Anti-Obesity Activities of Standardized Ecklonia stolonifera Extract in 3T3-L1 Preadipocytes and High-Fat-Diet-Fed ICR Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Quantitation of Dieckol in the Ecklonia stolonifera Extract by HPLC-PDA
2.3. Animal Experiments
2.4. Biochemical Analysis
2.5. Histological Analysis
2.6. Cell Culture
2.7. Cell Viability
2.8. ORO Staining and NBT Assay
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Amounts of Dieckol in the Ecklonia stolonifera Extract
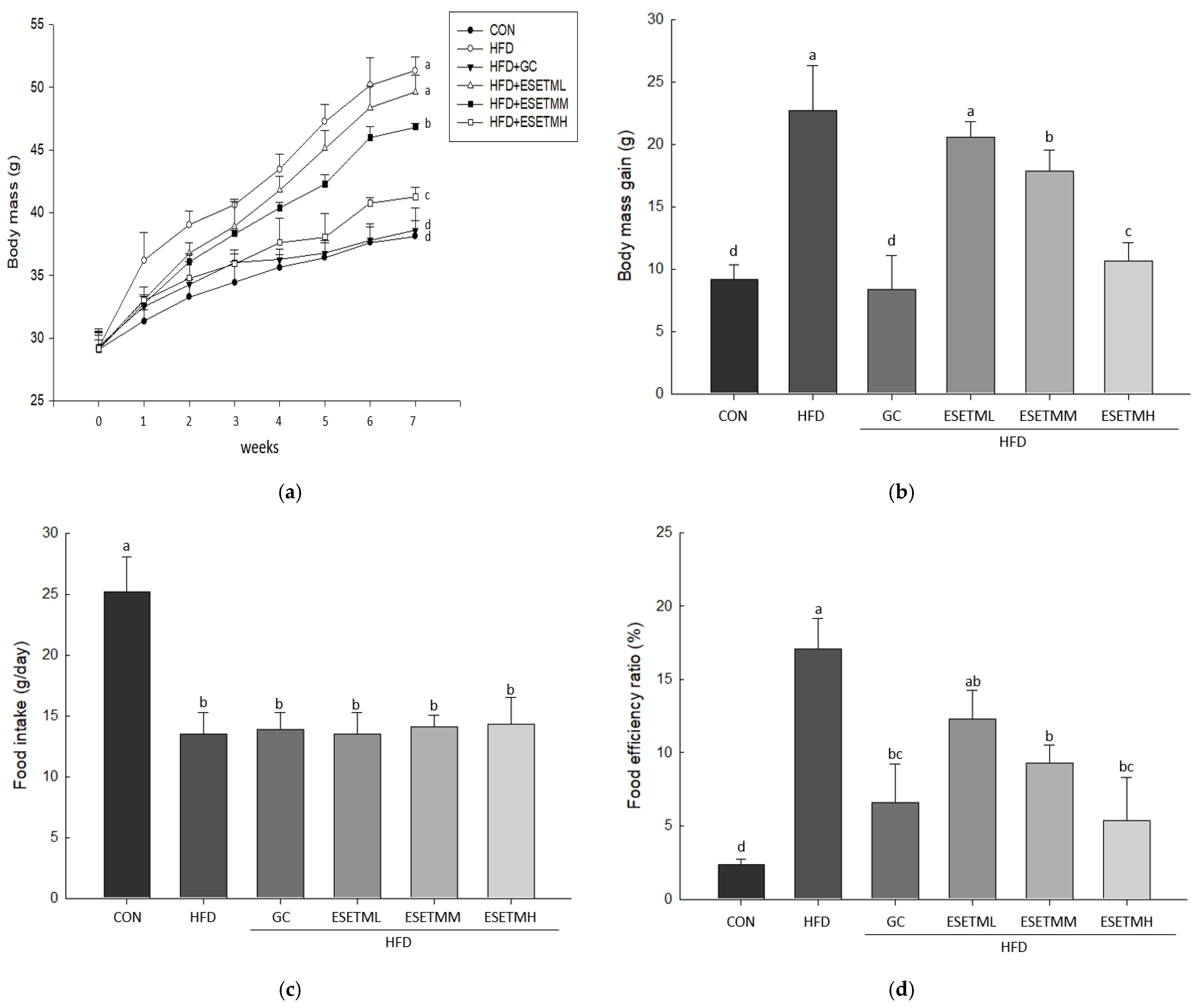
3.2. ESETM Prevents Development of Obesity in HFD-Fed Obese Mice
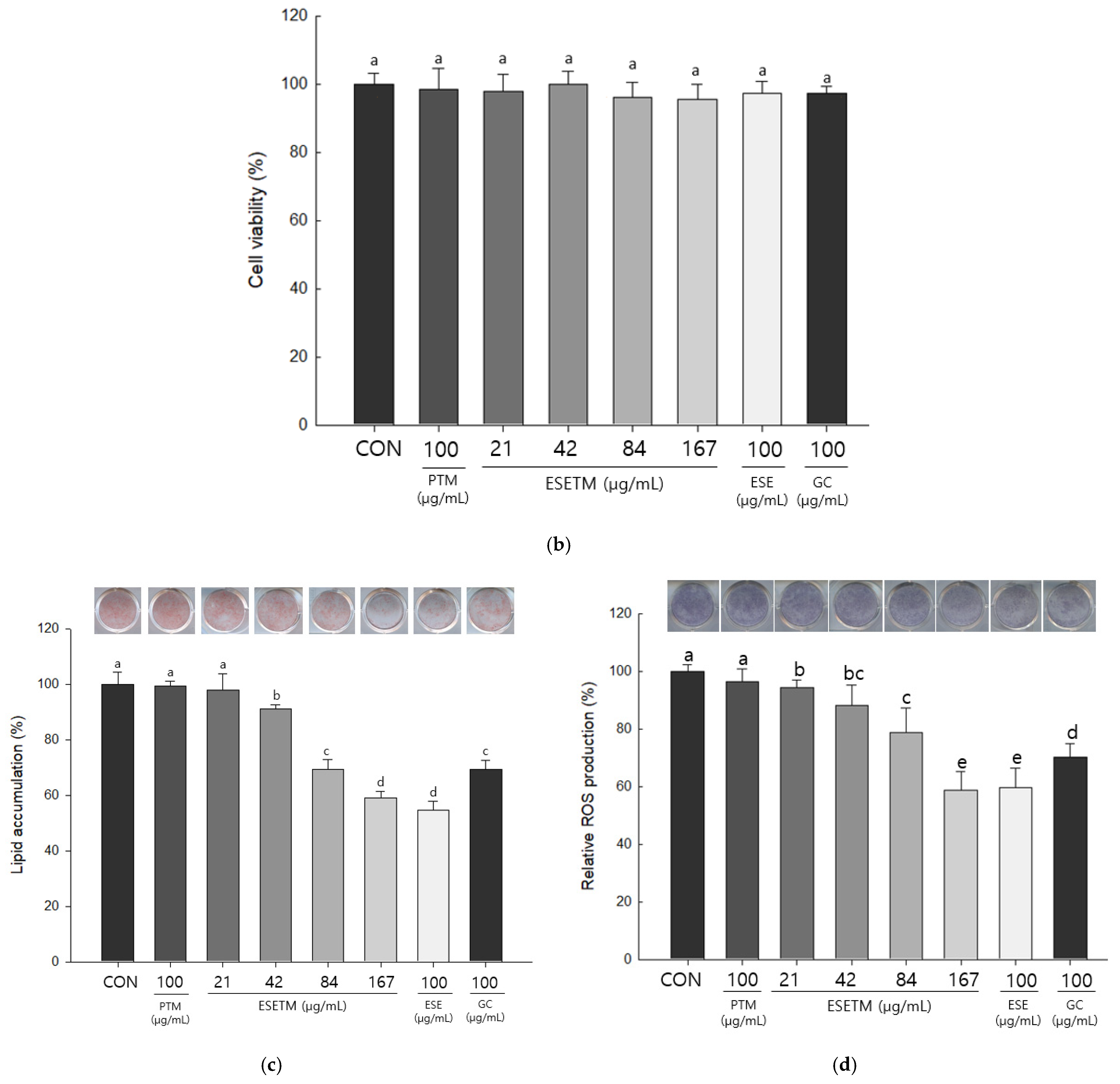
3.3. ESETM Inhibits Lipid Accumulation and ROS Production
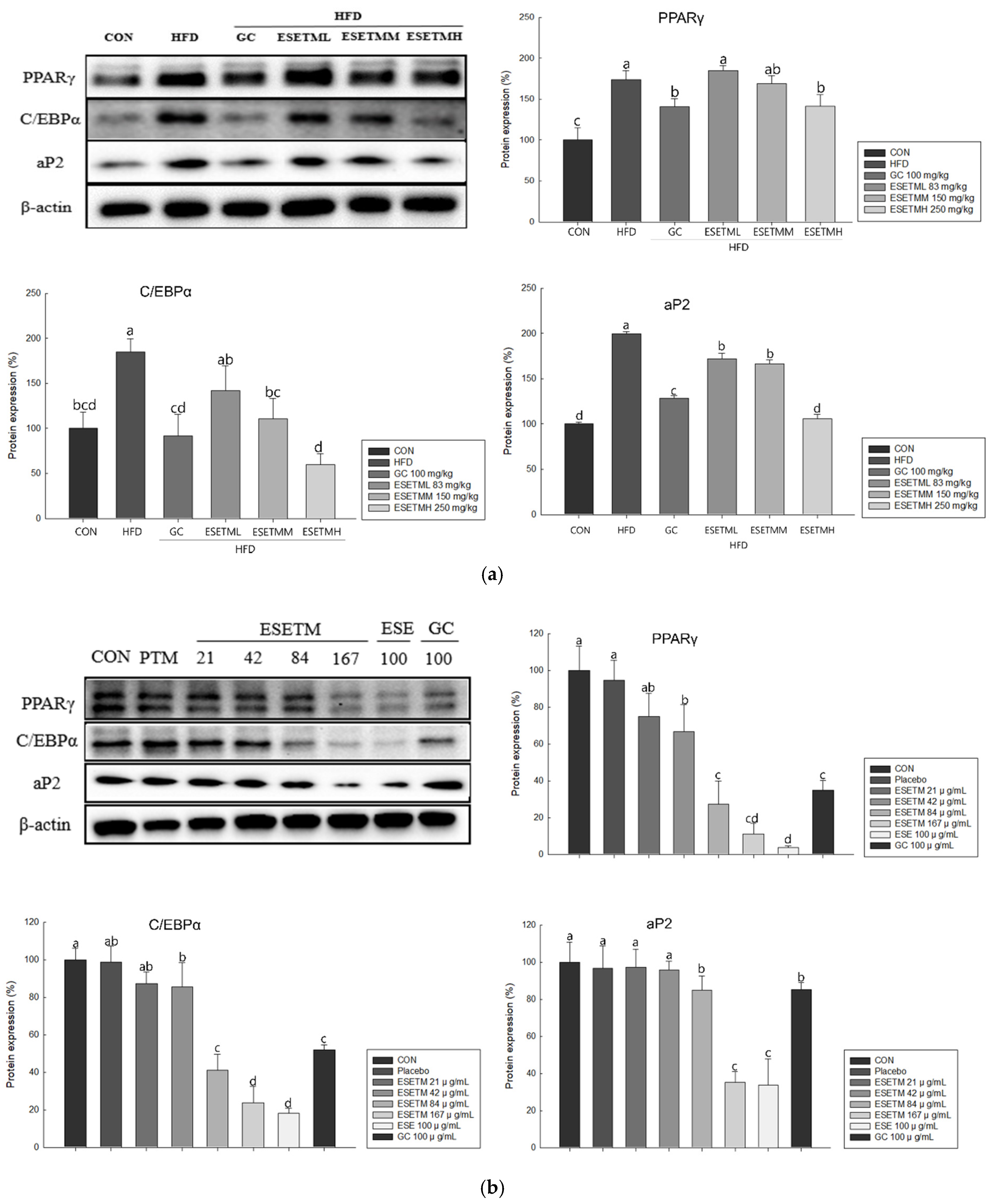
3.4. ESETM Reduces Adipogenesis in the WAT of HFD-Fed Obese Mice and Adipocytes
3.5. ESETM Reduces Lipogenesis in HFD-Fed Obese Mice and Adipocytes
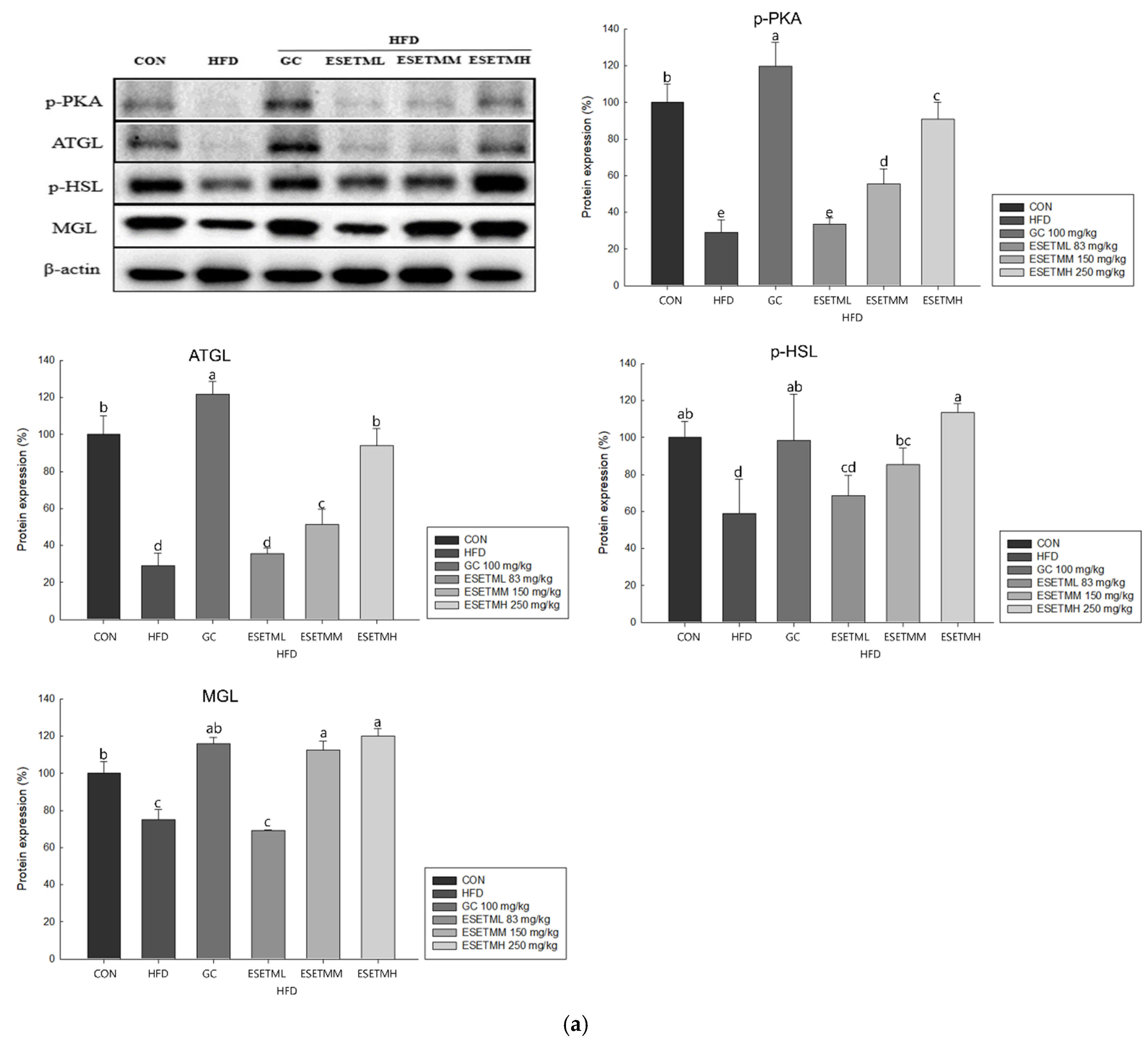
3.6. ESETM Stimulates Lipolysis in HFD-Fed Obese Mice and Adipocytes
3.7. ESETM Promotes Thermogenesis Browning in HFD-Fed Obese Mice and Adipocytes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Spiegelman, B.M.; Flier, J.S. Obesity and the regulation of energy balance. Cell 2001, 104, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Ballinger, A.; Peikin, S.R. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002, 440, 109–117. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Tschop, M.H.; Wilding, J.P. Anti-obesity drugs: Past, present and future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Cheung, B.M.Y.; Cheung, T.T.; Samaranayake, N.R. Safe of antiobesity drugs. Ther. Adv. Drug. Saf. 2013, 4, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.K.; Lee, W.S.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR -gamma in 3T3-L1 adipocytes and anti-proliferatory effect through AMPKalpha-COX-2 in cancer cells. J. Agric. Food Chem. 2009, 57, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef]
- de Sa, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional regulation of adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Gale, S.E.; Frolov, A.; Han, X.; Bickel, P.E.; Cao, L.; Bowcock, A.; Ory, D.S. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J. Biol. Chem. 2006, 281, 11082–11089. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Rui, B.B.; Tang, L.Y.; Hu, C.M. Lipin family proteins-key regulators in lipid metabolism. Ann. Nutr. Metab. 2015, 66, 10–18. [Google Scholar] [CrossRef]
- Koh, Y.K.; Lee, M.Y.; Kim, J.W.; Kim, M.; Moon, J.S.; Lee, Y.J.; Kim, K.S. Lipin1 is a key factor for the maturation and maintenance of adipocytes in the regulatory network with CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma 2. J. Biol. Chem. 2008, 283, 34896–34906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, J.M. Demonstrated and inferred metabolism associated with cytosolic lipid droplets. J. Lipid Res. 2009, 50, 2148–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechner, R.; Kienesberger, P.C.; Haemmerle, G.; Zimmermann, R.; Lass, A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 2009, 50, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Lee, J.H.; Han, J.S.; Kong, J.; Kim, J.B. Pka subunit balance plays a key role in lipolysis. FASEB J. 2017, 31, 770.15. [Google Scholar]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef]
- LO, K.A.; Sun, L. Turning WAT into BAT: A review on regulators controlling the browning of white adipocytes. Biosci. Rep. 2013, 33, e00065. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, H.R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-A y mice. Plant Foods Hum. Nutr. 2008, 63, 163. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, K.; Chei, S.; Oh, H.J.; Lee, K.P.; Lee, B.Y. Ecklonia stolonifera extract suppresses lipid accumulation by promoting lipolysis and adipose browning in high-fat diet-induced obese male mice. Cells 2020, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Oh, H.J.; Kim, J.; Lee, K.P.; Han, X.; Lee, O.H.; Lee, B.Y. Effects of Ecklonia stolonifera extract on the obesity and skeletal muscle regeneration in high-fat diet-fed mice. J. Funct. Foods 2021, 82, 104511. [Google Scholar] [CrossRef]
- Bang, C.Y.; Byun, J.H.; Choi, H.K.; Choi, J.S.; Choung, S.Y. Protective effects of Ecklonia stolonifera extract on ethanol-induced fatty liver in rats. Biomol. Ther. 2016, 24, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goorden, S.M.; Buffart, T.E.; Bakker, A.; Buijs, M.M. Liver disorders in adults: Alt and ast. Ned. Tijdschr. Voor Geneeskd. 2013, 157, A6443. [Google Scholar]
- Bolsoni-Lopes, A.; Alonso-Vale, M.I.C. Lipolysis and lipases in white adipose tissue–An update. Arch. Endocrinol. Metab. 2015, 59, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.A.; Roemmich, J.N.; Claycombe, K.J. Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. Nutr. Metab. 2016, 13, 24. [Google Scholar] [CrossRef] [Green Version]
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, D.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Periwal, V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Chou, Y.C.; Ho, C.T.; Pan, M.H. Immature Citrus reticulata extract promotes browning of beige adipocytes in high-fat diet-induced C57BL/6 mice. J. Agric. Food Chem. 2018, 66, 9697–9703. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 138, 1764–1785. [Google Scholar] [CrossRef]
- Hu, X.; Tao, N.; Wang, X.; Xiao, J.; Wang, M. Marine-derived bioactive compounds with anti-obesity effect: A review. J. Funct. Foods 2016, 21, 372–387. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Wan-Loy, C.; Siew-Moi, P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.P.; Baskaran, V. Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice. Algal Res. 2021, 54, 102187. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3t3-l1 adipocytes by downregulating c/ebpalpha and ppargamma. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.T.; Yoon, S.H. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J. Proteome Res. 2011, 10, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Clement, E.; Attane, C.; Muller, C.; Nieto, L. A new role for extracellular vesicles: How small vesicles can feed tumors’ big appetite. J. Lipid Res. 2018, 59, 1793–1804. [Google Scholar] [CrossRef] [Green Version]
- Houten, S.M.; Wanders, R.J. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.J.; Jin, H.; Lee, K.; Song, J.H.; Chei, S.; Oh, H.J.; Oh, J.H.; Lee, B.Y. Cardamonin suppresses lipogenesis by activating protein kinase a-mediated browning of 3t3-l1 cells. Phytomed. Int. J. Phytother. Phytopharm. 2019, 65, 153064. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
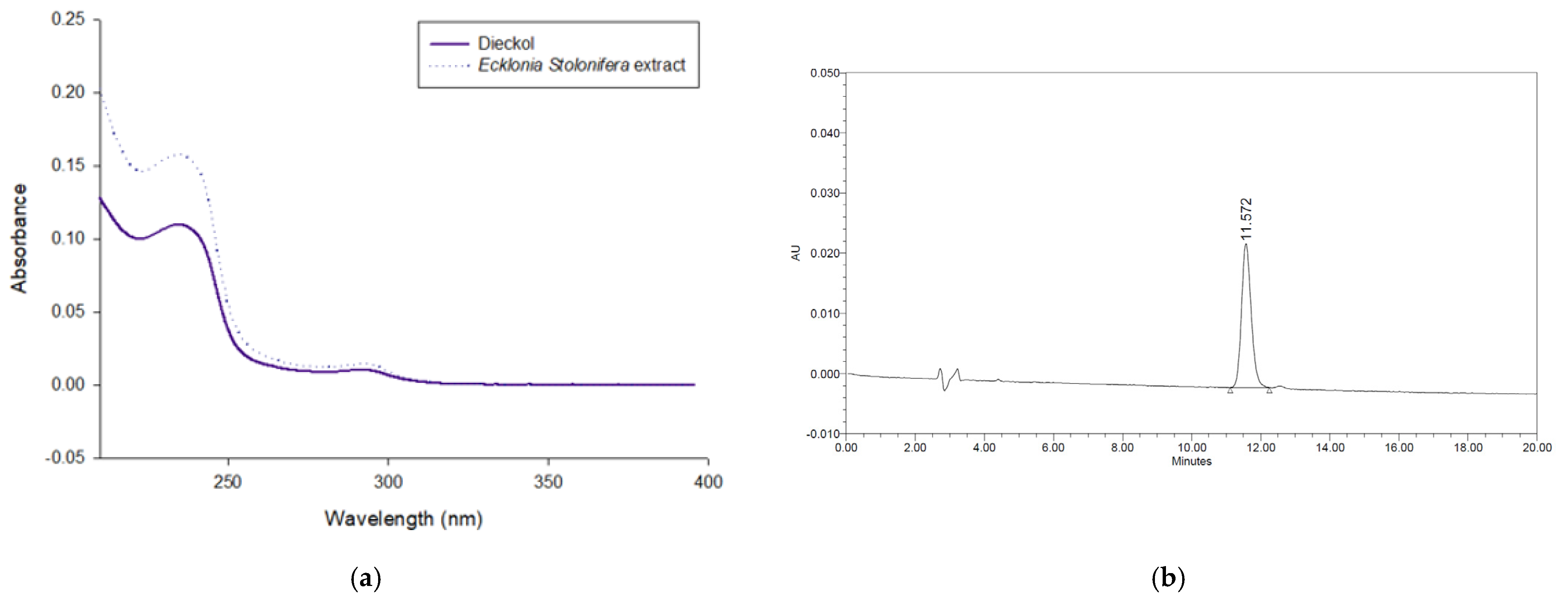





| Compound | Regression Equation | R2 | Linear Range (μg/mL) | RSD (%) (n = 3) | Contents of Dieckol in ESE (mg/g) | Contents of Dieckol in ESETM (mg/g) |
|---|---|---|---|---|---|---|
| Dieckol | y = 4628.6x − 1764.6 | 0.999 | 3.13–100 | 0.34–2.35 | 27.42 ± 0.66 | 18.65 ± 0.52 |
| CON | HFD | HFD + GC | HFD + ESETML | HFD + ESETMM | HFD + ESETMH | |
|---|---|---|---|---|---|---|
| WAT (mg) | 843.17 ± 86.08 e | 2867.67 ± 179.88 a | 1487.87 ± 134.10 d | 2332.87 ± 111.24 b | 1996.90 ± 211.67 c | 1563.60 ± 246.85 d |
| Liver (mg) | 1126.50 ± 63.01 e | 2229.33 ± 19.59 a | 1298.13 ± 9.97 d | 1732.47 ± 144.97 b | 1586.93 ± 97.93 c | 1292.70 ± 29.53 d |
| Kidney (mg) | 549.73 ± 1.16 a | 542.07 ± 71.05 a | 559.03 ± 17.76 a | 552.10 ± 38.06 a | 575.60 ± 33.08 a | 569.57 ± 21.29 a |
| Spleen (mg) | 123.50 ± 9.44 b | 151.70 ± 4.65 a | 122.80 ± 19.19 b | 121.60 ± 13.08 b | 125.43 ± 2.03 b | 123.80 ± 5.29 b |
| CON | HFD | HFD + GC | HFD + ESETML | HFD + ESETMM | HFD + ESETMH | |
|---|---|---|---|---|---|---|
| TG (mg/dL) | 55.33 ± 6.75 d | 137.90 ± 20.97 a | 74.92 ± 24.42 bc | 105.26 ± 16.57 b | 89.99 ± 10.07 b | 74.93 ± 8.92 bc |
| TC (mg/dL) | 157.73 ± 19.48 d | 211.67 ± 7.65 a | 191.10 ± 1.56 ab | 182.64 ± 18.51 b | 182.99 ± 12.16 b | 170.77 ± 9.49 bc |
| LDL-C (mg/dL) | 23.48 ± 3.10 b | 35.19 ± 1.78 a | 29.19 ± 1.53 ab | 29.87 ± 5.90 ab | 29.24 ± 4.07 ab | 25.34 ± 3.14 b |
| HDL-C (mg/dL) | 196.46 ± 2.42 a | 133.76 ± 11.94 c | 186.35 ± 10.78 ab | 179.29 ± 6.41 b | 180.56 ± 8.90 b | 184.45 ± 3.76 ab |
| AST (IU/L) | 75.52 ± 2.34 c | 118.49 ± 5.67 a | 78.50 ± 4.32 bcd | 89.58 ± 3.33 b | 83.99 ± 1.42 bc | 75.70 ± 6.31 b |
| ALT (IU/L) | 43.86 ± 2.75 c | 75.91 ± 15.39 a | 42.17 ± 7.41 c | 67.00 ± 6.43 ab | 59.86 ± 7.03 b | 54.73 ± 4.16 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Choi, S.-I.; Men, X.; Lee, S.-J.; Jin, H.; Oh, H.-J.; Kim, E.; Kim, J.; Lee, B.-Y.; Lee, O.-H. Anti-Obesity Activities of Standardized Ecklonia stolonifera Extract in 3T3-L1 Preadipocytes and High-Fat-Diet-Fed ICR Mice. Appl. Sci. 2022, 12, 5115. https://doi.org/10.3390/app12105115
Han X, Choi S-I, Men X, Lee S-J, Jin H, Oh H-J, Kim E, Kim J, Lee B-Y, Lee O-H. Anti-Obesity Activities of Standardized Ecklonia stolonifera Extract in 3T3-L1 Preadipocytes and High-Fat-Diet-Fed ICR Mice. Applied Sciences. 2022; 12(10):5115. https://doi.org/10.3390/app12105115
Chicago/Turabian StyleHan, Xionggao, Sun-Il Choi, Xiao Men, Se-Jeong Lee, Heegu Jin, Hyun-Ji Oh, Eunjin Kim, Jongwook Kim, Boo-Yong Lee, and Ok-Hwan Lee. 2022. "Anti-Obesity Activities of Standardized Ecklonia stolonifera Extract in 3T3-L1 Preadipocytes and High-Fat-Diet-Fed ICR Mice" Applied Sciences 12, no. 10: 5115. https://doi.org/10.3390/app12105115
APA StyleHan, X., Choi, S.-I., Men, X., Lee, S.-J., Jin, H., Oh, H.-J., Kim, E., Kim, J., Lee, B.-Y., & Lee, O.-H. (2022). Anti-Obesity Activities of Standardized Ecklonia stolonifera Extract in 3T3-L1 Preadipocytes and High-Fat-Diet-Fed ICR Mice. Applied Sciences, 12(10), 5115. https://doi.org/10.3390/app12105115







