Abstract
Novel tetragonal matrix Ba0.5−xLn0.5NaxF2.5−x with x = 0.08, doped by Yb3+, Ho3+, Er3+, was synthesized by molten salt synthesis (MSS) from nitrate flux. XRD data show that the tetragonal phase with a = 4.122(1) Å, c = 17.672(1) Å is stable in an argon atmosphere up to 960 °C. Luminescence spectra recorded in 500–900 nm and 1050–1700 nm upon 974 nm pumping demonstrated the characteristic luminescence at 1550 nm (4I13/2 → 4I15/2) for Er3+ and 1150 nm (5I6 → 5I8) for Ho3+. The relative thermal sensitivity (Sr) at 296–316 K were 0.3%×K−1 and 5.5%×K−1 in shortwave infrared (SWIR) and visible range, respectively. Synthesized luminophores can be used as dual-range optical temperature sensors, which simultaneously operate in visible and SWIR ranges.
1. Introduction
Luminescent thermometry is an effective way to display the temperature in the analyzed objects. The main advantage of luminescent thermometry is the possibility of contactless temperature measurement [1,2,3,4,5]. This approach is efficiently applied in medicine, biology, detecting catalytic reactions, and other areas, where contact measurement is inconvenient or inapplicable [6,7,8,9].
Luminescent nanoprobes are widely used quantum dots [10], organic dyes [11], polymers [12], DNA or protein-conjugated systems [13], transition metal-based materials, or materials doped with rare-earth ions [14]. The latter has many advantages, such as high photostability and narrow luminescence peaks with large shifts between excitation and emission. Moreover, these materials are also capable of converting near-infrared (NIR) excitation to emission in the ultraviolet (UV), visible, or NIR range via a multiphoton excitation process known as upconversion. This feature makes upconverting materials interesting for biological applications where NIR excitation offers several advantages, such as higher penetration depth in biological tissue, negligible autofluorescence, and lower photodamage in comparison to UV.
Temperature measuring using rare-earth (Ln) ions luminescence [15] is based on two main approaches. The first approach is based on the measurement of luminescence intensity ratio (LIR) from two different energy levels that are thermally coupled [16,17]. The energy gap between the thermally coupled levels is so small that, according to Boltzmann’s law, an increase in temperature can lead to an increase in the population of the overlying state. In the luminescence spectrum, an increase in the shorter-wavelength peak will be observed.
The second approach was proposed by Sekiyama et al. [18] and is based on temperature-dependent non-resonant energy transfer. In this case, a matrix is doped with three rare-earth ions [19,20]. One of them (usually Yb3+) acts as a donor, absorbs exciting radiation, and transfers it to two other acceptor ions. Acceptor ions are selected in such way that one of the energy transfers is resonant, its probability does not depend on temperature and this signal is used as a reference. The second transfer is non-resonant and with increasing temperature, its probability increases (phonon-assisted energy transfer). This approach was realized for Yb3+-Er3+-Ho3+-doped NaYF4 nanoparticles and allows thermometry in the SWIR region.
Pumping was carried out into the absorption band Yb3+ (7F7/2 → 5F5/2), followed by energy redistribution between Ho3+ and Er3+ ions as a result of non-radiative energy transfer. In this case, the energy transfer of Yb3+ → Ho3+ is phonon assisted, as a result of which the luminescence intensity of Ho3+ ions at the transition 5I6→5I8 will depend on temperature, and the energy transfer of Yb3+ → Er3+ is resonant, as a result of which the luminescence of Er3+ ions at the transition 4I13/2→ 4I15/2 depends on temperature weakly [21]. This makes it possible to measure the relative coefficient of temperature sensitivity by the ratio of the integral intensities of the luminescence bands. The obtained thermal sensitivity was in the range of 1.34–1.87%·°C in the physiological temperature range.
To date, the active search for new thermal sensors continues. The main tasks are to expand the range of operating temperatures and the range of excitation and emission wavelengths, as well as to increase thermal sensitivity. The sensitivity between 0.5 and 1.5% K–1 at around 300 K is typically for Er3+ [22]. In the paper [23], it was proposed to use silica coating to expand the thermometry range to 900 °C; the relative sensitivity was from 1.02% K–1 at 300 K to 0.13% K–1 at 900 °C. The use of Nd3+ ions allows a shift of the operating wavelength range into NIR and obtains a relative sensitivity of 1.1% K−1 for NaYF4:0.1%Nd3+ [24]. For Nd3+/Tm3+/Yb3+/Gd3+ four-doped NaYF4 nanoparticles the relative sensitivity was significantly higher and reached 2.89% K−1 in the temperature range of 333–553 K [25].
Thus, using thermally coupled levels, taking into account resonant and non-resonant energy transfer channels from the sensitizer, it is possible to expand the range of wavelengths in which thermometry can be performed, as well as increase the thermal sensitivity.
Fluoride matrices such as NaYF4 [26], NaGdF4 [27], and MF2, where M = Ca, Sr, Ba [28], have proven to be effective photonics materials [29]. It is known that an increase in matrix molecular weight leads to a decrease in multi-phonon relaxation and usually increases the luminescence energy yield [30,31]. In this connection, the purpose of the study is to search for new “heavy” matrices based on fluorides and recognize their spectral and luminescent characteristics for luminescent nanothermometers. As part of the study, a method was developed for the synthesis of a new “heavy” matrix Ba0.5−xLn0.5NaxF2.5−x (x = 0.08) and phosphors based on it for testing as a dual-band luminescent thermometer.
2. Materials and Methods
2.1. Synthesis of Luminophores
We synthesized the samples by crystallization from a solution in a sodium nitrate melt according to the method [32]. Sodium fluoride (Reagent grade, Chimmed) was used as a fluorinating agent, and sodium nitrate (Reagent grade, Chimmed) was used as a solvent. The reagents were used without additional purification steps. The weights of the initial reagents are given in Table S1.
The synthesis is described by the following reaction equation:
(1 − x)Ba(NO3)2 + xLn(NO3)3 × nH2O + (2 + x)NaF → Ba1−xLnxF2+x↓ + (2 + x)NaNO3 + nxH2O↑.
The samples of barium nitrate (specialty grade 10-2, Reachem) and nitrate hydrates of rare earth elements (99.99% purity in terms of cation impurity, Lanhit) were homogenized in an agate mortar for 10 min and then added to the samples of fluoride and sodium nitrate. After homogenization, the mixture was transferred to a porcelain glazed crucible and annealed at a temperature of 500 °C for 1 h with a heating rate of 10 °C per minute. After natural cooling to room temperature, the sinter was removed from the crucible and washed three times with double-distilled water in a polypropylene reactor until a negative reaction of a solution of diphenylamine in sulfuric acid to nitrate anions. The samples were dried under an IR lamp at a temperature of 40–60 °C for 6 h.
2.2. Characterization of Luminophores
The samples were characterized by X-ray phase analysis (XRD) (Bruker, D8 Advance with CuKa irradiation, Karlsruhe, Germany), scanning electron microscopy (SEM) (Carl Zeiss, Nvision 40, Oberkochen, Germany), energy dispersion analysis (EDX) (Oxford Instruments, X-Max 80 mm2, Abingdon, UK), differential scanning calorimetry (DSC) (Netzsch, STA 449 F3 Jupiter, Selb, Germany). All samples were analyzed as prepared.
2.3. Temperature-Dependent Luminescence Spectra Measurements
Luminescent spectroscopy of the samples was performed on an installation consisting of a laser with a wavelength of 974 nm, a spectrometer (StellarNet, DWARF-Star) in the SWIR range of 1000–1700 nm, a spectrometer (BIOSPEC, LESA-01-BIOSPEC) in the visible range of 300–900 nm, and a thermal infrared camera (CEDIP, JADE MWIR SC7300M) for temperature measurements in the range from 24 to 44 °C [21]. Heating was carried out using a Primelab PL-R-basic H heated magnetic stirrer (Primelab, Mytischci, Russia) with 0.1 °C temperature heating sampling. Luminescence intensity ratio (LIR) was used to quantify the dependence of luminescence spectra on temperature in the SWIR range:
where I is the integral luminescence intensity calculated as the area under the luminescence peak in the corresponding wavelength range. For SWIR the area was calculated under the peak in the 1100–1250 nm range for Ho3+ 5I6 → 5I8 transition and the 1450–1650 nm range for Er3+ 4I13/2 → 4I15/2 transition. In visible range we use luminescence in green range, 520–570 nm range, which corresponds to Er3+ 2H11/2, 4S3/2 → 4I15/2, and in red range, 625–675 nm, which probably includes both the Er3+ and Ho3+ luminescence, transitions 4F9/2 → 4I15/2 and 2F5 → 5I8, correspondingly.
LIR = IHo/IEr
The relative coefficient of temperature sensitivity (Sr) was calculated using the following formula:
3. Results
In the course of our work, we synthesized samples in the BaF2-GdF3 system doped with Yb, Ho, and Er. The nominal composition and composition determined according to the EDX data of the samples obtained, as well as the practical yield of the synthesis reaction are shown in Table 1. EDX results show that the crystal lattice of synthesized samples contains sodium in an amount of up to 8 mol. %, regardless of the content of the rare earth element. Apparently, this value corresponds to solubility limit of sodium in this solid solution. The content of rare earth elements was overestimated compared to the initial charge, and the content of barium was underestimated.

Table 1.
The nominal composition of the samples, the composition according to EDX data, and the practical yield of the sample synthesis reaction.
Figure 1 shows the X-ray diffraction patterns of prepared samples.
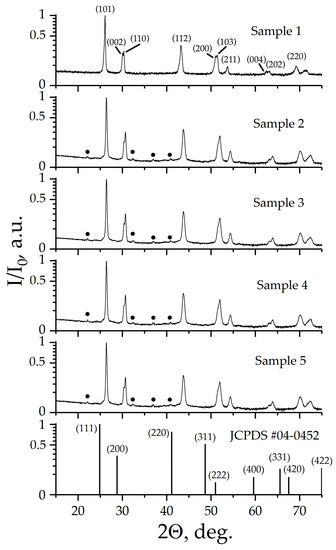
Figure 1.
XRD patterns of prepared samples. •—superstructural reflections.
According to X-ray diffraction patterns, Samples 1–5 are single phase, and have a structure derived from the fluorite structure in which BaF2 crystallizes. The X-ray pattern of Sample 1 is characterized by a fine splitting of the main X-ray diffraction reflexes and is indicated in the tetragonal crystallographic system in a space symmetry group (SSG) I4. X-ray pattern of Samples 2–5 also indexed in SSG I4. It is worth noting that X-ray diffraction patterns of Samples 2–5 contain superstructural peaks, which are well described when the crystal lattice parameter “c” is increased three times. The Supplementary Materials, Tables S2–S6, show the results of indexing of X-ray patterns. For all samples, the estimated values of the coherent scattering regions D were more than 100 nm. The lattice parameters of Samples 1–5 and the calculated X-ray density (Dx) values are given in Table 2.

Table 2.
Crystal lattice parameters and sample density.
SEM micrographs were obtained for all samples (Figure 2).
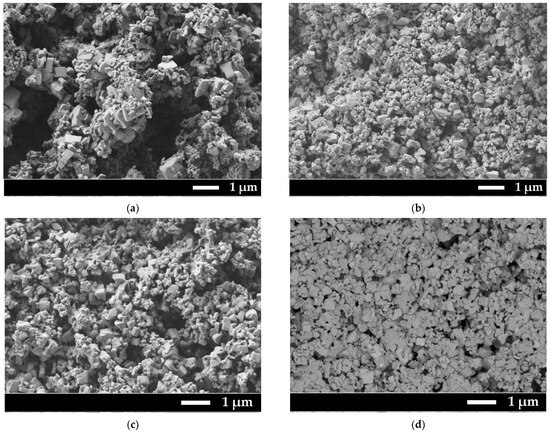
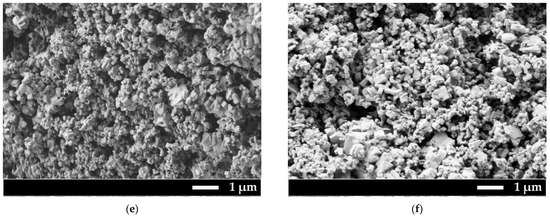
Figure 2.
SEM microphotography of prepared samples. (a) Sample 1; (b) Sample 2; (c) Sample 3; (d) Sample 3 (detector of backscattered electrons was used for elemental contrast); (e) Sample 4; (f) Sample 5.
All powders have submicron sizes with a bimodal particle size distribution. The first maximum is about 200 nm, and the second is about 700 nm. Histograms of particle size distribution can be found in Supplementary Materials, Figure S1. Detection of backscattered electrons showed that the particles in Sample 3 are homogeneous in composition, there is no contrast in microphotographs. It has to be mentioned that corrosion of the glazed porcelain crucible and additional phases in SEM microphotographs were not observed. The presence of aluminum or silicon as the main elements of the crucible was not detected in EDX analysis. Buchinskaya et al. [33] also showed that there is no interaction between the sodium nitrate melt and the porcelain glazed crucible.
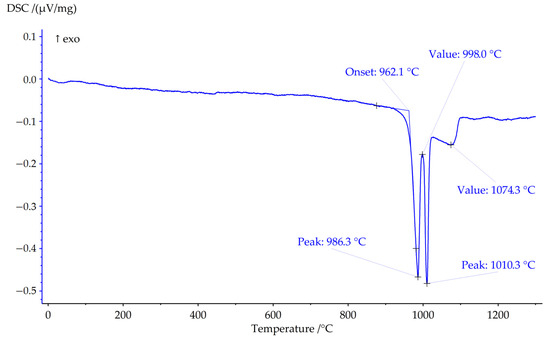
Figure 3.
DSC curve of Sample 3 in argon at heating rate 20 K×min−1.
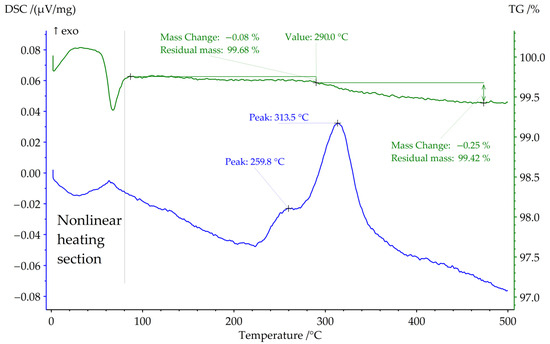
Figure 4.
DSC curve of Sample 3 in air at heating rate 20 K×min−1.
DSC analysis of Sample 3 in argon atmosphere was made up to 1300 °C (Figure 3). Peaks with maximums at 986 °C and 1010 °C correspond to phase reactions, and the effect with a maximum at 1074 °C corresponds to the end of melting of the sample (liquidus point). DSC data show that Sample 3 is stable in the flow of argon until 962.5 °C.
Thermal stability in air also was investigated (Figure 4). Sample 3 starts to lose weight at approximately 290 °C. The endothermal peak around 80 °C may be attributed to dehydration of adsorbed water. There are two exothermal peaks that could be attributed to the chemical reaction of pyrohydrolysis [34]. However, the X-ray pattern of Sample 3 did not change after this DSC experiment.
Figure 5 shows the luminescence spectra in the visible range for Sample 5 at different temperatures (Figure 5a) and in the SWIR range for Samples 3 and 5 at a temperature of 296 K (Figure 5b).
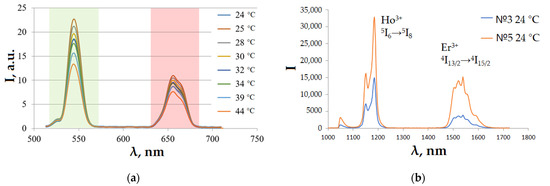
Figure 5.
Luminescence spectra (a) Sample № 5, visible range; (b) Sample №3 and 5, SWIR range.
The peak in the SWIR region at 1150 nm wavelength corresponds to Ho3+ (5I6 → 5I8), and the peak at 1550 nm corresponds to Er3+ (4I13/2 → 4I15/2).
The temperature dependences of the ratio of the intensities of the red and green luminescence bands for the visible range of the spectrum are shown in Figure 6a, and the intensity ratios of the erbium and holmium luminescence bands for the SWIR range are shown in Figure 6b.
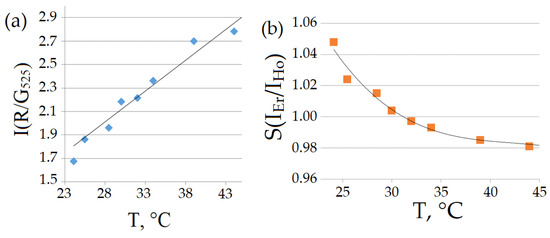
Figure 6.
Temperature dependences of the ratio of the integral intensities of the luminescence bands (LIR) for Sample 5. (a) The wavelengths are 625–675 nm and 520–570 nm. (b) The wavelengths are 1450–1650 nm and 1100–1250 nm.
From the dependences presented in Figure 6, the relative coefficients of temperature sensitivity in the temperature range from 24 to 44 °C are determined: Sr = 5.55%×K−1 in the visible range and Sr = 0.34%×K−1 in the SWIR range.
4. Discussion
According to the XRD data, the synthesized samples crystallize in a tetragonal crystallographic system with the spatial symmetry group I4. Comparing the data obtained with the well-known phase diagram of the BaF2-GdF3 system, as presented in Figure 7, makes it obvious that the samples prepared by us correspond to a high-temperature T phase having a similar composition [35]. This phase with a fluorite-derived structure is characterized by a tetragonal cell, SSG I4, with the parameter “c” increased three times. The cause of tetragonal distortion is the ordered arrangement of clusters of defects in a fluorite-type lattice. The synthesis of the phase of such a structure at a low synthesis temperature of 500 °C is apparently explained by the entry of sodium into the lattice of synthesized samples, leading to the stabilization of the high-temperature phase.
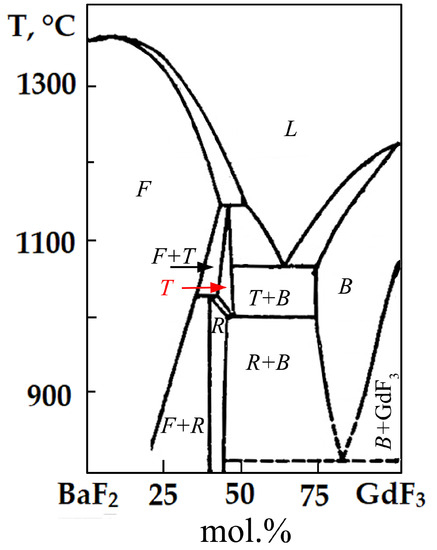
Figure 7.
Phase diagram of BaF2-GdF3 system. Phase designations: F—fluorite-type solid solution Ba1−xGdxF2+x; B—tysonite-type solid solution Gd1−yBayF3−y; R—trigonal fluorite-derivated phase on the base of Ba4Gd3F17 compound; T—tetragonal fluorite-derivated phase; L—liquid.
As the data in Figure 6 show, the luminescent thermometer synthesized by us can be used to detect temperature in both visible and SWIR ranges. At the same time, the sensitivity in the visible range is high and significantly higher than in the IR range. However, the obtained characteristic of our phosphor is consistent with the analogs described in the literature (Table 3).

Table 3.
Comparison of sensitivity of various luminescent IR thermometers in SWIR range.
5. Conclusions
The synthesis of a heavy matrix based on barium gadolinium fluoride with a density of more than 6 g/cm3 was developed as a result of this study. The entry of sodium into the composition of the samples was recorded using the EDX method. The presence of sodium stabilizes the high-temperature phase of the tetragonal crystallographic system with the general formula Ba0.5−xLn0.5NaxF2.5−x.
The relative coefficients of temperature sensitivity (Sr) in the temperature range 296–316 K are equal to Sr = 5.55%×K−1 in the visible range and Sr = 0.34%×K−1 in the SWIR range. The samples are not hygroscopic and are stable under air atmosphere up to 200 °C. Thus, a novel dual-range luminescent thermometer of the composition Ba0.5Gd0.45Na0.05F2.45: Yb, Ho, Er has been developed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13189999/s1, Figure S1: histogram of particle size distribution; Table S1: weights of initial reagents; Table S2: XRD data of Sample 1; Table S3: XRD data of Sample 2; Table S4: XRD data of Sample 3; Table S5: XRD data of Sample 4; Table S6: XRD data of Sample 5.
Author Contributions
Conceptualization, A.A.A., V.K.I. and P.P.F.; methodology, A.A.A., M.V.T. and D.V.P.; validation, S.V.K., V.K.I. and P.P.F.; investigation, A.A.A., L.A.P., D.V.P. and I.D.R.; resources, S.V.K.; writing—original draft preparation, A.A.A.; writing—review and editing, P.P.F.; visualization, A.A.A. and L.A.P., supervision, V.K.I.; project administration, P.P.F. and M.V.T.; funding acquisition, V.K.I. and P.P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant no. 22-13-00167. https://rscf.ru/project/22-13-00167/ (accessed on 1 September 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carlos, L.D.; Palacio, F. (Eds.) Thermometry at the Nanoscale: Techniques and Selected Applications; The Royal Society of Chemistry: Cambrige, UK, 2015; ISBN 978-1-84973-904-7. [Google Scholar]
- Chen, W.; Cao, J.; Hu, F.; Wei, R.; Chen, L.; Guo, H. Sr2GdF7:Tm3+/Yb3+ Glass Ceramic: A Highly Sensitive Optical Thermometer Based on FIR Technique. J. Alloys Compd. 2018, 735, 2544–2550. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, D.; Peng, Y.; Lu, Y.; Chen, X.; Li, X.; Ji, Z. A Review on Nanostructured Glass Ceramics for Promising Application in Optical Thermometry. J. Alloys Compd. 2018, 763, 34–48. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, Q.; Zhang, P.; Dong, Y.; Fang, F.; Jiang, J. Mechanistic Investigations on the Dramatic Thermally Induced Luminescence Enhancement in Upconversion Nanocrystals. J. Phys. Chem. C 2018, 122, 26142–26152. [Google Scholar] [CrossRef]
- Dramićanin, M.D. Trends in Luminescence Thermometry. J. Appl. Phys. 2020, 128, 040902. [Google Scholar] [CrossRef]
- Thimsen, E.; Sadtler, B.; Berezin, M.Y. Shortwave-Infrared (SWIR) Emitters for Biological Imaging: A Review of Challenges and Opportunities. Nanophotonics 2017, 6, 1043–1054. [Google Scholar] [CrossRef]
- Kamimura, M.; Matsumoto, T.; Umezawa, M.; Soga, K. Ratiometric Near-Infrared Fluorescence Nanothermometry in the OTN-NIR (NIR II/III) Biological Window Based on Rare-Earth Doped β-NaYF4 Nanoparticles. J. Mater. Chem. B 2017, 5, 1917–1925. [Google Scholar] [CrossRef]
- Geitenbeek, R.G.; Nieuwelink, A.-E.; Jacobs, T.S.; Salzmann, B.B.V.; Goetze, J.; Meijerink, A.; Weckhuysen, B.M. In Situ Luminescence Thermometry to Locally Measure Temperature Gradients during Catalytic Reactions. ACS Catal. 2018, 8, 2397–2401. [Google Scholar] [CrossRef]
- Meng, M.; Zhang, R.; Fa, X.; Yang, J.; Cheng, Z.; Qiao, X.; Ou, J.; Wurth, C.; Resch-Genger, U. Core–Shell NaYF4:Yb3+/Tm 3+@NaGdF4: Ce3+/Eu3+ Nanoparticles for Upconversion and Downconversion Dual-Mode Fluorescence-Based Temperature Sensing. ACS Appl. Nano Mater. 2022, 5, 9266–9276. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Y.; Wang, J.; Song, Z.; Shi, H.; Han, R.; Sha, Y.; Jiang, Y. Intracellular Temperature Sensing: An Ultra-Bright Luminescent Nanothermometer with Non-Sensitivity to PH and Ionic Strength. Sci. Rep. 2015, 5, 14879. [Google Scholar] [CrossRef]
- Yang, G.; Liu, X.; Feng, J.; Li, S.; Li, Y. Organic Dye Thermometry. In Thermometry at the Nanoscale: Techniques and Selected Applications; RSC Nanoscience & Nanotechnology; The Royal Society of Chemistry: Cambrige, UK, 2015; pp. 167–189. [Google Scholar]
- Zarrintaj, P.; Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Mozafari, M.; Sheiko, S.S.; Vatankhah-Varnoosfaderani, M.; Gutiérrez, T.J.; Saeb, M.R. Thermo-Sensitive Polymers in Medicine: A Review. Eur. Polym. J. 2019, 117, 402–423. [Google Scholar] [CrossRef]
- Donner, J.S.; Thompson, S.A.; Kreuzer, M.P.; Baffou, G.; Quidant, R. Mapping Intracellular Temperature Using Green Fluorescent Protein. Nano Lett. 2012, 12, 2107–2111. [Google Scholar] [CrossRef]
- Martins, J.C.; Skripka, A.; Brites, C.D.S.; Benayas, A.; Ferreira, R.A.S.; Vetrone, F.; Carlos, L.D. Upconverting Nanoparticles as Primary Thermometers and Power Sensors. Front. Photonics 2022, 3, 1037473. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Bu, Y.; Liu, C.-S.; Liu, T.; Yan, X. Optical Temperature Sensing of Rare-Earth Ion Doped Phosphors. RSC Adv. 2015, 5, 86219–86236. [Google Scholar] [CrossRef]
- Li, X.; Guo, H.; Wei, Y.; Guo, Y.; Lu, H.; Noh, H.M.; Jeong, J.H. Enhanced Up-Conversion in Er3+-Doped Transparent Glass-Ceramics Containing NaYbF4 Nanocrystals. J. Lumin. 2014, 152, 168–171. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Kuznetsov, S.V.; Konyushkin, V.A.; Nakladov, A.N.; Fedorov, P.P.; Carlos, L.D. Simultaneous Measurement of the Emission Quantum Yield and Local Temperature: The Illustrative Example of SrF2:Yb 3+/Er 3+ Single Crystals. Eur. J. Inorg. Chem. 2020, 2020, 1555–1561. [Google Scholar] [CrossRef]
- Sekiyama, S.; Umezawa, M.; Kuraoka, S.; Ube, T.; Kamimura, M.; Soga, K. Temperature Sensing of Deep Abdominal Region in Mice by Using Over-1000 Nm Near-Infrared Luminescence of Rare-Earth-Doped NaYF4 Nanothermometer. Sci. Rep. 2018, 8, 16979. [Google Scholar] [CrossRef]
- Runowski, M.; Goderski, S.; Przybylska, D.; Grzyb, T.; Lis, S.; Martín, I.R. Sr2LuF7:Yb3+–Ho3+–Er3+ Upconverting Nanoparticles as Luminescent Thermometers in the First, Second, and Third Biological Windows. ACS Appl. Nano Mater. 2020, 3, 6406–6415. [Google Scholar] [CrossRef]
- Runowski, M.; Bartkowiak, A.; Majewska, M.; Martín, I.R.; Lis, S. Upconverting Lanthanide Doped Fluoride NaLuF4:Yb3+-Er3+-Ho3+-Optical Sensor for Multi-Range Fluorescence Intensity Ratio (FIR) Thermometry in Visible and NIR Regions. J. Lumin. 2018, 201, 104–109. [Google Scholar] [CrossRef]
- Pominova, D.; Proydakova, V.; Romanishkin, I.; Ryabova, A.; Kuznetsov, S.; Uvarov, O.; Fedorov, P.; Loschenov, V. Temperature Sensing in the Short-Wave Infrared Spectral Region Using Core-Shell NaGdF4:Yb3+, Ho3+, Er3+@NaYF4 Nanothermometers. Nanomaterials 2020, 10, 1992. [Google Scholar] [CrossRef]
- Debasu, M.L.; Ananias, D.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Rocha, J.; Carlos, L.D. All-In-One Optical Heater-Thermometer Nanoplatform Operative From 300 to 2000 K Based on Er3+ Emission and Blackbody Radiation. Adv. Mater. 2013, 25, 4868–4874. [Google Scholar] [CrossRef]
- Geitenbeek, R.G.; Prins, P.T.; Albrecht, W.; Van Blaaderen, A.; Weckhuysen, B.M.; Meijerink, A. NaYF4:Er3+,Yb3+/SiO2 Core/Shell Upconverting Nanocrystals for Luminescence Thermometry up to 900 K. J. Phys. Chem. C 2017, 121, 3503–3510. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, K.; Marciniak, L. The Role of Nd3+ Concentration in the Modulation of the Thermometric Performance of Stokes/Anti-Stokes Luminescence Thermometer in NaYF4:Nd3+. Sci. Rep. 2023, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, J.; Jin, X.; Peng, Y.; Luo, J. A High-Sensitivity Optical Thermometer Based on Nd3+/Tm3+/Yb3+/Gd3+ Four-Doped NaYF4 Nanomaterials. J. Lumin. 2022, 246, 118807. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Mayakova, M.N.; Alexandrov, A.A.; Voronov, V.V.; Pominova, D.V.; Chernova, E.V.; Ivanov, V.K. Synthesis of NaYF4:Yb, Er up-Conversion Luminophore from Nitrate Flux. Nanosyst. Phys. Chem. Math. 2020, 11, 417–423. [Google Scholar] [CrossRef]
- Maurya, S.K.; Kushawaha, R.; Tiwari, S.P.; Kumar, A.; Kumar, K.; Da Silva, J.C.G.E. Thermal Decomposition Mediated Er3+/Yb3+ Codoped NaGdF4 Upconversion Phosphor for Optical Thermometry. Mater. Res. Express 2019, 6, 086211. [Google Scholar] [CrossRef]
- Alexandrov, A.A.; Mayakova, M.N.; Voronov, V.V.; Pominova, D.V.; Kuznetsov, S.V.; Baranchikov, A.E.; Ivanov, V.K.; Lysakova, E.I.; Fedorov, P.P. Synthesis of Upconversion Luminophores Based on Calcium Fluoride. Kondens. Sredy Mezhfaznye Granitsy Condens. Matter Interphases 2020, 22, 3–10. [Google Scholar] [CrossRef]
- Kuznetsov, S.V.; Alexandrov, A.A.; Fedorov, P.P. Optical Fluoride Nanoceramics. Inorg. Mater. 2021, 57, 555–578. [Google Scholar] [CrossRef]
- Basiev, T.T.; Orlovskii, Y.V.; Pukhov, K.K.; Sigachev, V.B.; Doroshenko, M.E.; Vorob’ev, I.N. Multiphonon Relaxation Rates Measurements and Theoretical Calculations in the Frame of Non-Linear and Non-Coulomb Model of a Rare-Earth Ion-L&and Interaction. J. Lumin. 1996, 68, 241–253. [Google Scholar] [CrossRef]
- Orlovskii, Y.V.; Basiev, T.T.; Pukhov, K.K.; Doroshenko, M.E.; Badikov, V.V.; Badikov, D.V.; Alimov, O.K.; Polyachenkova, M.V.; Dmitruk, L.N.; Osiko, V.V.; et al. Mid-IR Transitions of Trivalent Neodymium in Low Phonon Laser Crystals. Opt. Mater. 2007, 29, 1115–1128. [Google Scholar] [CrossRef]
- Fedorov, P.; Mayakova, M.; Alexandrov, A.; Voronov, V.; Kuznetsov, S.; Baranchikov, A.; Ivanov, V. The Melt of Sodium Nitrate as a Medium for the Synthesis of Fluorides. Inorganics 2018, 6, 38. [Google Scholar] [CrossRef]
- Buchinskaya, I.I.; Ivchenko, A.V. Solubility of calcium and strontium fluorides in a sodium nitrate melt and choosing a crucible material for working with their solution melts. Condens. Matter Interphases 2023, 25, 14–19. [Google Scholar] [CrossRef]
- Yonezawa, S.; Jae-Ho, K.; Takashima, M. Pyrohydrolysis of Rare-Earth Trifluorides in Moist Air. Solid State Sci. 2002, 4, 1481–1485. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Tkachenko, N.L. Phase Diagrams of BaF2-(Y, Ln)F3 Systems. J. Less-Common Met. 1982, 85, 155–170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).