Total Phenolic and Flavonoid Contents, and Preliminary Antioxidant, Xanthine Oxidase Inhibitory and Antibacterial Activities of Fruits of Lapsi (Choerospondias axillaris Roxb.), an Underutilized Wild Fruit of Nepal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents, Solvents, and Standard Drugs
2.2. Bacterial Strains
2.3. Collection and Identification of Plant Material
2.4. Extraction of C. axillaris Fruits
2.5. Determination of C. axillaris Dried Fruit Extract Yield
2.6. Phytochemical Screening
2.7. Quantitative Analysis of Total Flavonoid Content
2.8. Quantitative Analysis of Total Phenolic Content
2.9. Measurement of Total Carbohydrate Content
2.10. Evaluation of Antioxidant Activity
2.11. Xanthine Oxidase Inhibitory Activity
2.11.1. Preparation of Xanthine, Xanthine Oxidase (XO), and Sample Solution
2.11.2. Measurement of Xanthine Oxidase Inhibition
2.12. Antibacterial Activity Screening of C. axillaris Dried Fruit Extracts
2.12.1. Preparation of Plant Extracts and Filter Paper Discs
2.12.2. Development of Muller Hinton Agar (MHA) and Bacterial Strains Sub-Culturing
2.12.3. Preparation of Bacterial Suspension/Inoculums
2.12.4. Measurement of Zone of Inhibition (ZOI) against Bacterial Strains
2.12.5. Calculation of MIC and MBC
2.13. Statistical Analysis
3. Results and Discussion
3.1. Measurement of Extractive Yield Value
3.2. Phytochemical Screening
3.3. Quantitative Analysis of Total Phenol Content
3.4. Quantitative Analysis Total Flavonoid Content
3.5. Quantitative Analysis Total Carbohydrate Content
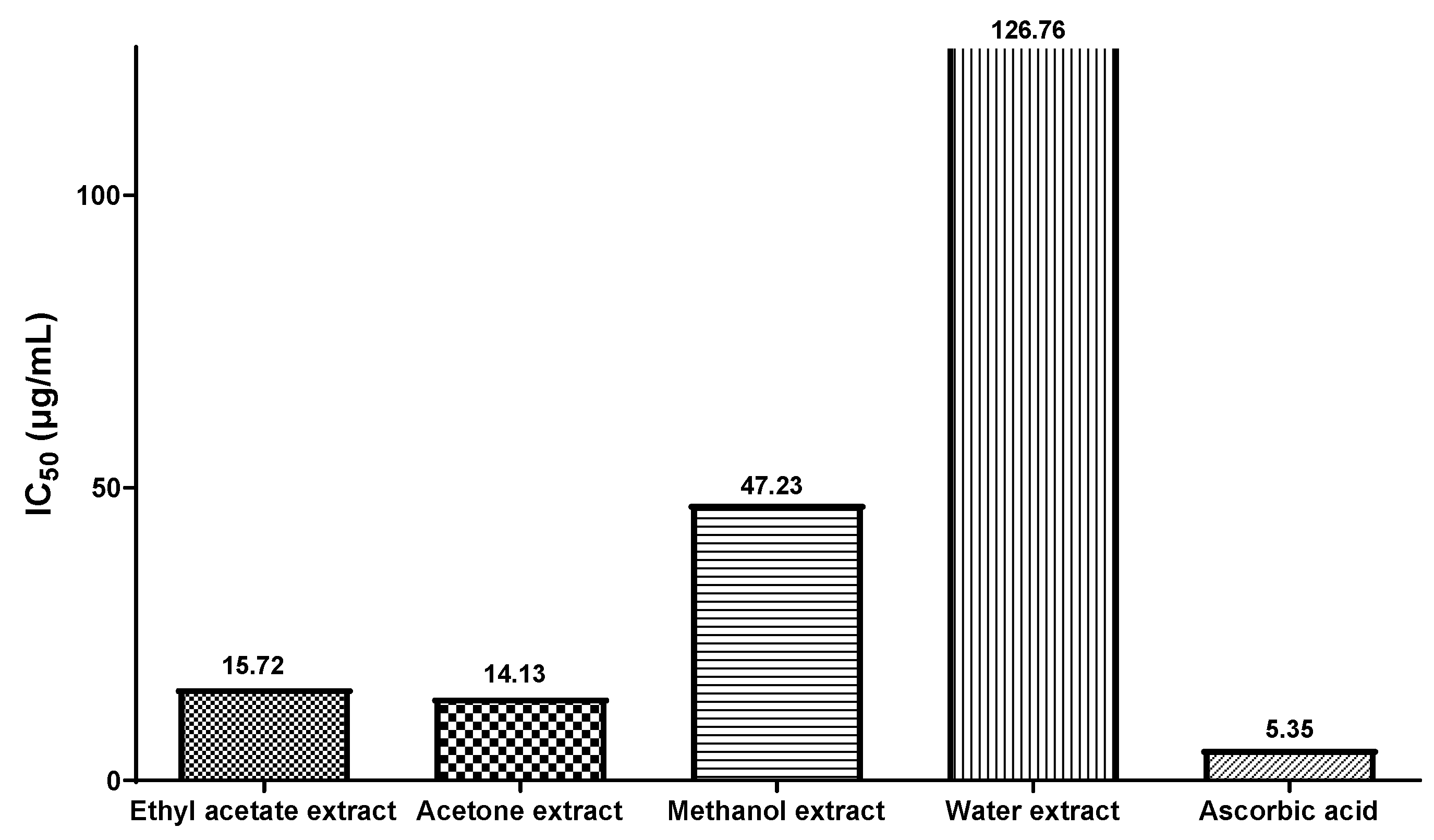
3.6. Evaluation of Antioxidant Potency by Scavenging DPPH Free Radicals
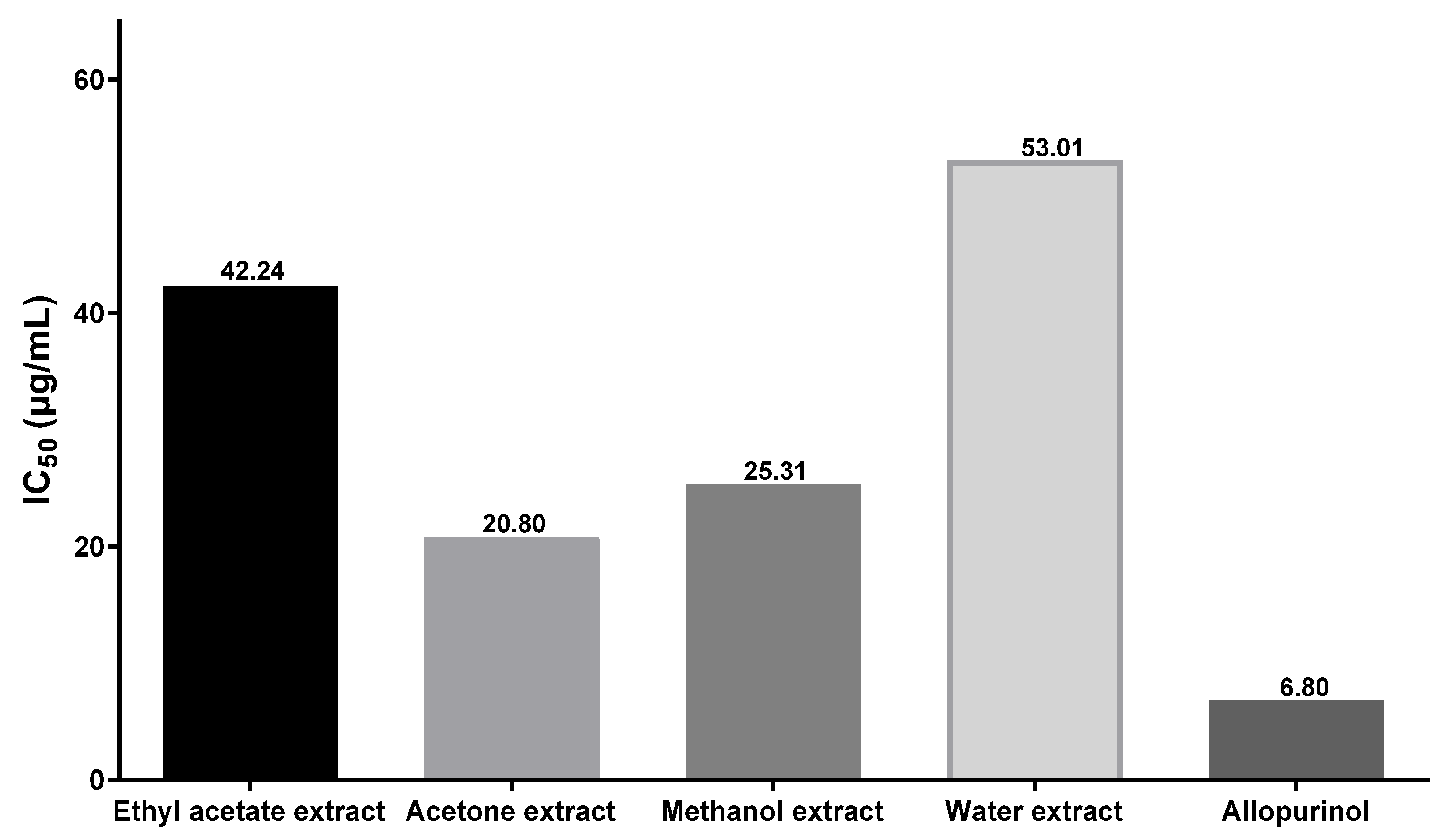
3.7. Measurement of Xanthine Oxidase Inhibitory Effect of C. axillaris Dried Extract
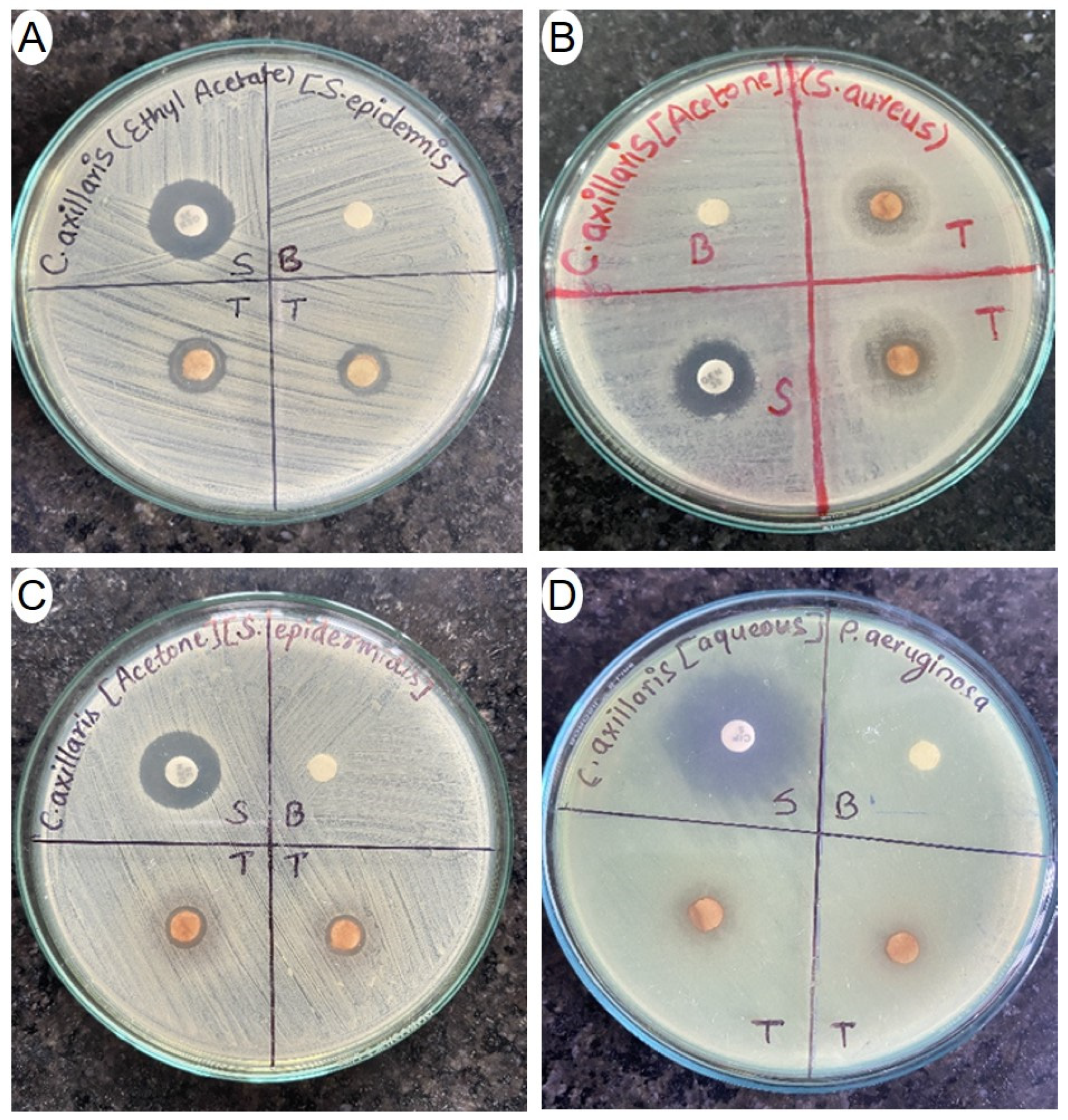
3.8. Evaluation of Antibacterial Effect of C. axillaris Dried Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lonkala, S.; Reddy, A.R. Antibacterial activity of Carica papaya leaves and Allium sativum cloves alone and in combination against multiple strains. Pharmacogn. J. 2019, 11, 600–602. [Google Scholar] [CrossRef]
- Naz, S.; Jabeen, S.; Ilyas, S.; Manzoor, F.; Aslam, F.; Ali, A. Antibacterial activity of Curcuma longa varieties against different strains of bacteria. Pak. J. Bot. 2010, 42, 455–462. [Google Scholar]
- Pandey, J.; Bhusal, S.; Nepali, L.; Khatri, M.; Ramdam, R.; Barakoti, H.; Giri, P.M.; Pant, D.; Aryal, P.; Rokaya, R.K.; et al. Anti-inflammatory activity of Artemisia vulgaris leaves, originating from three different altitudes of Nepal. Sci. World. J. 2021, 2021, 6678059. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Pandey, J.; Devkota, H.P. Bioactive chemical constituents and pharmacological activities of Ponciri fructus. Molecules 2023, 28, 255. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.; Poudel, P.; Devkota, H.P. Choerospondiasaxillaris (Roxb.) BLBurtt & AWHill. In Himalayan Fruits and Berries; Academic Press: Cambridge, MA, USA, 2023; pp. 61–69. [Google Scholar]
- Chhetry, A.K.; Dhakal, S.; Chaudhary, L.; Karki, K.; Khadka, R.B.; Chaudhary, G.P.; Bastola, T.; Poudel, A.; Aryal, P.; Pandey, J. Study of antibacterial activity of root bark, leaves, and pericarp extracts of Diploknema butyracea and evaluation of prospective antioxidant activity. J. Tropic. Med. 2022, 2022, 6814901. [Google Scholar] [CrossRef]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; et al. Development and application of a novel SPE-method for bioassay-guided fractionation of marine extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef]
- KC, S.K.; Müller, K. Medicinal plants from Nepal; II. Evaluation as inhibitors of lipid peroxidation in biological membranes. J. Ethnopharmacol. 1999, 64, 135–139. [Google Scholar]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomed 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Akinsulire, O.R.; Aibin, I.E.; Adenipekun, T.; Adelowotan, T.; Odugbemi, T. In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. Afr. J. Trad. Complement. Altern. Med. 2007, 4, 338–344. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Cincione, R.I.; Tocci, G.; Borghi, C. Clinical effects of xanthine oxidase inhibitors in hyperuricemic patients. Med. Princ. Pract. 2021, 30, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Tan, A. Novel 1, 2, 3-triazole compounds: Synthesis, In vitro xanthine oxidase inhibitory activity, and molecular docking studies. J. Mol. Struct. 2020, 121, 128060. [Google Scholar] [CrossRef]
- Singh, J.V.; Bedi, P.M.S.; Singh, H.; Sharma, S. Xanthine oxidase inhibitors: Patent landscape and clinical development (2015–2020). Expert. Opin. Therap. Pat. 2020, 30, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Liang, G.; Nie, Y.; Chang, Y.; Zeng, S.; Liang, C.; Zheng, X.; Xiao, D.; Zhan, S.; Zheng, Q. Protective effects of Rhizoma smilacis glabrae extracts on potassium oxonate-and monosodium urate-induced hyperuricemia and gout in mice. Phytomed 2019, 59, 152772. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, K.; Zhou, Y.; Xu, A.; Shi, S.; Liu, G.; Li, Z. Electroacupuncture treatment improves learning-memory ability and brain glucose metabolism in a mouse model of Alzheimer’s disease: Using morris water maze and micro-PET. Evid-Based Complement. Alt. Med. 2015, 2015, 142129. [Google Scholar] [CrossRef]
- Vasudeva, N.; Singla, P.; Das, S.; Sharma, S.K. Antigout and antioxidant activity of stem and root of Origanum majorana Linn. Am. J. Drug. Discov. Dev. 2014, 4, 102–112. [Google Scholar] [CrossRef]
- Umamaheswari, M.; AsokKumar, K.; Somasundaram, A.; Sivashanmugam, T.; Subhadradevi, V.; Ravi, T.K. Xanthine oxidase inhibitory activity of some Indian medical plants. J. Ethnopharmacol. 2007, 109, 547–551. [Google Scholar] [CrossRef]
- Irawan, C.; Utami, A.; Styani, E.; Putri, I.D.; Putri, R.K.; Dewanta, A.; Ramadhanti, A. Potential of ethanolic extract from ripe Musa balbisiana Colla fruit using ultrasound-assisted extraction as an antioxidant and anti-Gout. Pharmacogn. J. 2021, 13, 1332–1340. [Google Scholar] [CrossRef]
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Bnouham, M. Medicinal plants as a drug alternative source for the antigout therapy in Morocco. Scientifica 2020, 2020, 8637583. [Google Scholar] [CrossRef]
- Labh, S.N.; Shakya, S.R. Medicinal importance of Choerospondias axillaris (Roxb.) Burtt& Hill fruits in Nepal. J. Trop. Plant Res. 2016, 3, 463–469. [Google Scholar]
- Labh, S.N.; Shakya, S.R.; Kayasta, B.L. Extract of Medicinal lapsi Choerospondias axillaris (Roxb.) exhibit antioxidant activities during in vitro studies. J. Pharmacogn. Phytochem. 2015, 4, 194–197. [Google Scholar]
- Paudel, K.C.; Pieber, K.; Klumpp, R.; Laimer, M. Evaluation of Lapsi tree (Choerospondias axillaris, Roxb.) for fruit production in Nepal. Die Bodenkultur. 2003, 54, 3–9. [Google Scholar]
- Mann, S.; Chakraborty, D.; Biswas, S. An alternative perspective of an underutilized fruit tree Choerospondias axillaris in health promotion and disease prevention: A review. Food Biosci. 2022, 14, 101609. [Google Scholar] [CrossRef]
- Wangchuk, P.; Yeshi, K.; Jamphel, K. Pharmacological, ethnopharmacological, and botanical evaluation of subtropical medicinal plants of Lower Kheng region in Bhutan. Integr. Med. Res. 2017, 6, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Cui, C.B. One new and nine known flavonoids from Choerospondias axillaries and their in vitro antitumor, anti-hypoxia and antibacterial activities. Molecules 2014, 19, 21363–21377. [Google Scholar] [CrossRef] [PubMed]
- Jin, T. The clinical research of Guangzaofufang capsule on coronary disease. Chin. J. Ethnomed. Ethnopharm. 2011, 16, 12–14. [Google Scholar]
- Tang, W.; Eisenbrand, G. Chinese Drugs of Plant Origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine; Springer Science & Business Med: Berlin/Heidelberg, Germany, 2013; pp. 307–308. [Google Scholar]
- Van Sam, H.; Nanthavong, K.; Kessler, P.J. Trees of Laos and Vietnam: A field guide to 100 economically or ecologically important species. Blumea 2004, 49, 201–349. [Google Scholar] [CrossRef]
- Doanh, N.D.; Ham, N.N.; Tam, N.T.; Son, P.T.; van Dau, N.; Grabe, M.; Johansson, R.; Lindgren, G.; Stjernström, N.E.; Söderberg, T.A. The Use of a Water Extract from the Bark of Choerospondias axillaris in the Treatment of Second Decree Burns. Scand. J. Plast. Reconstr. Surg. Hand. Surg. 1996, 30, 139–144. [Google Scholar] [CrossRef]
- Dangal, A.; Timsina, P.; Dahal, S. A comprehensive review on study of physical, nutritional, and phytochemical characteristics as well as therapeutic activities of Choerospondias axillaris (lapsi). Food Biosci. 2023, 53, 102713. [Google Scholar] [CrossRef]
- Mann, S.; Sharma, A.; Sarkar, A.; Kharb, R.; Malhotra, R.; Datta, B.; Gupta, R.K.; Biswas, S. Evaluation of anti-inflammatory effects of Choerospondias axillaris fruit’s methanolic extract in synoviocytes and CIA rat model. Curr. Pharm. Biotechnol. 2020, 21, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.S.; Risinger, A.L.; Petersen, C.L.; Liang, H.; Grkovic, T.; O’Keefe, B.R.; Mooberry, S.L.; Cichewicz, R.H. Using the Cancer Dependency Map to Identify the Mechanism of Action of a Cytotoxic Alkenyl Derivative from the Fruit of Choerospondias axillaris. J. Nat. Prod. 2020, 83, 584–592. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Gao, Y.; Xing, Y.; Hou, J.; Tian, J. Preventive effect of total flavones of Choerospondias axillaries on ischemia/reperfusion-induced myocardial infarction-related MAPK signaling pathway. Cardiovasc. Toxicol. 2014, 14, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xia, Q.; Gao, Z. Total flavones of Choerospondias axillaris attenuate cardiac dysfunction and myocardial interstitial fibrosis by modulating NF-κB signaling pathway. Cardiovasc. Toxicol. 2015, 15, 283–289. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Chen, J.; Liu, C.; Li, T.; McClements, D.J.; Dai, T.; Liu, J. Antioxidant activity of proanthocyanidins-rich fractions from Choerospondias axillaris peels using a combination of chemical-based methods and cellular-based assay. Food Chem. 2016, 208, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, G.; Bhujel, D.; Rai, Y.K. Ethno-medicinally significant edible wild fruits of Sikkim Himalaya. J. Adv. Plant. Sci. 2020, 10, 45–57. [Google Scholar]
- Quang, C.M. Phytochemical and Pharmacological Evaluation of Choerospondias axillaris, a Vietnamese Medicinal Plant Used to TREAT Burns. Licentiate Thesis, Faculty of Pharmacy, Uppsala University, Uppsala, Sweden, 1994. Monograph 17. [Google Scholar]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropic. J. Pharma. Res. 2008, 7, 1019–1024. [Google Scholar]
- Evans, W.C. Trease and Evans’ Pharmacognosy International Edition E-Book; Elsevier Health Sciences: Amsterdam, The Netharlands, 2009. [Google Scholar]
- Gracelin, D.H.; Britto, A.J.; Kumar, B.J. Qualitative and quantitative analysis of phytochemicals in five Pteris species. Int. J. Pharm. Pharm. Sci. 2013, 5, 105–107. [Google Scholar]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Sharma, K.R.; Giri, G. Quantification of phenolic and flavonoid content, antioxidant activity, and proximate composition of some legume seeds grown in Nepal. Int. J. Food. Sci. 2022, 2022, 4629290. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Sharma, G.; Sapkota, B.; Adhikari, M.; Ghimire, S.; Poudel, P.; Jung, H.J. Screening of antioxidant, antibacterial, anti-adipogenic, and anti-inflammatory activities of five selected medicinal plants of Nepal. J. Exp. Pharmacol. 2023, 15, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, M.; Asokkumar, K.; Sivashanmugam, A.T.; Remyaraju, A.; Subhadradevi, V.; Ravi, T.K. In vitro xanthine oxidase inhibitory activity of the fractions of Erythrina stricta Roxb. J. Ethnopharmacol. 2009, 124, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Palani, T.; Shobha, K.; Thirunavukkarasu, P.; Hari, R. and in silico antigout arthritic activities of ethanolic and aqueous stem extracts of Cissus quadrangularis—A TLR2 and TLR4 receptor approach. J. Appl. Pharm. Sci. 2018, 8, 15–22. [Google Scholar]
- Owen, P.L.; Johns, T. Xanthine oxidase inhibitory activity of northeastern North American plant remedies used for gout. J. Ethnopharmacol. 1999, 64, 149–160. [Google Scholar] [CrossRef]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. In vitro antibacterial activities of methanolic extracts of fruits, seeds, and bark of Zanthoxylum armatum DC. J. Tropic. Med. 2020, 2020, 2803063. [Google Scholar] [CrossRef]
- Al Bratty, M.; Makeen, H.A.; Alhazmi, H.A.; Syame, S.M.; Abdalla, A.N.; Homeida, H.E.; Sultana, S.; Ahsan, W.; Khalid, A. Phytochemical, cytotoxic, and antimicrobial evaluation of the fruits of miswak plant, Salvadora persica L. J. Chem. 2020, 2020, 4251951. [Google Scholar] [CrossRef]
- Aziz, M.M.; Raza, M.A.; Saleem, H.; Wajid, M.; Bashir, K.; Ikram, M. Medicinal values of herbs and plants, importance of phytochemical evaluation and ethnopharmacological screening: An illustrated review essay. J. Pharm. Cosmet. Sci. 2014, 2, 6–10. [Google Scholar]
- Shaikh, J.R.; Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Baral, R.; Subedi, L.; Gurung, M.; Ojha, S.; Shrestha, B.; Chaudhary, D.; Jammarkattel, N. Phytochemical screening, free radical scavenging activity, in-vitro alpha-amylase enzyme and glucose diffusion inhibition activity of ethyl acetate and water extracts of selected medicinal plants of Nepal. Int. J. Herb. Med. 2021, 9, 18–27. [Google Scholar]
- Yen, G.C.; Duh, P.D.; Tsai, C.L. Relationship between antioxidant activity and maturity of peanut hulls. J. Agric. Food Chem. 1993, 41, 67–70. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Li, T.; Liu, C.; Liu, W.; Liu, J. Comparison of bioactivities and phenolic composition of Choerospondias axillaris peels and fleshes. J. Sci. Food Agric. 2016, 96, 2462–2471. [Google Scholar] [CrossRef]
- KC, Y.; Dangal, A.; Thapa, S.; Rayamajhi, S.; Chalise, K.; Shiwakoti, L.D.; Shiwakoti, R.; Katuwal, N. Nutritional, phytochemicals, and sensory analysis of Lapsi (Choerospondias axillaris) fruit leather. Int. J. Food Prop. 2022, 25, 960–975. [Google Scholar] [CrossRef]
- Pandey, Y.; Upadhyay, S.; Bhatt, S.S. Phytochemical constituent of some wild edible fruits of Sikkim Himalaya. J. Pharmacogn. Phytochem. 2018, 7, 1045–1047. [Google Scholar]
- Tharanathan, R.N.; Muralikrishna, G.; Salimath, P.V.; Rao, M.R. Plant carbohydrates-an overview. Plant Sci. 1987, 7, 81–155. [Google Scholar] [CrossRef]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T. Free-radical scavenging activity of wormwood (Artemisia absinthium L) extracts. J. Sci. Food Agric. 2005, 85, 265–272. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. [Google Scholar] [CrossRef]
- El-Tantawy, W.H. Natural products for the management of hyperuricaemia and gout: A review. Arch. Physiol. Biochem. 2021, 127, 61–72. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Mishra, A. In vitro and in silico interaction of faba bean (Viciafaba L.) seed extract with xanthine oxidase and evaluation of antioxidant activity as a therapeutic potential. Nat. Product. Res. 2019, 33, 2689–2693. [Google Scholar] [CrossRef]
- Kekilli, E.B.; Orhan, I.E.; Deniz, F.S.; Eren, G.; Emerce, E.; Kahraman, A.; Aysal, I.A. Erodium birandianum Ilarslan&Yurdak. shows anti-gout effect through xanthine oxidase inhibition: Combination of in vitro and in silico techniques and profiling of main components by LC-Q-ToF-MS. Phytochem. Lett. 2021, 43, 80–87. [Google Scholar]
- Chen, W.J.; Wu, Y.; Zhao, X.; Liu, S.; Song, F.R.; Liu, Z.Y.; Liu, Z.Q. Screening the anti-gout traditional herbs from TCM using an in vitro method. Chin. Chem. Lett. 2016, 27, 1701–1707. [Google Scholar] [CrossRef]
- Gupta, A.; Birhman, K.; Raheja, I.; Sharma, S.; Kar, H.K. Quercetin: A wonder bioflavonoid with therapeutic potential in disease management. Asian. Pac. J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Nutmakul, T. A review on benefits of quercetin in hyperuricemia and gouty arthritis. Saudi Pharm. J. 2022, 30, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Miyazawa, K.W.; Staurengo-Ferrari, L.; Mizokami, S.S.; Domiciano, T.P.; Vicentini, F.T.; Camilios-Neto, D.; Pavanelli, W.R.; Pinge-Filho, P.; Amaral, F.A.; Teixeira, M.M.; et al. Quercetin inhibits gout arthritis in mice: Induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology 2017, 25, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Abu-Gharbieh, E.; Shehab, N.G.; Almasri, I.M.; Bustanji, Y. Antihyperuricemic and xanthine oxidase inhibitory activities of Tribulus arabicus and its isolated compound, ursolic acid: In vitro and in vivo investigation and docking simulations. PLoS ONE 2018, 13, e0202572. [Google Scholar] [CrossRef]
- Lou, D.; Zhang, X.; Jiang, C.; Zhang, F.; Xu, C.; Fang, S.; Shang, X.; Zhang, J.; Yin, Z. 3β, 23-Dihydroxy-12-ene-28-ursolic Acid Isolated from Cyclocaryapaliurus Alleviates NLRP3 Inflammasome-mediated gout via PI3K-AKT-mTOR-dependent autophagy. Evid. Based Complement. Alt. Med. 2022, 2022, 5541232. [Google Scholar]
- Essawi, T.; Srour, M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef]
- Lin, J.; Opoku, A.R.; Geheeb-Keller, M.; Hutchings, A.D.; Terblanche, S.E.; Jäger, A.K.; Van Staden, J. Preliminary screening of some traditional zulu medicinal plants for anti-inflammatory and anti-microbial activities. J. Ethnopharmacol. 1999, 68, 267–274. [Google Scholar] [CrossRef] [PubMed]

| Extracts | Wt. of Crude Sample (g) | Wt. of Dry Extract (g) | Extraction Yield (%) |
|---|---|---|---|
| Ethyl acetate extract | 40 | 5.56 | 13.90 |
| Acetone extract | 40 | 8.58 | 21.45 |
| Methanol extract | 50 | 18.00 | 36.00 |
| Water extract | 50 | 25.10 | 50.20 |
| S.N | Test for | Test Used | Ethyl Acetate Extract | Acetone Extract | Methanol Extract | Water Extract |
|---|---|---|---|---|---|---|
| 1 | Alkaloids | Mayer’s, Hagner’s, and Dargendorff’s test | − | − | − | − |
| 2 | Carbohydrates | Molish’s test | − | + | + | + |
| 3 | Terpenoids | Salkowski test | + | + | + | + |
| 4 | Anthraquinones | Borntrager test | − | − | − | − |
| 5 | Saponins | Frothing test | + | + | + | − |
| 6 | Tannins | Gelatin test | + | + | + | − |
| 7 | Cardiac glycosides | Liebermann’s test | − | + | + | + |
| 8 | Flavonoids | Alkaline reagent test | + | + | + | + |
| 9 | Resins | Acetone water test | + | + | + | + |
| 10 | Proteins and amino acids test | Xanthoproteic test | − | + | + | + |
| 11 | Phytosterols | Salkowski’s test | − | − | − | − |
| 12 | Polyphenols | FeCl3 test | + | + | + | + |
| Extracts | Total Phenol Content (μg GAE/mg Dry Extract) | Total Flavonoid Content (μg QE/mg Dry Extract) | Total Carbohydrate Content (μg GE/mg Dry Extract) |
|---|---|---|---|
| Ethyl acetate extract | 88.67 ± 3.48 a | 256.57 ± 6.18 a | 67.26 ± 1.10 a |
| Acetone extract | 154.91 ± 0.16 b | 283.84 ± 7.73 b | 210.52 ± 1.75 b |
| Methanol extract | 112.28 ± 1.31 c | 101.12 ± 1.89 c | 239.71 ± 4.68 c |
| Water extract | 68.28 ± 2.49 d | 41.72 ± 2.40 d | 269.96 ± 0.96 d |
| Concentration (µg/mL) | % DPPH Radical Scavenging Activity | ||||||
|---|---|---|---|---|---|---|---|
| 7.8125 | 15.625 | 31.25 | 62.5 | 125 | 250 | 500 | |
| Ethyl acetate extract | 38.02 ± 0.6 | 49.20 ± 0.9 | 55.48 ± 0.1 | 65.76 ± 0.3 | 82.93 ± 0.1 | 87.17 ± 0.2 | 92.74 ± 0.2 |
| Acetone extract | 41.92 ± 1.2 | 51.18 ± 0.7 | 56.15 ± 0.1 | 65.05 ± 0.4 | 81.94 ± 0.2 | 88.87 ± 0.2 | 93.36 ± 0.2 |
| Methanol extract | 9.64 ± 0.2 | 11.94 ± 0.8 | 24.80 ± 0.5 | 58.17 ± 0.6 | 83.45 ± 0.3 | 83.96 ± 0.6 | 88.41 ± 0.5 |
| Water extract | 8.09 ± 0.6 | 8.84 ± 0.4 | 10.95 ± 0.2 | 30.47 ± 0.7 | 50.87 ± 1.2 | 68.92 ± 1.1 | 84.36 ± 0.9 |
| Concentration (µg/mL) | % Scavenged ± SD |
|---|---|
| 1 | 8.32 ± 0.21 |
| 2.5 | 25.89 ± 0.38 |
| 5 | 51.92 ± 0.76 |
| 10 | 91.34 ± 0.47 |
| Xanthine Oxidase Inhibition by C. axillaris Dried Fruit Extracts and Standard Drug Allopurinol | ||||
|---|---|---|---|---|
| Concentrations (µg/mL) | XO Inhibitory Activity (%) | |||
| 10 | 25 | 50 | 100 | |
| Alopurinol | 53.61± 0.47 | 66.78± 0.76 | 79.74 ± 1.69 | 96.67 ± 0.45 |
| Ethyl acetate | 31.55 ± 0.19 | 48.03 ± 0.80 | 58.50 ± 0.21 | 71.81 ± 1.87 |
| Acetone | 39.06 ± 0.23 | 54.79 ± 0.44 | 69.67 ± 0.21 | 82.25 ± 0.46 |
| Methanol | 34.05 ± 0.18 | 58.75 ± 0.61 | 64.37 ± 2.72 | 76.60 ± 1.88 |
| Water | 28.18 ± 0.17 | 41.85 ± 0.81 | 51.96 ± 1.84 | 67.13 ± 0.94 |
| Zone of Inhibition in mm (mean± SD) | ||||||
|---|---|---|---|---|---|---|
| Bacterial Strains | Ethyl Acetate Extract | Acetone Extract | Methanol Extract | Water Extract | Ciprofloxacin | Gentamicin |
| S. aureus | 10.33 ± 0.57 | 13.76 ± 0.82 | 8.90 ± 0.16 | 7.66 ± 0.45 | - | 14.98 ± 0.91 |
| S. epidermidis | 11.67 ± 0.57 | 9.33 ± 0.57 | 8.33 ± 0.57 | 9.00 ± 1.00 | - | 14.00 ± 0.70 |
| B. cereus | 9.66 ± 0.57 | 12.56 ± 0.51 | 8.23 ± 0.40 | 7.90 ± 0.48 | - | 15.36 ± 0.36 |
| S. pneumoniae | 8.00 ± 1.00 | 7.67 ± 0.57 | ND | ND | - | 20.00 ± 1.00 |
| P. aeruginosa | ND | ND | 8.00 ± 1.00 | 10.00 ± 1.00 | 24.00 ± 1.00 | - |
| K. pneumonia | ND | ND | 10.43 ± 1.30 | 11.56 ± 0.70 | 25.50 ± 1.04 | - |
| E. coli | 7.89 ± 0.91 | 8.36 ± 0.36 | 8.55 ± 0.31 | 11.00 ±1.50 | 22.00 ± 1.00 | - |
| S. enteritidis | ND | ND | ND | 10.82 ± 0.43 | 23.61 ± 1.00 | - |
| MIC and MBC Values of Samples (mg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Bacterial Strains | Ethyl Acetate Extract | Acetone Extract | Methanol Extract | Water Extract | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. epidermidis | 0.65 ± 0.22 | 1.30 ± 0.45 | 2.60 ± 0.91 | 4.16 ± 1.80 | 2.08 ± 0.92 | 4.16 ± 1.80 | 3.12 ± 0.00 | 5.21 ± 1.80 |
| S. aureus | 1.04 ± 0.36 | 2.08 ± 0.90 | 0.52 ± 0.22 | 0.78 ± 0.00 | 1.30 ± 0.45 | 3.12 ± 0.00 | 8.33 ± 3.60 | 16.66 ± 5.89 |
| B. cereus | 1.31 ± 0.36 | 2.60 ± 0.90 | 0.65 ± 0.22 | 1.30 ± 0.45 | 4.16 ± 1.80 | 5.2 ± 01.80 | 8.33 ± 3.60 | 12.5 ± 0.00 |
| S. pneumoniae | 1.040 ± 0.36 | 3.12 ± 0.00 | 5.20 ± 1.80 | 12.50 ± 0.00 | NT | NT | NT | NT |
| K. pneumonia | NT | NT | NT | NT | 4.66 ± 1.80 | 5.20 ± 1.80 | 1.04 ± 0.46 | 2.60 ± 0.90 |
| P. aeruginosa | NT | NT | NT | NT | 8.33 ± 3.60 | 10.41 ± 3.60 | 2.60 ± 0.90 | 4.17 ± 1.80 |
| E. coli | 4.16 ± 1.80 | 10.41 ± 3.60 | 8.33 ± 3.60 | 4.16 ± 1.80 | 8.33 ± 3.60 | 5.21 ± 1.81 | 1.30 ± 0.46 | 3.12 ± 0.00 |
| S. enteritidis | NT | NT | NT | NT | NT | NT | 4.17 ± 1.80 | 5.21 ± 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, S.; Bajracharya, S.; Thada, S.; Bakabal, A.; Khadka, R.B.; Devkota, H.P.; Pandey, J. Total Phenolic and Flavonoid Contents, and Preliminary Antioxidant, Xanthine Oxidase Inhibitory and Antibacterial Activities of Fruits of Lapsi (Choerospondias axillaris Roxb.), an Underutilized Wild Fruit of Nepal. Appl. Sci. 2023, 13, 8945. https://doi.org/10.3390/app13158945
Neupane S, Bajracharya S, Thada S, Bakabal A, Khadka RB, Devkota HP, Pandey J. Total Phenolic and Flavonoid Contents, and Preliminary Antioxidant, Xanthine Oxidase Inhibitory and Antibacterial Activities of Fruits of Lapsi (Choerospondias axillaris Roxb.), an Underutilized Wild Fruit of Nepal. Applied Sciences. 2023; 13(15):8945. https://doi.org/10.3390/app13158945
Chicago/Turabian StyleNeupane, Samikshya, Simran Bajracharya, Sanju Thada, Anita Bakabal, Ram Bahadur Khadka, Hari Prasad Devkota, and Jitendra Pandey. 2023. "Total Phenolic and Flavonoid Contents, and Preliminary Antioxidant, Xanthine Oxidase Inhibitory and Antibacterial Activities of Fruits of Lapsi (Choerospondias axillaris Roxb.), an Underutilized Wild Fruit of Nepal" Applied Sciences 13, no. 15: 8945. https://doi.org/10.3390/app13158945
APA StyleNeupane, S., Bajracharya, S., Thada, S., Bakabal, A., Khadka, R. B., Devkota, H. P., & Pandey, J. (2023). Total Phenolic and Flavonoid Contents, and Preliminary Antioxidant, Xanthine Oxidase Inhibitory and Antibacterial Activities of Fruits of Lapsi (Choerospondias axillaris Roxb.), an Underutilized Wild Fruit of Nepal. Applied Sciences, 13(15), 8945. https://doi.org/10.3390/app13158945










