A Novel Functional Refined Olive Oil, Enhanced with Orange Peel Extract, Modulates Postprandial LDL-Cholesterol Responses in Individuals at Cardiometabolic Risk: A Pilot Randomized, Controlled, Cross-Over Nutritional Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Recruiting and Screening
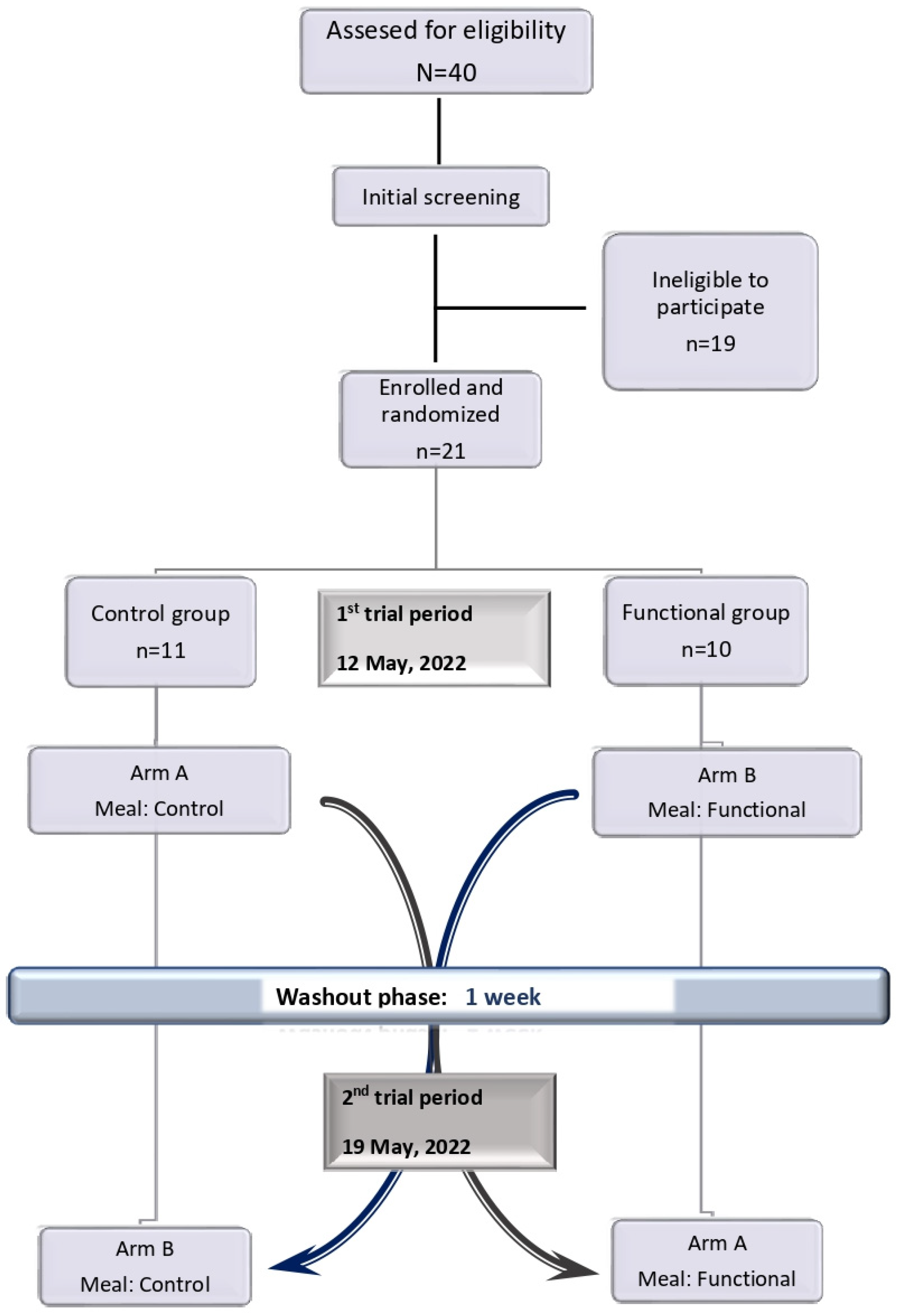
2.3. Experimental Design
2.4. Test Meals
2.5. Blood Sampling and Analysis
2.6. Statistical Analysis
2.6.1. Sample Size
2.6.2. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Postprandial Biochemical and Antioxidant Biomarkers
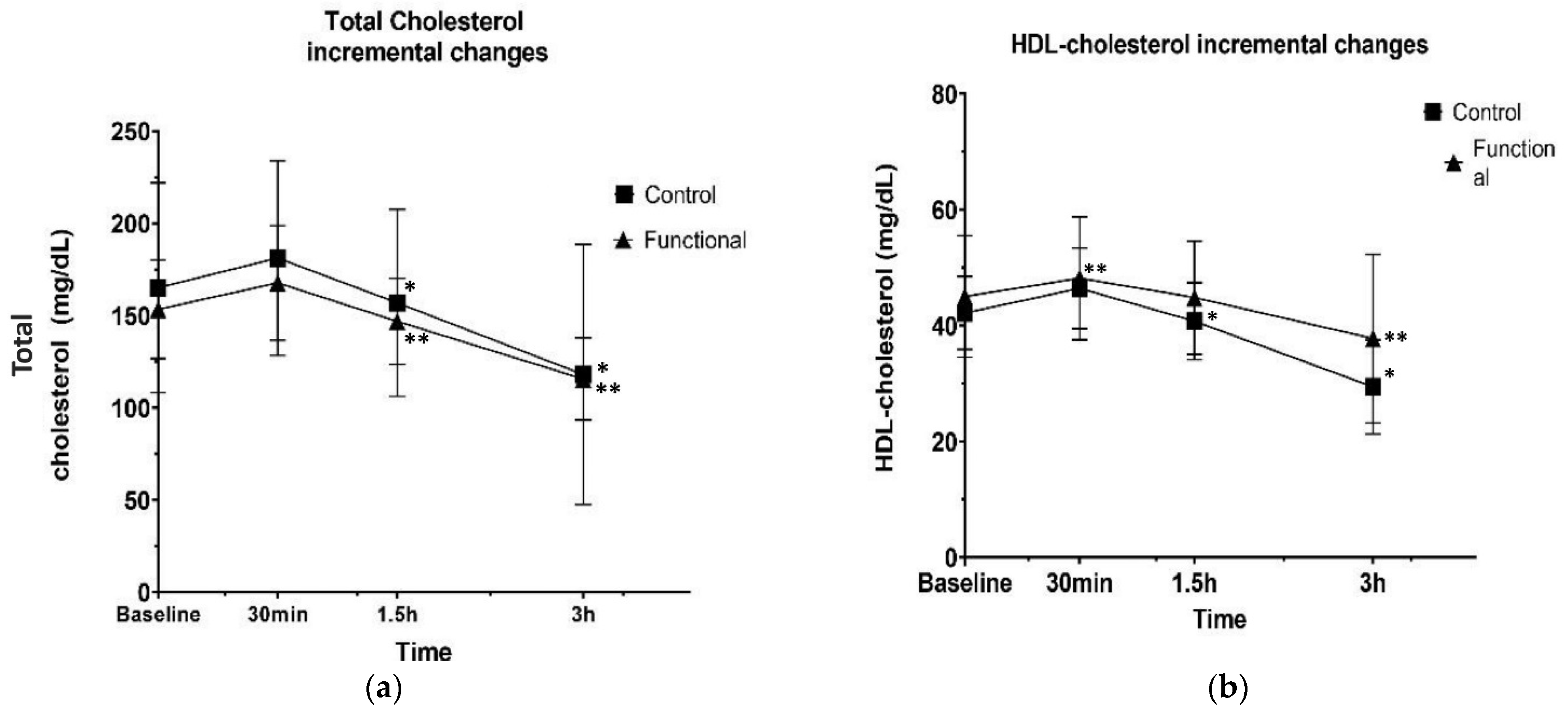
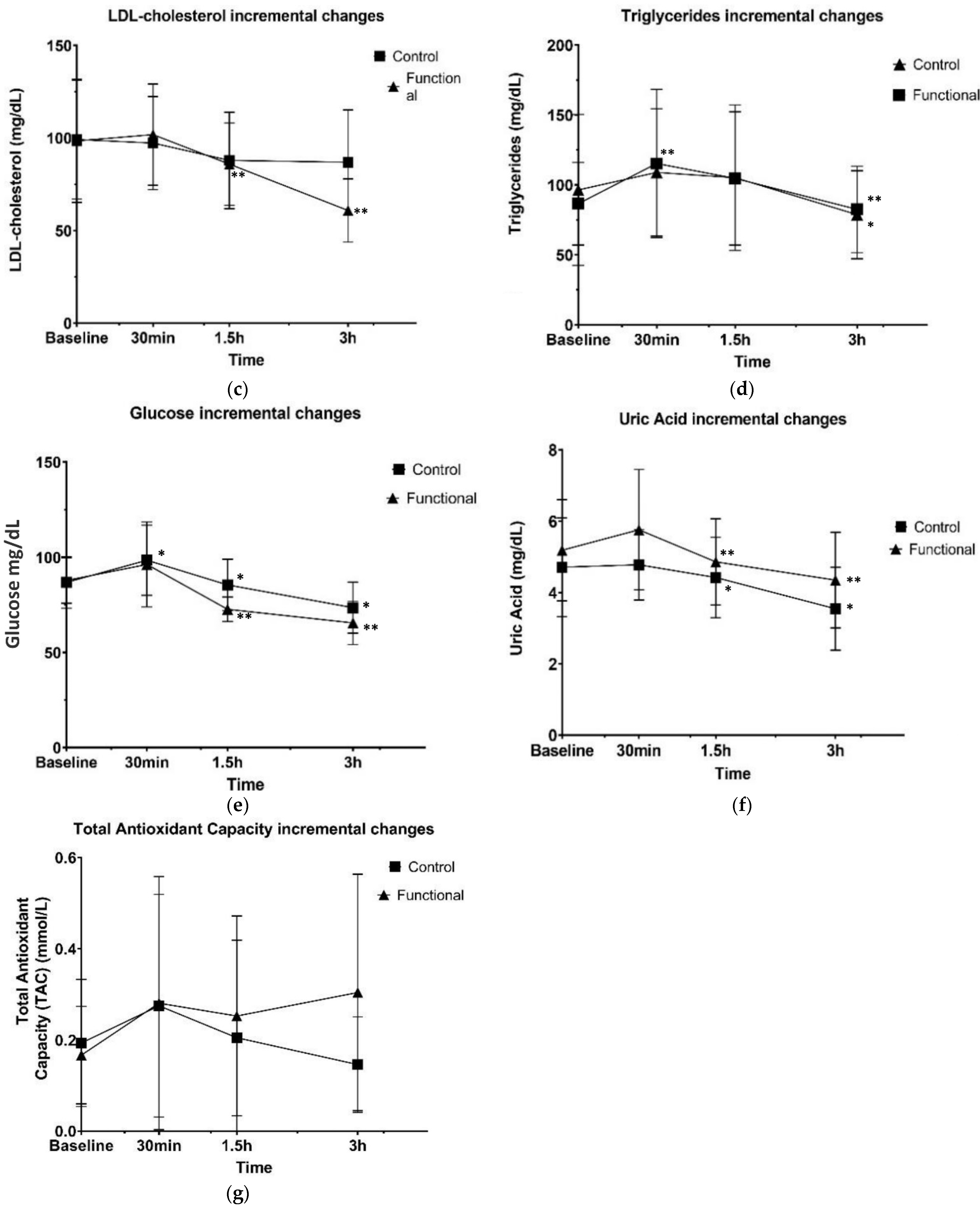
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Detopoulou, P.; Fragopoulou, E.; Nomikos, T.; Antonopoulou, S. Mediterranean Diet and the Postprandial State: A Focus on Lipemia, Glycemia, and Thrombosis; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Covas, M.I.; De La Torre, R.; Fitó, M. Virgin Olive Oil: A Key Food for Cardiovascular Risk Protection. Br. J. Nutr. 2015, 113, S19–S28. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Barricarte, A.; Ardanaz, E.; Moreno-Iribas, C.; Sánchez, M.J.; Molina-Montes, E.; Chirlaque, M.D.; Huerta, J.M.; Navarro, C.; et al. Olive Oil Intake and CHD in the European Prospective Investigation into Cancer and Nutrition Spanish Cohort. Br. J. Nutr. 2012, 108, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J.; Salvador, M.D.; Cancho-Grande, B.; Fregapane, G. State of the Art on Functional Virgin Olive Oils Enriched with Bioactive Compounds and Their Properties. Int. J. Mol. Sci. 2017, 18, 668. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Classification and Antioxidant Activity Evaluation of Edible Oils by Using Nanomaterial-Based Electrochemical Sensors. Int. J. Mol. Sci. 2023, 24, 3010. [Google Scholar] [CrossRef]
- Gomes, T.; Caponio, F.; Delcuratolo, D. Non-Conventional Parameters for Quality Evaluation of Refined Oils with Special Reference to Commercial Class Olive Oil. Food Chem. 2003, 83, 403–408. [Google Scholar] [CrossRef]
- Sánchez De Medina, V.; Priego-Capote, F.; Luque De Castro, M.D. Characterization of Refined Edible Oils Enriched with Phenolic Extracts from Olive Leaves and Pomace. J. Agric. Food Chem. 2012, 60, 5866–5873. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Correia, R.; Félix, S.; Ferreira, P.; Gordon, M.H. Effects of Enrichment of Refined Olive Oil with Phenolic Compounds from Olive Leaves. J. Agric. Food Chem. 2007, 55, 4139–4143. [Google Scholar] [CrossRef]
- Papagianni, O.; Moulas, I.; Loukas, T.; Magkoutis, A.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Trends in Food Innovation: An Interventional Study on the Benefits of Consuming Novel Functional Cookies Enriched with Olive Paste. Sustainability 2021, 13, 11472. [Google Scholar] [CrossRef]
- Katsa, M.E.; Nomikos, T. Olive Oil Phenolics and Platelets—From Molecular Mechanisms to Human Studies. Rev. Cardiovasc. Med. 2022, 23, 255. [Google Scholar] [CrossRef]
- Papagianni, O.; Delli, E.; Vasila, M.; Loukas, T.; Magkoutis, A.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. The Acute Effect of a Novel Miso-Type Sauce, Enhanced with a Carotenoid-Rich Extract from Fruit By-Products, on Postprandial Biomarkers of Oxidative Stress and Inflammation. Nutrients 2022, 14, 1316. [Google Scholar] [CrossRef]
- Dimina, L.; Mariotti, F. The Postprandial Appearance of Features of Cardiometabolic Risk: Acute Induction and Prevention by Nutrients and Other Dietary Substances. Nutrients 2019, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Loffredo, L.; Del Ben, M.; Angelico, F.; Nocella, C.; Petruccioli, A.; Bartimoccia, S.; Monticolo, R.; Cava, E.; Violi, F. Extra Virgin Olive Oil Improves Post-Prandial Glycemic and Lipid Profile in Patients with Impaired Fasting Glucose. Clin. Nutr. 2017, 36, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; Bermúdez, B.; López, S.; Abia, R.; Villar, J.; Muriana, F.J.G. Minor Compounds of Olive Oil Have Postprandial Anti-Inflammatory Effects. Br. J. Nutr. 2007, 98, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Daimiel, L.; Micó, V.; Valls, R.M.; Pedret, A.; Motilva, M.J.; Rubió, L.; Fitó, M.; Farrás, M.; Covas, M.I.; Solá, R.; et al. Impact of Phenol-Enriched Virgin Olive Oils on the Postprandial Levels of Circulating MicroRNAs Related to Cardiovascular Disease. Mol. Nutr. Food Res. 2020, 64, 2100670. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, M.; Bianchi, A.; Sanmartin, C.; Taglieri, I.; Venturi, F.; Testai, L.; Flori, L.; Calderone, V.; De Leo, M.; Braca, A.; et al. By-Products from Winemaking and Olive Mill Value Chains for the Enrichment of Refined Olive Oil: Technological Challenges and Nutraceutical Features. Foods 2020, 9, 1390. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Papagianni, O.; Loukas, T.; Magkoutis, A.; Kagoudi, M.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Postprandial Responses of Serum Cholesterol, Glucose and Plasma Antioxidant Activity, after Intake of an Innovative High Fat Mayonnaise-Based Appetizer, Enhanced with Olive Paste, in Healthy Volunteers. Life 2022, 12, 1385. [Google Scholar] [CrossRef]
- Papagianni, O.; Argyri, K.; Loukas, T.; Magkoutis, A.; Biagki, T.; Skalkos, D.; Kafetzopoulos, D.; Dimou, C.; Karantonis, H.C.; Koutelidakis, A.E. Postprandial Bioactivity of a Spread Cheese Enriched with Mountain Tea and Orange Peel Extract in Plasma Oxidative Stress Status, Serum Lipids and Glucose Levels: An Interventional Study in Healthy Adults. Biomolecules 2021, 11, 1241. [Google Scholar] [CrossRef]
- Khandouzi, N.; Zahedmehr, A.; Nasrollahzadeh, J. Effect of Polyphenol-Rich Extra-Virgin Olive Oil on Lipid Profile and Inflammatory Biomarkers in Patients Undergoing Coronary Angiography: A Randomised, Controlled, Clinical Trial. Int. J. Food Sci. Nutr. 2021, 72, 548–558. [Google Scholar] [CrossRef]
- Covas, M.I.; De La Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL Phenolic Content and LDL Oxidation Are Modulated by Olive Oil Phenolic Compounds in Humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef]
- Mezhal, F.; Oulhaj, A.; Abdulle, A.; Aljunaibi, A.; Alnaeemi, A. High Prevalence of Cardiometabolic Risk Factors amongst Young Adults in the United Arab Emirates: The UAE Healthy Future Study. BMC Cardiovasc. Disord. 2023, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.P.; Fagundes, M.B.; Guerra, D.R.; Leães, Y.S.V.; Speroni, C.S.; Robalo, S.S.; Emanuelli, T.; Cichoski, A.J.; Wagner, R.; Barin, J.S.; et al. Ultrasound Assisted Maceration for Improving the Aromatization of Extra-Virgin Olive Oil with Rosemary and Basil. Food Res. Int. 2020, 135, 109305. [Google Scholar] [CrossRef] [PubMed]
- Argyri, E.-A.; Piromalis, S.-P.; Koutelidakis, A.; Kafetzopoulos, D.; Petsas, A.S.; Skalkos, D.; Nasopoulou, C.; Dimou, C.; Karantonis, H.C. Olive Paste-Enriched Cookies Exert Increased Antioxidant Activities. Appl. Sci. 2021, 11, 5515. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Cardiovascular, E.S.C. SCORE2 Risk Prediction Algorithms: New Models to Estimate 10-Year Risk of Cardiovascular Disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Uylaşer, V.; Yildiz, G. The Historical Development and Nutritional Importance of Olive and Olive Oil Constituted an Important Part of the Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef]
- De La Torre-Carbot, K.; Chávez-Servín, J.L.; Jaúregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Nurmi, T.; Poulsen, H.E.; Gaddi, A.V.; Kaikkonen, J.; Zunft, H.F.; et al. Elevated Circulating LDL Phenol Levels in Men Who Consumed Virgin Rather than Refined Olive Oil Are Associated with Less Oxidation of Plasma LDL. J. Nutr. 2010, 140, 501–508. [Google Scholar] [CrossRef]
- Hernáez, Á.; Remaley, A.T.; Farràs, M.; Fernández-Castillejo, S.; Subirana, I.; Schröder, H.; Fernández-Mampel, M.; Muñoz-Aguayo, D.; Sampson, M.; Solà, R.; et al. Olive Oil Polyphenols Decrease LDL Concentrations and LDL Atherogenicity in Men in a Randomized Controlled Trial. J. Nutr. 2015, 145, 1692–1697. [Google Scholar] [CrossRef]
- Akiyama, S.; Katsumata, S.I.; Suzuki, K.; Nakaya, Y.; Ishimi, Y.; Uehara, M. Hypoglycemic and Hypolipidemic Effects of Hesperidin and Cyclodextrin-Clathrated Hesperetin in Goto-Kakizaki Rats with Type 2 Diabetes. Biosci. Biotechnol. Biochem. 2009, 73, 2779–2782. [Google Scholar] [CrossRef]
- Kandyliari, A.; Potsaki, P.; Bousdouni, P.; Kaloteraki, C.; Christofilea, M.; Almpounioti, K.; Moutsou, A.; Fasoulis, C.K.; Polychronis, L.V.; Gkalpinos, V.K.; et al. Development of Dairy Products Fortified with Plant Extracts: Antioxidant and Phenolic Content Characterization. Antioxidants 2023, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K. Plant-Derived Bioactives: Chemistry and Mode of Action, 1st ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Muhtadi; Haryoto; Azizah, T.; Suhendi, A.; Yen, K.H. Antidiabetic and Antihypercholesterolemic Activities of Citrus Sinensis Peel: In Vivo Study. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 382–385. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A Therapeutic Agent for Obesity. Drug Des. Devel. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef]
- Jacome-Sosa, M.; Parks, E.J.; Bruno, R.S.; Tasali, E.; Lewis, G.F.; Schneeman, B.O.; Rains, T.M. Postprandial Metabolism of Macronutrients and Cardiometabolic Risk: Recent Developments, Emerging Concepts, and Future Directions. Adv. Nutr. 2016, 7, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Schönknecht, Y.B.; Crommen, S.; Stoffel-Wagner, B.; Coenen, M.; Fimmers, R.; Holst, J.J.; Simon, M.C.; Stehle, P.; Egert, S. Acute Effects of Three Different Meal Patterns on Postprandial Metabolism in Older Individuals with a Risk Phenotype for Cardiometabolic Diseases: A Randomized Controlled Crossover Trial. Mol. Nutr. Food Res. 2020, 64, 1901035. [Google Scholar] [CrossRef]
- Bozzetto, L.; Della Pepa, G.; Vetrani, C.; Rivellese, A.A. Dietary Impact on Postprandial Lipemia. Front. Endocrinol. 2020, 11, 337. [Google Scholar] [CrossRef]
| Nutritional Composition of the Test Meals | Control | Functional |
|---|---|---|
| Energy (kcal) | 618.53 | 618.53 |
| Carbohydrates, total (g) | 50.03 | 50.03 |
| Carbohydrates, net (g) | 45.53 | 45.53 |
| Fat, total (g) | 45.90 | 45.90 |
| Protein (g) | 4.28 | 4.28 |
| Saturated fat (g) | 6.37 | 6.37 |
| Unsaturated fat (g) | 38.22 | 38.22 |
| Cholesterol (mg) | 0 | 0 |
| Dietary fiber, total (g) | 4.50 | 4.50 |
| Sugar, total (g) | 2.12 | 2.12 |
| Total phenolics (mg GAE/L) | 10.83 | 36.28 |
| Total antioxidant capacity (mmol Fe2+/L) | 0.20 | 0.51 |
| General Baseline Characteristics | |
| Number (N) | 21 (57% male) |
| Age (years) | 46 ± 10.6 |
| General Lifestyle Habits | |
| Smokers (N) | 18 |
| Anthropometric characteristics | |
| Height (cm) | 170.16 ± 6.93 |
| Weight (kg) | 85.64 ± 23.59 |
| Body mass index (kg/m2) | 29.29 ± 6.68 |
| Waist/hip circumference (index) | 0.86 ± 0.10 |
| Clinical characteristics | |
| Diastolic pressure (mm Hg) | 79.82 ± 11.06 |
| LDL-cholesterol (mg/dL) | 105.99 ± 42.91 |
| Triglycerides (mg/dL) | 99.10 ± 45.13 |
| Fasting glucose (mg/dL) | 93.68 ± 14.01 |
| Baseline | Δ 1 30 min | Δ 1 1.5 h | Δ 1 3 h | p Value Group Effect | p Value Time Effect | p Value Group × Time Interaction | ||
|---|---|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | Control | 165.08 ± 56.98 | 16.16 ± 11.43 | −24.33 ± 17.20 | −38.83 ± 27.45 | 0.613 | <0.001 | 0.857 |
| Functional | 166.33 ± 51.75 | 14.33 ± 10.13 | −20.83 ± 14.73 | −31.25 ± 22.09 | ||||
| HDL-cholesterol (mg/dL) | Control | 42.16 ± 6.32 | 12.50 ± 8.83 | −3.75 ± 2.65 | −26.41 ± 18.67 | 0.159 | <0.001 | 0.245 |
| Functional | 45.00 ± 10.47 | 28.75 ± 20.32 | −10.66 ± 7.54 | −22.16 ± 15.67 | ||||
| LDL-cholesterol (mg/dL) | Control | 99.71 ± 31.66 | 4.25 ± 3.00 | −5.66 ± 4.00 | −11.33 ± 8.01 | 0.012 | <0.001 | <0.001 |
| Functional | 96.70 ± 32.15 | 3.16 ± 2.23 | −3.33 ± 2.35 | −7.08 ± 5.00 | ||||
| Triglycerides (mg/dL) | Control | 96.41 ± 51.00 | −1.89 ± 1.34 | −9.36 ± 6.62 | −0.99 ± 0.70 | 0.997 | <0.001 | 0.589 |
| Functional | 86.58 ± 29.53 | 3.35 ± 2.37 | −15.91 ± 11.25 | −25.04 ± 17.70 | ||||
| Glucose (mg/dL) | Control | 86.63 ± 13.60 | 11.66 ± 8.24 | −13.00 ± 9.19 | −12.00 ± 8.48 | 0.164 | <0.001 | 0.162 |
| Functional | 89.73 ± 12.83 | 8.41 ± 5.95 | −23.58 ± 16.67 | −7.16 ± 5.06 | ||||
| Uric acid (mg/dL) | Control | 4.71 ± 1.38 | 0.06 ± 0.04 | −0.35 ± 0.25 | −0.87 ± 0.61 | 0.238 | <0.001 | 0.177 |
| Functional | 5.08 ± 1.39 | 0.57 ± 0.40 | −0.90 ± 0.63 | −0.51 ± 0.36 | ||||
| Total plasma antioxidant capacity (TAC) (mmol/L) | Control | 0.17 ± 0.13 | 0.08 ± 0.05 | −0.07 ± 0.04 | −0.05 ± 0.04 | 0.551 | 0.118 | 0.156 |
| Functional | 0.14 ± 0.10 | 0.11 ± 0.80 | −0.02 ± 0.02 | 0.05 ± 0.03 |
| p Value Δ 1 30 min | p Value Δ 1 1.5 h | p Value Δ 1 3 h | ||
|---|---|---|---|---|
| Total cholesterol (mg/dL) | Control | 0.24 | 0.02 *2 | 0.001 *2 |
| Functional | 0.12 | 0.03 **3 | 0.002 **3 | |
| HDL-cholesterol (mg/dL) | Control | 0.09 | 0.004 *2 | <0.001 *2 |
| Functional | 0.04 **3 | 0.05 | 0.01 **3 | |
| LDL-cholesterol (mg/dL) | Control | 0.66 | 0.06 | 0.89 |
| Functional | 0.54 | 0.01 **3 | <0.001 **3 | |
| Triglycerides (mg/dL) | Control | 0.19 | 0.58 | 0.01 *2 |
| Functional | 0.006 **3 | 0.20 | 0.18 | |
| Glucose (mg/dL) | Control | 0.02 *2 | 0.007 *2 | 0.002 *2 |
| Functional | 0.22 | 0.003 **3 | 0.04 **3 | |
| Uric acid (mg/dL) | Control | 0.78 | 0.02 *2 | 0.001 *2 |
| Functional | 0.20 | 0.05 | 0.002 **3 | |
| Total plasma antioxidant capacity (TAC) (mmol/L) | Control | 0.08 | 0.22 | 0.33 |
| Functional | 0.18 | 0.70 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagianni, O.; Kaloteraki, C.; Kandyliari, A.; Potsaki, P.; Bousdouni, P.; Almpounioti, K.; Ouzaid, C.; Mavrou, A.-K.; Panteli, V.; Loukas, T.; et al. A Novel Functional Refined Olive Oil, Enhanced with Orange Peel Extract, Modulates Postprandial LDL-Cholesterol Responses in Individuals at Cardiometabolic Risk: A Pilot Randomized, Controlled, Cross-Over Nutritional Intervention. Appl. Sci. 2023, 13, 8574. https://doi.org/10.3390/app13158574
Papagianni O, Kaloteraki C, Kandyliari A, Potsaki P, Bousdouni P, Almpounioti K, Ouzaid C, Mavrou A-K, Panteli V, Loukas T, et al. A Novel Functional Refined Olive Oil, Enhanced with Orange Peel Extract, Modulates Postprandial LDL-Cholesterol Responses in Individuals at Cardiometabolic Risk: A Pilot Randomized, Controlled, Cross-Over Nutritional Intervention. Applied Sciences. 2023; 13(15):8574. https://doi.org/10.3390/app13158574
Chicago/Turabian StylePapagianni, Olga, Chrysoula Kaloteraki, Aikaterini Kandyliari, Panagiota Potsaki, Panorea Bousdouni, Kalliopi Almpounioti, Camille Ouzaid, Anna-Kyriaki Mavrou, Vasiliki Panteli, Thomas Loukas, and et al. 2023. "A Novel Functional Refined Olive Oil, Enhanced with Orange Peel Extract, Modulates Postprandial LDL-Cholesterol Responses in Individuals at Cardiometabolic Risk: A Pilot Randomized, Controlled, Cross-Over Nutritional Intervention" Applied Sciences 13, no. 15: 8574. https://doi.org/10.3390/app13158574
APA StylePapagianni, O., Kaloteraki, C., Kandyliari, A., Potsaki, P., Bousdouni, P., Almpounioti, K., Ouzaid, C., Mavrou, A.-K., Panteli, V., Loukas, T., Magkoutis, A., Skalkos, D., Karantonis, H. C., & Koutelidakis, A. E. (2023). A Novel Functional Refined Olive Oil, Enhanced with Orange Peel Extract, Modulates Postprandial LDL-Cholesterol Responses in Individuals at Cardiometabolic Risk: A Pilot Randomized, Controlled, Cross-Over Nutritional Intervention. Applied Sciences, 13(15), 8574. https://doi.org/10.3390/app13158574










