Abstract
In the last decade, fresh-cut plants have become a more popular flavoring additive in food. It is important to find an effective method for ensuring the safety and quality of plant materials used as food additives. Ozonated water is being considered by the EFSA for approval as a cidal agent for plant protection. The objective of this study was to evaluate the effectiveness of ozonated water in improving the microbial safety of fresh-cut parsley leaves, with a particular focus on mesophilic and psychrotrophic bacteria and fungi. The yeasts and bacteria were identified with the MALDI-TOF MS system. Color changes on the surface of the parsley samples were measured in the CIE L*a*b trichromatic color model. The chemical composition of the essential oil was evaluated via gas chromatography with mass spectrometry (GCMS). The microbial level of the fresh leaves varied and depended on the season. The highest microbial levels were found in the leaves picked in the summer and autumn, at 104 to 106 CFU/g for fungi and 106 to 108 CFU/g for bacteria. Among the isolates with the highest isolation frequency, bacteria belonging to Pseudomonas fluorescens, Staphylococcus warneri, and Bacillus megaterium dominated. The dominant yeasts and molds were Candida sp., Rhodotorula sp., Cladosporium sp., and Fusarium sp. The conditions for water ozonation (ozone dose and time) were established for both mono- and mixed cultures. Time of 3 min, ozone content of 0.5, O3 mg/L or 1 min, and 1.5 mg of O3 mg/L were sufficient for a 90% reduction in the number of living microorganisms. Yeasts and bacteria were sensitive to ozone treatment, excluding P. fluorescens rods. The tested mold strains were the most resistant. However, it was noted that organic matter might reduce the decontamination effect. The effectiveness of ozonation was negatively influenced by organic compound content above 1%. Spectrophotometric measurements of parsley leaves after ozonation, especially after 3 min treatment at 1.5 O3 mg/L, revealed morphological changes. The CIELAB color space (L*a*b*) changed in the direction of lightness and yellowness; however, ΔE showed no statistically significant differences in comparison with the untreated leaves. In preliminary studies, no differences were noted in GLC-MS chromatograms for essential oils of parsley leaves before and after ozonation. The aroma of parsley treated with ozonated water was more intensely herbal than the control sample, probably due to the higher content of α and β phellandrene. The results of this study show that decontamination of parsley leaves by ozonated water containing 1.5 O3 mg/L in a closed 5 min process can effectively ensure the microbiological quality of fresh-cut parsley leaves. It can be concluded that ozone treatments in aqueous form appear to provide promising qualitative and quantitative results for the decontamination of this fresh-cut plant material. However, more work is necessary to study chemical and volatilome changes. Especially the sensory analyses should be conducted before and after ozone treatment.
1. Introduction
According to European Community standards, fresh-cut plants, which are essential to human health and wellbeing, are defined as minimally processed, ready-to-eat, fresh-cut products, i.e., subject to reduced technology for direct consumption without further handling or with minimal handling [1]. Post-harvest shelf life and microbial control strategies for these plants are often based on the use of chemical compounds that are harmful to both human health and the environment. Today, it is important to find effective alternatives to traditional technologies to ensure safety and quality.
Washing is the easiest way to clean plants, but it is unable to remove all microorganisms, some of which remain attached to the plant surfaces. Different decontamination methods have been described in the literature, where they are compared in terms of microbial decontamination efficacy and their effects on biochemical, aroma, and color properties [2]. Maintaining the desired visual and sensory values is very important for applications of the finished product and for consumers. The applied decontamination techniques should not strongly affect the color or the content of essential compounds, namely, volatile aroma compounds. A special type of treatment should be selected for sporulating microorganisms, which are particularly resistant to various environmental factors.
Currently, available decontamination technologies include gamma irradiation [3], high-temperature steaming [2,4], and chemical treatment with ethylene oxide [2]. However, these methods have toxicological consequences and may affect the composition, aroma, flavor, or color of the obtained product [4]. Therefore, other technologies are sought with equally good antimicrobial properties but fewer side effects. These include high hydrostatic pressure [5] and radiation using infrared [6], microwaves, or ultraviolet [2]. Such methods are not without disadvantages and limitations. For example, heat sterilization is unsuitable if thermally sensitive aroma and flavor components are present in the raw material [2]. Currently, there is no universal method for reducing the microbiological content of spices and herbs.
Ozonation may be considered one of the most promising treatments for the decontamination of fresh products in the food industry [2]. Ozone also seems to be a potential alternative disinfectant due to its mechanism of fast degradation of oxygen as well as the fact that it does not generate any residue after the process [7,8,9]. Moreover, ozonation has no need for thermal energy and can be generated at the time of use. Thus, it does not require any transport or chemical storage, which reduces the total operation cost on an industrial scale. In these respects, ozonation can be considered a green and environmentally friendly process [10]. In 1997, ozone (O3) was generally recognized as safe (GRAS), and from 2001, it has been fully approved by the U.S. Food and Drug Administration (FDA) as a sanitizing agent for direct contact with food. Therefore, it has received more attention from scientists and industry sectors [11]. Ozone technology improves food safety without compromising quality or endangering the environment [12]. Ozone, applied in either gaseous or aqueous phases [13], has been used as a postharvest treatment of fruits and vegetables with satisfactory results [14,15,16,17,18]. Ozonation of strawberries was found to delay the loss of weight and softening [19]. Similarly, ozone treatment extended shelf-life and retained the quality of fresh-cut celery [20] and lettuce [21] by reducing microbial contamination. Ozonation may also be an effective method of removing the residues of fenitrothion and benomyl pesticides from persimmon leaves [22]. Its microbial properties result from its high potential oxidizing capacity. Oxidation leads to the formation of primary species with high reactivity. However, microorganisms show varied sensitivity to ozone. Therefore, the process parameters should be optimized to the microbiological profile and the type of raw material. Particularly spore-forming Gram-positive bacteria and yeasts are more vulnerable than Gram-negative bacteria and molds [2,23,24]. Moreover, ozone is a highly oxidizing agent, which could possibly cause the decomposition of essential oils when used for long periods or in high concentrations. This is especially important in the case of spice plants used to improve aroma and taste [25].
In Europe, the use of gaseous ozone is controversial. The European Food Safety Authority (EFSA) is still validating the use of ozone for fresh food. The EFSA publication “Scientific opinions on the risks posed by pathogens in food of non-animal origin (Salmonella and Norovirus in leafy greens eaten raw as salads and berries)” suggests using ozone, as well as other sanitizing molecules, for decontamination of leaf vegetables and berries [26]. Another more recent EFSA document, “Outcome of the consultation with Member States and EFSA on the basic substance application for approval of ozone to be used in plant protection as a bactericide, fungicide, insecticide, nematicide, and viricide”, states that ozone should not be approved as a basic substance, due to its hazardous properties. However, ozonated water could be considered eligible for approval as a basic substance. Therefore, the assessment should be focused on ozonated water instead of gaseous ozone [27].
Parsley (Petroselinum crispum) is famous for its high content of bioactive compounds, such as phenols, flavonoids, vitamin C, chlorophylls, carotenoids, and minerals [28,29,30,31,32,33]. Parsley also shows significant antioxidant, antibacterial, and antifungal activities [33,34,35]. Parsley leaves were used by the ancient Greeks and Romans as a herb. Nowadays, parsley is cultivated all over the world and is widely used as a flavoring and aromatic food additive. Therefore, it is an interesting research object. Parsley leaves are used fresh or dried, and their slightly aromatic flavor is popular with fish, meats, soups, sauces, and salads [36]. In addition, parsley has a long history in traditional and herbal medicine. The plant has been used to alleviate menstrual pain and has anti-inflammatory properties [35]. However, the use of fresh parsley leaves may be associated with a significant increase in microbial risk as well as a decrease in shelf life due to the high number of microorganisms. Parsley is usually planted and grown under controlled conditions but is often irrigated using various organic fertilizers or contaminated water [37]. Therefore, similar to other leafy greens considered to promote healthier living, parsley has been frequently linked to outbreaks of foodborne diseases [38].
Parsley leaves may become contaminated by a wide range of microbes, which can cause spoilage and pose a public health risk [30,39,40]. The most common pathogens contaminating plant material are Salmonella spp., Bacillus cereus, Staphylococcus aureus, Clostridium perifingens, and Escherichia coli [2,3,41]. Contamination can occur both pre- and post-harvest (during cultivation, harvest, drying, transport, and storage) [3]. Sources of microorganisms include soil, water, dust, dirt, insects, fecal materials from animals, and even human excrement [41]. As a result, the microbiological compositions of herbs and spices vary depending on the climate, weather, and season.
The aim of this study was to evaluate the effectiveness of ozone water treatment on microbial decontamination of parsley leaves. There are few studies investigating the effects of different decontamination processes of fresh parsley leaves [42,43,44]. The effectiveness of ozonation was examined by microbiological and physico-chemical assays, with special attention to the color of the plant material.
2. Materials and Methods
2.1. Materials
Fresh parsley leaves were obtained by VegaDry, a spice producer purchased from local farmers in the Lodz area of Poland (51°46′36″ N 19°27′17″ E). A total of 50 g of representative samples (N = 4) were collected throughout the year (No. 1 in spring, No. 2 in summer, No. 3 in autumn, and No. 4 in winter).
2.2. Microbiological Contamination Assessment
The microbial quality of the fresh parsley leaves was examined via the plate count method. Determination of the total number of bacteria and fungi was carried out using 10 g of green parsley placed into 90 mL of 0.85% NaCl. The samples were homogenized (5 min, Masticator, IUL Instruments S.A., Barcelona, Spain). Next, ten-fold dilutions were prepared. The total number of bacteria was determined using TSA (Tryptic Soy Agar: Merck, Darmstadt, Germany) with (0.2%) nystatin, and the total number of fungi was determined using MEA (Malt Extract Agar, Merck, Darmstadt, Germany) with (0.1%) chloramphenicol. Incubation conditions were as follows: temp. 37 ± 2 °C for 24 h (mesophilic bacteria and fungi); temp. 15 ± 2 °C for 2–5 days (psychrotrophic bacteria and fungi). The results were expressed as the arithmetic mean of the colony-forming units per 1 g (CFU/g) of the tested material. Determination was made in three independent repetitions for each sample.
2.3. Identification of Microorganisms
All isolates of bacteria and fungi from the parsley samples were purified by the streak plate method, and the obtained pure cultures were identified. Preliminary identification of the bacterial isolates was performed using classical identification procedures [45,46] and included cell morphology, Gram-staining, sporulation, as well as Bactident oxidase, Bactident catalase, and Bactident L-alanine aminopeptidase tests (Merck, Darmstadt, Germany). The bacteria were grown at 30 ± 2 °C for 24 h on TSA (Tryptic Soy Agar: Merck, Darmstadt, Germany) and identified via the MALDI-TOF MS method (matrix-assisted laser desorption and ionization time-of-flight mass spectrometry). The AXIMA-iD Plus Confidence MALDI-TOF MS system (Kratos Analytical Ltd. and Shimadzu Corporation, Kyoto, Japan) was used with SARAMIS™ Premium software version 1.1 (Spectral ARchive And Microbial Identification System, bioMérieux, Marcy l’Étoile, France) and Escherichia coli DH5α (Takara Bio Inc., Shiga, Japan) cells as a calibrator and internal control for the identification process. Identification was performed according to the manufacturer’s recommended protocol (method of direct smear plus formic acid), as described in detail by [47]. Analysis was carried out in the SARAMIS linear positive mode with the following parameters: laser frequency 50 Hz; mass-to-charge ratio (m/z): 2.000–20.000 Da; laser power 90, profiles per sample 200; five shots accumulated per profile for each mass spectrum. The mass spectra were acquired and processed using Launchpad 2.9 software (Kratos Analytical Ltd. and Shimadzu Corporation, Kyoto, Japan). Identification was performed in triplicate for each strain. The results of identification were expressed as confidence score values (%) based on a comparative analysis of the obtained spectra with the reference spectra in the SARAMIS database. The isolates with mean confidence score values higher than 85% were considered as identified. Identification of selected strains of fungi (molds and yeast) was performed based on the ITS1/2 sequence of the rDNA region. The genomic DNA of each strain was extracted using a method described elsewhere [48]. The obtained nucleotide sequences of the studied microorganisms were analyzed and compared with the sequences deposited in the National Center for Biotechnology Information (NCBI) database using the BLASTN 2.13.0+ program.
2.4. Selection of Microorganisms
For all bacterial and fungal isolates, we determined the frequency of isolation in the pool of all isolated bacteria/fungi [%]. To assess the effectiveness of ozonation, the following isolates with the highest isolation frequency (>20%) were selected: bacteria Pseudomonas fluorescens; Staphylococcus warneri; Bacillus megaterium; yeasts Bullera alba; Rhodotorula babjevae; molds Cladosporium cladosporioides; and Epicoccum nigrum.
2.5. Ozonation
Ozonation was performed using the apparatus presented in Figure 1 in the following two variants: (A) ozonated water at concentrations of 0.5 mg/L and 1.5 mg/L with microbial isolates from parsley; (B) flowing ozonated water at a concentration of 0.5 mg/L with parsley. Ozonation was performed for the following variants of the experiments: (a) optimization of ozone concentration (0.5 mg/L and 1.5 mg/L) and water ozonation time (1 min, 3 min, 5 min, 10 min, 15 min) for 7 isolates from parsley (2 yeasts, 2 molds, 3 bacteria), as well as mixtures of bacteria, yeasts, and molds; (b) evaluation of the impact of organic pollutants in different concentrations (0.1%, 1%, 10%, 50%) on the efficiency of ozone treatment (0.5 g/L and 1.5 mg/L) for 7 isolates from parsley and their mixtures; (c) evaluation of treatment efficiency of rising parsley with ozonated water (ozone concentration 1.5 mg/L) in a closed circuit for different process times (3 min, 5 min, 10 min, 15 min). Each experiment was compared to a control sample without ozonation.

Figure 1.
System for ozonation. (a)—process flow diagram; (b)—photograph of the reactor for dissolution of ozone in water; O2—source of oxygen; H2O—source of water; MFC—mass flow controller; GEN—generator of ozone (BMT 803N, Stahnsdorf, Germany, BMT Messtechnik); Cin—measure of inlet ozone concentration (ozon analyzer BMT 964, Stahnsdorf, Germany, BMT Messtechnik); Cout—measure of outlet ozone concentration (ozon analyzer BMT 964, Stahnsdorf, Germany, BMT Messtechnik ); PP—peristaltic pump (Verderflex, Newtown, PA, USA, Verder Limited); WR—reactor—washing of parsley leaves; BR—bubble reactor—dissolution of ozone in water; SG—sintered glass.
The efficiency of ozonation was determined according to the reduction coefficient R using Formula (1):
where No is the number of microorganisms at time t = 0 before ozonation, and Nt is the number of microorganisms during ozonation or the number of microorganisms in the sample without the addition of organic pollutants. Decontamination was evaluated based on a scale described in the literature [49].
2.6. Evaluation of the Chemical Composition of Parsley Oil
The decontamination process should result in the appropriate microbiological purity while maintaining the product’s usable properties. The sensory properties of parsley depend on the chemical composition of the essential oil. Parsley oil was obtained in three rinsing cycles of 50 g of parsley in 1 L of water for 15 min with ozonated water containing 1.5 mg/L in a closed circuit. The reference material was parsley rinsed analogously in water (control sample). Parsley oil was obtained by distillation with steam in a Deryng apparatus (WPL, Gliwice, Poland). Distillation was carried out for 1.5 h with 150 g of material in 700 mL of water. The obtained oil was diluted to 1 mL with n-hexane and subjected to gas chromatography with mass spectrometry (GCMS). The composition of the volatile compounds in the vapor phase above the parsley was tested for unrinsed parsley, parsley rinsed for 3 min, and parsley rinsed for 15 min. Parsley (25 g) was rinsed in 1 L with ozonated water with an ozone concentration of 1.5 mg/L in a closed circuit. Then, 0.5 g of the dried parsley leaves was collected for the SPME study. Incubation of SPME fiber was conducted for 0.5 h at room temperature in a 20-mL vial with a membrane, using 50/30 μm DVB/CAR/PDMS, Stableflex (2 cm) fiber (Sigma-Aldrich, St. Louis, MO, USA).
2.7. Gas Chromatography with Flame Ionization Detector and Mass Spectrometry
Analyses were made in a Thermo Ultra GC Trace equipped with a flame ionization detector and Thermo DSQ II mass spectrometer (split flow) (Thermo Fisher Scientific, Waltham, MA, USA). A Rxi®-1 ms column (60 m × 0.25 mm × 0.25 µm film thickness) from Restek was used (Restek, Bellefonte, PA, USA). The MS temperatures were as follows: transfer line 280 °C and ion source 220 °C. The scan range was between 32 U and 450 U. The oven was programmed to heat up to 50 °C, hold there for 3 min, then perform a 4 °C/min ramp to 300 °C and hold there for 10 min. The injection quantity was 0.4 µL with a 45 mL/min split for oil samples and splitless for SPME. SPME fiber was injected for 3 min in splitless mode. After 20 s, the mode was changed to split mode with 20 mL/min flow. The analyte was identified based on electron spectra of the NIST 2011 library and on the Kovates index.
2.8. Color Change Assessment
Color changes on the surface of the parsley samples were measured in the CIE L*a*b trichromatic color model. An NH300 portable spectrophotometer (EnviSense, Lublin, Poland) was used with a horizontal construction and equipped with a silicon photodiode as a detector. The color change was measured after ozonation of the parsley samples in water (ozone concentration 0.5 mg/L and 1.5 mg/L) for 15 min, after rinsing with ozonated water in a closed circuit for 15 min (ozone concentration 1.5 mg/L) and after rinsing in non-ozonated water (control sample). Each variant was repeated 10 times in different areas of the parsley sample. The colorimeter was equipped with an 8-mm measuring aperture. Color coordinates representing lightness (L), red–green axis (a*), and yellow–blue axis (b*) were recorded. An average of 10 color readings were taken for each sample. The differences between treated and untreated samples were expressed as ΔL, Δa, and Δb. The total color changes ΔE after the ozonation processes were calculated using Formula (2),
where ΔE is the value of the color change relative to the reference sample; ΔL is the difference in lightness (white–black) between the tested and reference sample; Δa is the difference in green–red color between the tested and reference sample, and Δb is the difference in blue–yellow color between the tested and the reference sample.
Δ𝐸=√(Δ𝐿)2 + (Δ𝑎)2 + (Δ𝑏)2,
2.9. Statistical Analysis
The mean numbers of microorganisms in the parsley samples collected in different seasons, as well as their color parameters, were compared using a one-way analysis of variance (ANOVA) at a significance level of 0.05. When a statistical difference was detected (p < 0.05), the means were compared using Tukey’s post hoc procedure at a significance level of 0.05. Statistical analysis was carried out with Statistica 13.1 (Statsoft, Tulsa, OK, USA).
3. Results and Discussion
The numbers of mesophilic fungi in the parsley samples ranged from 1.10 × 104 to 2.53 × 105 CFU/g. The numbers of psychrotrophic fungi were higher: 2.36 × 104–1.24 × 106 CFU/g. The highest number of psychrotrophic fungi was found in the sample of parsley No. 3 (autumn). The lowest numbers of both psychrotrophic and mesophilic fungi were found in the sample of parsley No. 2 (summer). In the other samples, the numbers of fungi were at the level of 105 CFU/g. The numbers of mesophilic and psychrotrophic bacteria were 1.23 × 106–1.61 × 108 CFU/g and 1.10 × 107–5.45 × 107 CFU/g, respectively. The highest number of mesophilic bacteria was found in the sample of parsley No. 2 (summer). The sample the most contaminated with psychrotrophic bacteria was parsley No. 1 (spring) (Table 1).

Table 1.
Microbial contamination of parsley leaves.
The level of microbial contamination varied both quantitatively and qualitatively, depending not only on the season but also on the types of microorganisms. The highest levels of psychrotrophic and mesophilic fungi were determined for samples collected in autumn, statistically different from other seasons, and the smallest number of psychrotrophic fungi was observed for summer samples (Table 1). The highest results for mesophilic bacteria were the in the summer samples, which were statistically different from the samples collected in other seasons. Comparing the statistical differences between the number of examined groups of microorganisms, it was found that diversity was observed mainly between the numbers of bacteria (statistically higher) and the numbers of fungi (Table 1).
It is widely known that microbial processes are associated with seasonal environmental variations, which affect microbial activities. The levels of microbial contamination should be considered high and are comparable to other studies in which microbial contamination of parsley leaves reached levels of 104–105 CFU/g (fungi) and 106–108 CFU/g (bacteria) [38,50]. Studies of green plants indicate that 100% of parsley leaf samples are most contaminated with mesophilic bacteria and fungi [38]. High numbers of both fungi and bacteria may be found in both organic and conventionally grown vegetables [13]. It is worth noting that rinsing, trimming, and packaging can be ineffective at reducing microbial contamination [51,52] and may even contribute to secondary contamination.
Among the isolated and identified bacterial groups (Table 2), MALDI TOF analysis confirmed that the dominant bacterial species belonged to Pseudomonas fluorescens (Gram-negative rods, oxidase-positive), Klebsiella oxytoca and Acinetobacter johnsonii (Gram-negative rods, oxidase-negative), Staphylococcus warneri (Gram-positive cocci, oxidase-negative), and Bacillus megaterium (Gram-positive, spore-forming rods, oxidase-negative).

Table 2.
Fungi and bacteria genera isolated from parsley samples with isolation frequency (%).
All the tested samples from all four seasons were contaminated with yeasts and molds. The yeasts identified belonged to the species Bullera alba and Rhodotorula babjevae (respective frequencies: 27.4–54.8% and 11.3–40.2%). The dominant molds were from the strain Cladosporium cladosporioides (4.4–24.2%) and genera Alternaria (3.0–17.4%) and Geotrichum (10%). Molds from the species Epicoccum nigrum (2.1–8.5%) and genera Penicillium (2.2%) and Acremonium (0.4–0.6%) were isolated with less frequency (Table 2).
The presence in parsley leaves of molds from the genera Cladosporium, Aspergillus, Penicillium, Alternaria, Epicoccum, and Rhizopus has been described previously by other authors. The genus Cladosporium is the most often isolated mold. Previous studies have also reported a large variety of fungal genera in parsley compared to other green vegetables intended for direct consumption [38,53]. Fungi belong to the plant epiphytic microflora. However, their excessive development can both cause spoilage and have health effects for consumers due to the production of mycotoxins that are locally formed and may move inside the plant tissue [54,55].
There was less diversity of bacteria in the parsley samples than there was diversity of fungi. Five species were identified with the highest isolation frequency: Pseudomonas fluorescens; Klebsiella oxytoca; Acinetobacter johnsonii; Staphylococcus warneri; and Bacillus megaterium. All isolated bacteria belong to opportunistic pathogens and are common in soil and other natural environments. In their studies of parsley leaves, [38] also identified Enterococcus and rods belonging to Enterobacteriaceae.
Fungi and spore-forming bacteria are highly resistant to various techniques of disinfection. Due to the storage and use of raw parsley leaves as a food additive, it is important to develop successful methods to eradicate these microorganisms. In the present study, we used ozonated water to rinse the parsley. In the first experiment, we optimized the concentration of ozone in the water and the contact time with the suspension of microorganisms most commonly present in parsley (Figure 2, Table S1). Next, the influence of the organic matter on the effectiveness of ozonation was determined (Figure 3, Table S2).
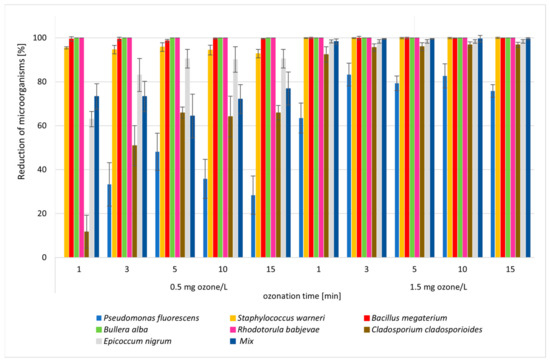
Figure 2.
Reduction in number of microorganisms during the ozonation process (1, 3, 5, 10, 15 min) with different ozone concentrations (0.5 O3 mg and 1.5 O3 mg/L).
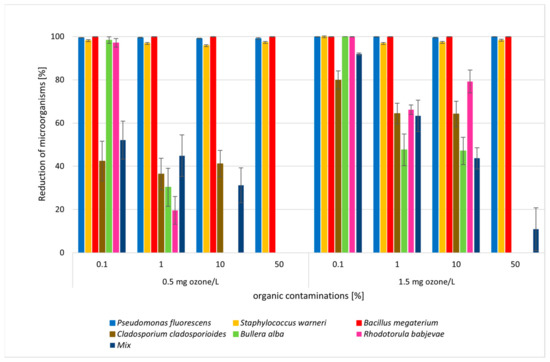
Figure 3.
Reductions in numbers of microorganisms after 15 min of ozonation with different concentrations of ozone (0.5 mg and 1.5 O3 mg/L) and organic compounds (0.1%, 1%, 10%, 50%).
The dose of ozone and the type of microorganism had a significant impact on ozonation efficiency, expressed as a reduction in the number of microorganisms isolated from parsley and in mixtures of microorganisms during ozonation under model conditions (Figure 2, Table S1). The ozone dose had the greatest impact on the efficiency of microbial inactivation. Significant statistical differences were noted for most microorganisms in the first minute of the ozonation process. A dose of 1.5 O3 mg/L resulted in a reduction in microorganisms of more than 0.5 O3 mg/L. Such a difference was not found for Bacillus megaterium bacteria or for microorganisms, which were completely eliminated during the ozonation process (Bullera alba, Rhodotorula babjevae) (Table S1). Statistically significant differences were found between the first and third minutes of the process at doses of both 0.5 O3 mg/L and 1.5 O3 mg/L. After this time, there were no significant statistical differences in the reduction in microorganisms for either dose of ozone. Therefore, it was concluded that the time of the process (1, 3, 5, 10, 15 min) was less important, especially from the fifth minute (Table S1). For most individual microorganisms and their mixtures, a high reduction of >90% was obtained for a dose of 1.5 O3 mg/L, with the exception of bacteria Pseudomonas fluorescens (R = 33–48%). Different sensitivity to ozonation was observed among the examined microbial isolates, from the most sensitive to the least sensitive microorganisms as follows: Bullera alba > Rhodotorula babjevae > Bacillus megaterium > Staphylococcus warneri > Epicoccum nigrum > Cladosporium cladosporioides > Pseudomonas fluorescens. The first four microorganisms were almost completely eliminated during ozonation (R > 95%). This is a satisfactory result because the yeast was the most often isolated fungi, and Bacillus megaterium is resistant to ozonation due to the production of spores. The lower sensitivity of molds is due to the high resistance of these microorganisms to antimicrobial agents (spore production, chemical composition, and thickness of the cell wall). Surprisingly, there was no elimination of Gram-negative Pseudomonas fluorescens in the parsley samples. Gram-negative rods of oxidase-positive bacteria were isolated at a frequency of 18–33% (Figure 2).
The presence of organic contaminations had a significant impact on the number of microorganisms during ozonation (Figure 3, Table S2). A concentration of 1% organic compounds (in the form of a nutrient medium) decreased the reduction in the number of microorganisms. This was especially important for yeasts and molds, where reduction decreased to levels of R = 19–66%. (Figure 3). Statistically significant differences were found in the reduction in C. cladosporioides mold depending on the number of organic contaminants. A concentration of 50% organic contaminants resulted in no reduction (Table S2). In addition, statistical differences in reduction were observed for this mold at an ozone dose of 1.5 mg/L with contamination levels in the medium of 0.1% and 1%. These observations also concerned B. alba and R. babjevae yeasts. There was no reduction after ozonation with 10% organic contamination. Statistically significant differences in reduction were observed for yeasts with 1% contamination (Table S2). Higher concentrations of organic compounds corresponded to the lower effectiveness of ozonation. However, the addition of organic compounds did not have a significant effect on the high efficiency of ozonation against the tested bacteria (R = 98–100%) (Figure 3). Statistical analysis showed no differences in the reduction in S. warneri bacteria at a dose of contamination above 1% (Table S2). A good effect reducing the number of Pseudomonas fluorescens was achieved after 15 min at both doses, 0.5 and 1.5 O3 mg/L. In the case of this strain, there was no statistically significant effect of the level of contamination on the efficiency of the ozonation process, which was slightly higher at a higher dose of ozone. Similarly, there were no differences relevant to the B. megaterium strain. The reduction was high at both doses, regardless of the level of contamination (Figure 3, Table S2).
Previous model studies have proven that high microbial inhibition rates can be obtained by aqueous and gaseous ozonation, including pathogens Shigella sonnei, Escherichia coli O157:H7, Salmonella Typhimurium, Yersinia enterocolitica, and natural microflora [23,56,57,58,59,60]. However, there have been few studies on parsley leaves. Our studies on parsley isolates confirmed the effectiveness of ozonation in removing microorganisms from water. On the other hand, ozone may have some harmful effects on different plant products, such as losses in color, antioxidant constituents, etc., due to its strong oxidizing activity [61,62,63]. Therefore, in the next stage of this study, we investigated the dose and ozonation time determined under model conditions (1.5 mg/L, 3, 5, 10, 15 min), simulating the process in an industrial plant. During ozonation, the reduction in bacteria and fungi on parsley was measured in relation to the control (before the process). Different weights of parsley were used (25, 50, 75, 100 g). The control sample was parsley rinsed in tap water. The results are presented in Figure 4 and Table S3.
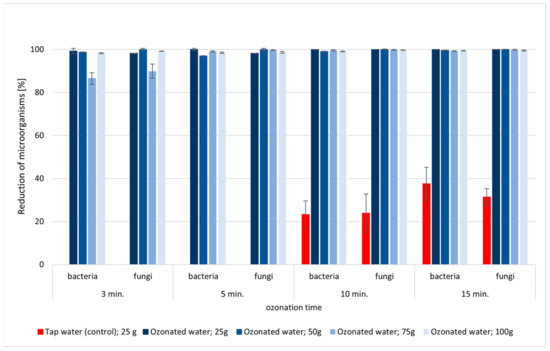
Figure 4.
Reduction in number of microorganisms (fungi and bacteria) in parsley samples rinsed with ozonated water (ozone concentration 1.5 mg/L) in a closed circuit (3, 5, 10, 15 min).
Satisfactory results were obtained after 3 min of ozonation. The reduction in the number of bacteria and fungi was at a high level of R = 85–100% (Figure 4). A statistically significant difference was found in the level of reduction in microorganisms (bacteria and fungi) between the third minute of the process and the next minutes of the ozonation process for all tested systems (Table S3). After 5 min of ozonation, bacteria and fungi were almost completely eliminated from the parsley. Moreover, it is worth noting that with a larger batch of plants (75 g parsley), the decontamination process should be extended to 5 min to achieve the same effect as with a smaller mass of plants. In this case, significant statistical differences were found in the fifth minute of ozonation compared to the previous time (Table S3). It is noteworthy that there was a lack of change in the number of microorganisms in the sample rinsed with tap water. Only after 10 min were about 23% of the microorganisms removed, and after 15 min of rinsing, the number of microorganisms decreased by 30% (Figure 4). Therefore, rinsing parsley leaves in ozonated water with an ozone concentration of 1.5 mg/L in a closed circuit for 5 min can be recommended for industrial processes.
The effects of treatment by washing with distilled, ozonated, and chlorinated water on the numbers of Escherichia coli and Listerina innocua bacteria on fresh parsley leaves have recently been studied by Karaca and Velioglu [44]. It was shown that microbial reduction with ozonation was approximately 1 log higher than the reduction obtained with chlorine. The same authors report that chlorine was more effective against E. coli than ozone (3.2 and 2.2 log reductions, respectively). There was no significant difference in L. innocua counts obtained with chlorine and ozone. In our study, the level of reduction was much higher, which resulted from the optimization of the dose and time of ozonation.
The effects of ozone and salicylic acid on the post-harvest quality of parsley bunches during storage have been studied by Ozturk and Koyuncu [64]. The authors used gaseous ozone at a dose of 2.14 mg/m3. After the process, the parsley batches were stored at 1 °C and 90% (±5%) relative humidity for 28 days. Salicylic acid was found to be a better way of retaining the sensory quality of the parsley, whereas ozone had a negative effect on appearance and taste. During storage after ozonation, the rate of respiration and ethylene production (indicators of metabolic activity) were suppressed in parsley treated with ozone gas. This indicates the inhibition of metabolic processes after ozonation using gaseous ozone.
Studies of other methods of parsley leaves storage (drying and freezing paired with gamma irradiation in doses 0.7–2.7 kGy) showed that a dose of 2.7 kGy could be applied to parsley to promote antibacterial activity against Gram-negative bacteria Escherichia coli and Salmonella Typhimurium [43]. However, this high dose of gamma irradiation may affect the bioactivity of parsley, as well as its content of ascorbic acid, polyphenols, and antiradical capacity.
Color is a very important quality parameter for edible leafy vegetables. The color changes in the parsley leaves after ozonation are presented in Table 3.

Table 3.
Color parameters determined as CIE L*a*b color change (ΔE) on the surface of ozonated leaf parsley after 15 min.
The effects of the ozone treatments on L* values (lightness) of the leaves were significant for samples ozonated with 0.5 O3 mg/L and 1.5 O3 mg/L in both experiments: in ozonated water as well in ozonated water in a closed circuit. The L value for 30 g of parsley with a dose of 1.5 O3 mg/L was statistically significantly higher. The average ΔL* value for all treatments was lower than for the control sample, except for the sample of parsley after ozonation with 1.5 O3 mg/L with 30 g of parsley, for which there were no statistically significant differences between the control parsley washed with water and parsley washed with ozonated water (Table 3). The higher L* values can be explained by the increase in light green color with treatment. These findings showing L* changes toward a lighter color are in agreement with reports from the previous studies. However, previous studies also observed that different treatments may delay the color change in vegetables and fruits [64,65,66]. For example, Ikeura and co-workers report that the treatment of persimmon leaves by ozone microbubble treatment had relatively little effect on leaf quality characteristics [35]. However, we observed that ozone treatment had no statistically significant effects on the a* value. It is known that the green color increases as the a* value moves to the negative (−) direction. In our experiments, we observed a tendency for the color to turn lighter green.
The unfavorable result in terms of maintenance of green color following treatment with ozone in comparison to the control samples may be attributed to its oxidizing properties. Other studies report that green crops became discolored or oxidized after ozonation at higher doses [33]. In our study, no statistically significant effects were observed in a* values after the ozone treatment of 30 g of parsley. The increase in b* values in the positive (+) direction indicates increased yellow color. The higher values of b* values in green vegetables can be explained by the degradation of chlorophyll with aging [67]. We observed a statistically significant increase in b* values only in the sample of 30 g of parsley ozonated with 1.5 mg O3 L−1 and an increase in b* values in the samples of 10 g and 25 g of parsley ozonated with 1.5 O3 mg/L and also in the sample of ozonated water in a closed circuit (Table 3). The effects of the ozone treatment on ΔE* values showed that ozone changed the color of the parsley. Higher doses of ozone resulted in more visible color changes. The changes were more visible (ΔE > 2) when we used 1.5 O3 mg/L (ΔE = 5.8–6.2). However, there were no differences in ΔE depending on the parsley mass, and the ΔE changes were not statistically significant compared to the control sample (Table 3).
In the decontamination process, both obtaining the appropriate microbiological purity and maintaining the product’s usable properties are expected. The sensory evaluation of parsley depends on the chemical composition of the essential oil and the superficial layer. Therefore, the chemical composition of parsley essential oils and volatile organic compounds in the headspace of plant material before and after the ozonation process were evaluated. The tests presented in Table 4 indicate that the raw material did not undergo any change during decontamination.

Table 4.
Chemical composition [%] of parsley essential oils and volatile organic compounds in the headspace of plant material before and after the ozonation process.
The major compounds found in parsley leaves are 1,5,8-p-menthatriene and β-phellandrene. These compounds are considered to make a major contribution to the sensory properties of parsley. Other compounds in parsley leaves include myristicin, α-,p-dimethylstyrene, β-myrcene, and apiole. In some cases, α- and β-pinene are also found [5]. In the present study, 1,5,8-p-menthatriene (2–26%), β-phellandrene (1.6–12%), and myristicin (0.4–15%) were detected in the parsley leaves. The climate conditions, plant species, growth stage, plant tissue, and date of sowing strongly affect the essential oil content of parsley leaves [5]. Aromatic plants may be used either fresh or dried, depending on the purpose. During different processes, such as drying and freezing, several changes occur, depending on the method and conditions of the process. Díaz-Maroto et al. [68] treated parsley leaves by drying them in the air at ambient temperature, at 45 °C, and by freeze-drying. They concluded that air drying at ambient temperature caused less favorable changes in the aromatic constituent profile and better sensory properties than the other two drying methods. Freezing resulted in a slight reduction in the β-phellandrene and 1,3,8-p-menthatriene concentrations in parsley leaves. Drying caused a large decrease in 1,3,8-p-menthatriene and an increase in β-phellandrene. The process of p-mentha-1,3,8-triene oxidation is responsible for changes in parsley aroma and has been reported to occur during the processes of drying and freezing [68].
In our study, there were no significant differences in the chemical compositions of the essential oils in the parsley samples before and after the ozonation process. In the sensory assessment, the smell of parsley treated with ozonated water was more intense than the control sample. In the headspace phase above the parsley, only eight volatile organic compounds were found before the process, and 14–16 compounds were found after ozonation, depending on the ozonation time. The distinct herbal aroma with a peppery sensation is mainly due to α and β phellandrene. The content of these compounds was between 37.0% and 52.6% of the total content of organic compounds. The preliminary results of chemical tests are promising, but they still require repetitions and normalized sensory analysis to be sure that the applied ozonation method minimizes quality and nutrient losses in parsley leaves.
4. Conclusions
Gaseous ozone should not be approved as a basic substance in the food industry due to its hazardous properties. However, according to the EFSA, ozonated water could be approved as a basic substance for decontamination processes. Therefore, our studies focused on using ozonated water instead of gaseous ozone. The results show that washing fresh-cut parsley leaves for 5 min with ozonated water at a dose of 1.5 O3 mg/L is an effective method for 98–100% reduction in all living microorganisms on parsley leaves. We also observed no statistically significant color differences in comparison to the untreated samples. Ozone treatments in aqueous form, with an appropriate concentration of ozone and applied for an appropriate contact time, appear to provide promising qualitative and quantitative results for the decontamination of this fresh-cut plant material. In further work, volatilome and sensory analyses should be conducted before and after ozone treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13158946/s1, Table S1: Number of microorganisms during 15-min ozonation process with different ozone concentrations (0.5 mg/L and 1.5 mg/L, Table S2: Number of microorganisms after 15-min ozonation process with different concentrations of ozone and organic compounds, Table S3: Number of microorganisms (fungi and bacteria) in parsley after rinsing with ozonated water (ozone concentration 1.5 mg/L) in a closed circuit over process time.
Author Contributions
Conceptualization, J.B., D.K., B.G., J.S., and K.Ś.; investigation, J.S., K.J., D.K., W.C.-W., M.M.-F., A.R., and W.L.; writing—original draft preparation, B.G. and W.C.-W.; writing—review and editing, B.G. and D.K.; supervision, B.G., D.K., K.Ś., and J.B.; resources, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding: The Agency for Restructuring and Modernisation of Agriculture (ARMA), Operation “Development of ozone treatment technology for spice plants”, M16 “Cooperation”, Rural Development Program 2014—grant number 202000026.DDD.6509.00054.2019.05.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Draft Guidance for Industry: Guide to Minimize Food Safety Hazards of Fresh-Cut Produce. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-guide-minimize-foodsafety-hazards-fresh-cut-produce (accessed on 7 July 2023).
- Mrozek-Sztela, A.; Rejda, P.; Wińska, K.A. Review of Hygienization Methods of Herbal Raw Materials. Appl. Sci. 2020, 10, 8268. [Google Scholar] [CrossRef]
- Gryczka, U.; Kameya, H.; Kimura, K.; Todoriki, S.; Migdał, W.; Bułka, S. Efficacy of low energy electron beam on microbial decontamination of spices. Radiat. Phys. Chem. 2020, 170, 108662. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kumar, V.; Khole, S.; Sanyal, B.; Murali, T.S.; Variyar, P.S. Radiation processing: An effective quality control tool for hygienization and extending shelf life of a herbal formulation, Amritamehari churnam. J. Radiat. Res. Appl. Sci. 2015, 9, 86–95. [Google Scholar] [CrossRef]
- Pinto, C.A.; Holovicova, M.; Habanova, M.; Lima, V.; Duarte, R.V.; Barba, F.J.; Saraiva, J.V. Effects of High Hydrostatic Pressure on Fungal Spores and Plant Bioactive Compounds. Encyclopedia 2022, 2, 1453–1463. [Google Scholar] [CrossRef]
- Atungulu, G.G.; Pan, Z. Microbial decontamination of nuts and spices. In Microbial Decontamination in the Food Industry; Novel Methods and Applications; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2012; pp. 125–162. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT-Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Gabler, F.M.; Smilanick, J.L.; Mansour, M.F.; Karaca, H. Influence of fumigation with high concentrations of ozone gas on postharvest gray mold and fungicide residues on table grapes. Postharvest Biol. Technol. 2010, 55, 85–90. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Application of ozone for the postharvest treatment of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef] [PubMed]
- İbanoğlu, Ş. Non-Thermal Food Processing Operations, Unit Operations and Processing Equipment in the Food Industry; Woodhead Publishing: Sawston, UK, 2023; pp. 1–14. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Effects of ozone treatments on microbial quality and some chemical properties of lettuce, spinach, and parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A review into the effectiveness of ozone technology for improving the safety and preserving the quality of fresh-cut fruits and vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef]
- O’Donnell, C.; Tiwari, B.K.; Cullen, P.J.; Rice, R.G. Ozone in Food Processing; Wiley and Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Palou, L.; Crisosto, C.H.; Smilanick, J.L.; Adaskaveg, J.E.; Zoffoli, J.P. Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biol. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Salvador, A.; Abad, I.; Arnal, L.; Martínez-Jávega, J.M. Effect of ozone on postharvest quality of persimmon. J. Food Sci. 2006, 71, S443–S446. [Google Scholar] [CrossRef]
- De Souza, L.P.; Faroni, L.R.D.A.; Heleno, F.F.; Cecon, P.R.; Gonçalves, T.D.C.; da Silva, G.J.; Prates, L.H.F. Effects of ozone treatment on postharvest carrot quality. LWT-Food Sci. Technol. 2018, 90, 53–60. [Google Scholar] [CrossRef]
- Luo, A.; Bai, J.; Li, R.; Fang, Y.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.; Liping, K. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Bolel, H.; Koyuncu, M.A.; Erbaş, D. The effects of ozone and fungicide treatments on the fruit quality changes of pomegranate during cold storage. J. Imaging Sci. Technol. 2019, 9, 1841–1850. [Google Scholar]
- Nadas, A.; Olmo, M.; Garci, J.M. Growth of Botrytis cinerea and strawberry quality in ozone-enriched atmo-spheres. J. Food Sci. 2003, 68, 1798–1802. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Yu, Z.; Gao, X. Preservation of fresh-cut celery by treatment of ozonated water. Food Control 2005, 16, 279–283. [Google Scholar] [CrossRef]
- Garcia, A.; Mount, J.R.; Davidson, P.M. Ozone and chlorine treatment of minimally processed lettuce. J. Food Sci. 2003, 68, 2747–2751. [Google Scholar] [CrossRef]
- Ikeura, H.; Hamasaki, S.; Tamaki, M. Effects of ozone microbubble treatment on removal of residual pesticides and quality of persimmon leaves. Food Chem. 2013, 138, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Dziugan, P.; Balcerek, M.; Binczarski, M.J.; Kregiel, D.; Kucner, M.; Kunicka-Styczynska, A.; Pielech-Przybylska, K.; Smigielski, K.; Witonska, I.A. Ozonation as an effective way to stabilize new kinds of fermentation media used in biotechnological production of liquid fuel additives. Biotechnol. Biofuels 2016, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of ozonation process on the microbiological and antioxidant status of raspberry (Rubus ideaeus L.) fruit during storage at room temperature. Agric. Food Sci. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Piechowiak, T.; Balawejder, M. Influence of drying temperature on the content of bioactive compounds in scots pine (Pinus sylvestris L.) Shoots as well as yield and composition of essential oils. Acta Univ. Cinbinesis Ser. E Food Technol. 2020, 1, 15–24. [Google Scholar] [CrossRef]
- EFSA Panel of Biological Hazards. Scientific Opinion on the risk posed by pathogens in food of non-animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). EFSA J. 2012, 11, 3025. [Google Scholar] [CrossRef]
- EFSA. Outcome of the consultation with Member States and EFSA on the basic substance application for approval of ozone to be used in plant protection as a bactericide, fungicide, insecticide, nematicide and viricide. EFSA Support. Publ. 2021, 18, EN-6659. [Google Scholar] [CrossRef]
- Opara, E.; Chochan, M. Culinary herbs and spices: Their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef] [PubMed]
- Otunola, G.A. Culinary Spices in Food and Medicine: An Overview of Syzygium aromaticum (L.) Merr. and L. M. Perry [Myrtaceae]. Front. Pharmacol. 2021, 12, 793200. [Google Scholar] [CrossRef] [PubMed]
- Topka, P.; Poliński, S.; Sawicki, T.; Szydłowska_Czerniak, A.; Tańska, M. Effect of Enriching Gingerbread Cookies with Elder (Sambucus nigra L.) Products on Their Phenolic Composition, Antioxidant and Anti-Glycation Properties, and Sensory Acceptance. Int. J. Mol. Sci. 2023, 24, 1493. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. Effect of freezing, drying and the duration of storage on the composition of essential oils of plain-leafed parsley [Petroselinum crispum (Mill.) Nym. ssp. neapolitanum Danert] and turnip-rooted parsley [Petroselinum crispum (Mill.) Nym. ssp. tuberosum (Bernh.) Crov.]. Flavour Fragr. J. 2010, 25, 28–34. [Google Scholar] [CrossRef]
- Dobricevic, N.; Sic Zlabur, J.; Voca, S.; Pliestic, S.; Galic, A.; Delic, A.; Fabek Uher, S. Bioactive compounds content and nutritional potential of different parsley parts (Petroselinum crispum Mill.). J. Cent. Eur. Agric. 2019, 20, 900–910. [Google Scholar] [CrossRef]
- Farah, H.; Elbadrawy, E.; Al-atoom, A.A. Evaluation of antioxidant and antimicrobial activities of ethanolic extracts of parsley (Petroselinum erispum) and coriander (Coriandrum sativum) plants grown in Saudi Arabia. Int. J. Adv. Res. 2015, 3, 1244–1255. [Google Scholar]
- Zhang, H.; Chen, F.; Wang, X.; Yao, H.Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res. Int. 2006, 39, 833–839. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activity. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U.; Luty, M.; Czyż, J. Effect of fortification with parsley (Peroselinum crispum Mill.) leaves on the nutraceutical and nutritional quality of wheat pasta. Food Chem. 2016, 190, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Tongesayi, T.; Tongesayi, S. Contaminated irrigation water and the associated public health risks. In Food, Energy, and Water; Elsevier: Amsterdam, The Netherlands, 2015; pp. 349–381. [Google Scholar] [CrossRef]
- Kowalska, B.; Szczech, M. Differences in microbiological quality of leafy green vegetables. Ann. Agric. Environ. Med. 2022, 29, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.M.; Hickey, D.K.; Alonso-Gomez, M.; Wilkinson, G.M. The microbiological quality of commercial herb and spice preparations used in the formulation of a chicken supreme ready meal and microbial survival following a simulated industrial heating process. Food Control 2011, 22, 616–625. [Google Scholar] [CrossRef]
- Kortei, N.K.; Djaba, B.T.; Tettey, C.O.; Agyemang, A.O.; Aninagyei, E.; Essuman, E.K.; Boakye, A.A.; Annan, T. Toxicogenic Fungi, Aflatoxins, and Antimicrobial Activities Associated with Some Spices and Herbs from Three Selected Markets in Ho Municipality, Ghana. Int. J. Food Sci. 2022, 2022, 7195890. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.J. Effects of Ozone Wash for Inactivation of S. Typhimurium and Background Microbiota on Lettuce and Parsley. J. Food Saf. 2013, 33, 273–281. [Google Scholar] [CrossRef]
- Cătunescu, G.M.; Rotar, I.; Vidican, R.; Bunghez, F.; Rotar, A.M. Gamma radiation enhances the bioactivity of fresh parsley (Petroselinum crispum (Mill.) Fuss Var. Neapolitanum). Radiat. Phys. Chem. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Effects of ozone and chlorine washes and subsequent cold storage on microbiological quality and shelf life of fresh parsley leaves. LWT-Food Sci Technol. 2020, 127, 109421. [Google Scholar] [CrossRef]
- Baron, E.J. Classification. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8406/ (accessed on 20 May 2023).
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms-From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef]
- Liszkowska, W.; Motyl, I.; Pielech-Przybylska, K.; Szulc, J.; Sypka, M.; Dziugan, P.; Berlowska, J. Plant biomass as a source of low-temperature yeasts. BioResources 2023, 18, 599–612. [Google Scholar] [CrossRef]
- Stepień, Ł.; Koczyk, G.; Waskiewicz, A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J. Appl. Genet. 2011, 52, 487–496. [Google Scholar] [CrossRef]
- Zając, I.; Szulc, J.; Gutarowska, B. The effect of ethylene oxide and silver nanoparticles on photographic models in the context of disinfection of photo albums. J. Cult. Herit. 2021, 51, 59–70. [Google Scholar] [CrossRef]
- Merlini, V.V.; Pena, F.D.; da Cunha, D.T.; de Oliveira, J.M.; Rostagno, M.A.; Antunes, A.E.C. Microbiological quality of organic and conventional leafy vegetables. J. Food Qual. 2018, 2018, 4908316. [Google Scholar] [CrossRef]
- Badosa, E.; Trias, R.; Pares, D.; Pla, M.; Montesinos, E. Microbiological quality of fresh fruit and vegetable products in Catalonia (Spain) using normalized plate counting methods and real time polymerase chain reaction (QPCR). J. Sci. Food Agric. 2008, 88, 605–611. [Google Scholar] [CrossRef]
- Rosberg, A.K.; Darlison, J.; Mogren, L.; Alsanius, B.W. Commercial wash of leaf vegetables do not significantly decrease bacterial load but leads to shifts in bacterial species composition. Food Microbiol. 2021, 94, 103667. [Google Scholar] [CrossRef]
- Kakde, U.; Kakde, H.U. Incidence of post-harvest disease and airborne fungal spores in a vegetable market. Acta Bot. Croat. 2012, 71, 147–157. [Google Scholar] [CrossRef][Green Version]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agriculture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Enikova, R.K.; Stoyonovska, M.R.; Karcheva, M.D. Mycotoxins in fruits and vegetables. J. IMAB 2020, 26, 3139–3143. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltran, D.; Chacon-Vera, E.; Gil, M.I. Effect of ozone on the inactivation of Yersinia enterocolitica and the reduction of natural flora on potatoes. J. Food Protect. 2006, 69, 2357–2363. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltran, D.; Allende, A.; Chacon-Vera, E.; Gil, M.I. Elimination by ozone of Shigella sonnei in shredded lettuce and water. Food Microbiol. 2007, 24, 492–499. [Google Scholar] [CrossRef]
- Selma, M.V.; Ibanez, A.M.; Allende, A.; Cantwell, M.; Suslow, T. Effect of gaseous ozone and hot water on microbial and sensory quality of cantaloupe and potential transference of Escherichia coli O157:H7 during cutting. Food Microbiol. 2008, 25, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Ibanez, A.M.; Cantwell, M.; Suslow, T. Reduction by gaseous ozone of Salmonella and microbial flora associated with fresh-cutcantaloupe. Food Microbiol. 2008, 25, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Hassenberg, K.; Frohling, A.; Geyer, M.; Schluter, O.; Herppich, W.B. Ozonated wash water for inhibition of Pectobacterium carotovorumon carrots and the effect on the physiological behaviour of produce. Eur. J. Hortic. Sci. 2008, 73, S37–S42. [Google Scholar]
- Allende, A.; Marin, A.; Buendia, B.; Tomas-Barberan, F.; Gil, M.I. Impact of combined postharvest treatments (UV-C light, gaseous O3, superat-mospheric O2 and high CO2) on health promoting compounds and shelf-life of strawberries. Postharvest Biol. Technol. 2007, 46, 201–211. [Google Scholar] [CrossRef]
- Vurma, M.; Pandit, R.B.; Sastry, S.K.; Yousef, A.E. Inactivation of Escherichia coli O157:H7 and natural microbiota on spinach leaves using gaseous ozone during vacuum cooling and simulated transportation. J. Food Protect. 2009, 72, 1538–1546. [Google Scholar] [CrossRef]
- Klockow, P.A.; Keener, K.M. Safety and quality assessment of packaged spinach treated with a novel ozone-generation system. LWT-Food Sci. Technol. 2009, 42, 1047–1053. [Google Scholar] [CrossRef]
- Ozturk, K.U.; Koyuncu, M.A. Effects of ozone and salicylic acid on post-harvest quality of parsley during storage. Biol. Agric. Hortic. 2021, 37, 183–196. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, Y. Exogenous salicylic acid inhibits browning of fresh–cut Chinese water chestnut. Food Chem. 2006, 94, 535–540. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Sci. Hortic. 2012, 142, 221–228. [Google Scholar] [CrossRef]
- Botondi, R.; DeSantis, D.; Bellincontro, A.; Vizovitis, K.; Mencarelli, F. Influence of ethylene inhibition by 1-methylcyclopropene on apricot quality, volatile production, and glycosidase activity of low- and high-aroma varieties of apricots. J. Agric. Food Chem. 2003, 51, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Maroto, C.M.; Viňas, G.M.A.; Cabezudo, D.M. Evaluation of the effect of drying on aroma of parsley by free choiceprofiling. Eur. Food Res. Technol. 2003, 216, 227–232. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).