EEM Fluorescence Spectroscopy Coupled with HPLC-DAD Analysis for the Characterization of Bud Derivative Dietary Supplements: A Preliminary Introduction to GEMMAPP, the Free Data-Repository from the FINNOVER Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Bud Derivative Manufacturing
2.4. The Chromatographic Characterization of BDs
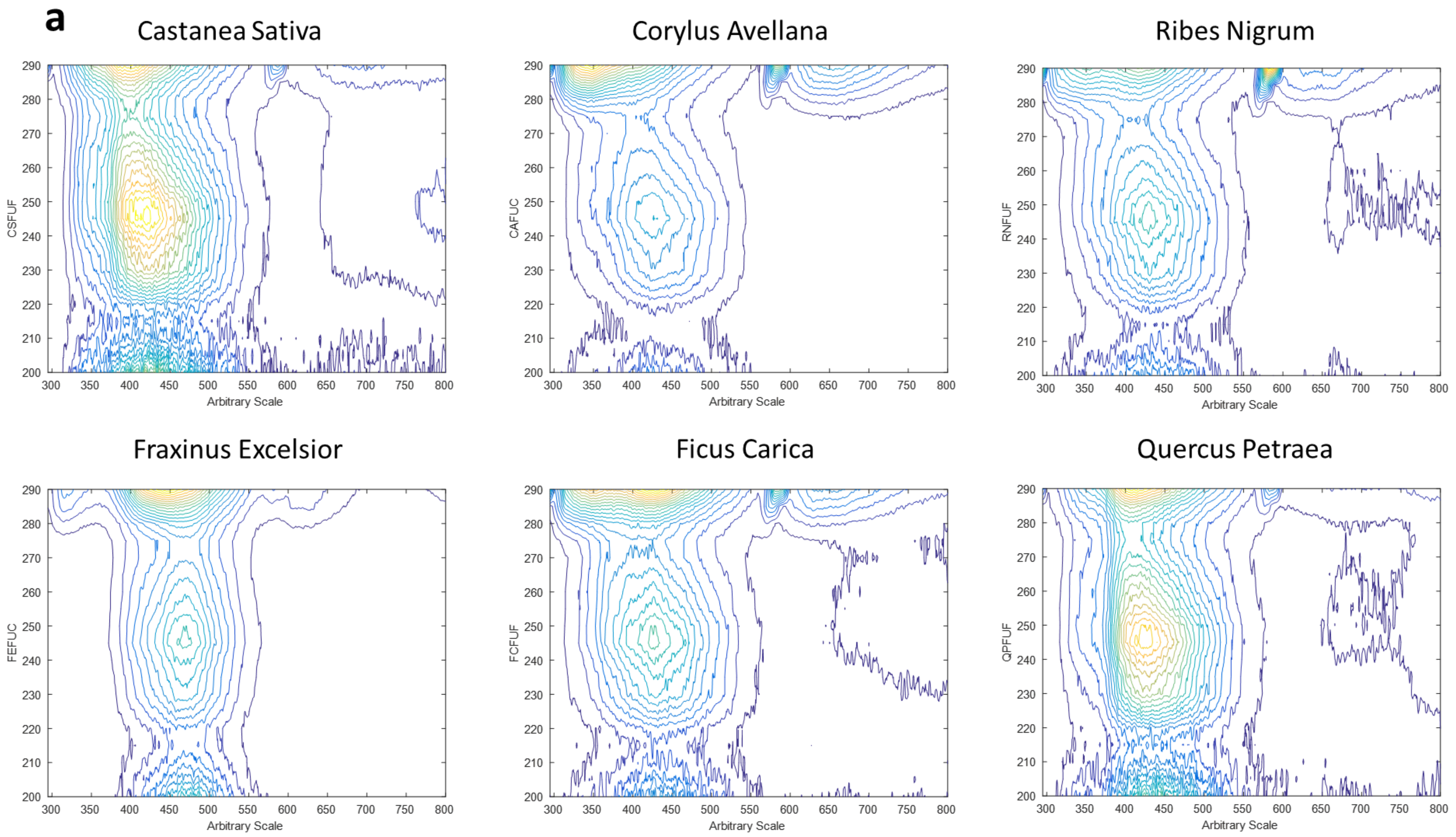
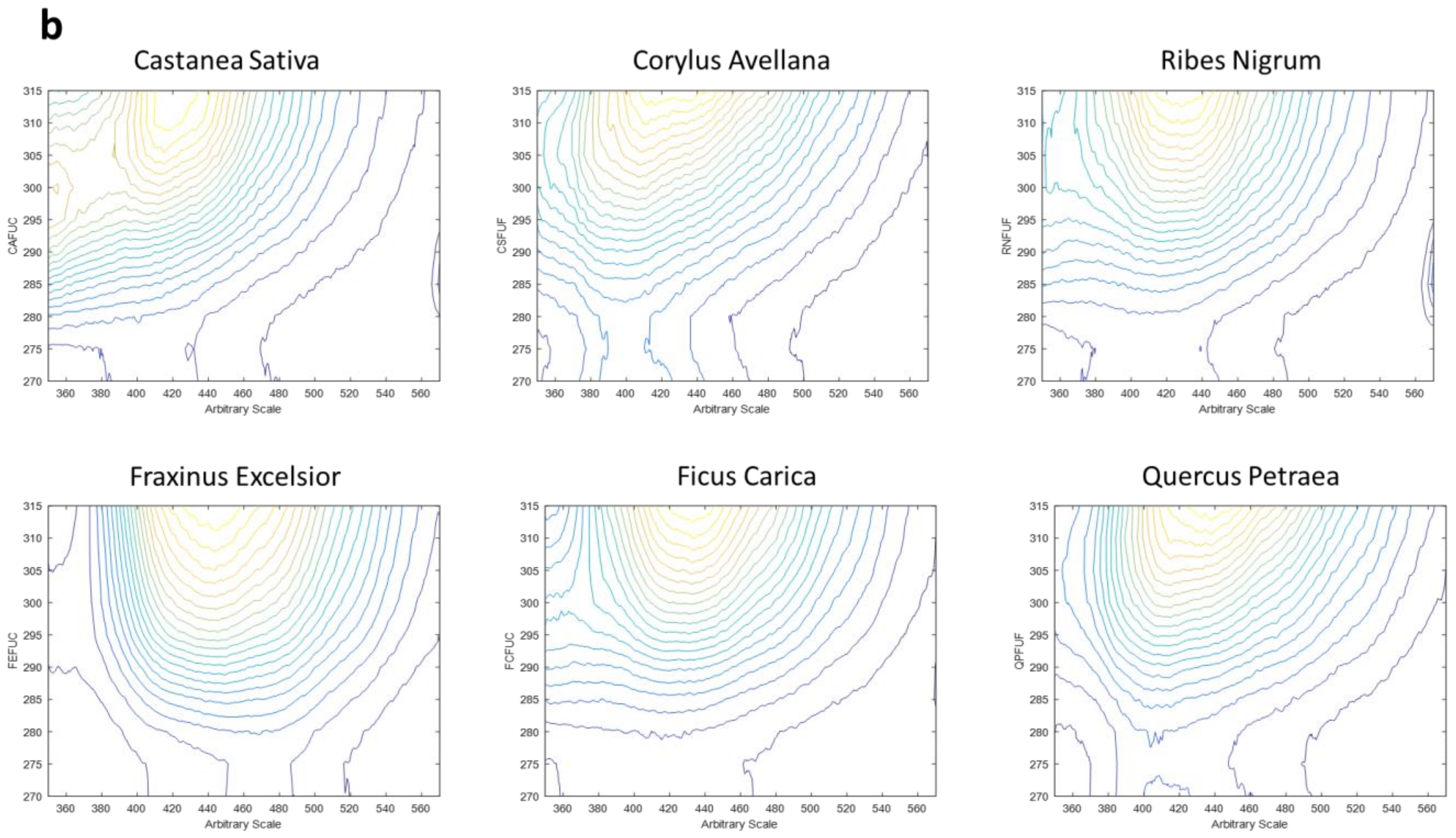
2.5. Three-Dimensional Fluorescence Spectroscopy
- Range 1, which takes almost the entire emission spectrum, presents emission wave-lengths from 295 to 800 nm (each 0.5 nm) and excitation wavelengths from 200 to 290 nm (each 5 nm).
- Range 2, which shows a smaller part of the emission spectrum but allows for the investigation of a different excitation range, presents emission wavelengths from 350 to 570 nm (each 0.5 nm) and excitation wavelengths from 270 to 315 nm (each 5 nm).
3. Results and Discussion
3.1. The Chromatographic Characterization of BDs
- -
- FE is very rich in phenolics and cinnamic acids (more than 40% of total phenolics, as shown in Figure 2) and in chlorogenic acid (489.94 ± 0.79 mg/100 g FW), followed by ferulic acid (295.28 ± 0.68 mg/100 g FW); it is also rich in catechins, especially epicatechin (270.51 ± 0.89 mg/100 g FW), and flavonols, especially hyperoside (242.26 ± 0.89 mg/100 g FW), and benzoic acids, especially ellagic acid (214.49 ± 0.69 mg/100 g FW), as shown in Figure 2. Fraxinus excelsior L. (FE) has been widely used in traditional medicine due to several claimed beneficial health effects including antioxidant, anti-inflammatory, anti-rheumatic and anti-pyretic activities [17]. Several findings from in vivo studies showed that the FE extract has hypoglycemic and anti-hyperlipidemic activities, providing anti-diabetic and anti-obesity effects [18,19].
- -
- QP is particularly rich in ellagic acid (231.56 ± 0.65 mg/100 g FW), catechins (especially epicatechin, 199.92 ± 0.46 mg/100 g FW) and quercitrin (188.45 ± 0.79 mg/100 g FW). The glyceric macerate of Quercus petraea L. (QP) is employed to regularize the action of the intestinal system, to counteract the state of asthenia and to fight against oxidative stress, helping to protect and preserve the well-being of the human organism [20].
- -
- CA is rich in ellagic acid (193.53 + 2.22 mg/100 g FW), quercetin (171.25 + 14.28 mg/100 g FW) and to a lesser extent, in catechins (about 100 mg/100 g FW), while cinnamic acids (less than 2%) are only represented by caffeic acid in trace amounts (<3 mg/100 g FW), as shown in Figure 2. Corylus avellana L. (CA), the common hazel plant, has been known since ancient times for its astringent and antiedema properties, vasoprotective activity and mild antimicrobial effect: in fact, it was used in traditional medicine for the treatment of edema, hemorrhoids, varicose veins and phlebitis [21]. Nowadays, its characteristic compounds, cyclic diarylheptanoids, are receiving increasing interest due to their remarkable health effects, including anti-inflammatory, anti-emetic, anti-influenza and estrogenic actions [22].
- -
- CS is characterized by high amounts of flavonols (e.g., quercetin and quercitrin, with about 30 mg/100 g FW) and benzoic acids (e.g., ellagic and gallic acids, with about 50–90 mg/100 g FW), followed by catechins, especially epicatechin (31.02 + 0.17 mg/100 g FW), as shown in Figure 2. Cinnamic acids were quantified in small amounts (less than 15 mg/100 g FW). Castanea sativa Miller (CS) is one of the most widely used herbal medicines thanks to its health-promoting activities, which are due to its high contents of bioactive compounds (polyphenols, organic acids, terpenes and vitamins) [6]. In fact, CS presents antioxidant and curative properties against both cardiovascular and urinary diseases (especially recurrent cystitis), and it is well known for its positive effects on stagnant and vascular fluids [23].
- -
- FC showed high values of catechins (75.79 + 1.04 mg/100 g FW for catechin and 191.56 + 1.13 mg/100 g FW for epicatechin), flavonols (e.g., quercitrin and hyperoside, with values of about 60–130 mg/100 g FW), and phenolic acids (e.g., coumaric and ellagic acids, with values of about 60 mg/100 g FW). Ficus carica L. (FC) was used in traditional medicine to cure various disorders such as gastrointestinal (dysentery, constipation, colic, ulcers and loss of appetite) and respiratory (coughs, sore throats and bronchial problems) thanks to its anti-inflammatory, antioxidant, antipyretic, antidiabetic, anthelmintic, antimicrobial and anti-carcinogenic activities [24].
- -
- RN presented a good quercetin and quercitrin contents (about 30–50 mg/100 g FW), ellagic acid (about 70 mg/100 g FW) and catechins (about 60–100 mg/100 g FW). Cinnamic acids were identified in amounts of about 5–20 mg/100 g FW. Ribes nigrum L. (RN) buds are used in medicinal preparations as anti-inflammatory agents for the treatment of dermal diseases, such as eczema and psoriasis, and for the healing of wounds [25,26]. R. nigrum BDs contain high polyphenol contents, especially cate-chins and phenolic acids, and are endowed with antioxidant and anti-inflammatory activities which have been proven to play an important role in the human health in the prevention of several chronic diseases [16,27].
3.2. Three-Dimensional Fluorescence Spectroscopy
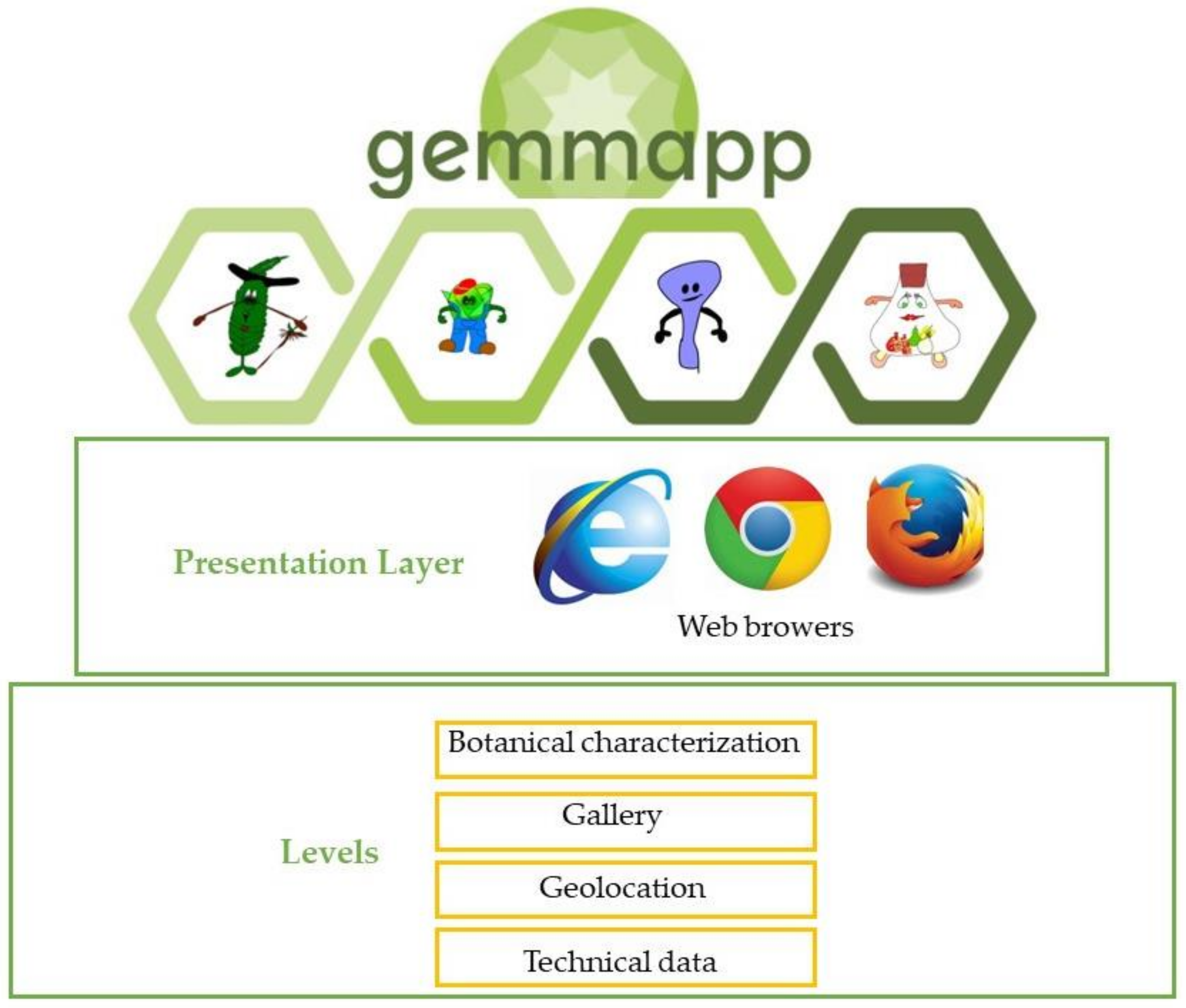
3.3. GEMMAPP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FINNOVER Interreg Alcotra Project 2017–2020. Available online: http://www.interreg-finnover.com/ (accessed on 2 April 2021).
- Turrini, F.; Donno, D.; Beccaro, G.L.; Pittaluga, A.; Grilli, M.; Zunin, P.; Boggia, R. Bud-derivatives, a novel source of polyphenols and how different extraction processes affect their composition. Foods 2020, 9, 1343. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopée Française. Codex Medicamentarius Gallicus, Codex Français: Monographie, Préparations Homéopathiques; Ordre National des Pharmaciens: Paris, France, 1965. Available online: http://ansm.sante.fr/Mediatheque/Publications/Pharmacopee-francaise-Plan-Preambule-index (accessed on 21 March 2023).
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds. Pharmaceuticals 2016, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud Extracts as New Phytochemical Source for Herbal Preparations-Quality Control and Standardization by Analytical Fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health, 1st ed.; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croatia, 2015; pp. 187–218. [Google Scholar]
- Turrini, F.; Donno, D.; Boggia, R.; Beccaro, G.L.; Zunin, P.; Leardi, R.; Pittaluga, A.M. An innovative green extraction and re-use strategy to valorize food supplement by-products: Castanea sativa bud preparations as case study. Food Res. Int. 2019, 115, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Allio, A.; Calorio, C.; Franchino, C.; Gavello, D.; Carbone, E.; Marcantoni, A. Bud extracts from Tilia tomentosa Moench inhibit hippocampal neuronal firing through GABAA and benzodiazepine receptors activation. J. Ethnopharmacol. 2015, 172, 288–296. [Google Scholar] [CrossRef]
- Calorio, C.; Donno, D.; Franchino, C.; Carabelli, V.; Marcantoni, A. Bud extracts from Salix caprea L. inhibit voltage gated calcium channels and catecholamines secretion in mouse chromaffin cells. Phytomedicine 2017, 36, 168–175. [Google Scholar] [CrossRef]
- Turrini, F.; Vallarino, G.; Cisani, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Boggia, R.; Pittaluga, A.; Grilli, M. Use of an Animal Model to Evaluate Anxiolytic Effects of Dietary Supplementation with Tilia tomentosa Moench Bud Extracts. Nutrients 2020, 12, 3328. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Kluwer Academic/Plenum: New York, NY, USA, 2007; pp. 63–94. [Google Scholar]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, Nutraceutical Properties, and Fluorescence Spectral Profiles of Bee Pollen Samples from Different Botanical Origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef]
- Sádecká, J.; Jakubíková, M. Varietal classification of white wines by fluorescence spectroscopy. J. Food Sci. Technol. 2020, 57, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Nowak, P.; Pawlak-Lemańska, K.; Sikorski, M. Characterization and Classification of Direct and Commercial Strawberry Beverages Using Absorbance–Transmission and Fluorescence Excitation–Emission Matrix Technique. Foods 2022, 11, 2143. [Google Scholar] [CrossRef]
- GEMMAPP. Available online: http://www.gemmapp.it/ (accessed on 20 March 2023).
- Donno, D.; Turrini, F.; Boggia, R.; Guido, M.; Gamba, G.; Mellano, M.G.; Riondato, I.; Beccaro, G.L. Sustainable Extraction and Use of Natural Bioactive Compounds from the Waste Management Process of Castanea spp. Bud-Derivatives: The FINNOVER Project. Sustainability 2020, 12, 10640. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project. Foods 2019, 8, 466. [Google Scholar] [PubMed]
- Naderi, M.; Torbati, M.; Azadmard-Damirchi, S.; Asnaashari, S.; Savage, G.P. Common ash (Fraxinus excelsior L.) seeds as a new vegetable oil source. LWT 2020, 131, 109811. [Google Scholar] [CrossRef]
- Ibarra, A.; Bai, N.; He, K.; Bily, A.; Cases, J.; Roller, M.; Sang, S. Fraxinus excelsior seed extract FraxiPureTM limits weight gains and hyperglycemia in high-fat diet-induced obese mice. Phytomedicine 2011, 18, 479–485. [Google Scholar] [CrossRef]
- Visen, P.; Saraswat, B.; Visen, A.; Roller, M.; Bily, A.; Mermet, C.; He, K.; Bai, N.; Lemaire, B.; Lafay, S.; et al. Acute effects of Fraxinus excelsior L. seed extract on postprandial glycemia and insulin secretion on healthy volunteers. J. Ethnopharmacol. 2009, 126, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Eaton, E.G.S.D.J.; Caudullo, G.; Oliveira, S.; De Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Public Office of the European Union: Luxembourg, 2016; pp. 162–163. [Google Scholar]
- Cerulli, A.; Masullo, M.; Montoro, P.; Hošek, J.; Pizza, C.; Piacente, S. Metabolite profiling of ‘green’ extracts of Corylus avellana leaves by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2018, 160, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Lauro, G.; Masullo, M.; Cantone, V.; Olas, B.; Kontek, B.; Nazzaro, F.; Bifulco, G.; Piacente, S. Cyclic Diarylheptanoids from Corylus avellana Green Leafy Covers: Determination of Their Absolute Configurations and Evaluation of Their Antioxidant and Antimicrobial Activities. J. Nat. Prod. 2017, 80, 1703–1713. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Bonvegna, L.; Bounous, G. Castanea spp. buds as a phytochemical source for herbal preparations: Botanical fingerprint for nutraceutical identification and functional food standardization. J. Sci. Food Agric. 2014, 94, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Barolo, M.I.; Ruiz Mostacero, N.; López, S.N. Ficus carica L. (Moraceae): An ancient source of food and health. Food Chem. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- Dvaranauskaite, A.; Venskutonis, P.R.; Raynaud, C.; Talou, T.; Viškelis, P.; Dambrauskiene, E. Characterization of steam volatiles in the essential oil of black currant buds and the antioxidant properties of different bud extracts. J. Agric. Food Chem. 2008, 56, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.O.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Marconi, V.; Bounous, G. Botanicals in Ribes nigrum bud-preparations: An analytical fingerprinting to evaluate the bioactive contribution to total phytocomplex. Pharm. Biol. 2013, 51, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Mok, D.K.W.; Chau, F.T. Chemical information of Chinese medicines: A challenge to chemist. Chemom. Intell. Lab. Syst. 2006, 82, 210–217. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L. Analytical strategies for fingerprinting of antioxidants, nutritional substances, and bioactive compounds in foodstuffs based on high performance liquid chromatography–mass spectrometry: An overview. Foods 2020, 9, 1734. [Google Scholar] [CrossRef]
- Fan, X.-H.; Cheng, Y.-Y.; Ye, Z.-L.; Lin, R.-C.; Qian, Z.-Z. Multiple chromatographic fingerprinting and its application to the quality control of herbal medicines. Anal. Chim. Acta 2006, 555, 217–224. [Google Scholar] [CrossRef]
- Feng, X.; Kong, W.; Wei, J.; Ou-Yang, Z.; Yang, M. Hplc fingerprint analysis combined with chemometrics for pattern recognition of ginger. Pharm. Biol. 2014, 52, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Carlen, C.; Ancay, A.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Analytical fingerprint and chemometrics as phytochemical composition control tools in food supplement analysis: Characterization of raspberry bud-preparations of different cultivars. J. Sci. Food Agric. 2016, 96, 3157–3168. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Turrini, F.; Anselmo, M.; Zunin, P.; Donno, D.; Beccaro, G.L. Feasibility of UV–VIS-Fluorescence Spectroscopy combined with pattern recognition techniques to authenticate a new category of plant food supplements. J. Food Sci. Technol. 2017, 54, 2422–2432. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multy-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Włodarska, K.; Pawlak-Lemańska, K.; Khmelinskii, I.; Sikorska, E. Explorative study of apple juice fluorescence in relation to antioxidant properties. Food Chem. 2016, 210, 593–599. [Google Scholar] [CrossRef]
- Rodrıguez-Delgado, M.A.; Malovana, S.; Perez, J.P.; Borges, T.; Montelongo, F.G. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Casale, M.; Pasquini, B.; Hooshyari, M.; Orlandini, S.; Mustorgi, E.; Malegori, C.; Turrini, F.; Ortiz, M.C.; Sarabia, L.A.; Furlanetto, S. Combining excitation-emission matrix fluorescence spectroscopy, parallel factor analysis, cyclodextrin-modified micellar electrokinetic chromatography and partial least squares class-modelling for green tea characterization. J. Pharm. Biomed. Anal. 2018, 159, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, D.M.; Mutavdžić, D.R.; Radotić, K.; Milivojević, J.M.; Maksimović, V.M.; Dragišić Maksimović, J.J. Phenolic profiling of 12 strawberry cultivars using different spectroscopic methods. J. Agric. Food Chem. 2020, 68, 4346–4354. [Google Scholar] [CrossRef] [PubMed]



| Experimental Conditions | |
|---|---|
| Extraction solvent | Mixture of glycerol and ethanol (95%) 50:50 w/w |
| Bud–solvent ratio | 1:20, dried weight (DW) |
| Time | 21 days of cold maceration |
| Cinnamic Acids | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeic Acid | Chlorogenic Acid | Coumaric Acid | Ferulic Acid | ||||||||
| Species | ID Code | Mean Value | SD | Mean Value | SD | Mean Value | SD | Mean Value | SD | ||
| Corylus avellana | CA | 2.53 | 0.13 | n.d. | / | n.d. | / | n.d. | / | ||
| Castanea sativa | CS | 1.61 | 0.02 | 14.23 | 0.29 | n.d. | / | 4.34 | 0.22 | ||
| Ficus carica | FC | n.d. | / | n.d. | / | 62.21 | 0.84 | n.d. | / | ||
| Fraxinus excelsior | FE | 43.81 | 0.80 | 489.94 | 0.79 | n.d. | / | 295.28 | 0.68 | ||
| Quercus petraea | QP | 5.08 | 0.65 | n.d. | / | 0.00 | 0.00 | n.d. | / | ||
| Ribes nigrum | RN | 22.48 | 0.04 | n.d. | / | 5.21 | 0.15 | n.d. | / | ||
| Flavonols | |||||||||||
| Hyperoside | Isoquercitrin | Quercetin | Quercitrin | Rutin | |||||||
| Species | ID Code | Mean Value | SD | Mean Value | SD | Mean Value | SD | Mean Value | SD | Mean Value | SD |
| Corylus avellana | CA | n.d. | / | n.d. | / | 171.25 | 14.28 | 83.46 | 4.98 | 79.50 | 2.14 |
| Castanea sativa | CS | 3.03 | 0.10 | n.d. | / | 30.64 | 0.23 | 29.01 | 0.82 | 1.54 | 0.12 |
| Ficus carica | FC | 63.67 | 0.94 | n.d. | / | 49.68 | 1.24 | 128.34 | 1.08 | 46.20 | 1.11 |
| Fraxinus excelsior | FE | 242.26 | 0.89 | n.d. | / | 176.88 | 0.63 | 79.93 | 1.00 | 0.00 | 0.00 |
| Quercus petraea | QP | 7.28 | 0.81 | n.d. | / | 27.91 | 0.38 | 188.45 | 0.79 | 0.00 | 0.00 |
| Ribes nigrum | RN | n.d. | / | n.d. | / | 49.53 | 0.49 | 30.86 | 0.85 | 17.25 | 0.22 |
| Benzoic Acids | Catechins | ||||||||||
| Ellagic Acid | Gallic Acid | Catechin | Epicatechin | ||||||||
| Species | ID code | Mean Value | SD | Mean Value | SD | Mean Value | SD | Mean Value | SD | ||
| Corylus avellana | CA | 193.53 | 2.22 | 46.29 | 2.54 | 48.53 | 2.89 | 49.19 | 2.83 | ||
| Castanea sativa | CS | 48.95 | 0.13 | 94.71 | 0.24 | 1.28 | 0.32 | 31.02 | 0.17 | ||
| Ficus carica | FC | 67.29 | 0.89 | n.d. | / | 75.79 | 1.04 | 191.56 | 1.13 | ||
| Fraxinus excelsior | FE | 214.49 | 0.69 | n.d. | / | 57.74 | 0.79 | 270.51 | 0.89 | ||
| Quercus petraea | QP | 231.56 | 0.65 | 52.03 | 0.64 | 94.83 | 0.39 | 199.92 | 0.46 | ||
| Ribes nigrum | RN | 69.66 | 0.08 | 0.31 | 0.09 | 95.88 | 0.26 | 59.83 | 0.37 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrini, F.; Donno, D.; Grasso, F.; Mustorgi, E.; Beccaro, G.L.; Guido, M.; Fior, T.; Grilli, M.; Pittaluga, A.; Boggia, R. EEM Fluorescence Spectroscopy Coupled with HPLC-DAD Analysis for the Characterization of Bud Derivative Dietary Supplements: A Preliminary Introduction to GEMMAPP, the Free Data-Repository from the FINNOVER Project. Appl. Sci. 2023, 13, 8679. https://doi.org/10.3390/app13158679
Turrini F, Donno D, Grasso F, Mustorgi E, Beccaro GL, Guido M, Fior T, Grilli M, Pittaluga A, Boggia R. EEM Fluorescence Spectroscopy Coupled with HPLC-DAD Analysis for the Characterization of Bud Derivative Dietary Supplements: A Preliminary Introduction to GEMMAPP, the Free Data-Repository from the FINNOVER Project. Applied Sciences. 2023; 13(15):8679. https://doi.org/10.3390/app13158679
Chicago/Turabian StyleTurrini, Federica, Dario Donno, Federica Grasso, Eleonora Mustorgi, Gabriele Loris Beccaro, Maddalena Guido, Teresa Fior, Massimo Grilli, Anna Pittaluga, and Raffaella Boggia. 2023. "EEM Fluorescence Spectroscopy Coupled with HPLC-DAD Analysis for the Characterization of Bud Derivative Dietary Supplements: A Preliminary Introduction to GEMMAPP, the Free Data-Repository from the FINNOVER Project" Applied Sciences 13, no. 15: 8679. https://doi.org/10.3390/app13158679
APA StyleTurrini, F., Donno, D., Grasso, F., Mustorgi, E., Beccaro, G. L., Guido, M., Fior, T., Grilli, M., Pittaluga, A., & Boggia, R. (2023). EEM Fluorescence Spectroscopy Coupled with HPLC-DAD Analysis for the Characterization of Bud Derivative Dietary Supplements: A Preliminary Introduction to GEMMAPP, the Free Data-Repository from the FINNOVER Project. Applied Sciences, 13(15), 8679. https://doi.org/10.3390/app13158679












