 g DW) | 4.53 16 | 3.6 12 | 2.82 16 | 7.16 16 |
| 3,4-O-Dicaffeoylquinic acid | (mg/100 g DW) | nd 15 | nd 15 | 10 15 | 1315 |
| 3,5-O-Dicaffeoylquinic acid | (mg/100 g DW) | 18 15 | nd 15 | 11 15 | 31 15 |
| 1,5-O-Dicaffeoylquinic acid | (mg/100 g DW) | 361 15 | 30 15 | 244 15 | 760 15 |
| 4,5-O-Dicaffeoylquinic acid | (mg/100 g DW) | Trace 15 | nd 15 | 6 15 | 8 15 |
| Flavonoids | |||||
| Naringenin-7-O-rutinoside | (mg/100 g DW) | nd 16 | - | 5.3 16 | 5.48 16 |
| Naringenin-7-O-glucoside | (mg/100 g DW) | nd 16 | - | nd 16 | nd 16 |
| Luteolin-7-O-rutinoside | (mg/100 g DW) | nd1 5 | 237 15 | 6 15 | 26 15 |
| Luteolin-7-O-glucuronide | (mg/100 g DW) | 18 15 | 217 15 | 15 15 | 18 15 |
| Luteolin-7-O-malonylglucoside | (mg/100 g DW) | nd 15 | 87 15 | nd 15 | 12 15 |
| Luteolin | (mg/100 g DW) | nd 15 | 23 15 | 9 15 | 7 15 |
| Apigenin-7-O-rutinoside | (mg/100 g DW) | nd 15 | nd 15 | nd 15 | nd 15 |
| Apigenin-7-O-glucuronide | (mg/100 g DW) | 20515 | nd 15 | 285 15 | nd 15 |
| Apigenin malonylglucoside | (mg/100 g DW) | 20 15 | nd 15 | 31 15 | nd 15 |
| Apigenin | (mg/100 g DW) | 5 15 | nd 15 | 10 15 | nd 15 |
| Quercitin | (mg/100 g DW) | 0.2 12 | 0.6 12 | - | 0.2 12 |
| Cyanidin 3,5-diglucoside | (mg/100 g DW) | 0.7 17 | - | - | - |
| Cyanidin 3-glucoside | (mg/100 g DW) | 10.18 17 | - | - | - |
| Cyanidin 3,5-malonyldiglucoside | (mg/100 g DW) | 16.41 17 | - | - | - |
| Cyanidin 3-(3″-malonyl) glucoside | (mg/100 g DW) | 2 17 | - | - | - |
| Cyanidin 3-(6″-malonyl) glucoside | (mg/100 g DW) | 58.56 17 | - | - | - |
| ABTS TEAC | (mg TE/g DW) | - | 17.47 18 | 14.96 18 | 11.97 18 |
| (μmol TE/g DW) | 39.24 19 | - | 32.7 19 | - | |
| DPPH | (mg TE/g DW) | - | 20.42 18 | 19.49 18 | 17.59 18 |
| (μg/mL) | 28.2 19 | - | 6.42 19 | - | |
| FRAP | (µmol Fe2+/g DW) | - | 195.14 18 | 170.78 18 | 121.5 18 |
| (μmol TE/mL) | 493.9 19 | - | 209.10 19 | - | |
| Total polyphenols | (mg GAE/g DW) | 19.31 20 | 10.59 18 | 10.73 18 | 8.89 18 |
| Type | Botanic Part | Experiment Design | Results | Ref. |
|---|---|---|---|---|
| Antioxidant activity | ||||
| In vitro | Head | Utilization of a hydroalcoholic extract of artichoke (0.18–1.44 μg/mL; 1–4 h) on Caco-2 human intestinal cells | Inhibition of LDL-C oxidation | [87] |
| In vitro | Stem | Application of extracts from artichoke stems (1 and 2 mg/mL) in LPS-stimulated human THP-1 macrophages for 1 h | ↓ ROS | [86] |
| ↓ IL-6, CCL2 | ||||
| In vitro | Leaf | Artichoke leaf extract (25, 50 and 100 µg/mL; 24 h) on endothelial cells and monocytes obtained from umbilical cords | ↓ Oxidized LDL | [85] |
| ↓ ROS | ||||
| In vivo | Leaf | Alloxan-induced diabetic rats treated with artichoke leaf extracts (two doses of 200–400 mg/kg bw) daily for 4 weeks | ↑ CAT, SOD and GSH in liver and kidney | [88] |
| ↓ α-amylase, TC, TG and LDL-C | ||||
| In vivo | Leaf | Cd-toxicity-induced rats treated with artichoke leaf extract (300 mg/kg bw) daily for 4 weeks | ↑ CAT, GSH and GPx in liver and kidney | [89] |
| ↓ Liver MDA and liver and kidney SOD | ||||
| In vivo | Head | ALD-induced mice treated with an ethanolic extract of artichoke (0.4, 0.8, and 1.6 g/kg bw) for 10 days | ↑ SOD and GSH levels | [90] |
| ↓ MDA | ||||
| In vivo | Leaf | Urolithiasis-induced rats treated with artichoke leaf extract (125, 250 and 500 mg/kg bw) for 28 days | ↓ MDA and GSH in kidney | [91] |
| Hepatoprotective action | ||||
| In vitro | Head | Application of artichoke water extract (100 µM; 24 h) in palmitate-induced insulin resistance HepG2 hepatocytes | ↓ Expression of PEPCK and G6Pase | [92] |
| Inhibition of GS phosphorylation | ||||
| In vitro | Head | Utilization of artichoke extract (400–1200 µM; 24 h) on rat hepatocytes and human HepG2 cells exposed to H2O2 | ↑ GSH | [93] |
| ↓ MDA | ||||
| In vivo | Leaf | Application of artichoke leaf extract (1.5 g/kg bw) in paracetamol-induced Sprague–Dawley rats for 14 days | ↓ ALT and AST | [94] |
| ↑ GSH and NO levels | ||||
| ↑ SOD, GST and GR | ||||
| In vivo | Leaf | Utilization of artichoke leaf extract (300 mg/kg bw) male Wistar rats after hepatic ischemia–reperfusion injury | ↓ ALT, AST, LDH, ALP and CK | [95] |
| ↑ SOD, CAT and GPx | ||||
| In vivo | Leaf | Application of hydroethanolic leaf extract of artichoke (300 mg/kg bw; 6 weeks) in rats after induction of toxicity with Pb | ↓ ALT, AST and ALP | [96] |
| ↓ TG, VLDL-C and MDA | ||||
| Clinical trial | Head | Type 2 diabetes and NAFLD adults received 300 mg of artichoke extract daily for 16 weeks | ↓ Serum ALT, AST, GGT and ALP | [97] |
| ↑ GPx and SOD levels | ||||
| Improvement in RHI and Frhi | ||||
| Clinical trial | Head | Adults with NAFLD treated with leaf artichoke extract (six tablets of 2700 mg) daily for 8 weeks | ↓ Serum ALT and AST | [98] |
| ↓ TG and TC | ||||
| Clinical trial | Leaf | Application of 600 mg artichoke extract in NAFLD adults for 8 weeks | ↓ ALT and AST | [99] |
| ↓ TG and TC, HDL-C and LDL-C | ||||
| ↓ Total bilirubin, portal vein diameter and liver size | ||||
| Prebiotic and anti-inflammatory effect | ||||
| In vitro | Head | Short-term colonic fermentation analysis after application of artichoke dry extract using an in vitro gut model | ↑ Acetate, propionate and total SCFA production | [100] |
| ↑ Lactobacillus spp. levels | ||||
| In vitro | By-products | SCFA and lactic acid production after in vitro fermentation by human fecal microbiota with artichoke by-products for 72 h | ↑ Acetate, propionate and total SCFA production | [101] |
| Consumption of 54.6% of substrate by the fecal bacteria | ||||
| In vitro | Leaf | Application of artichoke leaf extract on Caco-2 cells (0.001, 0.01 and 0.1 mg/mL; 16 h) after in vitro colonic fermentations | ↓ IL-1β and TNF-α | [102] |
| ↑ Antioxidant capacity | ||||
| In vivo | Leaf | TNBS-induced CD-1 mice treated with an intraperitoneal administration of artichoke leaf extract once per day for 4 days | ↓ TNF-α | [103] |
| Absence of significant side effects on the extra-intestinal manifestations related to IBD | ||||
| In vivo | Head | C57BL/6 mice with colitis treated with artichoke pectin used at two doses (40 and 80 mg/kg bw) for 15 days | ↓ TNF-α and ICAM | [104] |
| ↓ IL-1β and IL-6 | ||||
| Clinical trial | Head | Very-long-chain inulin extracted from globe artichoke (10 g/d) on healthy adults for 3 weeks | ↑ Bifidobacteria, Lactobacillus and Bacteroides–Prevotella | [105] |
| No differences in fecal SCFA production | ||||
| Hypoglycemic action | ||||
| In vivo | Leaf | Leaf aqueous extract (200 and 400 mg/kg bw) on streptozotocin-induced diabetic rats for 21 days | ↓ Serum glucose | [106] |
| ↓ TC, TG, LDL-C and VLDL-C | ||||
| ↓ Plasma MDA | ||||
| In vivo | Leaf | Dry leaf extract (0.2 g extract/kg bw) was given to Wistar diabetic rats for 30 days | ↓ Serum glucose | [107] |
| ↓ TC and TG | ||||
| ↓ Serum fructosamine | ||||
| In vivo | Head and leaf | Hypercholesterolemic and diabetic rats treated with artichoke heart and leaf extract (200, 400 and 600 mg/kg bw) for 4 weeks | ↓ Serum glucose | [18] |
| ↓ TC, LDL-C and TG | ||||
| Clinical trial | Head | Overweight and obese IFG patients treated with two daily oral doses of 500 mg of artichoke extract for 8 weeks | ↓ Serum glucose and insulin | [108] |
| ↓ ADAG and HOMA | ||||
| ↓ LDL-C | ||||
| Clinical trial | Head | Overweight and obese adults with newly detected IFG treated with three daily oral assumptions of film-coated tablets of 200 mg of artichoke extract for 8 weeks | ↓ FBG and glycosylated hemoglobin | [109] |
| ↓ HOMA and ADAG | ||||
| ↓ LDL-C and TC | ||||
| Clinical trial | Head | Type 2 diabetic patients treated daily with four wheat cookies containing 6 g of globe artichoke powder for 90 days | ↓ Serum glucose | [110] |
| ↓ TC, TG and LDL-C | ||||
| ↑ HDL | ||||
| Cardioprotective effect | ||||
| In vitro | Leaf | HCASMC incubated with a cytokine mixture and treated with an aqueous artichoke leaf extract (1–100 µg/mL; 6 h or 24 h) | Inhibition of iNOS induction by artichoke leaf extract | [72] |
| ↓ Cytokine-induced iNOS promoter activation and iNOS protein expression | ||||
| In vitro | Leaf | HepG2 cells incubated with 50–250 µg/mL of artichoke leaf extract for 48 h | ↑ Free cholesterol production | [111] |
| ↑ Bile acid production | ||||
| In vivo | Leaf | HFD-fed rats treated with leaf extract at two doses (200–400 mg/kg/bw) daily for 2 months | ↓ Serum TC, TG, LDH, ALT, MDA and AOPP | [112] |
| ↑ SOD, CAT and GPx | ||||
| Histological findings showed a cardioprotective effect | ||||
| ↑ Fatty acid oxidation inhibition | ||||
| In vivo | Leaf | Cholesterol-fed rats treated with 150, 300 or 600 mg/kg of leaf extract for 4 weeks | ↓ TC and oxidized-LDL | [113] |
| ↓ IL-1, IL-6, TNFα-, IFN-α and C-reactive protein | ||||
| In vivo | Stem | Diabetic male albino mice treated with artichoke floral stem extract (250 mg/kg bw) | ↓ Serum TC and TG | [114] |
| ↓ LDL-C and AIP | ||||
| In vivo | Head | HFD-fed hamsters treated with a 20% fiber by-product from artichoke for 3 weeks | ↓ Total fat, TC, TG and esterified cholesterol | [115] |
| ↑ Fecal excretion of total fat, TG and bile acids | ||||
| Clinical trial | Leaf | Patients with mild hypertension received 50 and 100 mg of artichoke juice concentrate for 12 weeks | ↓ SBP | [116] |
| ↓ DBP | ||||
| Clinical trial | Leaf | Daily leaf extract intake (twice a day, 250 mg) in adults with low HDL-cholesterol and mild hypercholesterolemia for 60 days | ↑ HDL-C and MCP-1 | [117] |
| ↓ ApoB/ApoA, total-C/HDL-C ratio and NK response | ||||
| Clinical trial | Head | Hypercholesterolemic patients treated with a 500 mg daily intake of dry extract of artichoke and beberis for 2 months | ↓ TC and LDL-C | [118] |
| ↓ Serum TG | ||||
| Neuroprotective effect | ||||
| In vitro | Head | Artichoke head extracts were subjected to antioxidant activity tests and AChE neuro-related assay | ↑ AChE inhibition | [119] |
| ↑ Inhibition of TMA-d9 production | ||||
| In vivo | Leaf | HFD-fed mice treated with artichoke leaf ethanol extract (1600 mg/kg bw) daily for 4 weeks | ↓ TNF-α, IL-1β, SOD and IL-10 in the striatum | [120] |
| ↓ ROS and RSN in hypothalamus, prefrontal cortex and striatum measured by DCFH | ||||
| ↓ NO production hypothalamus, prefrontal cortex and striatum | ||||
| In vivo | Leaf | AflatoxinB1-treated rats fed with artichoke leaf extract (100 mg/kg bw) for 42 days | ↓ AChE, MAO and NO | [121] |
| ↓ GSH, SOD, GST and GPx | ||||
| ↓ TBARS, XO and UA | ||||
| In vivo | Leaf | Fluoride-induced Nile tilapia treated with artichoke leaf extract (300 mg/kg bw) for 60 days | ↓ AChE | |
| ↓ Severity of disease in histopathological findings | [122] | |||
| ↓ MDA, glucose, cortisol and 8-OHdG levels | ||||
| In vivo | Head | Male rats induced with rotenone and treated with artichoke extract (200 mg/kg bw) for 20 days | ↑ DA tissue concentration and IL-6 | [123] |
| ↓ IL-1β, cas-3 and cyt-c | ||||
| Improvements in open locomotion duration, cataleptic state, balance beam and vertical pole performance | ||||
| In vivo | Leaf | Methanolic leaf extract supplementation (0.8 and 1.6 g/kg bw) in ameliorating DEN-induced BALB/c mice for 14 days. | ↓ Cas-3 and Bax levels | [124] |
| ↑ Klotho and PPARγ levels | ||||
| ↓ MDA and TOS | ||||
| Food Product | Formulation | Outcomes | Ref. | |
|---|---|---|---|---|
| Bakery and pastry products | Pasta | Addition of artichoke by-products (bracts and stems) extracted with UAE in traditional Italian fresh egg pasta (10%) | Higher TPC in the reformulated pasta and lower TPC loss after cooking | [169] |
| Extended shelf-life | ||||
| Bread | Replacement of durum wheat semolina with flour of artichoke by-products (bracts and stems) in bread (5%, 7.5% and 10%) | Visible effects on color, increasing a* and reducing b* and L* | [170] | |
| Higher water absorption as extract concentration increased | ||||
| Lower volume and higher hardness, but no moisture loss in 5 days of storage | ||||
| Crackers | Addition of leaf extract residue from artichoke in crackers (3–12%) | Decrease in product thickness and hardness | [171] | |
| Higher fiber content | ||||
| Decrease in overall acceptability | ||||
| Cake | Replacement of fat and wheat flour with artichoke bract powder in cake (20% and 40%) | Higher TPC and antioxidant activity | [172] | |
| Improvement in sensory properties and texture at optimum utilization ratio (replacement of 31.63% of fat and 16.43% of wheat flour) | ||||
| Cookies | Addition of fiber-rich powders from artichoke by-products (bracts and stems) in cookies (4%) | Similar overall acceptability to cookies formulated with CRF | [173] | |
| Similar behavior during storage to cookies formulated with the CRF | ||||
| Darker color than cookies formulated with CRF | ||||
| Meat and fish products | Sausage | Replacement of fat with artichoke bracts paste in beef sausages (25, 50% and 100% of fat) | Improvement in cooking loss, cooking yield and shrinkage | [174] |
| Similar sensory properties, especially in sample with 25% extract | ||||
| Lowest microbial count when replacing 100% of fat with extract | ||||
| Patties | Addition of artichoke by-product extract (external bracts) in beef patties (500 and 1000 ppm) | Higher TPC and DPPH values than in the control for 500 and 1000 ppm reformulations | [175] | |
| Lower TBARS values than in the control at the end of storage for 500 and 1000 ppm reformulations | ||||
| Inhibition of the viability of total aerobic psychrophilic bacteria, coliform bacteria and yeast mold | ||||
| Aubergine burgers | Addition of 1% and 3% of artichoke extract in aubergine burgers | Improvement in the attributes of off-odor, off-flavor and overall | [176] | |
| Observed microbial growth values did not exceed the recommended limit | ||||
| Lower intensity of color and firmness of the burger with 1% artichoke extract | ||||
| Sardines | Incorporation of 5% of artichoke leaf extract in marinated sardine filets | Improvement in oxidative stability and higher content of polyunsaturated fatty acids | [177] | |
| Decrease in total volatile basic nitrogen and trimethylamine during storage | ||||
| Greater color and appearance scores | ||||
| Dairy products | Yogurt | Addition of artichoke leaf extract in probiotic yogurt (0.5%) | Higher TPC and antioxidant activity | [178] |
| Faster acidity increase, shorter incubation time and greater final titrable acidity than control yogurts | ||||
| Decrease in overall consumer acceptability | ||||
| Cheese | Replacement of animal rennet with artichoke head extract (0.3%) in the manufacture of Gouda-type cheeses from bovine milk | No significant differences in chemical parameters of cheese analyzed depending on type of coagulant (bovine or vegetable) | [80] | |
| No significant organoleptic differences in cheese depending on type of coagulant | ||||
| Higher level of casein-degrading products |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuso, P.; Quizhpe, J.; Rosell, M.d.l.Á.; Peñalver, R.; Nieto, G. Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara scolymus L.) and Artichoke By-Products: A Review. Appl. Sci. 2024, 14, 4940. https://doi.org/10.3390/app14114940
Ayuso P, Quizhpe J, Rosell MdlÁ, Peñalver R, Nieto G. Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara scolymus L.) and Artichoke By-Products: A Review. Applied Sciences. 2024; 14(11):4940. https://doi.org/10.3390/app14114940
Chicago/Turabian StyleAyuso, Pablo, Jhazmin Quizhpe, María de los Ángeles Rosell, Rocío Peñalver, and Gema Nieto. 2024. "Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara scolymus L.) and Artichoke By-Products: A Review" Applied Sciences 14, no. 11: 4940. https://doi.org/10.3390/app14114940
APA StyleAyuso, P., Quizhpe, J., Rosell, M. d. l. Á., Peñalver, R., & Nieto, G. (2024). Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara scolymus L.) and Artichoke By-Products: A Review. Applied Sciences, 14(11), 4940. https://doi.org/10.3390/app14114940








