Comparative Analysis of Cooling Methods for Dynamic Infrared Thermography (DIRT)-Based Skin Cancer Diagnosis
Abstract
:1. Introduction
1.1. Skin Cancer
1.2. Skin Cancer and Infrared Thermography
1.3. Skin-Mimicking Phantoms
1.4. Previous Research on Skin-Cooling Techniques for Dynamic Infrared Thermography
2. Materials and Methods
2.1. Skin-Cooling Techniques
2.1.1. Evaporative Cooling
2.1.2. Conductive Cooling
2.1.3. Convection Cooling
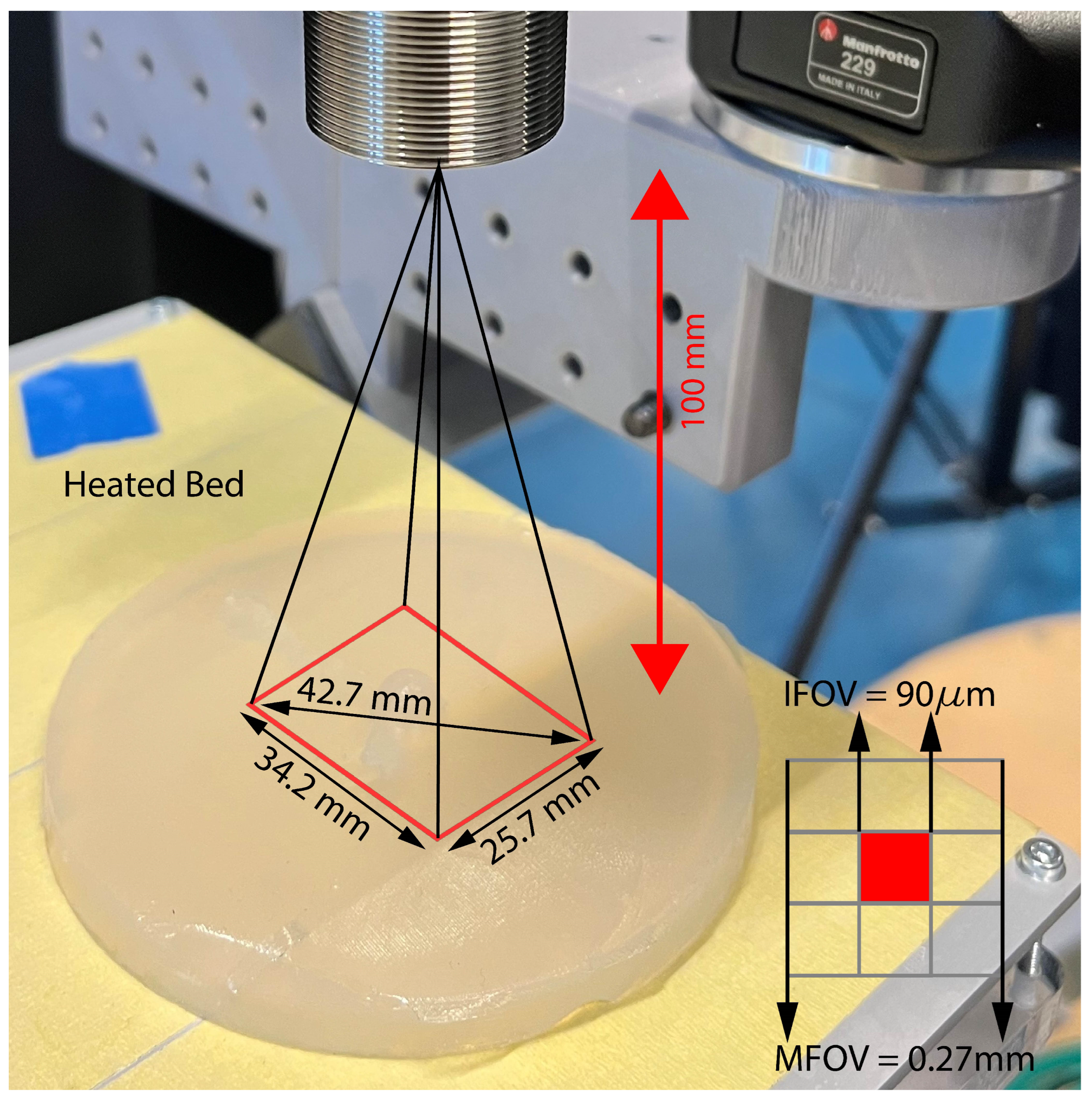
2.2. Experimental Setup
2.2.1. Infrared Camera
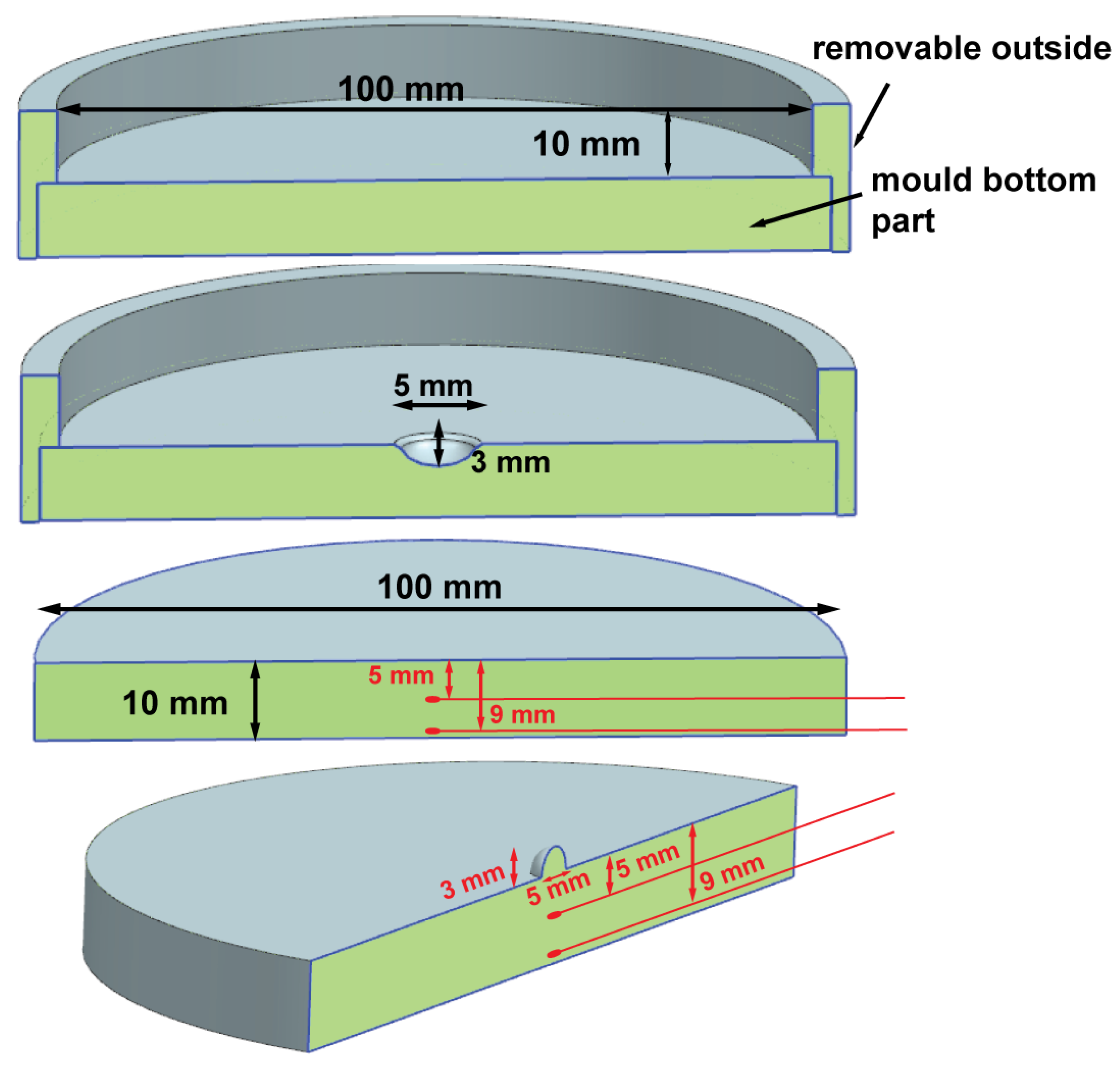
2.2.2. Skin Phantom
2.3. Measurement Protocol
2.4. Image Sequence
2.5. Decision Matrix
3. Results
3.1. Agar Skin Phantom
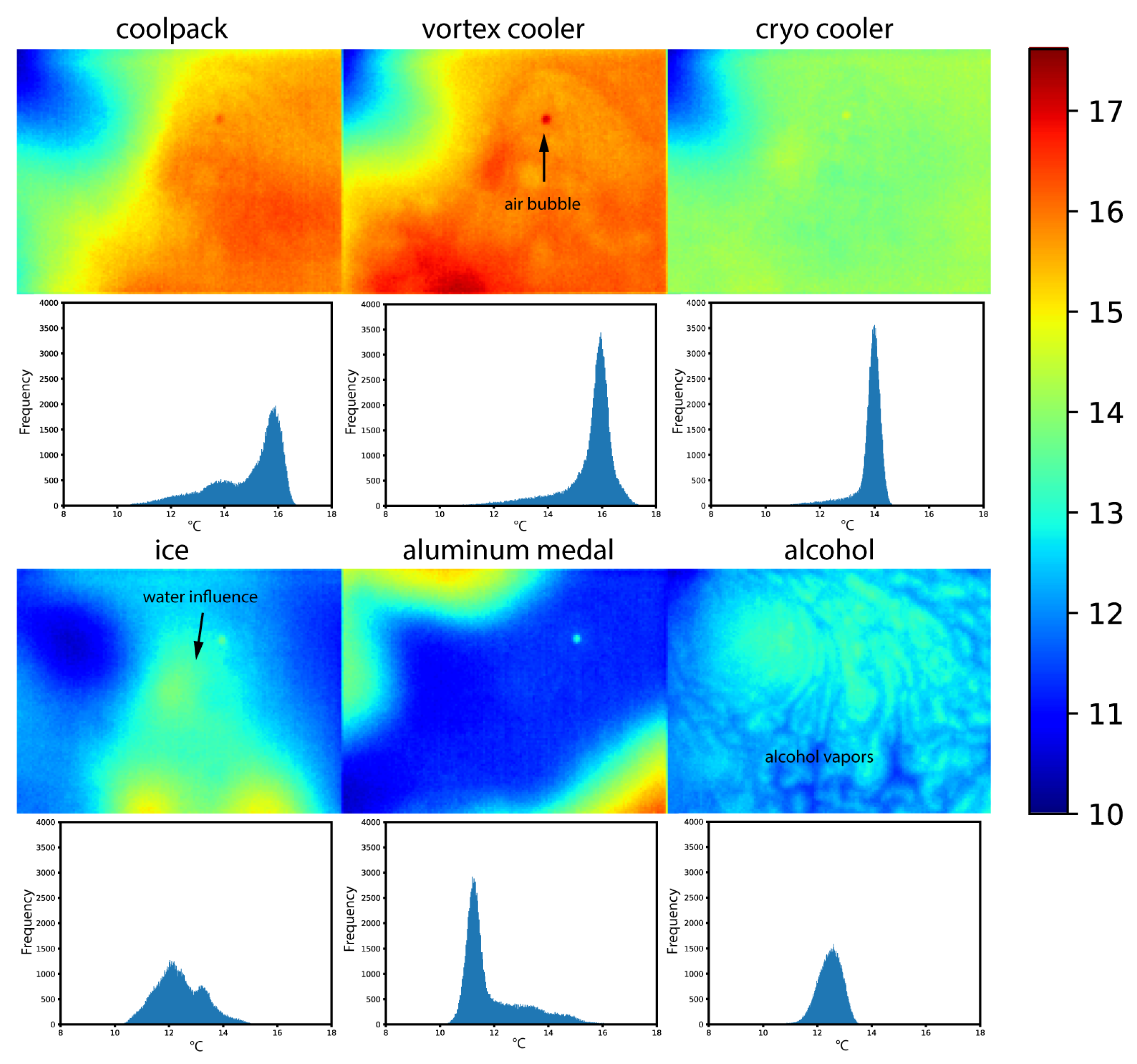
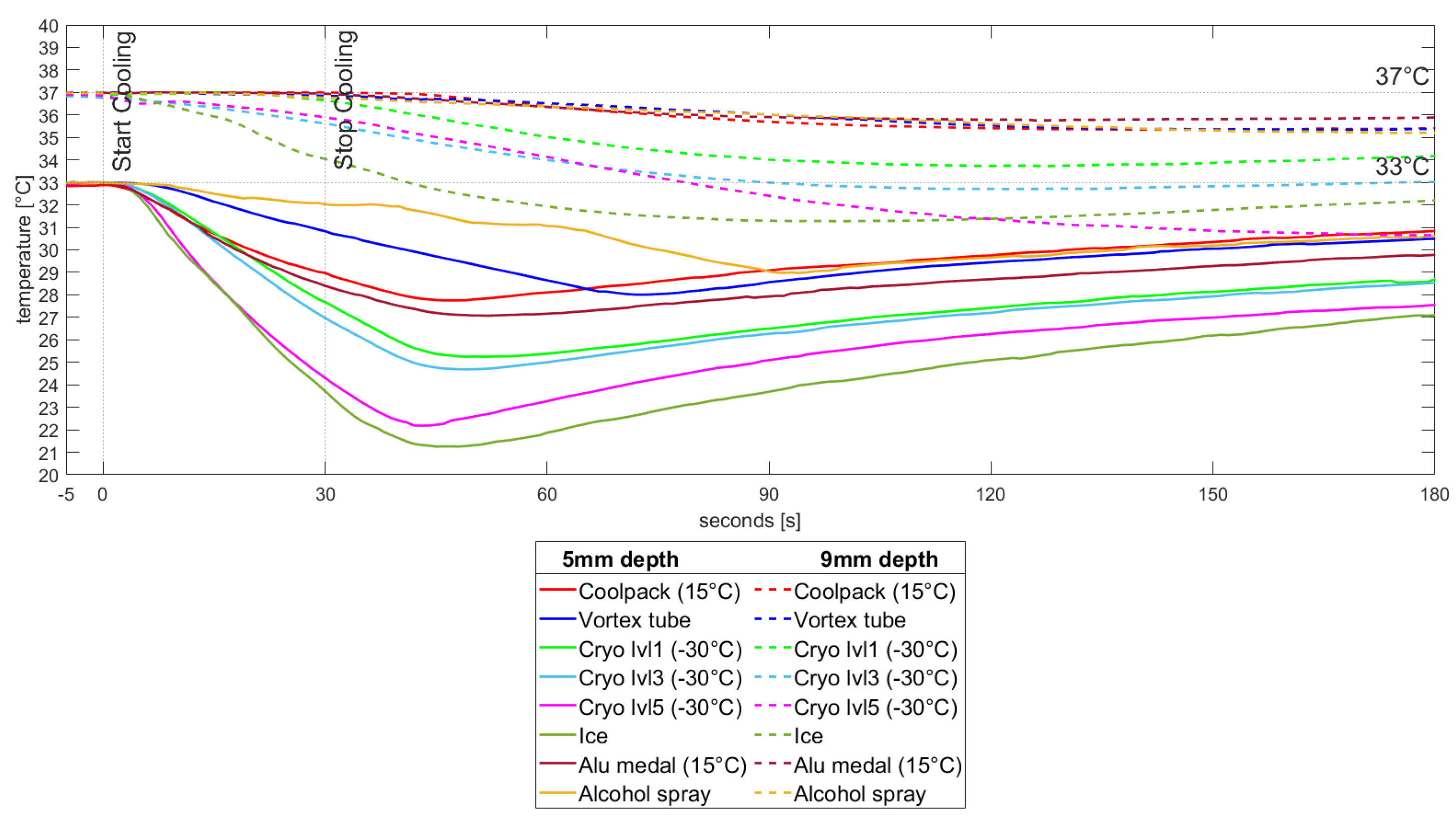
3.2. Temperature Measurements
3.3. Decision Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBSE | Total body skin examination |
| DIRT | Dynamic infrared thermography |
| CT | Computed Tomography |
| PET | Positron emission tomography |
| IRT | Infrared Thermography |
| NETD | Noise Equivalent Temperature Difference |
| NUC | Non-uniformity correction |
| IFOV | Instantaneous field of view |
| MFOV | Measurement field of view |
| RTD | Resistance Temperature Detector |
References
- World Health Organization. World Cancer Report: Cancer Research for Cancer Development; IARC: Lyon, France, 2020.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, N.; Waldmann, A.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Non-Melanoma Skin Cancer Incidence and Impact of Skin Cancer Screening on Incidence. J. Investig. Dermatol. 2014, 134, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Verstockt, J.; Verspeek, S.; Thiessen, F.; Tjalma, W.A.; Brochez, L.; Steenackers, G. Skin Cancer Detection Using Infrared Thermography: Measurement Setup, Procedure and Equipment. Sensors 2022, 22, 3327. [Google Scholar] [CrossRef]
- Johnson, M.M.; Leachman, S.A.; Aspinwall, L.G.; Cranmer, L.D.; Curiel-Lewandrowski, C.; Sondak, V.K.; Stemwedel, C.E.; Swetter, S.M.; Vetto, J.; Bowles, T.; et al. Skin cancer screening: Recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017, 4, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Nachbar, F.; Stolz, W.; Merkle, T.; Cognetta, A.B.; Vogt, T.; Landthaler, M.; Bilek, P.; Braun-Falco, O.; Plewig, G. The ABCD rule of dermatoscopy: High prospective value in the diagnosis of doubtful melanocytic skin lesions. J. Am. Acad. Dermatol. 1994, 30, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Brunssen, A.; Waldmann, A.; Eisemann, N.; Katalinic, A. Impact of skin cancer screening and secondary prevention campaigns on skin cancer incidence and mortality: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 129–139.e10. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.P.; Oliviero, M.; Kolm, I.; French, L.E.; Marghoob, A.A.; Rabinovitz, H. Dermoscopy: What’s new? Clin. Dermatol. 2009, 27, 26–34. [Google Scholar] [CrossRef]
- Rey-Barroso, L.; Peña-Gutiérrez, S.; Yáñez, C.; Burgos-Fernández, F.J.; Vilaseca, M.; Royo, S. Optical Technologies for the Improvement of Skin Cancer Diagnosis: A Review. Sensors 2021, 21, 252. [Google Scholar] [CrossRef]
- Kirimtat, A.; Krejcar, O.; Selamat, A. A Mini-review of Biomedical Infrared Thermography (B-IRT). In Bioinformatics and Biomedical Engineering; Lecture Notes in Computer Science; Rojas, I., Valenzuela, O., Rojas, F., Ortuño, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 11466, pp. 99–110. [Google Scholar] [CrossRef]
- Qi, H.; Diakides, N. Infrared Imaging in Medicine; Technical Report; University of Tennessee: Knoxville, TN, USA, 2007. [Google Scholar]
- Flores-Sahagun, J.H.; Vargas, J.V.C.; Mulinari-Brenner, F.A. Analysis and diagnosis of basal cell carcinoma (BCC) via infrared imaging. Infrared Phys. Technol. 2011, 54, 367–378. [Google Scholar] [CrossRef]
- Buzug, T.M.; Schumann, S.; Pfaffmann, L.; Reinhold, U.; Ruhlmann, J. Functional infrared imaging for skin-cancer screening. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology—Proceedings, New York, NY, USA, 30 August–3 September 2006; pp. 2766–2769. [Google Scholar] [CrossRef]
- Jiang, L.J.; Ng, E.Y.K.; Yeo, A.C.B.; Wu, S.; Pan, F.; Yau, W.Y.; Chen, J.H.; Yang, Y. A perspective on medical infrared imaging. J. Med. Eng. Technol. 2005, 29, 257–267. [Google Scholar] [CrossRef]
- Moustafa, A.M.N.; Muhammed, H.H.; Hassan, M. Skin Cancer Detection Using Temperature Variation Analysis. Engineering 2013, 5, 18–21. [Google Scholar] [CrossRef]
- Chen, H.; Wang, K.; Du, Z.; Liu, W.; Liu, Z. Predicting the thermophysical properties of skin tumor based on the surface temperature and deep learning. Int. J. Heat Mass Transf. 2021, 180, 121804. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Rotaru, G.M.; Derler, S.; Spano, F.; Camenzind, M.; Annaheim, S.; Stämpfli, R.; Schmid, M.; Rossi, R.M. Materials used to simulate physical properties of human skin. Skin Res. Technol. 2016, 22, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Patterson, M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef]
- Dabbagh, A.; Abdullah, B.J.J.; Ramasindarum, C.; Abu Kasim, N.H. Tissue-Mimicking Gel Phantoms for Thermal Therapy Studies. Ultrason. Imaging 2014, 36, 291–316. [Google Scholar] [CrossRef]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2009, 7, 229–258. [Google Scholar] [CrossRef]
- Ohmae, E.; Yoshizawa, N.; Yoshimoto, K.; Hayashi, M.; Wada, H.; Mimura, T.; Suzuki, H.; Homma, S.; Suzuki, N.; Ogura, H.; et al. Stable tissue-simulating phantoms with various water and lipid contents for diffuse optical spectroscopy. Biomed. Opt. Express 2018, 9, 5792–5808. [Google Scholar] [CrossRef]
- Lamouche, G.; Kennedy, B.F.; Kennedy, K.M.; Bisaillon, C.E.; Curatolo, A.; Campbell, G.; Pazos, V.; Sampson, D.D. Review of tissue simulating phantoms with controllable optical, mechanical and structural properties for use in optical coherence tomography. Biomed. Opt. Express 2012, 3, 1381–1398. [Google Scholar] [CrossRef]
- Nordstrom, R.J. Phantoms as standards in optical measurements. In Proceedings of the Optical Diagnostics and Sensing XI: Toward Point-of-Care Diagnostics; and Design and Performance Validation of Phantoms Used in Conjunction with Optical Measurement of Tissue III, SPIE, San Francisco, CA, USA, 26 January 2011; Volume 7906, pp. 75–79. [Google Scholar] [CrossRef]
- Çetingül, M.P.; Herman, C. A heat transfer model of skin tissue for the detection of lesions: Sensitivity analysis. Phys. Med. Biol. 2010, 55, 5933–5951. [Google Scholar] [CrossRef]
- Koehler, M.J.; Vogel, T.; Elsner, P.; König, K.; Bückle, R.; Kaatz, M. In vivo measurement of the human epidermal thickness in different localizations by multiphoton laser tomography. Skin Res. Technol. 2010, 16, 259–264. [Google Scholar] [CrossRef]
- Birgersson, U.; Birgersson, E.; Nicander, I.; Ollmar, S. A methodology for extracting the electrical properties of human skin. Physiol. Meas. 2013, 34, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Krackowizer, P.; Brenner, E. Thickness of the human skin. Phlebologie 2008, 37, 83–92. [Google Scholar] [CrossRef]
- Sontheimer, R.D. Skin Is Not the Largest Organ. J. Investig. Dermatol. 2014, 134, 581–582. [Google Scholar] [CrossRef] [PubMed]
- Venus, M.; Waterman, J.; McNab, I. Basic physiology of the skin. Surgery 2010, 28, 469–472. [Google Scholar] [CrossRef]
- Yoo, H.; Hu, Y.; Kim, E. Effects of Heat and Moisture Transport in Fabrics and Garments Determined with a Vertical Plate Sweating Skin Model. Text. Res. J. 2000, 70, 542–549. [Google Scholar] [CrossRef]
- Wang, F.; del Ferraro, S.; Lin, L.Y.; Sotto Mayor, T.; Molinaro, V.; Ribeiro, M.; Gao, C.; Kuklane, K.; Holmér, I. Localised boundary air layer and clothing evaporative resistances for individual body segments. Ergonomics 2012, 55, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Dynamic Moisture Vapor Transfer Through Textiles: Part III: Effect of Film Characteristics on Microclimate Moisture and Temperature Changes. Text. Res. J. 1999, 69, 193–202. [Google Scholar] [CrossRef]
- Huang, J.; Holt, R.G.; Cleveland, R.O.; Roy, R.A. Experimental validation of a tractable numerical model for focused ultrasound heating in flow-through tissue phantoms. J. Acoust. Soc. Am. 2004, 116, 2451–2458. [Google Scholar] [CrossRef]
- Ambastha, S.; Pant, S.; Umesh, S.; Vazhayil, V.; Asokan, S. Feasibility Study on Thermography of Embedded Tumor Using Fiber Bragg Grating Thermal Sensor. IEEE Sensors J. 2020, 20, 2452–2459. [Google Scholar] [CrossRef]
- Çetingül, M.P.; Herman, C. Using Dynamic Infrared Imaging to Detect Melanoma: Experiments on a Tissue-Mimicking Phantom. In Volume 2: Biomedical and Biotechnology Engineering; ASMEDC: Vancouver, BC, Canada, 2010; pp. 139–147. [Google Scholar] [CrossRef]
- Kumari, C.; Kumar, A.; Sarangi, S.K.; Thirugnanam, A. An experimental and numerical study for cutaneous cryotherapy. Heat Mass Transf. 2021, 57, 147–163. [Google Scholar] [CrossRef]
- Cho, J.; Prasad, B.; Kim, J.K. Near-infrared laser irradiation of a multilayer agar-gel tissue phantom to induce thermal effect of traditional moxibustion. J. Innov. Opt. Health Sci. 2018, 11, 1850033. [Google Scholar] [CrossRef]
- Hou, S. Photo-Thermally Enhanced Temperature Gradient Gel Electrophoresis for DNA Separation. Ph.D. Thesis, Northeastern University, Boston, MA, USA, 2018. [Google Scholar]
- Zenzie, H.; Altshuler, G.; Smirnov, M.; Anderson, R. Evaluation of cooling methods for laser dermatology. Lasers Surg. Med. 2000, 26, 130–144. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Herman, C. Analysis of skin cooling for quantitative dynamic infrared imaging of near-surface lesions. Int. J. Therm. Sci. 2014, 86, 175–188. [Google Scholar] [CrossRef]
- Strąkowska, M.; Strzelecki, M.; Kaszuba, A. Novel methodology of medical screening using IR thermography. In Proceedings of the 2014 Signal Processing: Algorithms, Architectures, Arrangements, and Applications (SPA), Poznan, Poland, 22–24 September 2014; pp. 172–175. [Google Scholar]
- Buzug, T.; Schumann, S.; Pfaffmann, L.; Reinhold, U.; Ruhlmann, J. Skin-Tumour Classification with Functional Infrared Imaging. In Proceedings of the 8th IASTED International Conference on Signal and Image Processing, SIP 2006, Atlanta, GA, USA, 8–11 October 2006. [Google Scholar]
- Santa Cruz, G.A.; Bertotti, J.; Marín, J.; González, S.J.; Gossio, S.; Alvarez, D.; Roth, B.M.C.; Menéndez, P.; Pereira, M.D.; Albero, M.; et al. Dynamic infrared imaging of cutaneous melanoma and normal skin in patients treated with BNCT. Appl. Radiat. Isot. 2009, 67, S54–S58. [Google Scholar] [CrossRef] [PubMed]
- Çetingül, M.P.; Alani, R.M.; Herman, C. Quantitative Evaluation of Skin Lesions Using Transient Thermal Imaging. In Proceedings of the 2010 14th International Heat Transfer Conference, Washington, DC, USA, 8–13 August 2010; ASMEDC: Washington, DC, USA, 2010; Volume 1, pp. 31–39. [Google Scholar] [CrossRef]
- Xue, Y.; Arjomandi, M.; Kelso, R. A critical review of temperature separation in a vortex tube. Exp. Therm. Fluid Sci. 2010, 34, 1367–1374. [Google Scholar] [CrossRef]
- Godoy, S.E.; Hayat, M.M.; Ramirez, D.A.; Myers, S.A.; Padilla, R.S.; Krishna, S. Detection theory for accurate and non-invasive skin cancer diagnosis using dynamic thermal imaging. Biomed. Opt. Express 2017, 8, 2301–2323. [Google Scholar] [CrossRef]
- Godoy, S.E.; Ramirez, D.A.; Myers, S.A.; Von Winckel, G.; Krishna, S.; Berwick, M.; Padilla, R.S.; Sen, P.; Krishna, S. Dynamic infrared imaging for skin cancer screening. Infrared Phys. Technol. 2015, 70, 147–152. [Google Scholar] [CrossRef]
- Magalhaes, C.; Vardasca, R.; Rebelo, M.; Valenca-Filipe, R.; Ribeiro, M.; Mendes, J. Distinguishing melanocytic nevi from melanomas using static and dynamic infrared thermal imaging. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1700–1705. [Google Scholar] [CrossRef]
- Gomboc, T.; Iljaž, J.; Wrobel, L.C.; Hriberšek, M.; Marn, J. Design of constant temperature cooling device for melanoma screening by dynamic thermography. Eng. Anal. Bound. Elem. 2021, 125, 66–79. [Google Scholar] [CrossRef]
- Bonmarin, M.; Gal, F.A.L. A lock-in thermal imaging setup for dermatological applications. Skin Res. Technol. 2015, 21, 284–290. [Google Scholar] [CrossRef]
- Bonmarin, M.; Le Gal, F.A. Chapter 31—Thermal Imaging in Dermatology. In Imaging in Dermatology; Hamblin, M.R., Avci, P., Gupta, G.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 437–454. [Google Scholar] [CrossRef]
- Burkes, S.A.; Patel, M.; Adams, D.M.; Hammill, A.M.; Eaton, K.P.; Randall Wickett, R.; Visscher, M.O. Infantile hemangioma status by dynamic infrared thermography: A preliminary study. Int. J. Dermatol. 2016, 55, e522–e532. [Google Scholar] [CrossRef] [PubMed]
- Cryo 6 Brochure—Information about the Zimmer Skin Cooling Chiller. Available online: https://zimmerusa.com/products/cryo-therapy/cryo-6/cryo-6-brochure/ (accessed on 28 May 2023).
- Armisén, R.; Gaiatas, F. Agar. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 82–107. [Google Scholar] [CrossRef]
- In ’t Hout, F.E.M.; Haydu, L.E.; Murali, R.; Bonenkamp, J.J.; Thompson, J.F.; Scolyer, R.A. Prognostic Importance of the Extent of Ulceration in Patients With Clinically Localized Cutaneous Melanoma. Ann. Surg. 2012, 255, 1165. [Google Scholar] [CrossRef] [PubMed]
- Grande Sarpa, H.; Reinke, K.; Shaikh, L.; Leong, S.P.L.; Miller, J.R.I.; Sagebiel, R.W.; Kashani-Sabet, M. Prognostic Significance of Extent of Ulceration in Primary Cutaneous Melanoma. Am. J. Surg. Pathol. 2006, 30, 1396. [Google Scholar] [CrossRef] [PubMed]
- Ullman, D.G. The Mechanical Design Process, 4th ed.; McGraw-Hill Series in Mechanical Engineering; McGraw-Hill Higher Education: Boston, MA, USA, 2010. [Google Scholar]
- Tague, N.R. The Quality Toolbox, 2nd ed.; ASQ Quality Press: Milwaukee, WI, USA, 2005. [Google Scholar]
- Figueiredo, A.A.A.; do Nascimento, J.G.; Malheiros, F.C.; da Silva Ignacio, L.H.; Fernandes, H.C.; Guimaraes, G. Breast tumor localization using skin surface temperatures from a 2D anatomic model without knowledge of the thermophysical properties. Comput. Methods Programs Biomed. 2019, 172, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, M.; Gershenson, J. Dynamic Vascular Imaging Using Active Breast Thermography. Sensors 2023, 23, 3012. [Google Scholar] [CrossRef]
- Sadeghi, M.; Boese, A.; Maldonado, I.; Friebe, M.; Sauerhering, J.; Schlosser, S.; Wehberg, H.; Wehberg, K. Feasibility test of Dynamic Cooling for detection of small tumors in IR thermographic breast imaging. Curr. Dir. Biomed. Eng. 2019, 5, 397–399. [Google Scholar] [CrossRef]

| Heat Transfer Type | Cooling Method | Temperature |
|---|---|---|
| Conductive cooling | ||
| aluminum medal (50 × 20 mm) | 15 °C | |
| cooled gel pack | 15 °C | |
| Evaporative cooling | ||
| alcohol spray | / | |
| Convective cooling | ||
| Ranque–Hilsch vortex tube | 5 °C | |
| Zimmer Cryo 6 cooler | −30 °C |
| Conductive Cooling | Evaporative Cooling | Convective Cooling | ||||||
|---|---|---|---|---|---|---|---|---|
| Criteria | Rating Scale | Coefficient | Aluminum Medal | Gel Pack | Ice | Alcohol Spray | Vortex Tube | Zimmer Cryo 6 |
| Obstructing view of camera during/after cooling | 1 = high 2 = moderate 3 = low | 8 | 2 | 2 | 1 | 1 | 3 | 3 |
| Noise exposure | 1 = high 2 = moderate 3 = low | 3 | 3 | 3 | 3 | 3 | 1 | 2 |
| consumables/additional equipment (e.g., pressured air (air compressor), ice (ice cube maker), alcohol, fridge) | 1 = high 2 = moderate 3 = low | 2 | 1 | 1 | 1 | 1 | 1 | 3 |
| Use in clinical/hospital setting (e.g., sterilizable/disinfectable) | 1 = low 2 = moderate 3 = high | 4 | 2 | 2 | 1 | 1 | 3 | 3 |
| Repeatability (e.g., constant temperature, pressure, flow…) | 1 = low 2 = moderate 3 = high | 9 | 2 | 2 | 2 | 1 | 2 | 3 |
| Price | 1 = high 2 = moderate 3 = low | 1 | 3 | 3 | 3 | 3 | 2 | 1 |
| Workload per patient (e.g., disinfecting, number of actions, application difficulty, measurement time…) | 1 = high 2 = moderate 3 = low | 6 | 1 | 1 | 1 | 1 | 2 | 3 |
| Patient comfort (e.g., applicable on whole body, discomfort, duration…) | 1 = low 2 = moderate 3 = high | 5 | 2 | 2 | 2 | 1 | 3 | 3 |
| Uniform cooling | 1 = low 2 = moderate 3 = high | 10 | 2 | 1 | 1 | 1 | 3 | 3 |
| Cooling efficiency | 1 = low 2 = moderate 3 = high | 7 | 2 | 2 | 3 | 1 | 2 | 3 |
| Total: | 20 | 19 | 18 | 14 | 22 | 27 | ||
| Total weighted: | 106 | 96 | 91 | 63 | 132 | 160 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verstockt, J.; Thiessen, F.E.F.; Hoorens, I.; Brochez, L.; Steenackers, G. Comparative Analysis of Cooling Methods for Dynamic Infrared Thermography (DIRT)-Based Skin Cancer Diagnosis. Appl. Sci. 2023, 13, 10105. https://doi.org/10.3390/app131810105
Verstockt J, Thiessen FEF, Hoorens I, Brochez L, Steenackers G. Comparative Analysis of Cooling Methods for Dynamic Infrared Thermography (DIRT)-Based Skin Cancer Diagnosis. Applied Sciences. 2023; 13(18):10105. https://doi.org/10.3390/app131810105
Chicago/Turabian StyleVerstockt, Jan, Filip E. F. Thiessen, Isabelle Hoorens, Lieve Brochez, and Gunther Steenackers. 2023. "Comparative Analysis of Cooling Methods for Dynamic Infrared Thermography (DIRT)-Based Skin Cancer Diagnosis" Applied Sciences 13, no. 18: 10105. https://doi.org/10.3390/app131810105
APA StyleVerstockt, J., Thiessen, F. E. F., Hoorens, I., Brochez, L., & Steenackers, G. (2023). Comparative Analysis of Cooling Methods for Dynamic Infrared Thermography (DIRT)-Based Skin Cancer Diagnosis. Applied Sciences, 13(18), 10105. https://doi.org/10.3390/app131810105







