Abstract
The American cranberry is a perennial North American fruit plant that is grown successfully on commercial plantations in Poland. The purpose of this study was to recognize filamentous fungi that colonize roots, leaves, and fruits without visible disease symptoms. Pure fungal cultures were isolated from disinfected plant fragments in agar media and identified by sequencing common taxonomic DNA markers such as the ITS region, the TEF-1α, or RPB2 genes. Of the 141 isolates studied, 59% were identified as closely related to soil saprotrophs. They were classified primarily as showing the greatest similarity to type strains of Trichoderma amoenum, Trichoderma dorothopsis, Paraphaeosphaeria sporulosa, and Penicillium murcianum. Additionally, isolates that are most similar to strains of Penicillium crustosum, Aspergillus flavus, and Aspergillus versicolor that produced mycotoxins were detected. The fungi identified as closest to Alternaria geophila, Alternaria senecionicola, Paraphoma radicina, Pestalotiopsis unicolor, Pestalotiopsis scoparia, and Neopestalotiopsis spp., whose hosts are plants other than American cranberry, represented 33.81% of the isolates tested. Only 7.2% of the isolates corresponded to the species of Physalospora vaccinia, Diaporthe vaccinii, and Diaporthe eres, known cranberry pathogens. The results of this study can be used to identify latent plant infection and potential disease risks.
1. Introduction
The Ericaceae family includes two types of cranberry plants that grow in Poland: European cranberry (Vaccinium oxycoccos L.) and American cranberry (Vaccinium macrocarpon Aiton). The European cranberry is found in northern and central Europe, northern Asia, and North America. The American cranberry, which is cultivated in Poland as a domesticated anthropophyte, is native to North America, from Texas to Alaska. Cranberries are among some of the most important medicinal plants. They are mainly known for their effectiveness in preventing urinary tract and bladder infections and can be used in cosmetology, medicine, and cuisine. However, only the American cranberry is of significant economic importance. The fruits of this plant have strong antioxidant and antimicrobial properties, high levels of pectin, minerals (such as potassium, calcium, phosphorus, magnesium, and iodine) and vitamins (including A, C, B1, B2, and B6). Furthermore, fruits contain tannins, organic acids (mainly malic and citric), and polyphenols, such as proanthocyanidins, anthocyanins, flavanols, and phenolic acids [1,2]. It can be grown in raised peat bogs, transitional peat bogs with regulated soil pH, and light mineral soils. It requires soil with a pH value of 3.5 to 4.0 and can tolerate high groundwater levels and periodic flooding, especially in winter and spring, which protects it from frost [3]. The first commercial plantations were established in existing wetlands and bogs in the early 1800s in the United States of America [4]. Today, the largest commercial plantations in Europe are located in the UK, the Netherlands, Germany, Switzerland, Latvia, Belarus, Estonia, Romania, and Poland, where cranberries are grown in artificial wetlands constructed on mineral soils, pure sand, or peat with a wide range of organic components. [5,6]. In southern-eastern Poland, the largest plantation in the European Union was created, with a current area of 42 ha, where the cultivars ‘Stevens’, ‘Pilgrim’, ‘Ben Lear’, and ‘Norman Le Munyon’ are grown [7].
The economic success of cranberry cultivation is based on the risk of pathogen infections. Shear et al. [8] assigned the fungi found in American cranberry plants to three groups: fungi recognized as the main causes of fruit rot, fungi known to cause diseases of the cranberry vine, and fungi of minor commercial importance found sporadically on leaves, stems, and decayed fruits. In the data from the literature, the greatest attention is paid to pathogenic fungi belonging to the first two groups, which can infect plants early in the growing season or during flowering. Some of them can remain latent until the fruit ripens [9,10]. In Central Europe, the most common pathogens associated with fruit yield, quality loss, and limited economic profits are Botrytis cinerea, Colletotrichum acutatum, Fusicoccum putrefaciens, Diaporthe vaccinii, Pestalotia vaccinii, Discosia artocreas, Physalospora vaccinii, Fusarium spp., Verticillium spp., and Phytophthora spp. [10,11]. Infected fruits can show soft rot, are reddish brown and mushy, and often disintegrate in the hand [12]. Furthermore, from cranberry fruits can be isolated typical saprotrophic fungi of the genus Penicillium and Aspergillus that can cause damage and loss of fruit quality during storage. Their reservoirs may be other host plants or decaying organic matter [11,13,14]. The fungi that inhabit leaves can also cause visible infection symptoms, such as chlorosis, colored spots, necrosis, wilting and dying of shoots, leaves, and stolons [12], and latent infections, or some of them can show the ability to control the growth of various plant pathogens. Among them were identified members of species such as Pestalotiopsis sydowiana, Alternaria alternata, Epicoccum nigrum, Pestalotia rhododendri, Gibberella stilboides, Phialophora cyclaminis, Sordaria fimicola, Umbelopsis isabellina, Boeremia exigua, Phoma leveillei, Phoma medicaginis, Cladosporium cladosporioides, Trichoderma hamatum, Paraphoma chrysanthemicola, Phialophora cinerescens, Davidiella macrocarpa, Ramularia spp., and Erysiphe spp. [10,15]. Cranberries can also be exposed to microorganisms that occur in irrigation waters, such as Phomopsis vaccinii, Synchronoblastia crypta, Phytophthora cinnamomi, and Phytophthora megasperma [3,4,16]. Infection can manifest itself in poor root system development, a small number of fruits, discoloration or loss of leaves, and plant death [17].
To ensure plant health, it is also important to monitor latent infections, where pathogens are present in plants, but disease symptoms are not observed. The reason for this phenomenon may be the lower susceptibility of the cultivars grown or environmental conditions that are not conducive to the development of infection at a given time. Therefore, the objective of this study was to analyze the occurrence of potentially pathogenic microorganisms that can colonize the roots, leaves, and fruits of American cranberry plants grown in one of the largest commercial plantations in central Europe without visible disease symptoms.
2. Materials and Methods
2.1. Plants
In September, American cranberry plants (V. macrocarpon Aiton) of the dessert variety ‘Stevens’ were harvested from a commercial plantation located in the southern part of Poland (50°70′ N, 21°91′ E). The cultivation was carried out on sandy soil after the natural accumulation level of the soil was removed. The one-hectare fields were located in artificially formed polders. The plants were in their first, second, and third years after planting and did not show visible symptoms of the disease.
2.2. Isolation of Filamentous Fungi from American Cranberry Plants
Seven whole plants with ripe fruits were randomly sampled from the plantation after the first, second, and third year after planting and transported to the laboratory at a temperature of +8 °C. Roots, leaves, and fruits were used to isolate the filamentous fungi that inhabit them and are capable of growing under laboratory conditions. Universal peptone-glucose agar medium with rose bengal, which enables the growth of a large group of molds and yeast, and selective solid media for fungi of the genera Fusarium, Botrytis, Rhizoctonia, and Penicillium were used. To do this, the plants were washed with tap water, disinfected for 15 min in 2.0% sodium hypochlorite, and then rinsed five times in sterile distilled water. Ten 1 cm long fragments of roots, leaves, and fruits were placed on agar media in Petri dishes with a diameter of 9.0 cm. The following media were used for the isolation of fungi from root fragments: universal Martin’s Rose Bengal agar (MRB) containing glucose 10 g, pepton tryptone 5.0 g (BTL, Łódź, Poland), KH2PO4 1.0 g, MgSO4 × 7 H2O 0.5 g, rose bengal dye 0.05 g, streptomycin 0.03 g, and agar 16 g in 1 L of water; Ko and Hora medium (K&H) for the species Rhizoctonia solani contained gallic acid 0.4 g, K2HPO4 1 g, MgSO4 × 7 H2O 0.5 g, KCl 0.5 g, FeSO4 × 7 H2O 0.01 g, NaNO2 0.2 g, chloramphenicol 0.05 g, streptomycin 0.05 g, metalaxyl M 0.07 g, mankozeb 1.18 g (Ridomil Gold® MZ 67.8 WG, Syngenta Crop Protection AG, Basel, Switzerland), prochloraz 0.005 g (Mirage 450EC® fungicide, Makhteshim Chemical Works Ltd., Beer Sheva, Israel), and agar 16 g in 1 L of water [18,19]; Nash and Snyder medium (N&S) for the genus Fusarium contained peptobak (BTL) 15 g, KH2PO4 1 g, MgSO4 × 7 H2O 0.5 g, chloramphenicol 0.1 g, pentachloronitrobenzene (PCNB) 0.1 g, streptomycin 0.05 g, malachite green dye 0.0025 g, and agar 16 g in 1 L of water [20]. For the isolation of fungi from leaves and fruits, there were MRB, N&S. B. cinerea (BSM) medium included glucose 2 g, tannic acid 5 g, NaNO3 0.1 g, K2HPO4 0.1 g, MgSO4 × 7 H2O 0.2 g, KCl 0.1 g, chloramphenicol 0.2 g, PCNB 0.02 g, mancozeb 0.016 g (Penncozeb 80WP, Cerexagri SAS, Plaisir, France), rose bengal dye 0.05 g, and agar 16 g in 1 L of water [21], and medium for the genus Penicillium (PRYES) contained peptone 5 g, yeast extract 3 g, sucrose 15 g, chloramphenicol 0.05 g, tetracycline 0.05 g, PCNB 1 g, Bengal rose 0.025 g, and agar 16 g in 1 L of water [22]. The incubation was carried out for 2 weeks at 26 °C ± 2 °C. After this time, the isolates were repicked in the appropriate agar medium and incubated under the same conditions as the pure cultures. The fungi were identified by DNA sequence analysis.
2.3. Identification of Fungal Isolates Colonizing American Cranberry Plants
The fungi isolated from roots, leaves, and fruits were identified by analyzing one of the following DNA taxonomic markers: internal transcribed spacer 1 with the 5.8S gene and internal transcribed spacer 2 (ITS), RNA polymerase II subunit B (RPB2), or translation elongation factor 1 alpha (TEF-1α). The ITS region was used as the primary taxonomic marker to identify the isolates tested. The secondary marker genes RPB2 and TEF-1α were used for isolates for which the ITS region showed 100% similarity to strains of more species. For isolates of the genus Trichoderma, the TEF-1α gene was selected as a secondary marker, while for the genera Penicillium and Alternaria, it was RPB2. The selection criterion was the availability of more reference sequences in the NCBI database. The following primers were used: ITS1F (5′-TCCGTAGGTGAACCTGCGG), ITS4R (5′-TCCTCCGCTTATTGATATGC), and ITS5F (5′-GGAAGTAAAAGTCGTAACAAGG) for the ITS regions [23], TEF1-728F (5′-CATCGAGAAGTTCGAGAAGG) and TEF1LLrev (5′-AACTTGCAGGCAATGTGG) for the TEF-1α genes [24], and fRPB2-5F (5′-GAYGAYMGWGATCAYTTYGG) and fRPB2-7cR (5′-CCCATRGCTTGYTTRCCCAT) for the RPB2 genes [25]. Genomic DNA was extracted using the Syngen Fungi DNA Mini Kit according to the manufacturer’s instructions (Syngen Biotech, Wrocław, Poland).
The polymerase chain reaction (PCR) mixture contained 4 μL of 5× HOT FIREPol Blend Master Mix with 7.5 mM MgCl2 (Solis BioDyne, Tartu, Estonia), 5 pmol μL−1 of forward and reverse primers, and from 1.0 to 10 ng μL−1 of genomic DNA in a total volume of 20 μL.
The amplification was carried out under the following conditions: initial DNA denaturation and polymerase activation at 95 °C for 15 min, followed by 29 cycles of denaturation at 95 °C for 20 s, annealing at 55 °C for 30 s (for the pair of primers ITS1F—ITS4R), at 58 °C for 30 s (for ITS5F—ITS4R and TEF1-728F—TEF1-LLR), and at 51 °C for 30 s (for RPB2-5F—RPB2-7cR), elongation at 72 °C for 60 s (for ITS1F—ITS4R and ITS5F—ITS4R), for 30 s (for ITS3F and ITS4R), for 90 s (for TEF1-728F—TEF1-LLR and RPB2-5F—RPB2-7cR), and final elongation at 72 °C for 10 min. The effectiveness of PCR was verified using a 1.2% agarose electrophoresis gel with ethidium bromide. The products obtained were purified using the Syngen Gel/PCR Mini Kit (Syngen Biotech, Poland) following the manufacturer’s instructions and, then, used as a template for the sequencing reaction, which was carried out by Genomed S.A. in Warszawa, Poland.
Single reads were aligned using the DNAStar software (version 9.1) package (DNAStar Lasergene, Inc., Madison, WI, USA). The experimental taxonomic nucleotide databases of the National Center for Biotechnology Information (NCBI) were used to compare the isolate consensus sequences with the sequences of species-type strains deposited in the NCBI using the Basic Local Alignment Search Tool (BLAST). The taxonomic position of the isolates and the percent identity were determined by pairwise sequence alignment based on BLAST. These results were then verified by building phylogenetic trees [26] using ClustalW in MegAlign DNAStar software (version 9.1) (DNAStar Lasergene, USA) and the maximum likelihood calculation algorithm. The reliability of the tree was assessed by performing bootstrap analyses of 1000 resamples. The results of the phylogenetic analysis are presented in figures. The names of the isolates tested and the most similar reference strains are highlighted in bold font.
3. Results
To identify 141 fungal isolates originating from roots, leaves, and fruits of 1-, 2-, and 3-year-old American cranberry plants, DNA sequences of universal taxonomic markers such as the ITS region, RPB2, or TEF-1α gene were analyzed. The number of isolates obtained on universal Martin’s Rose Bengal agar and selective media for the genus Penicillium, Botrytis, Fusarium, and Rhizoctonia is presented in Table 1.

Table 1.
Numbers of filamentous fungi isolated from roots, leaves, and fruits of 1-, 2-, and 3-year-old plants of American cranberry.
3.1. Identification of Fungi That Colonize the Roots of American Cranberry
3.1.1. One-Year-Old Plants
Of the 19 isolates obtained from 1-year-old plants, the most prevalent were fungi belonging to the Aspergillaceae family (Eurotiales, Eurotiomycetes). Seven of these isolates were identified as Penicillium arizonense, P. granatense, P. yarmokense, P. murcianum, P. radiatolobatum, P. canescens, P. jensenii, and P. janczewskii with 100% similarity in the ITS region to the reference strains. An analysis of the secondary marker RPB2 gene allowed the identification of six of these isolates as the most similar to P. murcianum CBS 161.81, with a percentage identity of 97.37%. An isolate was recognized as closest to P. radiatolobatum CBS 340.79 with a similarity level of 99.46% (Figure 1). Among the remaining fungi, one was 100% similar to Penicillium chrysogenum CBS 306.48 (Figure 2), and two were 99.62% and 99.63% similar to Penicillium pulvillorum CBS 280.39 (Figure 3) by an analysis of the ITS sequence.
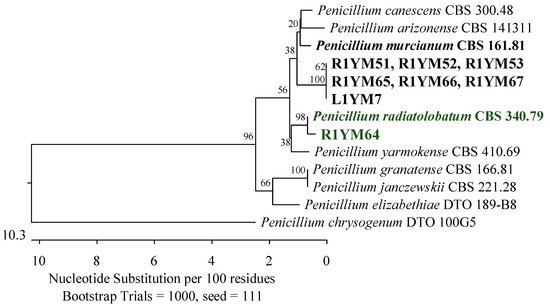
Figure 1.
Phylogenetic tree of fungi isolated from American cranberry most closely related to Penicillium murcianum and Penicillium radiatolobatum constructed from partial sequences of the RPB2 gene (674 bp). Isolates with the name prefix R1YM were obtained from roots and with the prefix L1YM from the leaves of 1-year-old plants on Martin’s Rose Bengal agar.
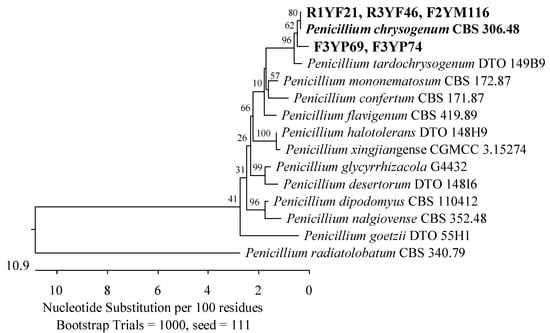
Figure 2.
The phylogenetic tree of fungi originated from American cranberry, most closely related to Penicillium chrysogenum, constructed with partial sequences of the RPB2 gene (674 bp). Isolates from roots of 1-year-old (name prefix R1YF) and 3-year-old plants (R3YF) were obtained in the medium for Fusarium, from fruits of 2-year-old plants (F2YM) on Martin’s Rose Bengal agar and from fruits of 3-year-old plants (F3YP) in a medium for Penicillium.
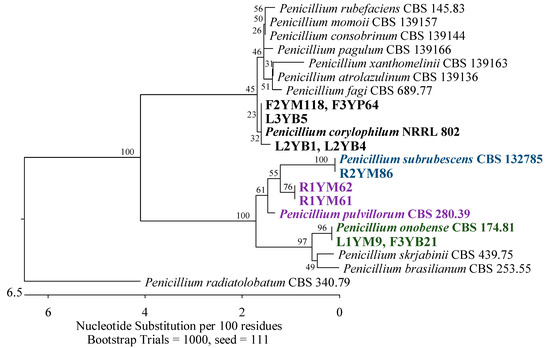
Figure 3.
Phylogenetic tree of fungi isolated from American cranberry most closely related to Penicillium corylophilum, P. subrubescens, P. pulvillorum, and P. onobense constructed with partial sequences of the ITS region (503 bp). Isolates from roots (name prefix R1YM) and leaves (L1YM) of 1-year-old plants and fruits of 2-year-old plants (F2YM) were obtained on Martin’s Rose Bengal agar. Isolates from leaves of 2-year-old plants (L2YB) and 3-year-old plants (L3YB) and fruits of 3-year-old plants (F3YB) were found in the medium for Botrytis. In the Penicillium medium, isolates were detected from fruits of 3-year-old plants (F3YP).
Fungi from the Didymosphaeriaceae and Phaeosphaeriaceae families (Pleosporales, Dothideomycetes) were also found in the roots of the youngest plants. Three isolates had ITS sequences that were 100% identical to Paraphaeosphaeria sporulosa CBS 824.68 (Figure 4), and another three had sequences that were 99.68–99.80% similar to Paraphoma radicina CBS 111.79 (Figure 5).
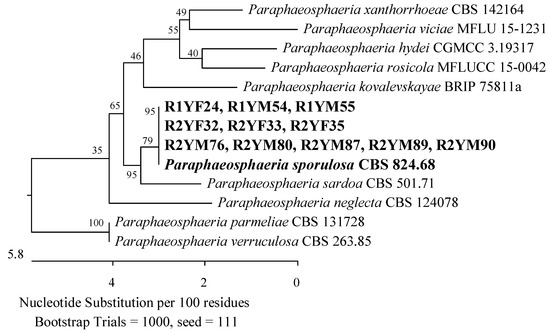
Figure 4.
The phylogenetic tree of American cranberry isolates most similar to Paraphaeosphaeria sporulosa constructed with partial sequences of the ITS region (540 bp). Isolates from roots of 1-year-old (name prefix R1YF) and 2-year-old (R2YF) plants were found in Fusarium medium and on Martin’s Rose Bengal agar (R1YM and R2YM).
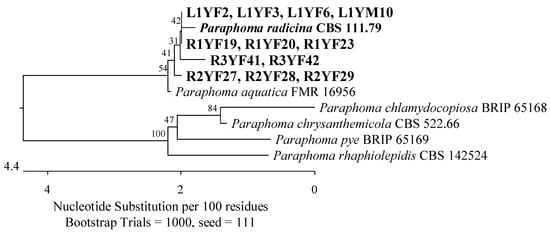
Figure 5.
The phylogenetic tree of isolates originated from American cranberry, most similar to Paraphoma radicina constructed with partial sequences of the ITS region (469 bp). In Fusarium medium, isolates were obtained from roots of 1-year-old (name prefix R1YF), 2-year-old (R2YF), and 3-year-old plants (R3YF), and leaves of 1-year-old plants (L1YF). The L1YM isolate was obtained from the leaves of 1-year-old plants on Martin’s Rose Bengal agar.
The roots of 1-year-old plants were also found to contain members of the Ophiocordycipitaceae family (Hypocreales, Sordariomycetes). Two isolates were identified as closest to Purpureocillium lilacinum ATCC 10114 (Figure 6). Furthermore, an isolate with an ITS sequence of 95.47% similarity to the type strain of Hapsidospora irregularis CBS 824.68 (Hypocreales, incertae sedis) was discovered.
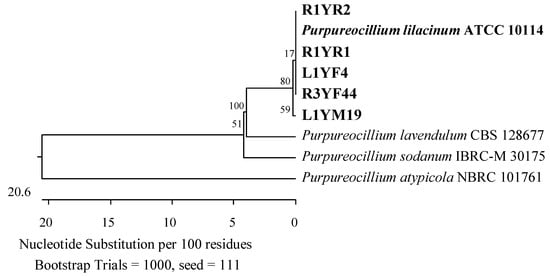
Figure 6.
Phylogenetic tree of isolates from American cranberries most similar to Purpureocillium lilacinum constructed using partial sequences of the ITS region (513 bp). Isolates from roots of 3-year-old plans (name prefix R3YF) and leaves of 1-year-old plants (L1YF) were found in the medium for the genus Fusarium, from roots of 1-year-old plants (R1YR) in Rhizoctonia medium, and leaves of 1-year-old plants (L1YM) on Martin’s Rose Bengal agar.
3.1.2. Two-Year-Old Plants
The main groups of 28 fungi from the roots of 2-year-old plants were assigned to the Hypocreaceae, Nectriaceae (Hypocreales), and Sporocadaceae (Xylariales) families of the class Sordariomycetes. Eleven of them were recognized as Trichoderma canadense, T. gamsii, T. dorotheae, T. neokoningii, T. texanum, T. amoenum, T. dorothopsis, T. caribbaeum var. aequatoriale, or T. caribbaeum var. caribbaeum with 100% identity of the ITS region simultaneously with reference strains from each of these species. An analysis of the TEF-1α gene revealed that its closest taxonomic position was T. amoenum YMF 1.06209 or T. dorothopsis HZA5, with sequence identities of 95.67–98.06% and 95.25–97.72%, respectively (Figure 7). The remaining three isolates were identified by analyzing the ITS region. Two of them showed a greater similarity to Trichoderma koningii ATCC 64262 (99.68–99.84%) and one to Trichoderma scalesiae CBS 120069 (99.60%) (Figure 8).
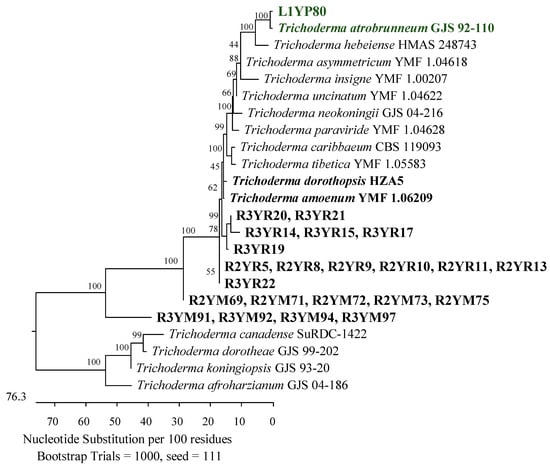
Figure 7.
Phylogenetic tree of American cranberry isolates closest to Trichoderma atrobrunneum, T. amoenum, and T. dorothopsis constructed with partial sequences of the TEF-1α gene (1191 bp). The isolates from the roots of 2-year-old plants (name prefix R2YR) and 3-year-old plants (R3YR) were obtained in Rhizoctonia medium and Martin’s Rose Bengal agar (R2YM and R3YM) and from the leaf of a 1-year-old plant in Penicillium medium (L1YP).
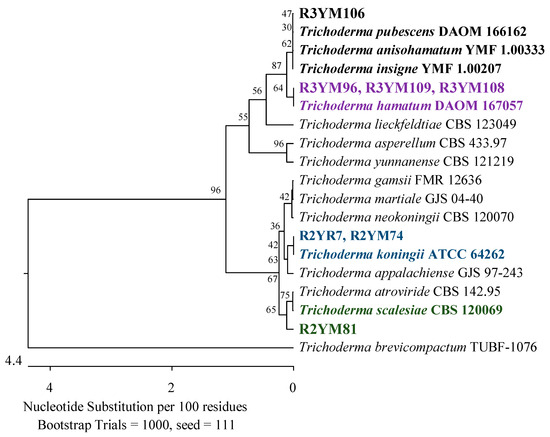
Figure 8.
The phylogenetic tree of American cranberry isolates most similar to Trichoderma insigne, T. pubescens, T. anisohamatum, T. hamatum, T. koningii, and T. scalesiae constructed with partial sequences of the ITS region (542 bp). Isolates from roots of 2-year-old plants (name prefix R2YR) were found in Rhizoctonia medium and from roots of 2-year-old plants (R2YM) and 3-year-old plants (R3YM) on Martin’s Rose Bengal agar.
Furthermore, an isolate exhibited 100% identity in the ITS region with Fusarium foetens CBS 110286 (Figure 9), and the second to two strains of the genus Pestalotiopsis; Pestalotiopsis unicolor MFLUCC 12-0276 (99.63%) and Pestalotiopsis scoparia CBS 176.25 (99.66%) (Figure 10).
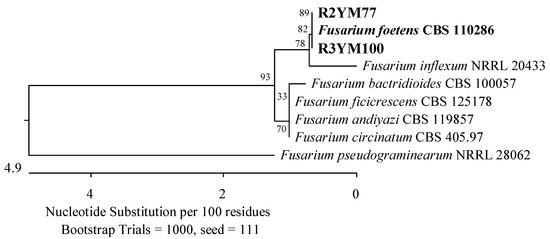
Figure 9.
Phylogenetic tree of American cranberry isolates the most similar to Fusarium foetens constructed with partial sequences of the ITS region (406 bp). Isolates from roots of 2-year-old plants (name prefix R2YM) and 3-year-old plants (R3YM) were obtained on Martin’s Rose Bengal agar.
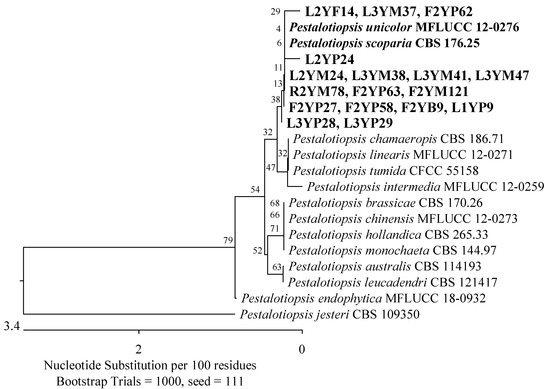
Figure 10.
Phylogenetic tree of American cranberry isolates closest to Pestalotiopsis unicolor and Pestalotiopsis scoparia constructed with partial sequences of the ITS region (530 bp). On Martin’s Rose Bengal agar, isolates were obtained from leaves of 2-year-old plants (name prefix L2YM) and 3-year-old plants (L3YM), roots of 2-year-old plants (R2YM) and fruits of 2-year-old plants (F2YM), in Penicillium medium from leaves of 1-year-old plants (L1YP), 2-year-old (L2YP) and 3-year-old plants (L3YP) and fruits of 2-year-old plants (F2YP), in Botrytis medium from fruits of 2-year-old plants (F2YB), and in Fusarium medium from leaves of 2-year-old plants (L2YF).
The roots of 2-year-old plants revealed the presence of the Phaeosphaeriaceae and Didymosphaeriaceae families, which were represented by three isolates with ITS regions related to P. radicina CBS 111.79 with 99.63–99.68% identity (Figure 5) and eight isolates that correspond 100% to P. sporulosa CBS 824.68 (Figure 4). The last isolate showed 100% similarity in the ITS region to Penicillium subrubescens CBS 132785 (Figure 3).
3.1.3. Tree-Year-Old Plants
Twenty-one fungal isolates were obtained from the roots of the oldest plants tested. The dominant family was Hypocreaceae, represented by 11 isolates that were identified by an analysis of the TEF-1α gene as closest to T. amoenum YMF 1.06209 (95.02–98.06%) and T. dorothopsis HZA5 (94.80–97.72%) (Figure 7). An analysis of the ITS region revealed that three other fungi of this genus were most similar (99.50–100%) to Trichoderma hamatum DAOM 167057, and one was 99.83% similar simultaneously to Trichoderma insigne YMF 1.00207, Trichoderma pubescens DAOM 166162, and Trichoderma anisohamatum YMF 1.00333 (Figure 8). Furthermore, two isolates of Nectriaceae and Sporocadaceae were detected with 100% similarity to F. foetens CBS 110286 (Figure 9) and P. lilacinum ATCC 10114 (Figure 6), and a fungus was identified as Neopestalotiopsis sp.
Of the remaining isolates obtained, two were identified by an analysis of the ITS region as the most similar to P. radicina CBS 111.79 (99.38–99.45%) of the Phaeosphaeriaceae family (Figure 5), while an analysis of the RPB2 gene showed that an isolate was most similar to P. chrysogenum CBS 306.48 (100%) of the Aspergillaceae family (Figure 2).
3.2. Identification of Fungi That Colonize Leaves of American Cranberry
3.2.1. One-Year-Old Plants
Seventeen isolates were obtained from the leaves of the youngest plants, most of which belonged to the Phaeosphaeriaceae and Didymellaceae families (Pleosporales, Dothideomycetes). The isolates were analyzed using the ITS region. Four of them showed 99.63–99.82% agreement with P. radicina CBS 111.79 (Figure 5), two 99.81% with Didymella prosopidis CBS 136414 (Figure 11), and one 100% with Epicoccum tritici MFLUCC 16-0276 and Epicoccum layuense CGMCC 3.18362 (Figure 12). An isolate was assigned to the genus Preussia (Sporormiaceae, Pleosporales, Dothideomycetes).
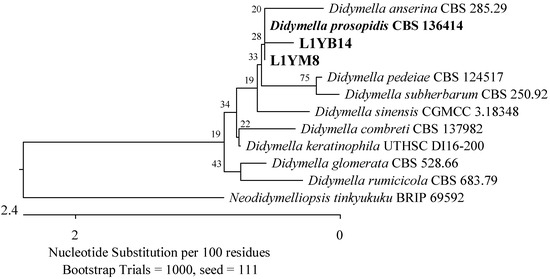
Figure 11.
Phylogenetic tree of American cranberry isolates closest to Didymella prosopidis constructed with partial sequences of the ITS region (460 bp). Isolates from leaves of 1-year-old plants were obtained in Botrytis medium (name prefix L1YB) and Martin’s Rose Bengal agar (L1YM).
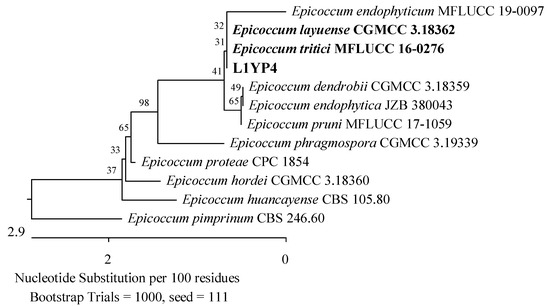
Figure 12.
Phylogenetic tree of the American cranberry isolate, most similar to Epicoccum tritici and Epicoccum layuense constructed with partial sequences of the ITS region (460 bp). The L1YP isolate was obtained from the leaves of 1-year-old plants in Penicillium medium.
The second taxonomic group comprised the Hypocreaceae (Hypocreales), Sporocadaceae, and Apiosporaceae (Xylariales) families of the Sordariomycetes class. This group included two isolates with 99.68% ITS region similarity to P. lilacinum ATCC 10114 (Figure 6), one with 99.84% to P. scoparia CBS 176.25 and P. unicolor MFLUCC 12-0276 (Figure 10), one with 100% to Apiospora guangdongensis ZHKUCC 23-0004 (Figure 13), and single members of the genera Neopestalotiopsis and Discosia. Furthermore, an isolate was identified as closest to Trichoderma atrobrunneum GJS 92-110 (99.76%) by analyzing the TEF-1α gene (Figure 7).
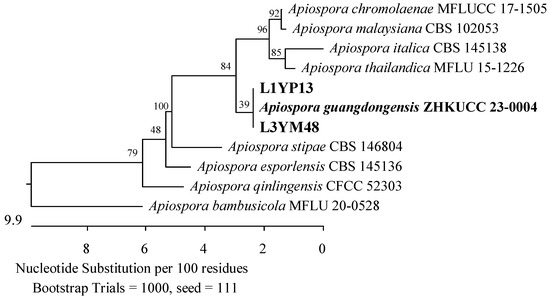
Figure 13.
Phylogenetic tree of American cranberry isolates most closely related to Apiospora guangdongensis constructed with partial sequences of the ITS region (543 bp). Isolates from leaves of 1-year-old plants were found in the medium for Penicillium (name prefix L1YP) and 3-year-old plants (L3YM) on Martin’s Rose Bengal agar.
3.2.2. Two-Year-Old Plants
The taxonomic groups of 20 fungi isolated from 2-year-old plants were mainly the Hypocreaceae and Sporocadaceae families of the class Sordariomycetes, which were also found in the youngest plants. Additionally, the Diaporthaceae family (Diaporthales) was present. Three isolates were 100% identical to Diaporthe vaccinii CBS 160.32, while two others were 98.72% Diaporthe biguttusis CGMCC 3.17081 and 99.81% Diaporthe eres CAA756 (simultaneously), which is not recognized as a strain type of D. eres species (Figure 14). Furthermore, three isolates showed a similarity of 99.33–99.66% in the ITS region with P. unicolor MFLUCC 12-0276 and P. scoparia CBS 176.25 (Figure 10), and an isolate was identified as Neopestalotiopsis sp.
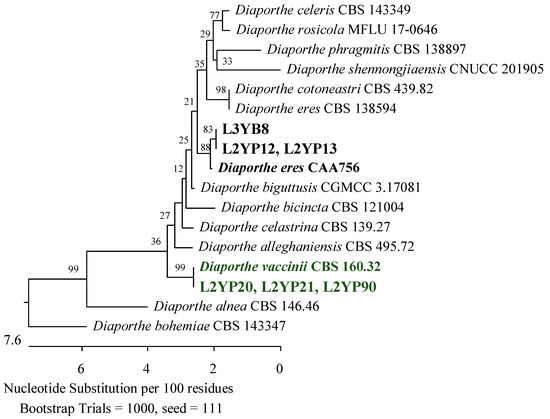
Figure 14.
Phylogenetic tree of American cranberry isolates most similar to Diaporthe eres and D. vaccinii constructed with partial sequences of the ITS region (470 bp). Isolates from leaves of 2-year-old plants (name prefix L2YP) were found in Penicillium medium and from 3-year-old plants (L3YB) in the medium for Botrytis.
The next two isolates were identified as 99.82% similar to Penicillium corylophilum NRRL 802 (Figure 3), two 99.65% similar to Aspergillus fumigatus ATCC 1022 (Figure 15), one 100% Aspergillus sydowii CBS 593.65 (Figure 16), and another 100% similar to Aspergillus flavus ATCC 16883, A. pipericola DTO 228-H4 and A. aflatoxiformans CBS 143679 (simultaneously) from the Aspergillaceae family (Figure 17).
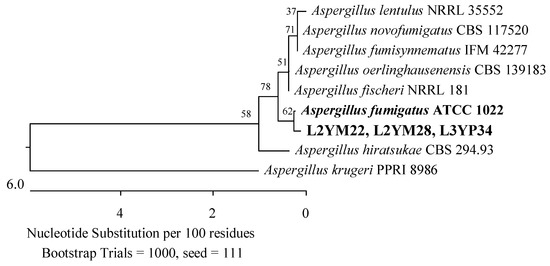
Figure 15.
Phylogenetic tree of American cranberry isolates most similar to Aspergillus fumigatus constructed with partial sequences of the ITS region (566 bp). Isolates from leaves of 2-year-old plants (name prefix L2YM) were found on Martin’s Rose Bengal agar and from 3-year-old plants in Penicillium medium (L3YP).
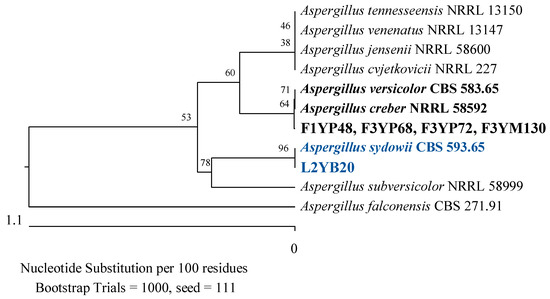
Figure 16.
Phylogenetic tree of American cranberry isolates most closely related to Aspergillus creber, A. versicolor, and A. sydowii constructed with partial sequences of the ITS region (461 bp). Isolates from fruits of 1-year-old plants (F1YP) and 3-year-old plants (F3YP) were found in Penicillium medium, 3-year-old plants (F3YM) on Martin’s Rose Bengal agar, and from the leaf of a 2-year-old plant (L2YB) in Botrytis medium.
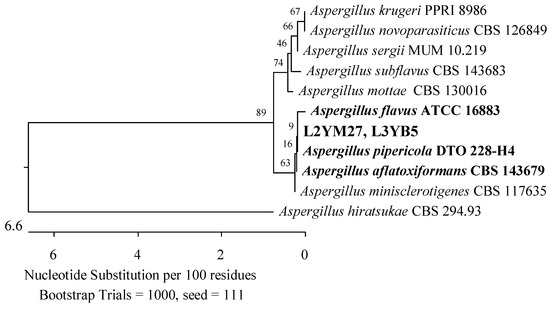
Figure 17.
Phylogenetic tree of American cranberry isolates most similar to Aspergillus flavus, A. pipericola, and A. aflatoxiformans constructed with partial sequences of the ITS region (554 bp). Isolates from leaves of 2-year-old plants (name prefix L2YM) were found on Martin’s Rose Bengal agar and 3-year-old plants (L3YB) in Botrytis medium.
The last taxonomic group detected in 2-year-old plants was the Pleosporaceae family (Pleosporales, Dothideomycetes). It was represented by four isolates recognized as 98.94% similar to Alternaria geophila CBS: 101.13 and Alternaria senecionicola CBS: 119545 based on an analysis of the RPB2 gene sequence (Figure 18).
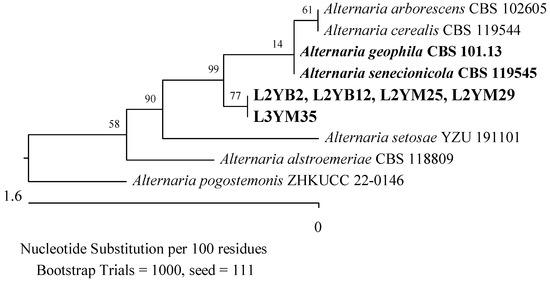
Figure 18.
Phylogenetic tree of American cranberry isolates most similar to Alternaria geophila and Alternaria senecionicola constructed with partial sequences of the RPB2 gene (753 bp). Isolates from leaves of 2-year-old plants (name prefix L2YM) and 3-year-old plants (L3YM) were found on Martin’s Rose Bengal agar and from leaves of 2-year-old plants (L2YB) in Botrytis medium.
3.2.3. Three-Year-Old Plants
In leaf samples from the oldest plants, 16 isolates were detected, with members of the Sporocadaceae family predominating. Based on the analysis of the ITS region, six of them were identified as the most similar to P. scoparia CBS 176.25 (99.66–100%) and P. unicolor MFLUCC 12-0276 (99.63%) (Figure 10), and two to Neopestalotiopsis spp. Furthermore, an isolate showed a 100% match with A. guangdongensis ZHKUCC 23-0004 of the Apiosporaceae family (Figure 13), the second 99.29% with Physalospora vaccinii CBS 143.22 of the Hyponectriaceae family (Figure 19), and the third, belonging to the Diaporthaceae family, had a 99.81% similarity to the D. eres strain CAA 756 (Figure 14).
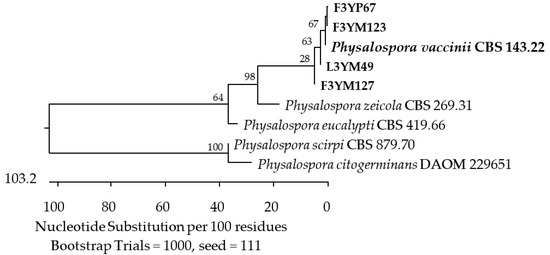
Figure 19.
Phylogenetic tree of American cranberry isolates most similar to Physalospora vaccinii constructed with partial sequences of the ITS region (572 bp). Isolates from fruits of 3-year-old plants were found on Martin’s Rose Bengal agar (name prefix F3YM) and in Penicillium medium (F3YP). The 3-year-old plant leaf isolate (L3YM) was found on Martin’s Rose Bengal agar.
Two additional fungal isolates were identified as belonging to the Aspergillaceae and Pleosporaceae families. One of them demonstrated 100% compatibility of the ITS sequence with P. corylophilum NRRL 802 (Figure 3), another with Penicillium crustosum FRR 1669 (Figure 20), a third with A. flavus ATCC 16883, A. pipericola DTO 228-H4, and A. aflatoxiformans CBS 143679 (Figure 17), and the last with A. fumigatus ATCC 1022 (Figure 15). Furthermore, an isolate was found to have 98.72% identity of the RPB2 gene sequence with A. geophila CBS 101.13 and A. senecionicola CBS 119548 (Figure 18).
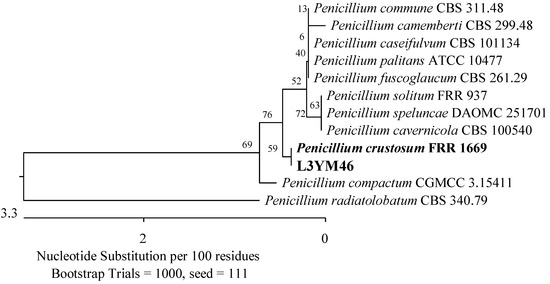
Figure 20.
Phylogenetic tree of the American cranberry isolate, most similar to Penicillium crustosum constructed with partial sequences of the ITS region (548 bp). The L3YM isolate was obtained from the leaf of a 3-year-old plant on Martin’s Rose Bengal agar.
3.3. Identification of Fungi Colonizing Fruits of American Cranberry
3.3.1. One-Year-Old Plants
For 1-year-old plants, only one isolate was obtained. It was identified as 100% similar to Aspergillus versicolor CBS 583.65 and Aspergillus creber NRRL 58592 (Figure 16).
3.3.2. Two-Year-Old Plants
In tissues of 2-year-old plants, nine fungi were found. There were representatives of the Sporocadaceae (Xylariales) and Cordycipitaceae (Hypocreales, Sordariomycetes). Members of the Sporocadaceae family were also detected in the roots and leaves, while members of the Cordycipitaceae family were present only in the fruits. Five of the fungi exhibited 99.65–100% similarity in the ITS region with P. scoparia CBS 176.25 and P. unicolor MFLUCC 12-0276 (Figure 10). Meanwhile, two of them showed 100% agreement with Simplicillium aogashimaense JCM 18167 (Figure 21).
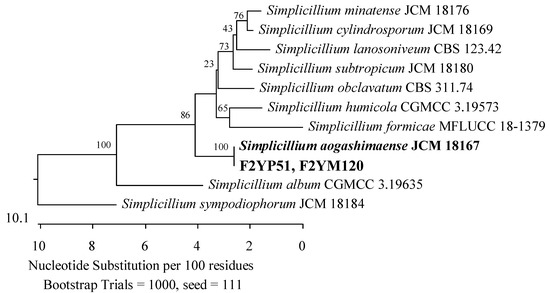
Figure 21.
Phylogenetic tree of American cranberry isolates most closely related to Simplicillium aogashimaense constructed with partial sequences of the ITS region (565 bp). Isolates from fruits of 2-year-old plants were found on Martin’s Rose Bengal agar (name prefix F2YM) and in Penicillium medium (F2YP).
3.3.3. Three-Year-Old Plants
Most of the isolates obtained from 3-year-old plants were assigned to the family Aspergillaceae. The sequences of the ITS region of three isolates were 100% similar to A. versicolor CBS 583.65 and A. creber NRRL 58592 (Figure 16). Furthermore, an isolate showed 100% agreement with P. corylophilum NRRL 802 and the second with P. onobense CBS 174.81 (Figure 3), while the RBP2 genes of two other fungi were 99.48% similar to P. chrysogenum CBS 306.48 (Figure 2).
The ITS region of the last three isolates showed a similarity of 98.93–99.3% to P. vaccinii CBS 143.22 of the Hyponectriaceae family (Figure 19).
3.4. Fungal Growth on Semi-Selective Agars
In this study, 46.5% of filamentous fungi were isolated using Martin’s Rose Bengal agar, a universal medium that supported the growth of representatives of all identified genera, except Diaporthe, Epicoccum, and Hapsidospora. Then, 18.3% of the fungi were isolated using a selective medium for fungi of the genus Penicillium. Most of them were identified as members of the genera Pestalotiopsis and Diaporthe. Furthermore, isolates belonging to the genera Penicillium (especially those most similar to P. chrysogenum and P. corylophilum), Aspergillus (A. fumigatus and A. versicolor), Trichoderma (T. atrobrunneum), Simplicillium, Physalospora, Apiospora, Epicoccum, Diaporthe (D. vaccinii), and Preussia were also obtained. The selective medium for the genus Fusarium allowed the growth of 14.8% of the isolates, which were mainly recognized as the genus Paraphoma and Paraphaeosphaeria. Additionally, fungi of the genera Pestalotiopsis, Purpureocillium, Penicillium (P. chrysogenum), and Hapsidospora were also able to grow in this medium.
None of the identified fungi belonged to the genera Rhizoctonia and Botrytis. However, 11.2% of the isolates were obtained in a selective medium for R. solani. They were classified as the closest to T. amoenum, T. dorothopsis, T. koningii, and P. lilacinum. The last one used was the B. cinerea selective medium that allowed the growth of 9.1% of the isolates. They were classified into genera of Alternaria, Didymella, Diaporthe (D. eres), Neopestalotiopsis, Pestalotiopsis, Penicillium (P. corylophilum, P. onobense), and Aspergillus (A. fumigatus, A. sydowii).
4. Discussion
The purpose of this study was to identify filamentous fungi that inhabit American cranberry plants that grow in one of the largest commercial plantations in central Europe. The isolates tested were obtained from disinfested fragments of roots, leaves, and fruits of 1-, 2-, and 3-year-old plants that did not show visible disease symptoms. Identification was performed through a sequence analysis of the ITS region, which includes fragments ITS1 and ITS2 separated by the 5.8S gene and is located between the 18S rDNA and the 28S rDNA. This region is widely accepted as the universal primary DNA barcode marker [27]. If satisfactory identification was not achieved by analyzing the ITS region, one of the following secondary markers was used: DNA-directed RNA polymerase II subunit B (RPB2) or translation elongation factor 1 alpha (TEF-1α) [26]. According to Nilsson et al. [28], approximately 20% of fungal DNA sequences deposited in public databases can be misidentified at the species level. Therefore, the search for reference strains that were most similar to tested isolates was restricted to data sets that contained type material described as holotypes, neotypes, lectotypes, or isotypes. The exceptions were A. geophila and P. sporulosa species, for which these types of nomenclature were not included in the NCBI database.
The dominant group of the 141 isolates tested was soil-borne saprotrophs and endophytes. Thirty-five percent of them were included in the genera Trichoderma, Paraphaeosphaeria, Penicillium, and Aspergillus. Most Trichoderma isolates came from roots of 2- and 3-year-old plants. Their taxonomic positions were determined by analyzing the sequence of the TEF-1α gene and showed 95.25% to 98.06% identity with strains of T. amoenum and T. dorothopsis. These species are exotic to central Europe and have been poorly described in the literature. The first strains of T. dorothopsis were found in China, in soil from the Yangtze River basin, and in the rhizosphere of chili plants [29]. Meanwhile, T. amoenum originated in the soil in Yunnan province [30]. The other species not yet recorded in Europe is T. scalesiae, which was found in tropical regions of the Galapagos Islands, the Philippines, and Indonesia [31,32,33]. The sequence of the ITS region of a fungus isolated from the root of the tested cranberry was 99.66% similar to that of this species.
The remaining Trichoderma isolates showed the highest similarity to cosmopolitan species, such as T. koningii, T. atrobrunneum, T. insigne, and T. hamatum. These fungi have been frequently isolated from soil, grass, sand, decaying plants and the rhizosphere [34,35,36,37,38,39,40,41]. They are known for their antibiotic activity, secretion of cell-wall-degrading enzymes, and stimulation of plant growth. Among them, only strains of T. hamatum have been reported on the leaves of American cranberry plants that grow in a botanical garden in Poland [15].
The common fungi in the tested plants were also members of the genus Paraphaeosphaeria. The isolates obtained from roots of 1- and 2-year-old plants showed the greatest similarity to P. sporulosa. This species grouped strains from tissues of different plants, including Festuca spp., Fragaria sp., Malus sylvestris, Picea abies, Triticum aestivum, Clematis sp., Solanum tuberosum, Rosa canina, and Opuntia spp. [42]. According to Carrieri et al. [43], some of them showed antibiotic activity against Salmonella enterica. In fruit tissues, single isolates most similar to S. aogashimaense were also found. This species has a broad host spectrum and can inhibit the growth of pathogens such as Puccinia triticina, P. hordei, P. coronata, Bipolaris sorokiniana, Alternaria alternata, Curvularia trifolii, Blumeria graminis, and Diplodia corticola [44,45,46,47].
Molds of the genera Penicillium and Aspergillus, which cause diseases of vegetables and fruits during storage, were the second group of fungal contaminations and represented 23.89% of microorganisms. It consisted of isolates obtained mainly from the roots of annual plants, which showed between 97.08% and 99.59% similarity in the RPB2 gene sequences with P. murcianum, P. arizonense, P. canescens, and P. radiatolobatum. The remaining isolates were obtained from all parts of the plants and identified as most similar to P. pulvillorum, P. onobense, P. corylophilum, P. chrysogenum, P. crustosum, and P. subrubescens. The isolates of Aspergillus, which showed the greatest similarity to A. flavus, A. fumigatus, A. versicolor, A. sydowii, and A. creber, were obtained from leaves and fruits. The fungi of both genera are very common in different climate zones and have been isolated from soil, rhizosphere, decaying organic matter, water, dust, damp building materials, and indoor environments [48,49,50,51,52]. They have also been found to be contaminants in cereal grains, frozen fruits, acid liquids, cheese, eggs, walnuts, and almonds [53,54,55,56,57]. Furthermore, P. crustosum, as well as A. flavus, A. fumigatus, and A. versicolor, are known to be sources of bioactive secondary metabolites with neurotoxic, cytotoxic, mutagenic, teratogenic, and carcinogenic properties, as well as opportunistic pathogens that cause aspergillosis in immunosuppressed patients [56,57,58,59,60,61].
Of the isolates tested, 33.81% were fungi found in various habitats around the world but not previously associated with American cranberry plants. This group included isolates of the genera Alternaria, Apiospora, Paraphoma, Didymella, Fusarium, Pestalotiopsis, Neopestalotiopsis, and Epicoccum. The fungi detected in leaves and fruits had the greatest similarity to P. unicolor and P. scoparia. Based on data from the literature, among members of this genus, Pestalotiopsis sydowiana infected the leaves of American cranberries grown in Poland [15], while Pestalotiopsis maculans was found in the shoots of plants grown in New Zealand [62]. In addition, individual isolates closely related to endophytic A. guangdongensis have been detected. This species has been described in asymptomatic leaves of Wurfbainia villosa in Guangdong province, China [63]. In leaf samples, isolates most similar to A geophila and A. senecionicola were also found. These fungi cause tomato stem canker and potato leaf blight [64,65]. According to the literature, A. alternata was identified on the leaves of cranberry plants in a Kraków botanical garden [15].
The isolates obtained from the roots were found to be more similar to the species P. radicina, which had previously been detected in the roots of Prunus cerasus, M. sylvestris, Picea obovata, Lycopersicon esculentum, and Medicago sativa [66,67], to the species E. tritici, which was described as pathogenic for T. aestivum [68], and to the species of D. prosopidis identified in Prosopis sp. in South Africa, Narcissus tazetta in Iran, and Gastrolobium celsianum endemic in Australia [69,70]. The roots of the tested plants were also contaminated with pathogenic microorganisms belonging to the Fusarium oxysporum species complex. Miller et al. [62] identified these fungi as one of the dominant pathogens in the shoots and roots of cranberry plants grown in New Zealand. Sinkevičienė et al. [11] isolated them from the twigs, leaves, and fruits of V. oxycoccos grown in Lithuania. However, we have only found isolates closest to F. foetens, which have been described as pathogenic for Begonia x hiemalis plants grown in greenhouses in several countries, including the Netherlands, the United States, Canada, Japan, Germany, France, Norway, and the Czech Republic [71].
In this study, only 7.19% of all isolates were identified as the most similar to the American cranberry pathogens described in the literature. The most significant isolates were those closest to P. vaccinii [72,73]. According to Oudemans et al. [9], this species can infect host plants at the beginning of the growing season, during flowering, and, then, remain latent until the fruits ripen. It is commonly found in native habitats in the temperate climate of North America and is isolated from the stems, leaves, and fruits of plants grown in the United States. These pathogens have also been detected in Central Europe, where cranberry plants are grown in seedlings originating in North America. P. vaccinii was also found to be one of the dominant fungi in V. macrocarpon plants in Latvia and was isolated from V. oxycoccos in Lithuania [11]. It is important to note that infections with this pathogen do not always cause fruit damage, and fungi can also be isolated from healthy berries. In this study, P. vaccinii was found in the fruits and leaves of 3-year-old plants without visible disease symptoms.
The tested plants were also infested with fungi of the D. eres species complex, which are cosmopolitan phytopathogens in tropical and temperate ecosystems [10]. Among them, D. vaccinii, which causes shoot death and necrosis, as well as sticky fruit rot from cultivated plants such as Vaccinium corymbosum, V. macrocarpon, Vaccinium vitis, V. myrtillus, and V. oxycoccos, is included in the European list of quarantine pathogens [74]. This fungus has been detected in several European countries, including Latvia, Lithuania, Germany, the Netherlands, the United Kingdom, Romania, and Poland [10,75]. The leaves of the tested plants were contaminated with isolates most similar to this species, D. biguttusis, and D. eres, which are also found in Europe as causes of stem necrosis, fruit rot, and leaf necrosis [74,76].
5. Conclusions
The results obtained in this work indicate that soilborne saprophytes were the dominant group of isolates that colonized the roots, leaves, and fruits of American cranberry plants grown in a commercial plantation in Poland, while the number of fungi described in the literature as typical pathogens did not exceed 10% of all isolates tested.
Fungi of the genus Trichoderma were isolated mainly from root samples. Among them, isolates were found that showed the greatest similarity of the DNA fragments tested to those described only in China strains of species T. amoenum and T. dorothopsis, as well as T. hamatum and T. koningii, which can be found in different climatic zones and are known plant growth stimulators. Furthermore, fungi closely related to strains of P. sporulosa, P. murcianum, P. lilacinum, and P. radicina were also discovered.
Leaf-colonized fungi were identified as more similar to cosmopolitan species with a wide range of host plants, such as P. scoparia, P. unicolor, P. radicina, A. geophila, and Neopestalotiopsis spp. Additionally, members of the D. eres species complex, which are known to be quarantine fungi in American cranberry plants, as well as isolates most similar to A. flavus and A. fumigatus, which are known producers of toxic secondary metabolites, were found.
The isolates that contaminated the fruits were dominated by fungi with the greatest similarity to P. scoparia, P. unicolor, the toxigenic A. versicolor, and P. vaccinii, which is a serious pathogen of the American cranberry.
Current knowledge of the fungi that inhabit the tissues of American cranberry plants is derived from studies of isolates obtained from plants that exhibit disease symptoms. The identification of filamentous fungi presented in this work, for which colonization of leaves, roots, and fruits was not associated with the appearance of the disease, can contribute to the detection of latent infections and the evaluation of potential risks that may occur in a given plantation, depending on the environmental conditions and the plant cultivars grown. Additionally, an understanding of the relationships between the tested plant and fungi expands our knowledge of the host range and distribution limits of some species around the world.
Author Contributions
Conceptualization, M.P.O. and E.G.M.; methodology, M.P.O. and A.K.; formal analysis, M.P.O.; data curation, M.P.O.; writing—original draft, M.P.O.; writing—review & editing, E.G.M.; supervision, S.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
The APC is financed by the Wroclaw University of Environmental and Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results are collected at the Laboratory of Biogeochemistry and Environmental Microbiology at the Wrocław University of Environmental and Life Sciences in Wroclaw. The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lacombe, A.; Wu, V.C.H.; Tyler, S.; Edwards, K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010, 139, 102–107. [Google Scholar] [CrossRef]
- Viskelis, P.; Rubinskiene, M.; Jasutiene, I.; Sarkinas, A.; Daubaras, R.; Cesoniene, L. Anthocyanins, antioxidant and antimicrobial properties of American cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J. Food Sci. 2009, 74, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Polashock, J.J.; Vaiciunas, J.; Oudemans, P.V. Identification of a new phytophthora species causing root and runner rot of cranberry in New Jersey. Phytopathology 2005, 95, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Kour, K.; Bakshi, P.; Sharma, R.M. Cranberry (Vaccinium macrocarpon Ait. L). In Cultivate Minor Temperate Fruit Scientifically; Ghosh, S.N., Kumar, A., Eds.; Jaya Publishing House: New Delhi, India, 2019; pp. 128–181. [Google Scholar]
- Szwonek, E.; Maciorowski, R.; Koziński, B.; Smolarz, K.; Sas-Paszt, L.; Bryk, H.; Derkowska, E.; Estabrooks, E. Initial growth and yield structure of selected cultivars of cranberry (Vaccinium macrocarpon Ait.) cultivated on mineral soils. Folia Hort. 2016, 28, 77–86. [Google Scholar] [CrossRef]
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit physical features, phenolic compounds profile and inhibition activities of cranberry cultivars (Vaccinium macrocarpon) compared to wild-grown cranberry (Vaccinium oxycoccos). Plant Foods Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Koniarski, M.; Sitarek, M. Assessment of the growth, flowering and yielding intensity of cranberry. Zagadnienia Doradz. Rolniczego. 2022, 108, 63–74. [Google Scholar]
- Shear, C.L.; Stevens, N.E.; Bain, H.F. Fungous Diseases of the Cultivated Cranberry; US Department of Agriculture: Washington, DC, USA, 1931; Volume 258, pp. 1–58. [Google Scholar]
- Oudemans, P.V.; Caruso, F.L.; Strech, A.W. Cranberry fruit roti in the Northeast: A complex disease. Plant Dis. 1998, 82, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Michalecka, M.; Bryk, H.; Seliga, P. Identification and characterization of Diaporthe vaccinii Shear causing upright dieback and viscid rot of cranberry in Poland. Eur. J. Plant Pathol. 2016, 148, 595–605. [Google Scholar] [CrossRef]
- Sinkevicienė, J.; Sinkeviciute, A.; Cesoniene, L.; Daubaras, R. Fungi present in the clones and cultivars of European cranberry (Vaccinium oxycoccos) grown in Lithuania. Plants 2023, 12, 2360. [Google Scholar] [CrossRef]
- Karnkowski, W. Diaporthe vaccinii Shear. Zagrożenie dla Upraw Borówek i Żurawiny w Polsce. 2020. Available online: www.piorin.gov.pl (accessed on 15 April 2024).
- Olatinwo, R.O.; Hanson, E.J.; Shilder, A.M.C. A first assessment of the cranberry fruit rot complex in Michigan. Plant Dis. 2003, 87, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Doyle, V.P.; Oudemans, P.V.; Rehner, S.A.; Litt, A. Habitat and host indicate lineage identity in Colletotrichum gloeosporioides s.l. from wild and agricultural landscapes in North America. PLoS ONE 2013, 8, e62394. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, M.; Kwiatkowska, J.; Duda-Franiak, K. Micromycetes on ericaceous plant leaves. Acta Mycol. 2015, 50, 1055. [Google Scholar] [CrossRef][Green Version]
- Oudemans, P.V. Phytophthora species associated with cranberry root rot and surface irrigation water in New Jersey. Plant Dis. 1999, 83, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sabaratnam, S.; Dicarlo, A.; Fitzpatrick, S.; Forge, T. Cranberry dieback disorder: A new and emerging threat to cranberry production in British Columbia. Acta Hortic. 2009, 810, 417–424. [Google Scholar] [CrossRef]
- Castro, C.; Davis, J.R.; Wiese, M.V. Quantitative estimation of Rhizoctonia solani AG-3 in soil. Phytopathology 1988, 78, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Vincelli, P.C.; Beaupre, C.M.S. Comparison of media for isolating Rhizoctonia solani from soil. Plant Dis. 1989, 73, 1014–1017. [Google Scholar] [CrossRef]
- Nash, S.M.; Snyder, W.C. Quantitative estimations by plate counts of propagules of the bean root rot fusarium in field soils. Phytopathology 1962, 52, 567–572. [Google Scholar]
- Edwards, S.G.; Seddon, B. Selective media for the specific isolation and enumeration of Botrytis cinerea conidia. Lett. Appl. Microbiol. 2001, 32, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C. A selective and indicative medium for groups of Penicillium viridicatum producing different mycotoxins in cereals. J. Appl. Microbiol. 1983, 54, 409–416. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D.; Sninsky, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansai, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Ryberg, M.; Kristiansson, E.; Abarenkov, K.; Larsson, K.H.; Koljalg, U. Taxonomic reliability of DNA sequences in public sequence databases: A fungal perspective. PLoS ONE 2006, 1, e59. [Google Scholar] [CrossRef] [PubMed]
- Tomah, A.A.; Alamer, I.S.A.; Li, B.; Zhang, J.Z. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biol. Control 2020, 145, 104261. [Google Scholar] [CrossRef]
- Zheng, H.; Qiao, M.; Lv, Y.; Du, X.; Zhang, K.Q.; Yu, Z. New species of Trichoderma isolated as endophytes and saprobes from Southwest China. J. Fungi 2021, 7, 467. [Google Scholar] [CrossRef] [PubMed]
- Jaklitsch, W.M.; Samuels, G.J.; Dodd, S.L.; Lu, B.S.; Druzhinina, I.S. Hypocrea rufa/Trichoderma viride: A reassessment, and description of five closely related species with and without warted conidia. Stud. Mycol. 2006, 56, 135–177. [Google Scholar] [CrossRef] [PubMed]
- Aban, J.L. In vitro growth-promoting properties of non-dominant root symbiotic fungi (ND-RSF) from Drynaria quercifolia L. and their effects on PSB Rc10 Rice (Oryza sativa L.). Philipp. J. Sci. 2020, 149, 695–706. [Google Scholar] [CrossRef]
- Munir, E.; Yurnaliza; Lutfia, A.; Hartanto, A. Isolation and characterization of phosphate solubilizing activity of endophytic fungi from Zingiberaceous species. Online J. Biol. Sci. 2022, 22, 149–156. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Komon-Zelazowska, M.; Irina, S.; Druzhinina, I.S. Fungal genus Hypocrea/Trichoderma: From barcodes to biodiversity. J. Zhejiang Univ. Sci. B. 2008, 9, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, S.; Okhovvat, S.M.; Javan-Nikkhah, M.; Vagvolgyi, C.; Khosravi, V.; Kredics, L. Biological control of Rhizoctonia solani AG1-1A, the causal agent of rice sheath blight with Trichoderma strains. Phytopathol. Mediterr. 2010, 49, 287–300. Available online: http://www.jstor.org/stable/26458654 (accessed on 29 September 2023).
- Mulaw, B.T.; Kubicek, C.P.; Druzhinina, I.S. The rhizosphere of Coffea Arabica in its native highland forests of Ethiopia provides a niche for a distinguished diversity of Trichoderma. Diversity 2010, 2, 527–549. [Google Scholar] [CrossRef]
- Mghalu, M.J.; Tsuji, T.; Kubo, N.; Kubota, M.; Hyakumachi, M. Selective accumulation of Trichoderma species in soils suppressive to radish damping-off disease after repeated inoculations with Rhizoctonia solani, binucleate Rhizoctonia and Sclerotium rolfsii. J. Gen. Plant Pathol. 2007, 73, 250–259. [Google Scholar] [CrossRef]
- Sariah, M.; Choo, C.W.; Zakaria, H.; Norihan, M.S. Quantification and characterisation of Trichoderma spp. from different ecosystems. Mycopathologia 2005, 159, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.T.; Zhuang, W.Y. Three Trichoderma species new to China. Mycosystema 2016, 35, 1008–1017. [Google Scholar] [CrossRef]
- Tang, G.T.; Li, Y.; Zhou, Y.; Zhu, Y.H.; Zheng, X.J.; Chang, X.L.; Zhang, S.R.; Gong, G.T. Diversity of Trichoderma species associated with soil in the Zoige alpine wetland of Southwest China. Sci. Rep. 2022, 12, 21709. [Google Scholar] [CrossRef]
- Baroncelli, R.; Da Lio, D.; Vannacci, G.; Sarrocco, S. Genome resources for the endophytic fungus Paraphaeosphaeria sporulosa. Mol. Plant Microbe. Interact. 2020, 33, 1098–1099. [Google Scholar] [CrossRef]
- Carrieri, R.; Borriello, G.; Piccirillo, G.; Lahoz, E.; Sorrentino, R.; Cermola, M.; Bolletti Censi, S.; Grauso, L.; Mangoni, A.; Vinale, F. Antibiotic activity of a Paraphaeosphaeria sporulosa—Produced diketopiperazine against Salmonella enterica. J. Fungi 2020, 10, 83. [Google Scholar] [CrossRef]
- Costa, D.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Cork oak endophytic fungi as potential biocontrol agents against Biscogniauxia mediterranea and Diplodia corticola. J. Fungi 2020, 6, 287. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, S.E.; Caradus, J.R.; Johnson, L.J. Fungal endophyte diversity from tropical forage grass Brachiaria. Plant Ecol. Divers. 2018, 11, 611–624. [Google Scholar] [CrossRef]
- Wilson, A.; Cuddy, W.S.; Park, R.F.; Harm, G.F.; Priest, M.J.; Bailey, J.; Moffitt, M.C. Investigating hyperparasites as potential biological control agents of rust pathogens on cereal crops. Australas. Plant Pathol. 2020, 49, 231–238. [Google Scholar] [CrossRef]
- Zhu, M.; Duan, X.; Cai, P.; Li, Y.; Qiu, Z. Deciphering the genome of Simplicillium aogashimaense to understand its mechanisms against the wheat powdery mildew fungus Blumeria graminis f. sp. tritici. Phytopathol. Res. 2022, 4, 16. [Google Scholar] [CrossRef]
- Ammar, H.A.; Ezzat, S.M.; Elshourbagi, E.; Elshahat, H. Titer improvement of mycophenolic acid in the novel producer strain Penicillium arizonense and expression analysis of its biosynthetic genes. BMC Microbiol. 2023, 23, 135. [Google Scholar] [CrossRef]
- Marfetán, J.A.; Vélez, M.L.; Comerio, R.; Gallo, A.; Romero, S. Rhizospheric Penicillium and Talaromyces strains in Austrocedrus chilensis native forests: Identification and evaluation of biocontrol candidates against the pathogen Phytophthora austrocedri. J. Plant Prot. Res. 2023, 63, 239–253. [Google Scholar] [CrossRef]
- Visagie, C.M.; Frisvad, J.C.; Houbraken, J.; Visagie, A.; Samson, R.A.; Jacobs, K. A re-evaluation of Penicillium section Canescentia, including the description of five new species. Persoonia 2021, 46, 163–187. [Google Scholar] [CrossRef]
- Mansouri, S.; Houbraken, J.; Samson, R.A.; Frisvad, J.C.; Christensen, M.; Tuthill, D.E.; Koutaniemi, S.; Hatakka, A.; Lankinen, P. Penicillium subrubescens, a new species efficiently producing inulinase. Antonie Van Leeuwenhoek 2013, 103, 1343–1357. [Google Scholar] [CrossRef]
- Monmi Pangging, M.; Nguyen, T.T.T.; Lee, H.B. Seven new records of Penicillium species belonging to section Lanata-Divaricata in Korea. Mycobiology 2021, 49, 363–375. [Google Scholar] [CrossRef]
- Demjanová, S.; Jevinová, P.; Pipová, M.; Regecová, I. Identification of Penicillium verrucosum, Penicillium commune, and Penicillium crustosum isolated from chicken eggs. Processes 2020, 9, 53. [Google Scholar] [CrossRef]
- Tournas, V.H.; Niazi, N.S.; Kohn, J.S. Fungal presence in selected tree nuts and dried fruits. Microbiol. Insights 2015, 8, MBI-S24308. [Google Scholar] [CrossRef] [PubMed]
- Al-Bedak, O.A.; Abdel-Sater, M.A.; Abdel-Latif, A.M.A.F.; Abdel-Wahab, D.A. Aspergillus creber and A. keveii, two new records as endophytes from wild medicinal plants in Egypt. J. Multidiscip. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Piontek, M.; Łuszczyńska, K.; Lechów, H. Occurrence of the toxin-producing Aspergillus versicolor Tiraboschi in Residential Buildings. Int. J. Environ. Res. Public Health 2016, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Jurjevic, Z.; Peterson, S.W.; Horn, B.W. Aspergillus section Versicolores: Nine new species and multilocus DNA sequence based phylogeny. IMA Fungus 2012, 3, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Frisvad, J.C.; Sondergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between fungal species and water-damaged building materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Moldes-Anaya, A.; Rundberget, T.; Fæste, C.K.; Eriksen, G.S.; Bernhoft, A. Neurotoxicity of Penicillium crustosum secondary metabolites: Tremorgenic activity of orally administered penitrem A and thomitrem A and E in mice. Toxicon 2012, 60, 1428–1435. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Latgé, J.P. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef]
- Miller, S.A.; Patel, N.; Stanley, C.J. Cranberry pests and diseases in New Zealand. Acta Hortic. 2006, 715, 509–512. [Google Scholar] [CrossRef]
- Liao, C.; Senanayake, I.C.; Dong, W.; Chethana, K.W.T.; Tangtrakulwanich, K.; Zhang, Y.; Doilom, M. Taxonomic and phylogenetic updates on Apiospora: Introducing four new species from Wurfbainia villosa and grasses in China. J. Fungi 2023, 9, 1087. [Google Scholar] [CrossRef]
- Mamgain, A.; Roychowdhury, R.; Tah, J. Alternaria pathogenicity and its strategic controls. Res. J. Biol. 2013, 1, 1–9. [Google Scholar]
- Landschoot, S.; Vandecasteele, M.; De Baets, B.; Höfte, M.; Audenaert, K.; Haesaert, G. Identification of A. arborescens, A. grandis, and A. protenta as new members of the European Alternaria population on potato. Fungal. Biol. 2017, 121, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liang, Q.W.; Nzabanita, C.; Li, Y.Z. Paraphoma root rot of alfalfa (Medicago sativa) in Inner Mongolia, China. Plant Pathol. 2020, 69, 231–239. [Google Scholar] [CrossRef]
- De Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 2010, 102, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Iliff, G.J.; Mukherjee, R.; Gruszewski, H.A.; Schmale, D.G., III; Jung, S.; Boreyko, J.B. Phase-change-mediated transport and agglomeration of fungal spores on wheat awns. J. R. Soc. Interface 2022, 19, 2021087. [Google Scholar] [CrossRef] [PubMed]
- Keirnan, E.C.; Tan, Y.P.; Laurence, M.H.; Mertin, A.A.; Liew, E.C.Y.; Summerell, B.A.; Shivas, R.G. Cryptic diversity found in Didymellaceae from Australian native legumes. MycoKeys 2021, 78, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Baaji, Y.; Farokhinejad, R.; Mehrabi-Koushki, M. New host and record of Didymella prosopidis from Iran. Mycologia Iranica 2022, 9, 117–121. [Google Scholar] [CrossRef]
- Schroers, H.J.; Baayen, R.P.; Meffert, J.P.; De Gruyter, J.; Hooftman, M.; O’Donnell, K. Fusarium foetens, a new species pathogenic to begonia elatior hybrids (Begonia x hiemalis) and the sister taxon of the Fusarium oxysporum species complex. Mycologia 2004, 96, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Cinget, B.; Labbe, C.; Asselin, Y.; Belanger, R.R. New insights into the fungal diversity of cranberry fruit rot in Quebec farms through a large-scale molecular analysis. Plant. Dis. 2021, 106, 215–222. [Google Scholar] [CrossRef]
- Polashock, J.J.; Caruso, F.L.; Oudemans, P.V.; Mc Manus, P.S.; Crouch, J.A. The North American cranberry fruit rot fungal community: A systematic overview using morphological and phylogenetic affinities. Plant Pathol. 2009, 58, 1116–1127. [Google Scholar] [CrossRef]
- Lombard, L.; van Leeuwen, G.C.M.; Guarnaccia, V.; Polizzi, G.; van Rijswick, P.C.J.; Rosendahl, K.C.H.M.; Gabler, J.; Crous, P.W. Diaporthe species associated with Vaccinium, with specific reference to Europe. Phytopathol. Mediterr. 2014, 53, 287–299. Available online: https://www.jstor.org/stable/43871781 (accessed on 29 September 2023).
- Jovaisiene, Z. Diaporthe vaccinii. Diagnostics Bull. OEPP/EPPO 2009, 39, 18–24. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.; Lin, Y.; Fu, Y.; Zhu, F.; Luo, C. Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci. Biology 2021, 10, 179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).