Abstract
Inflammation and oxidative stress are known to be major factors in muscle atrophy. The objective of this study was to evaluate whether the antioxidant activity of Ishige sinicola ethanol extract (ISE) and fractions from ISE could prevent lipopolysaccharide (LPS)-induced muscle atrophy in C2C12 myotubes. IS was extracted with ethanol and fractionated with five organic solvents. Then, ISE and five fractions from ISE were used to evaluate the total antioxidant activity and the protective effect of LPS-induced muscle atrophy in C2C12 myotubes. The ISE and butanol (BuOH) fraction showed higher total antioxidant activity and higher total phenol content than other fractions of ISE. The ISE and BuOH fraction significantly attenuated the LPS-induced diameter of C2C12 myotubes as well as the mRNA and protein expression levels of the muscle-specific E3 ubiquitin ligases. The mRNA expression of forkhead box O type 3α was stimulated by LPS, which was suppressed by the BuOH fraction but not ISE. Furthermore, ISE and the BuOH fraction significantly reduced LPS-stimulated gene expression of pro-inflammatory cytokines and inflammation-inducible enzymes, which was mediated by through the inhibition of the p38/extracellular signal-regulated kinase signaling pathway. Thus, ISE exerts a protective effect against muscle atrophy in LPS-induced C2C12 myotubes through the antioxidant activity and anti-inflammatory effects of ISE.
1. Introduction
Skeletal muscle accounts for more than 40% of the total body weight in healthy adults [1,2]. Also, skeletal muscle plays essential roles, including regulating physical performance, promoting basal energy metabolism, and serving as a reservoir of essential substrates such as glucose and amino acids [1,2,3,4]. However, various pathological conditions such as aging, malnutrition, muscle injury, and an inactive lifestyle, as well as other acquired diseases such as cancer, diabetes, and sepsis, can stimulate the progression of loss of muscle mass and function into muscle atrophy. Muscle atrophy is defined as a decrease in muscle mass and strength due to an imbalance between protein synthesis and degradation, which can lead to muscle wasting. Therefore, muscle atrophy has been indicated as a major public health concern because of its increased prevalence and how it reduces health-related quality of life [3].
Studies have shown that increased circulating endotoxin levels and persistent systemic inflammation can be observed in patients with disease-related skeletal muscle atrophy, suggesting a crucial role of inflammation in the process of muscle atrophy [2]. In particular, lipopolysaccharides (LPS), a major pathogenic infectious agent of Gram-negative bacteria, activate the ubiquitin-proteasome system and the autophagy-lysosome system. Both systems can cause muscle damage as well as an innate immunity that triggers skeletal muscle protein degradation [3,5]. Thus, as systemic inflammation control can be essential for muscle atrophy, natural bioactive materials possessing potent anti-inflammatory properties combined with antioxidant activity are attractive resources for the management of muscle atrophy.
Ishige sinicola (IS), edible brown algae, has been distributed along the coast of Jeju Island in Republic of Korea, Japan, and China. Studies have demonstrated that IS has antioxidant, anti-inflammatory, antibacterial, osteoblast differentiating, and hair growth-promoting effects. Furthermore, IS has a high content of octaphlorethol A, a type of phlorotannin which has antioxidant, anti-diabetic, anti-inflammatory, and anti-melanogenesis effects [6,7,8,9]. However, whether IS has protective effects on inflammation-induced skeletal muscle atrophy with antioxidants in C2C12 myotubes has never been determined. In this study, IS was extracted with ethanol and fractionated with five organic solvents: Hex (n-hexane), CHCl3 (chloroform), EtOAc (ethyl acetate), BuOH (butanol), and H2O (water), and the antioxidant activity was evaluated. Based on the antioxidant activity of ISE and five fractions of ISE, we selected one of the fractions of ISE with the best antioxidant activity to determine for the first time whether the ISE and its fraction could prevent LPS-induced muscle atrophy in C2C12 myotubes.
2. Materials and Methods
2.1. Chemical Reagents
Folin–Ciocalteu’s phenol reagent, phloroglucinol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), and ferric chloride hexahydrate were purchased from Roche (Basel, Switzerland). For cell experiments, Dulbecco’s modified Eagle’s medium (DMEM, high glucose), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromid (MTT), LPS (Escherichia coli O26:B6), and Giemsa stain modified solution were purchased from Sigma-Aldrich. Penicillin-streptomycin solution was purchased from Hyclone (Logan, UT, USA). Fetal bovine serum (FBS) and the bicinchoninic acid (BCA) protein assay kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Preparation of the Extraction and Fractions
The dried IS was purchased from the local market of Parajeju (Jeju Island, Republic of Korea). The seaweed was ground using a blender and stored at −20 °C. The seaweed was extracted three times, with 100% ethanol for 7 days, 100% ethanol for 3 days, and 70% aqueous ethanol at room temperature. The three extracts were combined and filtered using a filter paper (F1093 grand, Chmlab, Barcelona, Spain) and concentrated using the rotary vacuum evaporator (Büchi Labortechnik AG, Flawil, Switzerland) at 40 °C. The extract was dissolved with deionized water and fractionated by liquid–liquid partitioning using different polarity solvents. The aqueous crude extract was fractionated two or three times with equal volume of Hex, CHCl3, EtOAc, BuOH, and H2O. The fractions were concentrated using the rotary vacuum evaporator (Büchi Labortechnik AG) to quantify the yield for each fraction.
2.3. Total Phenolic Content
The total phenolic amount of ISE and five fractions of ISE was determined using Folin–Ciocalteu method [10]. Briefly, 10 µL of ISE or each fraction (1000 µg/mL) was mixed with 130 µL of distilled water and 10 µL of Folin–Ciocalteu reagent in a 96-well plate. After a 6 min reaction, 100 µL of 7% sodium carbonate solution was subsequently added into mixture and incubated at room temperature for 90 min. The absorbance of blue-colored samples was measured at 750 nm using a microplate reader (Hangzhou Allsheng Instruments Co., Ltd., Hangzhou, China). The total phenolic content (TPC) was expressed as mg phloroglucinol equivalents (PAE)/g dry weight of the ISE or fractions of ISE compared to a calibration curve produced with standard phloroglucinol.
2.4. DPPH Radical Scavenging Activity
DPPH radical scavenging activity was determined as previously described [10]. Briefly, the DPPH solution was prepared by mixing 7.89 mg of DPPH and 200 µL of 80% aqueous methanol to make a concentration of 0.1 mM. This solution was diluted with 80% aqueous methanol to an absorbance of 0.700 ± 0.020 at 517 nm using a microplate reader (Hangzhou Allsheng Instruments Co., Ltd.). A total of 5 µL ISE, or each fraction of ISE, was mixed with 295 µL DPPH solution and incubated at room temperature in the dark for 30 min. The absorbance was measured at 517 nm using a microplate reader (Hangzhou Allsheng Instruments Co., Ltd.). 80% aqueous methanol and vitamin C were used as the blank and the standard, respectively. DPPH radical scavenging activity was expressed as mg vitamin C equivalents (VCE)/g of dry weight of the ISE or fractions of ISE.
2.5. ABTS Radical Scavenging Assay
ABTS radical scavenging activity was measured as previously described [10]. In brief, the ABTS radical solution was prepared by mixing 1.0 mM AAPH and 2.5 mM ABTS in 100 mL of phosphate-buffered solution (PBS) and incubated at 80 °C for 40 min in the dark to generate the ABTS radical. The radical solution was filtered using a 0.45 µm PVDF filter and diluted with PBS to an absorbance of 0.650 ± 0.020 at 734 nm using a microplate reader (Hangzhou Allsheng Instruments Co., Ltd.). A total of 5 µL of ISE and each fraction of ISE was mixed with 245 µL ABTS radical solution and incubated at 37 °C in the dark for 10 min. Then, the absorbance was measured at 734 nm using a microplate reader (Hangzhou Allsheng Instruments Co., Ltd.). ABTS radical scavenging activity was expressed as mg vitamin C equivalents (VCE)/g of dry weight of the ISE or fractions of ISE.
2.6. Ferric Reducing Antioxidant Power Assay
The ferric-reducing antioxidant power (FRAP) of the ISE and fractions of ISE was measured as described previously with slight modifications [10]. The FRAP solution was prepared by mixing a 300 mM acetate buffer (pH 3.6), 10 mM TPTZ solution (in 40 mM HCl), and 20 mM ferric chloride hexahydrate (FeCl3·6H2O) solution at a ratio of 10:1:1 (v/v), respectively. The FRAP solution was kept at 37 °C until use. A total of 6 µL of ISE or each fraction of ISE was mixed with a 200 µL FRAP solution and incubated at 37 °C for 4 min. The absorbance was measured at 593 nm using a microplate reader (Hangzhou Allsheng Instruments Co., Ltd.). Distilled water and ferrous sulphate (FeSO4, 0.1 mM to 1 mM) were used as the blank and the standard, respectively. FRAP was expressed as mM FeSO4 equivalents (FSE)/g of dry weight of the ISE or fractions of ISE.
2.7. Cell Culture and Differentiation
C2C12 cells, a murine myoblast cell line, were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured and maintained in high-glucose DMEM supplemented with 10% FBS and antibiotics (100 units/mL penicillin A and 100 µg/mL streptomycin) at 37 °C under a 5% CO2 humidified atmosphere. When C2C12 cells reached 90% confluence, the growth medium was changed to a differentiation medium (DM) consisting of high-glucose DMEM, 2% FBS, and antibiotics to induce differentiation into the myotube. Differentiation was continued for 4 days and the DM was refreshed every 2 days. After 4 days, fully differentiated C2C12 myotubes was treated with 1 μg/mL LPS and an ISE or BuOH fraction at 0–100 µg/mL for 48 h.
2.8. Cell Viability
Cell viability was determined with a colorimetric MTT assay. C2C12 myotubes were treated with various concentrations (0–100 µg/mL) of ISE and BuOH fractions for 48 h. After sample treatment, the MTT solution (500 μg/mL) was added to the cells and incubated at 37 °C for 1 h. Subsequently, the MTT solution was removed and insoluble formazan dye was dissolved using dimethyl sulfoxide. Absorbance at 570 nm was determined spectrophotometrically by using a BioTek Cytation 5 Image reader (Winooski, VT, USA).
2.9. C2C12 Myotube Diameter Measurement
The analysis of the diameter of C2C12 myotubes was performed via Giemsa staining. After the indicated treatments, C2C12 myotubes were washed with PBS and fixed with 100% methanol for 10 min. Myotubes were incubated with the Giemsa staining solution for 10 min at room temperature and washed three times with distilled water to measure the morphological changes of C2C12 cells. Myotube diameters were estimated by a BioTek Cytation 5 Image reader (Winooski) at ×20 magnification. The diameters of three different sites in each myotube were determined using ImageJ software (ImageJ bundled with 64-bit Java 8, National Institutes of Health, Bethesda, MD, USA), using at least 100 myotubes in 10 random fields. The results were expressed as percentages of the diameter in the control group.
2.10. Quantitative Real Time PCR (qRT-PCR)
Total RNA was extracted from C2C12 myotubes using homemade Trizol reagent according to the manufacturer’s instructions. The RNA (1 µg) was reverse-transcribed into cDNA with a Compact cDNA Synthesis Kit (Smart Gene, Daejeon, Republic of Korea) in a GeneAmp PCR System 9700 (PerkinElmer, Inc., Waltham, MA, USA). qRT-PCR was conducted using the SYBR Green Q-PCR Master Mix (Smart Gene) in a QuantStudio™ 1 Real-Time PCR system (Thermo Fisher Scientific) under the standard thermal cycle conditions: polymerase activation for 2 min at 95 °C followed by 40 cycles of denaturation for 5 s at 95 °C, then annealing/extension for 30 s at 60 °C. The list of primers is provided in Table 1. All mRNA levels were normalized using the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. Relative expression values were calculated using 2−ΔΔCt method.

Table 1.
Primers for real-time PCR amplification of gene expression.
2.11. Western Blot Analysis
C2C12 myotubes for Western blot analysis were treated with LPS (1 µg/mL) and ISE or BuOH fractions (50, 75 and 100 µg/mL) for 24 or 48 h. C2C12 myoblasts were lysed using a CETi lysis buffer (TransLab, Daejeon, Republic of Korea). The lysate protein concentrations were measured using the Pierce™ BCA protein assay kit. Equal amounts of protein in each sample were separated by 8% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and PVDF membranes. The membranes were blocked using a blocking buffer at room temperature for 1 h, and then incubated at 4 °C overnight with primary antibodies against muscle atrophy F-box (MAFbx; also known to atrogin-1), muscle RING-finger 1 (MuRF1), inducible NO synthase (iNOS), cyclooxygenase-2 (COX2), phospho-p38 (p-p38), total-p38 (t-p38), p-extracellular signal-regulated kinase (p-ERK), t-ERK, and β-Actin (1:1000 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA and Cell Signaling Technology, Danvers, MA, USA). After washing three times in Tris-buffered saline containing 0.1% Tween 20, the membranes were incubated with goat anti-mouse and goat anti-rabbit IgG-conjugated secondary antibodies (1:10,000 dilution, Bethyl Laboratories Inc., Montgomery, TX, USA) for 1 h at room temperature. Proteins were detected with an ECL detection solution and visualized with the ImageQuant LAS 500 (GE Health Care, Chalfont Saint Giles, UK). The relative intensity of each protein band was quantified using Image Studio Lite Western Blot Quantification version 5.2 software and normalized to that of the corresponding loading control.
2.12. Statistical Analysis
One-way analysis of variance (ANOVA) with Tukey’s post-hoc test was performed using Graphpad 9.0 (GraphPad Software La Jolla, CA, USA). p values less than 0.05 were considered statically significant. All data were expressed as mean ± standard deviation.
3. Results
3.1. Total Phenolic Content
Phenolic compounds in plants have a hydroxyl substituted benzene ring, which can have interesting biological activities such as antioxidant, anti-inflammatory, antiproliferative, and antibacterial activity [10,11]. We measured the TPC of ISE and five different fractions—Hex, CHCl3, EtOAc, BuOH, and H2O (Table 2). The TPC of ISE and the five different fractions of ISE range from 18.21 to 286.25 mg PGE/g of dry weight. BuOH (188.44 ± 11.77) had the highest TPC, followed by EtOAc (88.01 ± 9.82), H2O (77.58 ± 3.08), ISE (76.29 ± 8.95), CHCl3 (20.38 ± 0.99), and Hex (10.16 ± 0.00). The BuOH fraction showed the highest TPC compared to other fractions of ISE.

Table 2.
Extraction yield, total anti-oxidant capacity, and total phlorotannin content of the ISE and its fractions.
3.2. Antioxidant Capacity
We determined the antioxidant capacity using ABTS, DPPH, and FRAP assays, which are known to be the most commonly used methods to evaluate total antioxidant capacity (Table 2). For ABTS radical scavenging activity, the BuOH fraction showed the highest ABTS radical scavenging capacity among the five fractions of ISE, with 286.25 ± 14.61 mg VCE/g of dry weight, followed by EtOAc (120.32 ± 8.14), H2O (109.26 ± 8.94), CHCl3 (25.40 ± 3.86), and Hex (18.21 ± 4.43). Furthermore, ISE showed the third highest ABTS radical scavenging capacity, with 119.26 ± 9.28 mg VCE/g of dry weight compared to the five fractions of ISE. The DPPH radical scavenging capacity was the highest in BuOH (82.14 ± 12.13), followed by EtOAc (53.29 ± 6.84), ISE (44.85 ± 5.96), H2O (36.76 ± 3.49), CHCl3 (12.21 ± 1.36), and Hex (11.63 ± 0.38). In the case of FRAP activity, BuOH (0.40 ± 0.05) had the highest, followed by EtOAc (0.15 ± 0.01), H2O (0.16 ± 0.01), ISE (0.12 ± 0.01), CHCl3 (0.05 ± 0.01), and Hex (0.04 ± 0.00). We further conducted the Pearson correlation analysis to estimate the association between the TPC and three antioxidant assays (Table 3). The TPC assay was significantly positively correlated with the ABTS (r = 1.00, p < 0.01), DPPH (r = 0.98, p < 0.01), and FRAP (r = 0.99, p < 0.01) assays, which indicated that polyphenol compounds in ISE have strong total antioxidant capacity.

Table 3.
Pearson’s correlation between TPC and three total antioxidant capacity results in ISE and its fractions.
3.3. Effect of ISE on LPS-Induced C2C12 Myotube Atrophy
Compounds with antioxidant properties are known to have the ability to delay muscle atrophy [12]. We first investigated the protective effects of ISE on muscle atrophy in LPS-induced C2C12 myotubes. When the cytotoxicity of ISE was measured at 0–100 µg/mL on C2C12 myotubes, cells viability was not significantly changed at 100 µg/mL ISE (Figure 1a). Thus, the following experiments were performed at ISE concentrations under 100 µg/mL. LPS significantly decreased the diameter of C2C12 myotubes, which were significantly reversed by ISE (Figure 1b,c). The maximal effect was observed at 100 µg/mL of ISE, which increased myotube diameter by 46.5% compared to the LPS-treated myotubes. Forkhead box O type 3α (FoxO3α) transcription factors induce muscle atrophy by upregulating muscle-specific E3 ubiquitin ligases, such as MAFbx and MuRF1 [13]. The mRNA expression levels of MAFbx and MuRF1 were significantly increased by LPS, whereas only 75 and 100 µg/mL of ISE significantly decreased MAFbx and MuRF1 expression levels (Figure 1d). Furthermore, increases in the protein expression of MAFbx and MuRF1 by LPS were markedly suppressed by ISE (Figure 2). However, ISE did not alter the mRNA expression of FoxO3α in LPS-induced C2C12 myotubes.
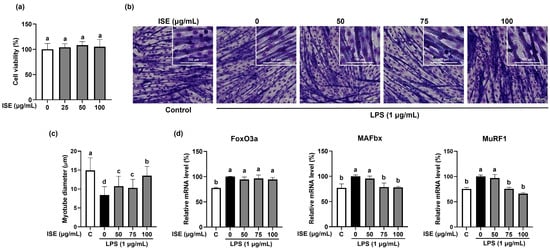
Figure 1.
Inhibitory effects of ISE on muscle atrophy in LPS-induced C2C12 myotubes. C2C12 myotubes were treated with LPS (1 µg/mL) and ISE (50, 75, and 100 µg/mL) for 48 h. (a) Cell viability of ISE in C2C12 myotubes. C2C12 myotubes were treated with 0–100 µg/mL of ISE to measure the cell viability. (b) Giemsa staining was performed to visualize C2C12 myotube morphology (magnification 20×, scale bar = 100 μm). (c) Myotube diameters (μm) were observed from randomly selected fields and were quantified using the ImageJ program. The mRNA expression of (d) FoxO3a, MAFbx, and MuRF1 was analyzed by RT-PCR. GAPDH was used as internal controls. Bars with a different letter are significantly different from the control (p < 0.05).
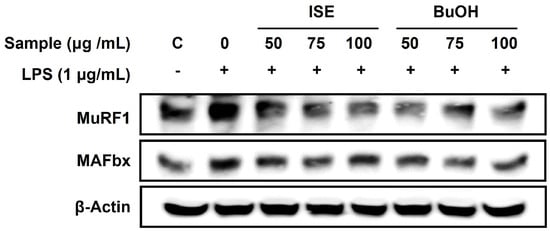
Figure 2.
Effects of ISE and BuOH on the protein expression of muscle-specific E3 ubiquitin ligases in LPS-induced C2C12 myotubes. C2C12 myotubes were treated with LPS (1 µg/mL) and ISE or BuOH fraction of ISE (50, 75, and 100 µg/mL) for 48 h. The protein expression of MAFbx and MuRF1 was analyzed using Western blot analysis. β-Actin was employed as a loading control.
3.4. Effect of BuOH Fraction on LPS-Induced C2C12 Myotube Atrophy
As the BuOH fraction showed the highest TPC and total antioxidant capacity among the five fractions from ISE, we further confirmed the effect of the BuOH fraction on muscle atrophy in LPS-induced C2C12 myotubes. We measured the cell viability of the BuOH fraction in C2C12 myotubes and found that more than 95% of cells were viable when treated with 0–100 µg/mL of BuOH fraction (Figure 3a). Reduced myotube diameter by LPS was significantly increased by the BuOH fraction at 75 and 100 µg/mL (Figure 3b,c). The diameter of LPS-treated myotubes was reduced by 63.6% compared to the control (LPS-untreated myotubes), while 75 and 100 µg/mL of the BuOH fraction increased by 43.5% and 56.5%, respectively, compared to the LPS-treated myotubes. The gene expression levels of FoxO3α, MAFbx, and MuRF1 were significantly increased by LPS, and were significantly reduced by the BuOH fraction (Figure 3d). Furthermore, LPS-stimulated protein expression levels of MAFbx and MuRF1 were noticeably attenuated by the BuOH fraction (Figure 2).
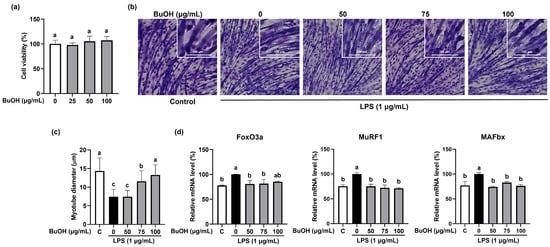
Figure 3.
Inhibitory effects of the BuOH fraction from ISE on muscle atrophy in LPS-induced C2C12 myotubes. C2C12 myotubes were treated with LPS (1 µg/mL) and the BuOH fraction of ISE (50, 75, and 100 µg/mL) for 48 h. (a) Cell viability of BuOH fraction in C2C12 myotubes. C2C12 myotubes were treated with 0–100 µg/mL of BuOH fraction to measure the cell viability. (b) Giemsa staining was performed to visualize C2C12 myotube morphology (magnification 20×, scale bar = 100 μm). (c) Myotube diameters (μm) were observed from randomly selected fields and were quantified using the ImageJ program. The mRNA expression of (d) FoxO3a, MAFbx, and MuRF1 was analyzed by RT-PCR. GAPDH was used as internal controls. Bars with a different letter are significantly different from control (p < 0.05).
3.5. Anti-Inflammatory Effects of ISE and BuOH Fraction through Inhibition of p38/ERK Signaling Pathway in LPS-Induced C2C12 Myotube Atrophy
As inflammation is known to be a major factor in skeletal muscle atrophy [14], we measured the expression of pro-inflammatory cytokines and inflammation-inducible enzymes in LPS-induced C2C12 myotube atrophy. LPS significantly increased the expression of pro-inflammatory cytokines (interleukin-6 (IL-6) and IL-1β) and inflammation-inducible enzymes (iNOS and COX2) (Figure 4a,b) in C2C12 myotubes. ISE significantly reduced IL-1β and COX2 mRNA expression, but not IL-6 and iNOS mRNA expression. On the other hand, the BuOH fraction significantly reduced both the mRNA expression of pro-inflammatory cytokines and inflammation-inducible enzymes. In particular, ISE markedly abolished the LPS-stimulated protein expression of iNOS and COX2 in C2C12 myotubes (Figure 5a). However, the BuOH fraction markedly reduced the LPS-stimulated iNOS protein expression in C2C12 myotubes, but not COX2 protein expression. Furthermore, since LPS-stimulated gene expression of pro-inflammatory cytokines and inflammation-inducible enzymes are mainly activated by the mitogen-activated protein kinase (MAPK) signaling pathway [15], we further investigated the effect of both the ISE and the BuOH fraction on the inhibition of the p38/ERK pathway. LPS markedly increased the phosphorylation of p38 and ERK, which was markedly attenuated by the ISE and the BuOH fraction, but had no dose-dependent effects (Figure 5b,c).
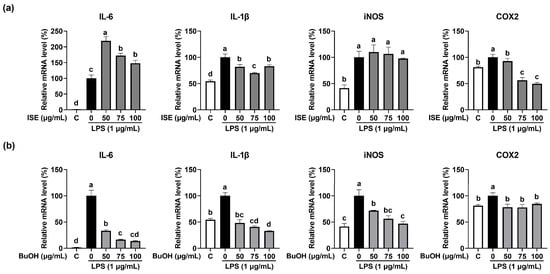
Figure 4.
Effects of ISE and the BuOH fraction of ISE on the expression of pro-inflammatory cytokines and inflammation-inducible enzymes in LPS-treated C2C12 myotubes. C2C12 myotubes were treated with LPS (1 µg/mL) and the (a) ISE or (b) BuOH fraction of ISE (50, 75, and 100 µg/mL) for 24 h. The mRNA expression of IL-6, IL-1β, iNOS, and COX2 was determined using RT-PCR. GAPDH was used as the internal controls. Bars with a different letter are significantly different from the control (p < 0.05).
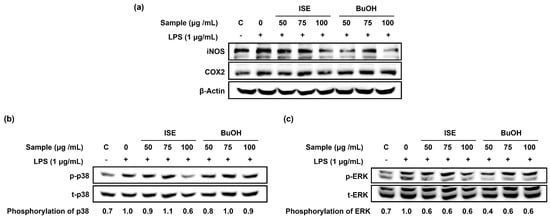
Figure 5.
Effects of ISE and BuOH on the protein expression of inflammation-inducible enzymes and phosphorylation of p38 and ERK in LPS-induced C2C12 myotubes. C2C12 myotubes were treated with LPS (1 µg/mL) and ISE, or the BuOH fraction of ISE (50, 75, and 100 µg/mL) for 24 h. (a) The protein expression of iNOS and COX2 was analyzed by Western blot analysis. β-Actin was employed as a loading control. (b) The protein expression of p-p38 and t-p38 was analyzed by Western blot analysis. Phosphorylation p38 was calculated as a ratio of p-p38/t-p38 and expressed relative to LPS. (c) The protein expression of p-ERK and t-ERK was analyzed by Western blot analysis. Phosphorylation ERK was calculated as a ratio of p-ERK/t-ERK and expressed relative to LPS.
4. Discussion
Since the regulation of inflammation and oxidative stress is essential for muscle atrophy, seaweeds with potent anti-inflammatory and antioxidant activity are an attractive natural resource for the management of muscle atrophy [14,16]. Studies have demonstrated IS exerts anti-inflammatory and antioxidant properties. ISE and its active compounds suppressed pro-inflammatory cytokines and nitric oxide production by inhibiting the MAPK/nuclear factor κB (NF-κB) signaling pathway in LPS-induced RWA 264.7 macrophages [7,17]. Also, IS methanol extract showed high hydrogen peroxide and DPPH free radical scavenging activity [10,18]. ISE has inhibitory effects on inflammatory cytokines and oxidative stress and therefore likely exert muscle atrophy effects in LPS-induced C2C12 myotubes. In this study, we found that ISE and the BuOH fraction exert anti-muscle atrophy effects in C2C12 myotubes by attenuating LPS-induced expression of muscle-specific E3 ubiquitin ligases and pro-inflammatory through inhibition of the p38/ERK MAPK signaling pathway, which was accompanied by high TPC and total antioxidant activity.
Skeletal muscle atrophy depends on the relative balance between protein degradation and synthesis [19]. When muscle atrophy occurs, protein breakdown exceeds protein synthesis. Protein synthesis is mainly regulated through activation of the mTOR pathway. The mTOR signaling pathway is mediated by mTORC1 (mTOR complex 1), which regulates protein synthesis, and mTOR complex 2 (mTORC2), which regulates cell survival and metabolism. mTORC1 is activated through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/Akt) pathway to increase protein synthesis by phosphorylating the downstream targets, 70 kDa ribosomal protein S6 kinase 1 (70S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). On the other hand, the primary regulatory mechanism of protein degradation in skeletal muscle is the ubiquitin-proteasome pathway [19]. Muscle-specific E3 ubiquitin ligases, such as MAFbx and MuRF1, are instrumental in the processes underlying protein degradation and are highly stimulated by the transcription factor FoxO3α [20,21]. In skeletal muscle cells, muscle-specific E3 ubiquitin ligases are activated by dephosphorylation of FoxO3α, which influences its transcriptional activity [22]. In this study, the BuOH fraction attenuated the LPS-induced mRNA expression of MAFbx and MuRF1 in C2C12 myotubes by significantly suppressing the transcription factor FoxO3a. In addition, the BuOH fraction increased myotube diameter against LPS-induced myotube atrophy, which might be associated with the suppression of MAFbx and MuRF1 expression by the BuOH fraction. Thus, the anti-muscle-atrophy effect of the BuOH fraction is likely mediated by inhibiting FoxO3α activation. These results suggest that the BuOH fraction of ISE can prevent the development of muscle atrophy.
Although ISE significantly reduced LPS-induced MAFbx and MuRF1 mRNA and protein expression in C2C12 myotubes, the effect on FoxO3a expression was minimal. However, the BuOH fraction significantly attenuated the expression of MAFbx and MuRF1 through inhibiting the expression of FoxO3a. The reason for the different effects of ISE and BuOH fractions could be due to the higher TPC and total antioxidant capacity of the BuOH fraction compared to ISE. Antioxidants can potentially prevent skeletal muscle atrophy by inhibiting reactive oxygen species (ROS) production [12]. High cellular ROS levels increase the expression of proteins required for the ubiquitin-proteasome system [12]. Furthermore, studies have shown that strong antioxidant properties and high polyphenol content are closely related to inhibition of muscle atrophy-related ubiquitin ligases. Polyphenols, such as catechin and quercetin, have been shown to prevent clinorotation-induced expression of MAFbx and MuRF1 in C2C12 myotubes [23]. Rutin, one of the flavonoids, prevented dexamethasone-induced muscle loss in C2C12 myotubes and mouse models by controlling FOXO3-dependent signaling [24]. Didrovaltrate, an active compound of Valeriana fauriei, has ROS scavenging activity and has inhibited dexamethasone-induced muscle atrophy in C2C12 myotubes and C57BL/6 mice [25]. The higher TPC and antioxidant capacity of the BuOH fraction compared to ISE may contribute to the repressed expression of muscle-specific E3 ubiquitin ligases genes by the BuOH fraction in LPS-induced C2C12 myotubes. Thus, the study to find bioactive compounds of the BuOH fraction from ISE with potent muscle atrophy effects is warranted to test this possibility.
Inflammation is an important factor that causes skeletal muscle atrophy [14]. Pro-inflammatory cytokines can trigger muscle atrophy mediated by the activating ubiquitin-proteasome and NF-κB/MAPK signaling pathways [26,27]. MAPKs including ERK, c-Jun N-terminal kinase, and p38 regulate MAFbx and MuRF1 expression of muscles under oxidative stress and inflammatory conditions. In particular, elevated phosphorylation of p38 and ERK induces overexpression of MAFbx and MuRF1 in C2C12 myotubes [28]. Also, studies have demonstrated that systemic administration of LPS can stimulate the expression of MAFbx and MuRF1 through an increase of NF-κB activity and its downstream inflammatory mediator, such IL-6, IL-1β, and COX2 [26,29]. In the present study, we observed that ISE and the BuOH fraction significantly decreased the expression of pro-inflammatory cytokines and inflammation-inducible enzymes in LPS-induced C2C12 myotubes. Also, ISE and the BuOH fraction noticeably attenuated the LPS-stimulated increase in protein expression of phosphorylation of p38 and ERK in C2C12 myotubes. Thus, the decreased mRNA expression of IL-6, IL-1β, iNOS, and COX2 by ISE and the BuOH fraction is mediated through inhibition of the p38/ERK MAPK signaling pathway. This can contribute to the suppressed expression of muscle atrophy-related ubiquitin ligases by ISE and the BuOH fraction in LPS-induced C2C12 myotubes. Also, since oxidants produced by infiltrating immune cells can directly cause muscle damage, the effect of ISE and the BuOH fraction on muscle atrophy is likely accompanied by the antioxidant and anti-inflammatory effects of ISE and BuOH fraction.
5. Conclusions
In summary, the present study provides the first evidence that ISE and the BuOH fraction from ISE has a protective effect on muscle atrophy in LPS-induced C2C12 myotubes. ISE and the BuOH fraction inhibited LPS-induced myotube atrophy by suppressing muscle-specific E3 ubiquitin ligase genes through the inactivation of FoxO3α in C2C12 myotubes. Also, ISE and BuOH not only exhibit high total antioxidant capacity, but also suppress inflammatory mediator expression through the inhibition of p38 and ERK phosphorylation, which likely contributes to the inhibitory effect of ISE and BuOH fractions on muscle atrophy. However, further studies are required to investigate bioactive compounds of the BuOH fraction from ISE with a potent muscle atrophy effect. Therefore, our findings suggest that ISE has excellent potential as a nutraceutical material for the prevention and treatment of muscle atrophy.
Author Contributions
Conceptualization, S.G.L. and M.-B.K.; methodology, M.-B.K., H.L. and C.L.; software, M.-B.K., H.L. and C.L.; validation, M.-B.K., H.L. and C.L.; formal analysis, H.L. and C.L.; investigation, H.L. and C.L.; resources, S.G.L.; data curation, M.-B.K., H.L. and S.G.L.; writing—original draft preparation, M.-B.K., H.L., Y.T. and S.G.L.; writing—review and editing, M.-B.K., H.L., C.L., Y.T. and S.G.L.; visualization, M.-B.K. and H.L.; supervision, S.G.L.; project administration, S.G.L.; funding acquisition, M.-B.K. and S.G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Future Fisheries Food Research Center of the Ministry of Oceans and Fisheries of the Republic of Korea, grant number 201803932 to S.G.L. and by funds from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, grant number 202300247455 to M.-B.K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ceci, R.; Maldini, M.; Olson, M.E.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Duranti, G. Moringa oleifera leaf extract protects C2C12 myotubes against H2O2-induced oxidative stress. Antioxidants 2022, 11, 1435. [Google Scholar] [CrossRef]
- Fang, W.Y.; Tseng, Y.T.; Lee, T.Y.; Fu, Y.C.; Chang, W.H.; Lo, W.W.; Lin, C.L.; Lo, Y.C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Q.; Zhang, X.; Zhu, X.; Dong, X.; Shen, L.; Zhang, S.; Niu, L.; Chen, L.; Zhang, M. l-Arginine alleviates LPS-induced oxidative stress and apoptosis via activating SIRT1-AKT-nrf2 and SIRT1-FOXO3a signaling pathways in C2C12 myotube cells. Antioxidants 2021, 10, 1957. [Google Scholar] [CrossRef] [PubMed]
- KC, P.B.; Kang, B.S.; Jeoung, N.H. Butyrate Ameliorates Lipopolysaccharide-induced Myopathy through Inhibition of JNK Pathway and Improvement of Mitochondrial Function in C2C12 Cells. J. Life Sci. 2021, 31, 464–474. [Google Scholar]
- Fang, W.-Y.; Lin, C.-L.; Chang, W.-H.; Chang, C.-H.; Huang, Y.-C.; Tsai, Y.-H.; Chang, F.-R.; Lo, Y.-C. Protective Effects of the Chalcone-Based Derivative AN07 on Inflammation-Associated Myotube Atrophy Induced by Lipopolysaccharide. Int. J. Mol. Sci. 2022, 23, 12929. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kim, K.-N.; Lakmal, H.C.; Kim, E.-A.; Wijesinghe, W.; Yang, X.; Heo, S.-J.; Jeon, Y.-J. Octaphlorethol A isolated from Ishige foliacea prevents and protects against high glucose-induced oxidative damage in vitro and in vivo. Environ. Toxicol. Pharmacol. 2014, 38, 607–615. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, G.-R.; Lee, D.-H.; XU, X.; Kim, G.-E.; Cho, K.-S.; Park, S.-H.; Kim, K.-B.-W.-R.; Kim, M.-J.; Ahn, D.-H. Anti-inflammatory effects of Ishige sinicola ethanol extract in LPS-induced RAW 264.7 cell and mouse model. Korean J. Food Preserv. 2017, 24, 1149–1157. [Google Scholar] [CrossRef][Green Version]
- Kim, K.-N.; Yang, H.-M.; Kang, S.-M.; Kim, D.; Ahn, G.; Jeon, Y.-J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Moon, S.-H.; Jeon, B.-T.; Lee, D.H.; Jeon, Y.-J. Octaphlorethol A: A potent α-glucosidase inhibitor isolated from Ishige foliacea shows an anti-hyperglycemic effect in mice with streptozotocin-induced diabetes. Food Funct. 2014, 5, 2602–2608. [Google Scholar] [CrossRef]
- Baek, S.H.; Cao, L.; Jeong, S.J.; Kim, H.-R.; Nam, T.J.; Lee, S.G. The comparison of total phenolics, total antioxidant, and anti-tyrosinase activities of Korean Sargassum species. J. Food Qual. 2021, 2021, 6640789. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K. Can antioxidants protect against disuse muscle atrophy? Sports Med. 2014, 44, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Motohashi, N.; Uezumi, A.; Fukada, S.-i.; Yoshimura, T.; Itoyama, Y.; Aoki, M.; Miyagoe-Suzuki, Y.; Takeda, S.i. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J. Clin. Investig. 2007, 117, 2468–2476. [Google Scholar] [CrossRef]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W. Inflammation: Roles in skeletal muscle atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Maeshige, N.; Hirayama, Y.; Yamaguchi, A.; Ma, X.; Uemura, M.; Kondo, H.; Fujino, H. Pulsed ultrasound prevents lipopolysaccharide-induced muscle atrophy through inhibiting p38 MAPK phosphorylation in C2C12 myotubes. Biochem. Biophys. Res. Commun. 2021, 570, 184–190. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Chun, Y.-S.; Kim, J.-K.; Lee, J.-O.; Lee, Y.-J.; Ku, S.-K.; Shim, S.-M. Curcumin ameliorated oxidative stress and inflammation-related muscle disorders in C2C12 myoblast cells. Antioxidants 2021, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Manzoor, Z.; Koo, J.-E.; Moon, S.-R.; Byeon, S.-H.; Yoo, E.-S.; Kang, H.-K.; Hyun, J.-W.; Lee, N.-H.; Koh, Y.-S. Monoolein, isolated from Ishige sinicola, inhibits lipopolysaccharide-induced inflammatory response by attenuating mitogen-activated protein kinase and NF-κB pathways. Food Sci. Biotechnol. 2017, 26, 507–511. [Google Scholar] [CrossRef]
- Heo, S.-J.; Cha, S.-H.; Lee, K.-W.; Cho, S.-M.K.; Jeon, Y.-J. Antioxidant activities of chlorophyta and phaeophyta from Jeju Island. Algae 2005, 20, 251–260. [Google Scholar] [CrossRef]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.-H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L. Regulation of autophagy and the ubiquitin–proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef]
- Pomiès, P.; Blaquière, M.; Maury, J.; Mercier, J.; Gouzi, F.; Hayot, M. Involvement of the FoxO1/MuRF1/Atrogin-1 signaling pathway in the oxidative stress-induced atrophy of cultured chronic obstructive pulmonary disease myotubes. PLoS ONE 2016, 11, e0160092. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Ohkawa, S.; Li, H.; Roberts-Wilson, T.K.; Price, S.R. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010, 24, 2660. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, D.I.I.; Hirasaka, K.; Nakao, R.; Kohno, S.; Kagawa, S.; Abe, T.; Harada-Sukeno, A.; Okumura, Y.; Nakaya, Y.; Terao, J. Polyphenols prevent clinorotation-induced expression of atrogenes in mouse C2C12 skeletal myotubes. J. Med. Investig. 2009, 56, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.-S.; Lee, W.K.; Lee, S.-J.; Lee, S.Y.; Seo, J.-H.; Kim, E.J.; Choe, Y.-I.; Kim, S.G.; Yoo, J.-I. Rutin Prevents Dexamethasone-Induced Muscle Loss in C2C12 Myotube and Mouse Model by Controlling FOXO3-Dependent Signaling. Antioxidants 2023, 12, 639. [Google Scholar] [CrossRef]
- Kim, Y.I.; Lee, H.; Nirmala, F.S.; Seo, H.-D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Antioxidant activity of Valeriana fauriei protects against dexamethasone-induced muscle atrophy. Oxidative Med. Cell. Longev. 2022, 2022, 3645431. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef]
- Ha, S.-E.; Bhagwan Bhosale, P.; Kim, H.-H.; Park, M.-Y.; Abusaliya, A.; Kim, G.-S.; Kim, J.-A. Apigetrin Abrogates Lipopolysaccharide-Induced Inflammation in L6 Skeletal Muscle Cells through NF-κB/MAPK Signaling Pathways. Curr. Issues Mol. Biol. 2022, 44, 2635–2645. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.-J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.-J. Sabinene prevents skeletal muscle atrophy by inhibiting the MAPK–MuRF-1 pathway in rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef]
- Huang, N.; Kny, M.; Riediger, F.; Busch, K.; Schmidt, S.; Luft, F.C.; Slevogt, H.; Fielitz, J. Deletion of Nlrp3 protects from inflammation-induced skeletal muscle atrophy. Intensive Care Med. Exp. 2017, 5, 3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).