Abstract
Right after water, tea is one of the most popular beverages in the world. The composition of a tea drink is determined by, among other things: the degree of fermentation and the fineness of the tea leaves, as well as the brewing time and temperature. The purpose of this study was to verify the hypothesis that the extraction of selected elements from dried tea during infusion preparation was improved by using ultrasound under laboratory conditions. The effect of increasing the extraction time was also analyzed. The effectiveness of ultrasound was evaluated by measuring the content of selected elements in the extract of teas that were treated with ultrasound compared to a control group. The effectiveness of the application of ultrasound was evaluated by measuring the content of individual elements at intervals of 1 min, 5 min and 10 min comparing the application of ultrasound with the classical technique of tea brewing. In addition, the results were related to measurements of the amounts of overall concentrations of selected ions after the dried tea mineralization procedure. Samples of extracts and mineralizates were analyzed for elemental composition using an ICP-OES iCAP Dual 6500 Thermo® spectrometer. The use of ultrasound was shown to have a positive effect on the extraction process, and the efficiency of the process depended on the tea variety and the extraction time. The percentage of extraction relative to the results of the total amount of extracted ions was also calculated. Analyzing the effect of ultrasound on the leaching of minerals from tea leaves during infusion preparation is another step toward optimizing the tea brewing extraction process and a way to improve the nutritional value of tea infusions as a functional beverage base. The application of ultrasound in the extraction process of tea infusions may be a good solution to support traditional methods of extracting infusions for both research and technological purposes.
1. Introduction
The infusion of tea leaves (Camellia sinensis) is one of the most widely consumed beverages in the world [1]. About 80% of the world’s tea consumption is the black variety [2,3,4]. A classification of teas can be made according to the degree of fermentation [5,6,7,8]. There are: black teas (fully fermented), red teas (30–50% fermented), green teas (unfermented) and white teas (unfermented) [9,10,11]. Tea contains more than a dozen groups of compounds in its composition, while their content depends on various factors, including variety, type of cultivation, region of origin and even the location of the leaves on the branch [12,13,14,15,16]. Compounds found in tea include tannins, nitrogenous compounds, essential oils, fiber, purine alkaloids, pigments, vitamins and minerals [17,18]. Tannins make up 13–30% of the dry weight of tea and are among the most important compounds found in tea. They are a mixture of polyphenolic compounds, which include tannins and catechins and their derivatives, such as tannin, quercetin, rutin, catechin, flavonoids and saponins [19]. Tannins contained in tea are divided into two groups, hydrolyzing tannins, i.e., gallo- and elagotannins, and non-hydrolyzing catechin tannins, which are categorized as flavonoid compounds. Most of those present in tea are catechins that have at least four hydroxyl groups [20]. The catechins in tea leaf extract have a role in inhibiting the formation of free radicals [21]. Catechins are more potent oxidants than ascorbic acid or tocopherol [22]. Catechins are the main antioxidants behind the health benefits of tea [23,24,25]. They are water-soluble, colorless compounds that impart bitterness and astringency to brews. They affect the taste, aroma and color of infusions [26,27]. Studies have proven the anticancer properties of tea flavonols, including quercetin and myricetin [28].
Tea infusion in its composition has many macro- and micronutrients. There are 6.5% mineral compounds in tea. Depending on the species and soil composition, such components as chromium, calcium, magnesium, iron, aluminum, manganese, copper, zinc, molybdenum, sodium, phosphorus, potassium, fluorine, selenium, silicon and iodine can be identified [29,30,31,32,33]. The aforementioned compounds in tea play an important role in the nutrition of human tissues [34,35]. However, the most important of these are the macronutrients that, along with tea, can become an excellent base for functional beverages, namely, Na, Ca, Mg and K. Sodium in isotonic drinks is not only intended to replenish losses due to sweating but also perhaps facilitate water absorption. Water in the gastrointestinal tract is taken up through cells by simple diffusion. In addition, it can also be absorbed by active sodium transport, which means that, in the presence of sodium, the body absorbs more water [36,37]. Sodium and potassium are very important, especially in isotonic drinks, the consumption of which is recommended during prolonged exercise, as well as to replenish deficiencies created after exercise and in situations of exposure to significant dehydration resulting from external temperature or intense exercise. The main function of sodium is to maintain optimal fluid volume and blood pressure, and it is also responsible, along with potassium, for proper nerve transmission in the process of muscle contraction (including cardiac muscles) [38,39,40]. Calcium is the main building block of bone and is essential for proper blood clotting. It is also responsible for nerve conduction and muscle contraction [41,42,43,44,45,46]. Magnesium, like calcium, is a building block of bone and is responsible for blood clotting and cardiac muscle contraction. It is also a catalyst for many enzymes [47,48,49,50]. As indicated by studies [51,52,53,54], a progressive increase in Na and Cl in sweat is observed during exercise, while the loss of K, Mg and Ca, and amino acids decreases.
Nowadays, in addition to conventional aqueous extraction using higher temperatures or longer process times, scientists are also investigating alternative extraction methods, such as supercritical carbon dioxide extraction [55], assisted by microwave radiation (MAE), which uses the phenomenon of direct absorption of microwave radiation by the particles of a substance and is effective because of the way heat energy is transferred. Microwaves deliver energy directly to the particle of the chemical component, without energy loss due to convection or conduction.
Less conventional methods include extraction mediated by superheated water, a pulsed electric field and electrowetting [56]. Ultrasound extraction is one method to increase the process efficiency. The use of ultrasound against chemical and physical phenomena differs dramatically from conventional extraction methods. The advantage of using ultrasound lies in higher yields and quality, faster processing times (sustained, repetitive processes can be performed to a maximum of a few minutes), reduced high costs, and low chemical as well as physical hazards. The UAE (ultrasonic-assisted extraction) method is popular for obtaining proteins, fats, aromatic compounds or essential oils. Wanting to achieve satisfactory extraction process performance for plant raw material, it is necessary to normalize ultrasound operating parameters such as time, pH, solubility and polarity [57]. In the extraction process, samples are subjected to agitation with a given extraction solvent and then to the action of ultrasound.
The aim and novelty of the study is to select and identify the optimum extraction times for three types of tea—three of each type. In addition, UAE was used to determine the effect of this factor on the value of extracted ions. The teas used belonged to three species, i.e., black, green and white, and were purchased on the commercial market. The parameter of ultrasound frequency and power was chosen to improve extraction. These parameters followed earlier studies by the authors. In addition, an extended extraction time parameter from 1 to 10 min was used each time to plot the extraction growth curve.
2. Materials and Methods
2.1. Tea Samples
The study material consisted of nine high-quality types of tea, three varieties of black (Nepal Everest, Ceylon Kenilworth and Black Vietnam), green (Fog Green Tea, Sencha Kyoto Organic and Yunnan Green) and white (White Monkey, Yin Zhen Hunan and Pai Mu Tan Rose) leaf teas purchased on the commercial market (Table 1).

Table 1.
Overview of analyzed teas by region of origin and basic analytical parameters.
Analyses of water content, ash content and protein content were performed based on standard testing procedures, i.e., [58,59,60].
2.2. Assessment of the Total Dissolved Solids and Electrical Conductivity
The samples were assessed using TDS&EC meter (MW802 Pro, Milwaukee, Wisconsin, USA). It was applied to measure such factors as total dissolved solids (TDS) and electrical conductivity (EC). The value of TDS makes it possible to determine the degree of overall water mineralization and is expressed in ppm. TDS < 50—corresponds to water with very low level of mineralization, TDS 50–500—water with low level of mineralization, TDS 500–1500—water with moderate level of mineralization and TDS > 1500—water with high level of mineralization [37].
2.3. Procedure for Measuring the Total Ion Content of Dried Tea
Micro and macro elements from solid material (tea leaves) were analyzed using ICP-EOS method (Thermo iCAP Dual 6500 with horizontal plasma, Schaumburg, IL, USA). Samples of 0.5 g of each of the 9 teas were mineralized in 65% HNO3 under high pressure, in 3 lab replicates. Samples were weighed and placed in Teflon dishes, then topped up with 8 mL of nitric acid and sealed. One blank sample—pure HNO3—was used for each group of nine samples. The samples were mineralized using the temperature rise algorithm specified for biological samples, never exceeding 220 °C. The procedure was carried out in an Ethos One microwave digestion system (Milestone, Bologna, Italy). The vessels were opened after the digestion process and the acid samples were brought to room temperature. They were then topped up with water to a volume of 50 mL. The detection threshold obtained for each element was no lower than 0.1 mg kg−1 (assuming a detection capacity of the measuring apparatus of more than 10 ppb). Measurements were carried out on an ICP-OES spectrometer (Thermo Fisher Scientific, Schaumburg, IL, USA) and the detection capability was determined both along and across the plasma flame (radially and axially). Before measuring each batch of samples, the equipment was calibrated using certified Merck models at concentrations of 10,000 mg l−1 (for macronutrients) and 1000 mg l−1 (for trace elements and metals). The measurement result for each element was corrected to account for the measurement of elements in the blank sample. In each case, a 3-point calibration curve was used for each element, with optical correction using the internal model method, in the form of yttrium and ytterbium ions, at concentrations of 2 mg l−1 and 5 mg l−1, respectively. The analytical methods were validated using two independent tests. A certified reference material (NIEST CRM 7: Tea Leaves) was used. A model addition method of known concentration was used to identify appropriate measurement lines and avoid possible interferences. The recoveries obtained for each element are shown in Table 2 below.

Table 2.
The recovery obtained for specific elements.
2.4. Extraction Procedure
Aqueous extracts were prepared by taking 10 g from each tea sample and weighing them into 1000 mL volumetric flasks. The teas were brewed in demineralized water with conductivity <0.05 µS/cm using a unified temperature, i.e., 100 °C to optimize the method. Extractions were performed in 3 laboratory replicates and in 3 time variants, i.e., 1, 5 and 10 min. After these times, 8 mL of extract was taken sequentially from each sample, which served as material for analysis. Similarly, ultrasound-assisted aqueous extracts were prepared, which were additionally sonicated using ultrasound extractor (BEM-150A, Beijing, China) apparatus in the parameter range of 22.5 kHz and at 100 W of power using a 6 mm sonotrode.
The sampled aqueous extracts and sonication-assisted aqueous extracts were subjected to analytical measurements for the content of selected ions, in an identical measurement procedure to the mineralizates, using an ICP-OES Thermo iCAP Dual 6500 (Thermo Fisher Scientific, Schaumburg, IL, USA.
2.5. Calculation of the Percentage of Extracted Ions
The procedure for calculating the real percentage of extracted ions was based on comparing the total amount (abundance) of ions in the dried tea, as measured on the spectrometer, and comparing it with the measured amounts of extracted ions after the traditional and ultrasound-assisted brewing procedures. The calculations made according to the formula: ((milligrams of ions after extraction)/(gram of tea leaves using for extraction))/((milligrams of ions after mineralization)/(gram of tea leaves using for mineralization)).
Each time, the calculations were reduced to the standardized amounts of the tea dried weight both during the mineralization process and during the extraction process.
2.6. Statistical Analysis of the Results
The statistical hypotheses related to the effects of tea type and magnetic factor were verified with ANOVA test with Tukey’s post-hoc test, at α = 0.05 and numbers of repetitions n = 3. Composition of chemical elements in the matrix was determined using ICP-OES apparatus (Schaumburg, IL, USA), Thermo iCAP Dual 6500 with horizontal plasma, and capacity for detection along and across the plasma flame (radial and axial). Before measuring each batch of 10 samples, calibration was performed using certified (Merck) models, with concentrations of 10,000 ppm for Ca, Mg, K and P; and 1000 ppm for Al, As, Cd, Cr, Cu, Fe, Na, Ni, Pb, S and Zn.
3. Results
As a first step, after the mineralization process and spectral determinations, the total amount of selected ions in each of the three types of white, green and black teas was estimated to determine the nominal total values of the parameter in question. The results of the ion content are shown in Table 3 below.

Table 3.
Results of the content of measured ions in dried tea.
The results presented clearly show that teas more abundant in ions, especially macronutrients, are black and green teas. Among the ions present in the highest amounts, potassium, phosphorus, calcium, sulfur, magnesium and sodium dominate. In contrast, the teas that are least saturated in ions are white teas. The differences in the amount of minerals are quite large because they are up to two times greater.
In general, it seems that the results at these levels are a good proxy for teas as a valuable source of micro- and macronutrients, especially considering the possibility of their use as an ionic base for functional beverages. The presence of osmotically active substances in solutions, among other things, of sodium and chlorine ions conditions the movement of water and ions across semipermeable cell membranes, causing electrolytes to move into cells or in the opposite direction [61].
Among the analyzed and measured components in dried tea, there are insignificant amounts of heavy and toxic metals. Among them, aluminum and nickel were the highest amounts. Aluminum in the present study increased with the length of brewing time. Excess amounts of this element can be harmful, but consuming a cup of tea a day does not have a toxic effect on the human body. The European Food Safety Authority (EFSA) sets the permissible weekly amount of aluminum from food at 1 mg/kg body weight. The aluminum content of tea infusions averages about 3 mg/10 g dried, ranging from 0.41 to 7.52 mg/10 g dried. This does not mean, of course, that the entire form of aluminum will enter into infusion or aqueous solution. This is because the tendency of aluminum migration depends on the form of the solvent and so the aluminum citrate forms become the most labile [62].
The ionic results after the extraction process are broken down into two sets of Table 4, Table 5 and Table 6. Below are the results of the four main macronutrients found in teas, i.e., Ca, Mg, Na and K, which appear to be the most important due to their value to the body as well as their use as an ionic base for functional beverages. The remaining micronutrients as well as heavy metals are shown in Supplementary Materials.

Table 4.
Results of the content of measured ions (four major macronutrients) in 3 black teas.

Table 5.
Results of the content of measured ions (four macronutrients) in 3 green teas.

Table 6.
Results of the content of measured ions (four macronutrients) in 3 white teas.
It was found that, for black teas, potassium was the dominant ion in the infusion regardless of extraction time and type. Both increased extraction time and the use of an additional factor in the form of sonic assisted extraction resulted in increases in the concentrations of the analyzed macronutrients. The proposed form of sonic-wave assisted extraction proved to be a good tool for assisting the amounts of extracted constituents and also reducing the extraction time itself. The average increase in mineral content oscillated between 27.25% for extended extraction time for traditional brewing and 21.97% for ultrasound-assisted brewing.
The average increase in mineral content oscillated between 11.22% for extended extraction time for traditional brewing and 5.22% for ultrasound-assisted brewing.
The results posted in the Supplementary Materials show that the ion with the highest concentration potential is phosphorus. From a nutritional point of view, it is of little or no importance. It participates in the production of energy, affects the development and function of bones, muscles and the nervous system. It has an important effect on maintaining acid-base balance in the body [63]. Phosphorus concentration in teas ranged from 38.40 mg/10 g for white tea 8 to 128.44 mg/10 g in black tea 1. Most of the teas analyzed are rich in phosphate ions, so they can be a source of them in the diet.
In addition, sizable amounts of sulfur were extracted. Sulfur is part of the structure of insulin for which it is essential for proper metabolism. It is a building block of sulfur amino acids such as cystine, cysteine and methionine [64]. The average value of sulfate concentrations determined in the teas was 14.37 mg/10 g and 10.75 mg/10 g in the extract, with a single ingestion of the extract from the teas tested posing no risk of exceeding the daily intake.
Heavy and toxic metals present in the extracts were aluminum and nickel. They have negative effects on the human body. Excess aluminum primarily affects the work of the human nervous system. Deposits of this element can accumulate in the brain, where they impair the work of the nervous system, block some of the enzymes and react with DNA in the cells of nervous tissues [65,66].
Considering the type of teas, white teas showed the lowest aluminum content, while the average value of nickel in black teas was 0.04 mg/10 g.
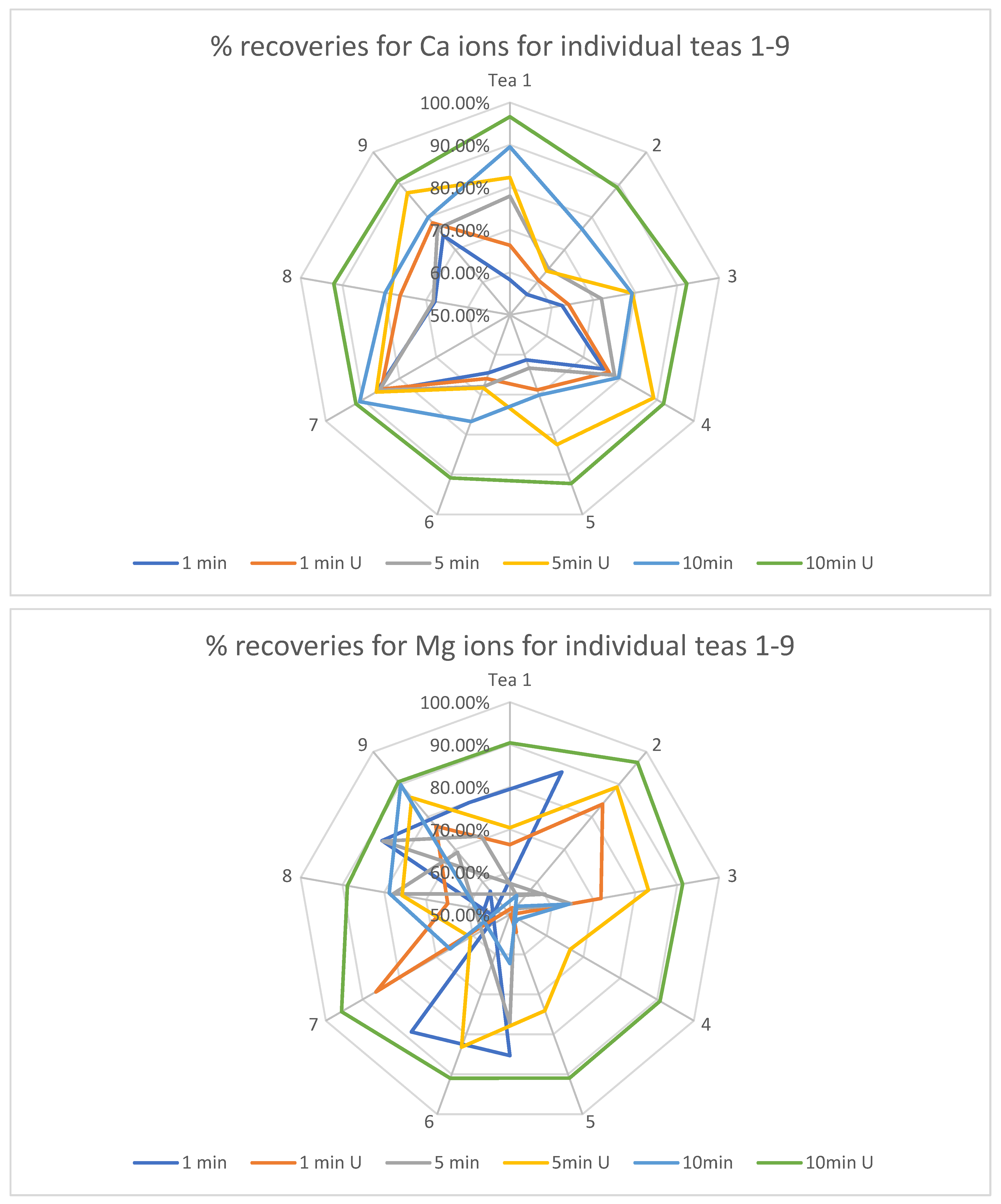
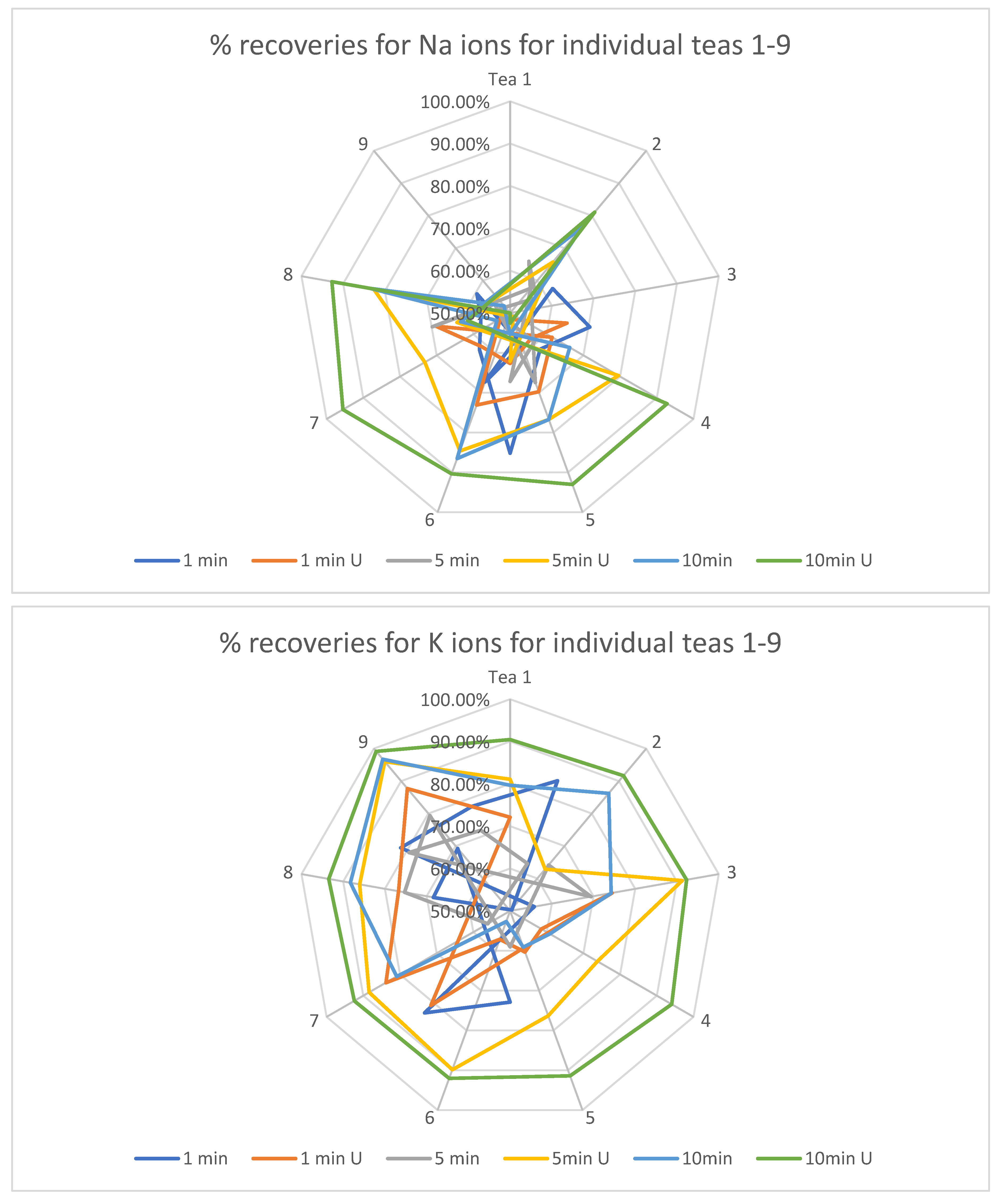
The next statistical step was to calculate the extraction recoveries for each type of tea, brewing time and booster factor. This calculation is shown in the radar charts for each macronutrient below and includes a comparison of the nominal average results for each of the four macronutrients after the extraction processes in relation to the results of the determinations from the mineralization process (i.e., the total amount of each ion in the dried tea). This calculation is possible due to the use of a weight conversion for mineralizates equal to the amount of extracted tea in the extraction process in 1 L of solvent.
The dominant trend appeared to be an improvement in the degree of extraction with an increase in the time of extracted infusions (Figure 1). This tendency is particularly clear for Ca, Mg and K ions. Sodium ions proved to be the least influenced by the factor in question, which may be due to their relatively lowest concentration among the analyzed primers and, consequently, the greatest susceptibility to measurement errors resulting from the inhomogeneity of the dried tea material. Another factor analyzed was the increase in extraction rate caused by the sonic factor. For each of the analyzed ions and each of the analyzed teas, this factor was of particular importance already from the lowest of the infusion times. This increase is especially evident for the ions with the highest ionic concentration, namely Mg, Ca and K.
Figure 1.
Percentages of extracted macronutrients for different extraction times along with the booster used.
4. Discussion
Studies on the effects of UAE on biological material are abundant [67,68,69]. Widely addressed is the issue of the effect of UAE on availability of biological content, such as flavonoids or polyphenols [70,71,72]. Herbal teas such as mint, chamomile and others are also often the raw material for research [73]. Ultrasound research can be included in the optimization of extraction processes [74,75]. Ultrasound may enable the dissolution of essential oils and other tea herbs, which affects the flavor and aroma of the tea.
Extraction of plant materials has been carried out by various extraction methods. Over the past 50 years, non-conventional methods have been developed that are more ecological and eco-friendly due to shorter operation time, less use of synthetic and organic chemicals, and better yield and quality of the extract. To increase the overall yield and selectivity of bioactive constituents from plant materials, ultrasound [76], pulsed electric field [77], microwave heating [78], enzymatic digestion [79], ohmic heating, supercritical fluids [80,81] and accelerated solvents (Wasilewski) have been investigated as unconventional methods.
In a number of scientific studies comparing different extraction techniques compared to ultrasound-assisted extraction, the advantage of ultrasound is clear. This is mentioned, among others, by [78] in a study comparing the efficiency of extraction of plant raw materials by means of unconventional extraction techniques, including but not limited to ultrasound. The raw materials for the study there were peppermint leaf (Menthae piperitae folium), sage leaf (Salviae folium) and dandelion root (Taraxaci radix). The results indicated that the use of ultrasonic waves results in an improvement in extraction efficiency, compared to a standard extraction using a Soxhlet apparatus, and that the total energy consumption is lower than in the case of ‘hot’ extraction. Notwithstanding the increase in yield of the key ingredient, the UV–Vis spectrum demonstrates that the concentration of other, as yet unidentified, substances in the extract is increased using this method. Ultrasound is a method of assisting extraction, whose applicability in the food industry is being extensively investigated as the authors [66] point out as a method for optimizing the extraction of bioactive compounds from the by-products of fruit and vegetable processing. Another example is the extraction of antioxidant polyphenols from green tea (Camellia sinensis), where improved extraction efficiency of antioxidant polyphenols from green tea was investigated by combining ultrasound-assisted extraction (UAE) and deep eutectic solvents. The effect of extraction parameters on total phenolic content (TPC) values was also investigated and the liquid-to-solid ratio, ultrasonic power and ultrasonic time were optimized. Optimal extraction conditions included a liquid-to-solid ratio of 36:1 (ml/g), an ultrasonic power of 461.5 W and an ultrasonic time of 21 min, with a highest TPC value of 243 ± 7 mg gallic acid equivalent (mg GAE)./g dry matter (sm), which was 13% higher than before optimization. Furthermore, under optimized extraction conditions, the tea polyphenolic extract showed higher antioxidant activity compared to conventional extraction methods. Similar results were obtained by the authors for ultrasound-assisted techniques with infusions. Raghunanth and Mallikarjunan [82] evaluated the effect of ultrasound at different times (10, 20, 30, 40, 50 and 60 min), amplitudes (0%, 10%, 30%, 50% and 70%) and solute/solvent ratios (1:25, 1:50, 1:75 and 1:100) on TPC extraction and radical absorption activity from cold-brewed black tea. The authors found that optimal extraction of bioactive compounds for cold-brewed black tea was achieved at ul amplitude of 69.9% with a solvent volume of 25 mL and a sonication time of 30 min at 4 °C. TPC was found to be 78.97 ± 1.85 mg GAE/g for 30 min in the ultrasonic brewing system, compared to 19.51 ± 0.76 mg GAE/g for 360 min in the conventional brewing system. The extraction of phenolic compounds increased fourfold compared to conventional cold brewing. However, the brewing time was reduced from 6 h to 30 min using the ultrasonic brewing method. Another example is the work of Horzic et al. [83] which studied the effect of ultrasound and conventional hot water extraction on the bioactive compounds of yellow tea. Maximum extraction of TPC was carried out at 67 °C for 30 min with 75% aqueous ethanol solution using an ultrasonic probe. Another example of extraction is grape tea, consumed by steeping tea leaves in boiling water. A traditional drink in China, grape tea has been treated with ultrasound to extract polyphenols. Maximum polyphenols were extracted from grape tea with 70% ethanol at 70 °C for 40 min using ultrasound [84]. The results of aqueous extraction are also reflected, thus analogous to the text discussed in Cheng’s text [85], where antioxidant capacity was assessed at 25–100 °C using steeping times from 5 to 720 min.
However, there are few examples in research of the use of UAE on biological material to extract ions into solution. An example of the use of assisted extraction in the study of ion extraction intensification is provided by, among others, the research of Zagula [76]; the assisting factor was a magnetic field. It was observed that an alternating magnetic field had different effects on the extraction of selected metal ions from dry tea leaves into different black tea infusions. Of all the chemical elements, potassium was found to migrate most rapidly when an alternating magnetic field was applied. There was a more than twofold increase in potassium ion extraction efficiency in two of the three tea brands. Variable magnetic field-assisted extraction also significantly improved Ca, Mg and P ion content in the final infusions of all three tea brands. Another extracted active substance analyzed was caffeine. Its content in conventionally extracted black teas ranged from 209.2 to 221.6 mg/L and in green teas from 243.2 to 244.7 mg/L.
For the studies presented in this manuscript and the results of all three teas tested, increasing the steeping time resulted in the extraction of more ions for 10 of the 10 analyzed ions. Of the three teas tested, the ion that it was possible to extract in the highest amount was potassium. This element came from extracts treated with ultrasound for 10 min. Here, it is worth pointing out that it is not necessarily the time of 10 min with UAE, where there is the highest amount of ion that was successfully extracted, that will be the most optimal and economical. For example, the potassium content of tea number 1 contained 220.22 mg/10 g of this ion. The results of the analysis of the ion content of infusion tea No. 1 show that extraction for 1 min assisted by ultrasound yielded nearly 253% of the potassium ion concentration compared to the extraction conducted traditionally for 1 min. Subsequent measurements after extending the extraction time to 5 and 10 min, respectively, yielded results of 283% and 317%. Similar results were obtained in research papers that included the factor of extended tea infusion extraction time and the ultrasound-assisted extraction agent used [86,87]. The most pronounced upward trend was for ions with the highest concentrations. Studies by other authors indicate that ultrasound can be used not only to increase the extracted ions but also the previously mentioned bioactive compounds. The concentrations of nickel and aluminum increased progressively with brewing time; their concentrations were higher in teas extracted using the ultrasound method.
5. Conclusions
This article presents a study on the use of ultrasound for the extraction of ions from tea, an important issue in the context of extracting valuable chemical compounds from plant raw materials. The results presented in the article appear promising and open the door to further research into this extraction technique. Based on the research conducted, it can be concluded that yes, the use of ultrasound in the extraction process influenced the intensity of leaching of the studied ions from the tea leaves into the infusion, increasing the amounts of ions in the infusions. Given the advantages of ultrasound, it is worth determining the optimal extraction time based on the results used, depending on the ion. White teas were found to be the least abundant in minerals. The teas tested showed a tendency to significantly improve the amount of extracted ionic substances using ultrasound. In conclusion, the results presented in this paper suggest that ion extraction from tea using ultrasound can significantly improve the efficiency and productivity of the process. This is also worth considering for its application in the food and pharmaceutical industries, where plant ion extraction is commonly used. It seems very important from the point of view of technology and the economics of extracting ions into aqueous solutions to use an ultrasonic procedure, which significantly reduces the time of the extraction procedure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app132011392/s1, Table S1: Black teas; Table S2: Green teas; Table S3: White teas.
Author Contributions
Conceptualization, K.M. and G.Z.; methodology, G.Z.; software, B.S.; validation, A.S., B.S. and M.B.; formal analysis, K.M.; investigation, K.M.; resources, K.M.; data curation, K.M.; writing—original draft preparation, K.M.; writing—review and editing, G.Z.; visualization, K.M.; supervision, C.P.; project administration, C.P.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
The project was financed by the program of the Minister of Education and Science named “Regional Initiative of Excellence” in the years 2019–2023, project number 026/RID/2018/19, the amount of financing PLN 9 542 500.00.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and Its Consumption: Benefits and Risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Szefer, P. Herbata Jako Źródło Manganu w Codziennej Diecie Człowieka. Bromat. Chem. Toksykol. 2016, 3, 234–237. [Google Scholar]
- Jakubczyk, K.; Kupnicka, P.; Melkis, K.; Mielczarek, O.; Walczyńska, J.; Chlubek, D.; Janda-Milczarek, K. Effects of Fermentation Time and Type of Tea on the Content of Micronutrients in Kombucha Fermented Tea. Nutrients 2022, 14, 4828. [Google Scholar] [CrossRef]
- Hazimeh, D.; Massoud, G.; Parish, M.; Singh, B.; Segars, J.; Islam, M.S. Green Tea and Benign Gynecologic Disorders: A New Trick for an Old Beverage? Nutrients 2023, 15, 1439. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.; Godfrey, M.; Limeback, H.; Potter, W. Black Tea Source, Production and Consumption: An Assessment of Health Risks Associated with Fluoride Intake in New Zealand. J. Environ. Public Health 2017, 2017, 5120504. [Google Scholar] [CrossRef] [PubMed]
- Esfehani, M.; Ghasemzadeh, S.; Mirzadeh, M. Comparison of Fluoride Ion Concentration in Black, Green and White Tea. Int. J. Ayurvedic Med. 2019, 9, 263–265. [Google Scholar] [CrossRef]
- Klepacka, J.; Tońska, E.; Rafałowski, R.; Czarnowska-Kujawska, M.; Opara, B. Tea as a Source of Biologically Active Compounds in the Human Diet. Molecules 2021, 26, 1487. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Q.; Xie, F.; Jiang, J. Brick Tea Consumption and Its Relationship with Fluorosis in Tibetan Areas. Front. Nutr. 2022, 9, 1030344. [Google Scholar] [CrossRef]
- Li, X.; Smid, S.D.; Lin, J.; Gong, Z.; Chen, S.; Zhang, Y.; Hao, Z.; Lin, H.; Yu, X. Neuroprotective and Anti-Amyloid β Effects and Main Chemical Profiles of White Tea: Comparison with Green, Oolong and Black Tea. Molecules 2019, 24, 1926. [Google Scholar] [CrossRef]
- Yadav, K.C.; Parajuli, A.; Khatri, B.B.; Shiwakot, L.D. Phytochemicals and Quality of Green and Black Teas from Different Clones of the Tea Plant. J. Qual. Food 2020, 2020, 1–13. [Google Scholar]
- Brodziak-Dopierała, B.; Fischer, A. Analysis of Mercury Content in Various Types of Tea (Camellia sinensis) and Yerba Mate (Ilex Paraguariensis). Int. J. Environ. Res. Public Health 2022, 19, 5491. [Google Scholar] [CrossRef] [PubMed]
- Bhutia Pemba, H.; Ab Lepcha, R.; Tamang, D. Bioactive Compounds and Antioxidant Properties of Tea: Status, Global Research and Opportunities. J. Tea Sci. Res 2015, 5, 1–13. [Google Scholar]
- Li, X.; Zhu, X. Tea: Types, Production and Tradel. In Encyclical: Food Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 279–282. [Google Scholar]
- Hać-Szymańczuk, E.; Fiziar, M.; Cegiełka, A.; Piwowarek, K.; Misiura, S. Comparison of the Microbiological Quality of Black, Green and Red Teas, Activity on the Warsaw Market. Prob Noteb. Prog. Role Sci. 2017, 591, 33–42. [Google Scholar]
- Kj Dutta, S.; Chowdhury, P.; Sanjoy, D.; Jarin, A. Status and Productivity of Tea Plantations in the Chattogram Tea Valley in Bangladesh. Int. J. Biosci. 2021, 18, 251–260. [Google Scholar]
- Czarniecka-Skubina, E.; Korzeniowska-Ginter, R.; Pielak, M.; Sałek, P.; Owczarek, T.; Kozak, A. Consumer Choices and Habits Related to Tea Consumption by Poles. Foods 2022, 11, 2873. [Google Scholar] [CrossRef] [PubMed]
- Tm Agarwalb, S.; Maki, K.C. The Anti-Obesity Effect of Green Tea Catechins: A Mechanistic Review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar]
- Roy, R.B.; Tudu, B.; Pramanik, P.; Deca, H.; Tamuly, P.; Bandyopadhyay, R. Detection of Theaflavins in Black Tea Using a Molecularly Imprinted Nanocomposite Polyacrylamide-Graphite Electrode. Sens. Cylind. B Chem. 2017, 246, 840–847. [Google Scholar]
- Mr, H.; Gao, Y.; Tu, Y. Mechanisms of Weight Reduction by Black Tea Polyphenols. Molecules 2016, 21, 1659. [Google Scholar]
- Horžić, D.; Komes, D.; Belščak, A.; Ganić, K.K.; Iveković, D.; Karlović, D. The Composition of Polyphenols and Methylxanthines in Teas and Herbal Infusions. Food Chem. 2009, 115, 441–448. [Google Scholar] [CrossRef]
- Wereńska-Sudnik, M.; Chelmecka, I.; Wołoszyn, J.; Okruszek, A.; Haraf, G.; Okrusz, A. Wpływ Dodatku Proszku Zielonej Herbaty Na Jakość Wyrobów Podrobowych Przechowywanych w Warunkach Chłodniczych. Technologia 2016, 23, 60–71. [Google Scholar]
- Sharangi, A. Medicinal and Therapeutic Potentialities of Tea (Camellia sinensis L). Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Dias, T.R.; Tomas, G.; Teixeira, N.F.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. White Tea (Camellia sinensis (L.)): Antioxidant Properties and Beneficial Health Effects. Int. J. Food Sci. Nutr. Diet IJFS 2013, 2, 19–26. [Google Scholar]
- Dmowski, P.; Kosiorek, A. Antioxidant Properties of High-Quality Black Teas on the e-Commerce Market. Noteb. Sci. Acad. Plague Sci. Acad. Plague Gdyn. 2017, 99, 9–19. [Google Scholar]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Michalak-Majewska, M.W. Część 1. Znaczenie Żywieniowe. Nauka Przyr. Technol. 2011, 5, 1–11. [Google Scholar]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Grembecka, M.; Ciesielski, T.; Flaten, T.P.; Szefer, P. Evaluation of Macro- and Microelement Levels in Black Tea in View of Its Geographical Origin. Biol. Trace Elem. Res. 2017, 176, 429–441. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–A Fascinating Trace Element, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Milani, R.F.; Silvestre, L.K.; Morgano, M.A.; Cadore, S. Investigation of Twelve Trace Elements in Herbal Tea Commercialized in Brazil. J. Trace Elem. Med. Biol. 2019, 52, 111–117. [Google Scholar] [CrossRef]
- Fernandes, J.; Reboredo, F.H.; Luis, I.; Silva, M.M.; Simões, M.M.; Lidon, F.C.; Ramalho, J.C. Elemental Composition of Commercial Herbal Tea Plants and Respective Infusions. Plants 2022, 11, 1412. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzyńska, K. Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and Herb Water Extracts. Appl. Sci. 2023, 13, 1071. [Google Scholar] [CrossRef]
- Wyprostek, J.; Kowalski, R.; Pankiewicz, U.; Solarska, E. Determination of the Content of Selected Active Substances in Primary and Secondary Herbal Infusions by Means of UV-VIS and GC-MS Spectroscopic Analysis. J. Anal. Methods 2020, 2020, 8891855. [Google Scholar] [CrossRef]
- Mr, S.Y.; Tai, H.C.; Xl Tong, Y.; Zhang, L.J.F.; Lin, Z.H.X. Tea and Tea Drinking: China’s Outstanding Contribution to Humanity. Chin. Med. 2022, 17, 1–40. [Google Scholar]
- David, L. Water and Electrolyte Requirements: Effects of Exercise and Environmental Conditions. Clin. Sport Med. 1984, 3, 639–648. [Google Scholar] [CrossRef]
- Frączek, B.; Gacek, M.; Grzelak, A. Żywieniowe Wspomaganie Zdolności Wysiłkowych w Grupie Sportowców Wyczynowych. Probl. Hig. Epidemiol. 2012, 93, 817–823. [Google Scholar]
- Jarosz, M. Normy Żywienia dla Populacji Polskiej-Nowelizacja; Pol Health: Warsaw, Poland, 2012. [Google Scholar]
- Krupa, R. Zagrażające Życiu Stany Hipokaliemii-Przyczyny, Objawy, Powikłania Kardiologiczne, Zasady Postępowania. Choroby Serca Naczyń 2013, 10, 173–174. [Google Scholar]
- Zagroda, M.; Prystupa, A.; Mosiewicz, J. Zaburzenia Gospodarki Potasowej w Patogenezie i Leczeniu Napadowego Migotania Przedsionków. Fam. Med. Prim. Care Rev. 2013, 2, 205–206. [Google Scholar]
- Szeleszczuk, Ł.; Kuras, M. Znaczenie Wapnia w Metabolizmie Człowieka i Czynniki Wpływające na Jego Biodostępność w Diecie. Prospect. Pharm. Sci. 2014, 12, 16–22. [Google Scholar] [CrossRef]
- Tankeu, A.N.; Agbor, V.; Noubiap, J.J. Calcium Supplementation and Cardiovascular Risk: A Growing Concern. J. Clin. Hypertens. 2017, 19, 640–646. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.F.; Li, D.Y.; Chen, Y.C.; Zhao, L.J.; Xg Guo, Y.F.; Shen, J.; Lin, X.; Deng, J. The Good, the Bad and the Ugly of Calcium Supplementation: A Review of Calcium Intake for Human Health. Clin. Clin. Interv. Aging 2018, 13, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G. JM Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Shkembi, B.; Huppertz, T. Calcium Absorption from Foods: Food Matrix Effects. Nutrients 2021, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Puścion-Jakubik, A.; Staniaszek, G.; Brzozowska, P.; Socha, K. Quality of Calcium Food Supplements: Evaluation Compared to Manufacturers’ Declarations. Molecules 2022, 27, 8154. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal Absorption and Factors Affecting Magnesium Bioavailability–An Update. Curr. Nutr. Sci. Food 2017, 13, 260–278. [Google Scholar] [CrossRef]
- Blancquaert, L.; Vervaet, C.; Derave, W. Predicting and Testing Bioavailability of Magnesium Supplements. Nutrients 2019, 11, 1663. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Utyshenko, V.P.; Molchanov, M.; Beskaravayny, P.; Uversky, V.N.; Timchenko, M.A. Analyzing and Mapping Sweat Metabolomics by High-Resolution NMR Spectroscopy. PLoS ONE 2011, 6, e28824. [Google Scholar] [CrossRef]
- Dunstan, R.H.; Sparkes, D.L.; Dascombe, B.J.; Macdonald, M.M.; Evans, C.A.; Stevens, C.J.; Crompton, M.J.; Gottfries, J.; Franks, J.; Murphy, G.; et al. Sweat Facilitated Amino Acid Losses in Male Athletes during Exercise at 32–34 °C. PLoS ONE 2016, 11, e0167844. [Google Scholar] [CrossRef]
- Murphy, G.R.; Dunstan, R.H.; Macdonald, M.M.; Borges, N.; Radford, Z.; Sparkes, D.L.; Dascombe, B.J.; Roberts, T.K. Relationships between Electrolyte and Amino Acid Compositions in Sweat during Exercise Suggest a Role for Amino Acids and K+ in Reabsorption of Na+ and Cl- from Sweat. PLoS ONE 2019, 14, e0223381. [Google Scholar] [CrossRef] [PubMed]
- Sugajski, M.; Buszewska-Forajta, M.; Buszewski, B. Functional Beverages in the 21st Century. Beverages 2023, 9, 27. [Google Scholar] [CrossRef]
- Wolski, T.; Ludwiczuk, A. Ekstrakcja Produktów Naturalnych Gazami w Stanie Nadkrytycznym. Przemysł Chemiczny T 2001, 80, 286–289. [Google Scholar]
- Zhang, Q.; Lin, L.; Ye, W. Natural Product Extraction and Isolation Techniques: A Comprehensive Review. Chin. Med. 2018, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.; Alizadeh, N.; Salimi, S.; Haji-Ghasemi, T. Ultrasonic Assisted Extraction of Natural Pigments from Rhizomes of Curcuma longa, L. Progress in Color. Color. Coat. 2009, 2, 103–113. [Google Scholar]
- PN-ISO 1573:1996; Tea—Determination of Loss in Mass at 103 Degrees C. Polish Committee for Standardization: Warsaw, Poland, 1996.
- PN-ISO 1575:1996; Tea—Determination of Total Ash. Polish Committee for Standardization: Warsaw, Poland, 1996.
- PN-A-04018:1975/Az3:2002; Agricultural Food Products—Determination of Nitrogen by the Kjeldahl Method and Expressing as Protein. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Stasiuk, E.; Przybyłowski, P.; Tomczyk, M.; Olesiuk, J.; Dżugan, M. Ocena Jakości Napojów Izotonicznych Przygotowanych Samodzielnie Na Bazie Naturalnych Składników. Pol. J. Sports Med. 2015, 4, 169–177. [Google Scholar]
- Renke, G.; Almeida, V.B.P.; Souza, E.A.; Lessa, S.; Teixeira, R.L.; Rocha, L.; Sousa, P.L.; Starling-Soares, B. Clinical Outcomes of the Deleterious Effects of Aluminum on Neuro-Cognition, Inflammation, and Health: A Review. Nutrients 2023, 15, 2221. [Google Scholar] [CrossRef]
- Matwiejuk, A. Mineral Ingredients (Macro-and Microelements) Their Importance in Sports Nutrition. Sci. Ann. Univ. Phys. Educ. Tour. Bialystok 2009, 97, 97–99. [Google Scholar]
- Piskuła, P.; Astel, A. Rola Suplementacji w Zbilansowanym Żywieniu Człowieka. Cz. 2, Charakterystyka Oraz Skład Jonowy Ekstraktów z Herbat; LAB Laboratoria, Aparatura, Badania: Katowice, Poland, 2017; Volume 22, pp. 16–23. [Google Scholar]
- Łukasz, B.; Rybakowska, I.M.; Krakowiak, A.; Sein Anand, J. The Health Effects of Environmental and Occupational Exposure to Aluminum. Med. Pr 2020, 71, 79–88. [Google Scholar] [CrossRef]
- Katarzyna Dziubak, A.; Kręźlewicz, A. Poziomy Metali Szkodliwych Dla Zdrowia w Różnych Typach Żywności. Stud. Ecol. Bioethicae 2021, 19, 115–122. [Google Scholar]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the Pectin Production Process Using Novel Extraction Techniques: A Review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of Conventional and Ultrasound Assisted Extraction of Flavonoids from Grapefruit (Citrus paradisi L.) Solid Wastes. LWT—Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of Ultrasound-Assisted Extraction (UAE) of Flavonoids Compounds (FC) from Hawthorn Seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Pingret, D.; Fabiano-Tixier, A.-S.; Bourvellec, C.L.; Renard, C.M.G.C.; Chemat, F. Lab and Pilot-Scale Ultrasound-Assisted Water Extraction of Polyphenols from Apple Pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Perkowska, S.; Bazylak, B.; Wplyw, G. Warunkow Ekstrakcji Na Zawartosc Rozpuszczalnych Szczawianow w Wodnych Naparach Herbat Zielonych i Herbatek Ziolowych. Żywność Nauka Technol. Jakość 2010, 17, 107–121. [Google Scholar]
- Zurek, N.; Kapusta, I.; Cebulak, T. Wpływ Warunków Ekstrakcji Na Potencjał Przeciwutleniający Wyciągów z Kwiatów, Liści i Owoców Głogu (Crataegus x Macrocarpa L). Żywność Nauka Technol. Jakość 2020, 27, 130–141. [Google Scholar]
- Przybylska, A.; Stuper-Szablewska, K.; Matysiak, A.; Perkowski, J. Optymalizacja Warunków Ekstrakcji Związków Fenolowych Ogółem i Aktywności Przeciwutleniającej z Ziarna Pszenicy. Apar. Badaw. Dydakt. 2018, 23, 4–12. [Google Scholar]
- Sołtysiak, K. Ekstrakcja Związków Bioaktywnych z Ziaren Ryżu Wspomagana Ultradźwiękami. Politechnika Łódzka-Wydział Inżynierii Procesowej i Ochrony Środowiska, VII Seminarium Studenckie. Bezpieczeństwo w Inżynierii Procesowej; Wydawnictwo Politechniki Łódzkiej: Łódź, Poland, 2017. [Google Scholar]
- Zaguła, G.; Bajcar, M.; Saletnik, B.; Czernicka, M.; Puchalski, C.; Kapusta, I.; Oszmiański, J. Porównanie efektywności wodnej ekstrakcji substancji z suchych liści herbaty technikami ekstrakcji wspomaganej polem magnetycznym. Cząsteczki 2017, 22, 1656. [Google Scholar] [CrossRef]
- Tal-Figiel, B.; Figiel, W. Porównanie efektywności ekstrakcji surowców roślinnych za pomocą niekonwencjonalnych technik ekstrakcyjnych. Inż. Ap. Chem. 2010, 49, 15–16. [Google Scholar]
- Turek, A. Wpływ Kwasu Taninowego i Penicyliny Na Stabilność Białek Osierdzia. Eng. Biomater. 2007, 10, 84–86. [Google Scholar]
- Avilés-Betanzos, K.A.; Scampicchio, M.; Ferrentino, G.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Evaluation of the Capsaicinoid Extraction Conditions from Mexican Capsicum Chinense Var. Mayapan with Supercritical Fluid Extraction (SFE). Processes 2023, 11, 2272. [Google Scholar] [CrossRef]
- Paryjczak, T. Promowanie Zrównoważonego Rozwoju Przez Zieloną Chemię, Część 2. Probl. Ekorozwoju 2008, 3, 45–51. [Google Scholar]
- Raghunath, S.; Mallikarjunan, K. Optimization of Ultrasound-Assisted Extraction of Cold-Brewed Black Tea Using Response Surface Methodology. J. Food Process Eng. 2020, 43, 11. [Google Scholar] [CrossRef]
- Horžić, D.; Jambrak, A.R.; Belščak-Cvitanović, A.; Komes, D.; Lelas, V. Comparison of Conventional and Ultrasound Assisted Extraction Techniques of Yellow Tea and Bioactive Composition of Obtained Extracts. Food Bioproc. Technol. 2012, 5, 2858–2870. [Google Scholar] [CrossRef]
- Xie, K.; He, X.; Chen, K.; Chen, J.; Sakao, K.; Hou, D.-X. Antioxidant Properties of a Traditional Vine Tea, Ampelopsis Grossedentata. Antioxidants 2019, 8, 295. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yang, Y. Hot Water Extraction of Antioxidants from Tea Leaves–Optimization of Brewing Conditions for Preparing Antioxidant-Rich Tea Drinks. Molecules 2023, 28, 3030. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.-R.; Li, H.-B.; Wu, D.-T.; Geng, F.; Corke, H.; Wei, X.-L.; Gan, R.-Y. Ekstrakcja zielonych polifenoli przeciwutleniających z zielonej herbaty (Camellia sinensis). Przeciwutleniacze 2020, 9, 785. [Google Scholar] [CrossRef]
- Wyrostek, J.; Kowalski, R. Wpływ ultradźwięków i rozdrobnienia surowca na ekstrakcję związków fenolowych i flawonoidów z liści mięty pieprzowej i herbaty czarnej. Przemysł Chem. 2022, 101, 98–103. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).