A Dynamic In Vitro Model for Testing Intestinal Absorption of Different Vegetable Food Secondary Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
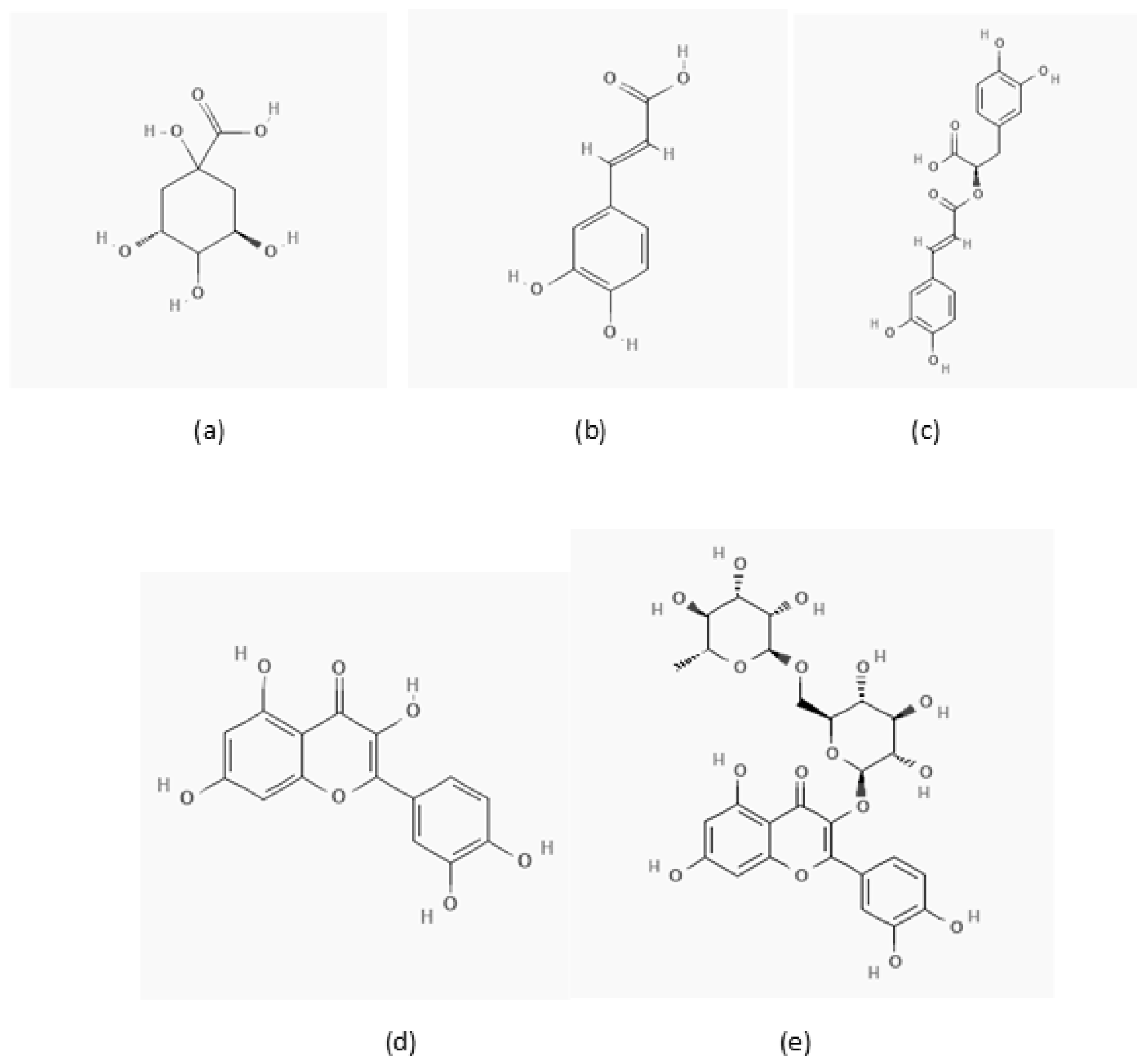
2.2. Sample Preparation
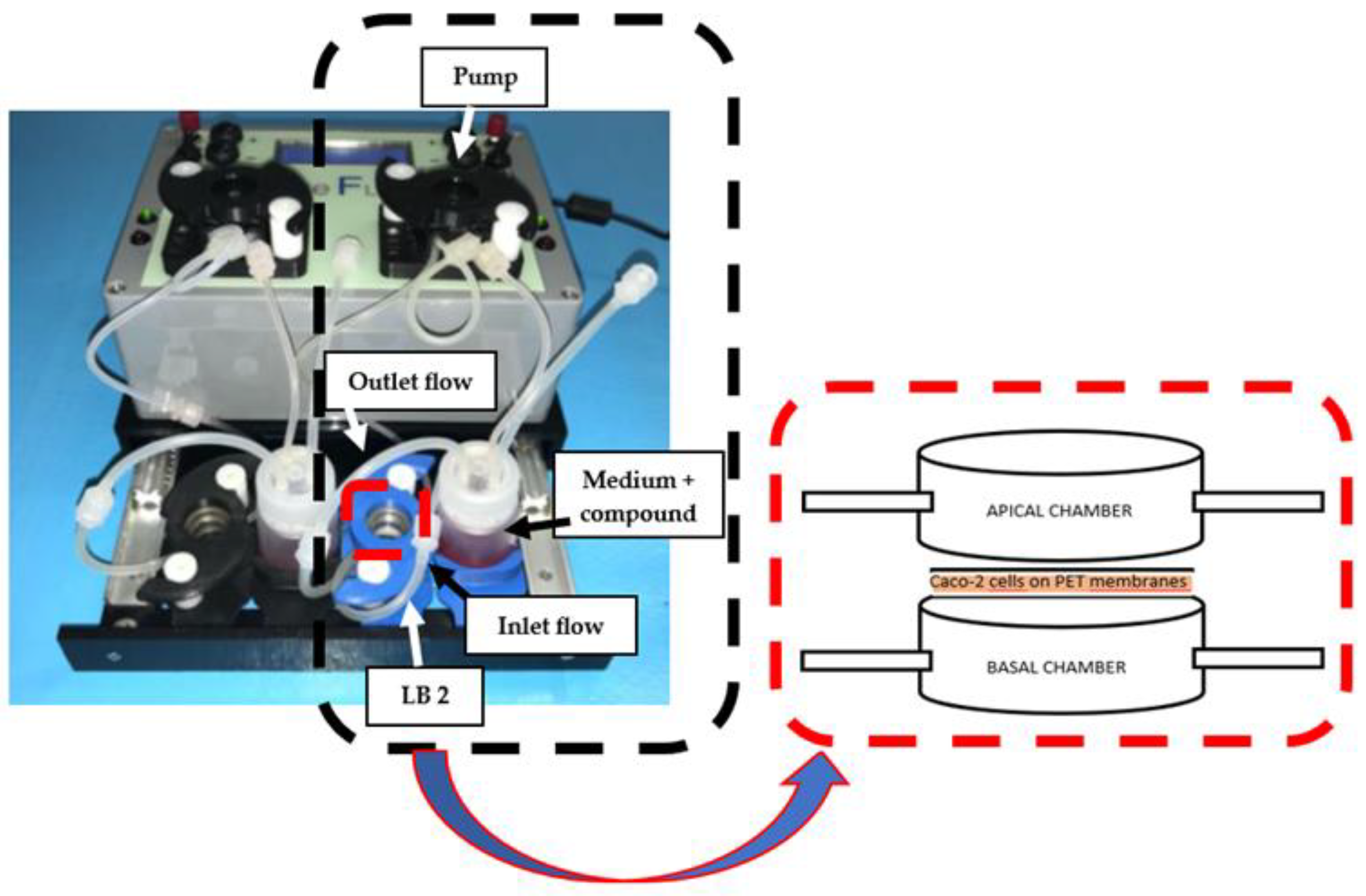
2.3. Dynamic Bioreactor Set-Up
2.4. Cell Cultures and Cell Viability Assays
2.5. RP-HPLC-DAD Analyses and Methods Validation
2.6. Statistical Analysis
3. Results and Discussion
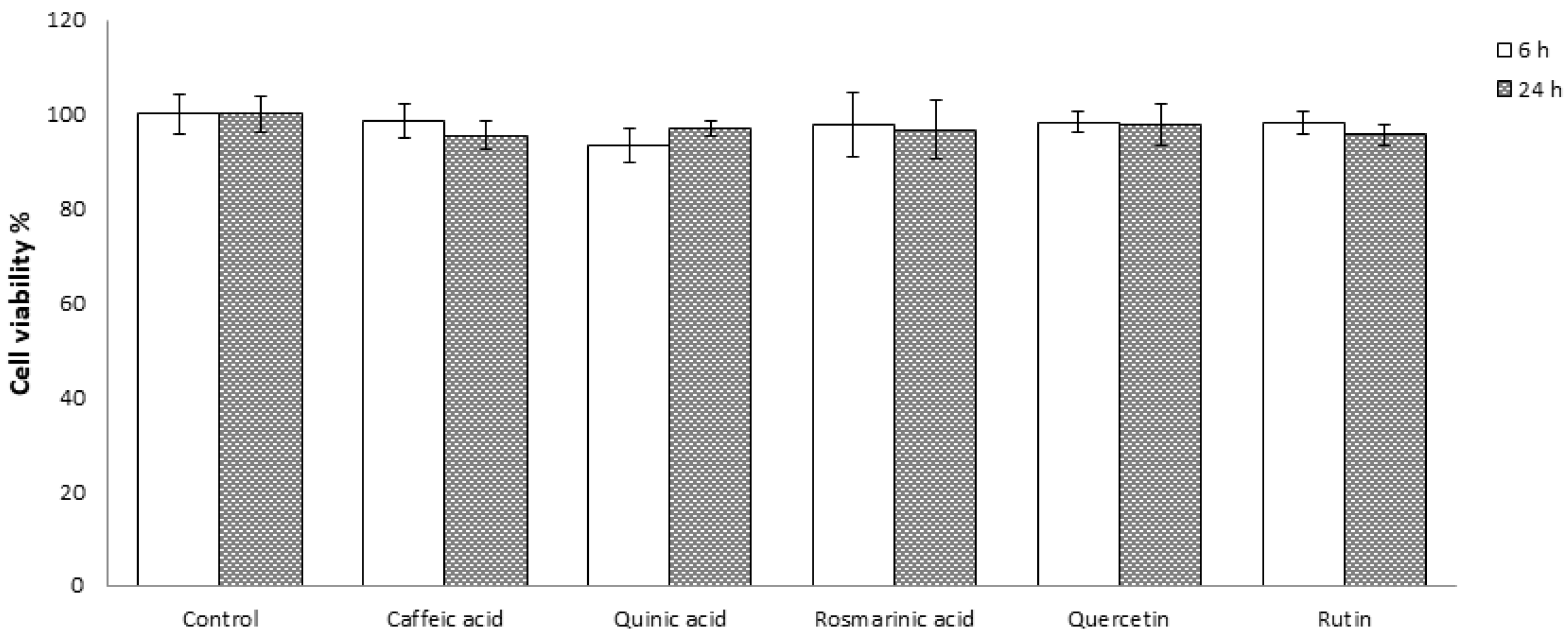
3.1. Effect of the Tested Molecules on Caco-2 Cells Viability
3.2. Absorption Evaluation Using the In Vitro Dynamic Intestinal Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunantha, K.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bio-Actives on Gut Health: In Vitro and Ex Vivo Models; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Lucas-González, R.; Viuda-Martos, M.; Pèrez-Alvarez, J.A.; Fernàndez-Lòpez, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Van De Wiele, T.R.; Oomen, A.G.; Wragg, J.; Cave, M.; Minekus, M.; Hack, A.; Cornelis, C.; Rompelberg, C.J.; De Zwart, L.L.; Klinch, B.; et al. Comparison of five in vitro digestion models to in vivo experimental results: Lead bioaccessibility in the human gastrointestinal tract. J. Environ. Sci. Health 2007, 42, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Colombo, R.; Ferron, L.; Frosi, I.; Papetti, A. Advances in static in vitro digestion models after the COST action Infogest consensus protocol. Food Funct. 2021, 12, 7619–7636. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. Infogest static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1040. [Google Scholar] [CrossRef]
- Cost-Infogest.eu. Available online: http://www.cost-infogest.eu (accessed on 27 March 2023).
- Egger, L.; Schlegel, P.; Baumann, C.; Stoffers, H.; Guggisberg, D.; Brügger, C.; Dürr, D.; Stoll, P.; Vergères, G.; Portmann, R. Physiological comparability of the harmonized INFOGEST in vitro digestion method to in vivo pig digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef]
- Hamzalıoğlu, A.; Gökmen, V. Investigations on the reactions of α-dicarbonyl compounds with amino acids and proteins during in vitro digestion of biscuits. Food Funct. 2016, 6, 109–114. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Bradkorb, A.; Cattenoz, T.; Clemente, A.; Comi, I.; et al. The harmonized INFOGEST in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Heredia, A.; Andrés, A. In vitro digestion models to assess lipolysis: The impact of the simulated conditions of gastric and intestinal pH, bile salts and digestive fluids. Food Res. Int. 2019, 125, 108511. [Google Scholar] [CrossRef] [PubMed]
- Wikman-Larhed, A.; Artursson, P. Co-cultures of human intestinal goblet (HT29-H) and absorptive (Caco-2) cells for studies of drug and peptide abs orption. Eur. J. Pharm. Sci. 1995, 3, 171–183. [Google Scholar] [CrossRef]
- Lennernäss, H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica 2007, 37, 1015–1051. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Patel, S.; Huang, C.; Paiva, A.; Sun, Y.; Barker, G.; Weller, H.; Shou, W. Comprehensive characterization and optimization of Caco-2 cells enabled the development of a miniaturized 96-well permeability assay. Xenobiotica 2022, 52, 742–750. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R.; Huis in’t Veld, J.H. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P.A. A Human Gastric Simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, 625–635. [Google Scholar] [CrossRef]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The design, operation, and application of a dynamic gastric model. Dissolution Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Huh, D.; Torisawa, Y.S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered physiological biomimicry: Organs-on-chips. Lab Chip 2012, 12, 2156–2164. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 23, 6. [Google Scholar] [CrossRef]
- Mazzei, D.; Giusti, S.; Sbrana, T.; Ahluwalia, A. Multicompartmental modular bioreactor as innovative system for dynamic cell cultures and co-cultures. In Bioreactors: Design, Properties and Applications; Antolli, P.G., Liu, Z., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 159–178. [Google Scholar]
- Giusti, S.; Sbrana, T.; La Marca, M.; Di Patria, V.; Martinucci, V.; Tirella, A.; Domenici, C.; Ahluwalia, A. A novel dual-flow bioreactor simulates increased fluorescein permeability in epithelial tissue barriers. Biotechnol. J. 2014, 9, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-M.; Kim, S. In vitro models of the small intestine for studying intestinal diseases. Front. Microbiol. 2022, 12, 767038. [Google Scholar] [CrossRef]
- Carton, F.; Malatesta, M. In vitro models of biological barriers for nanomedical research. Int. J. Mol. Sci. 2022, 23, 8910. [Google Scholar] [CrossRef] [PubMed]
- Fedi, A.; Viatle, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Cacopardo, L.; Costa, J.; Giusti, S.; Buoncompagni, L.; Meucci, S.; Corti, A.; Mattei, G.; Ahluwalia, A. Real-time cellular impedance monitoring and imaging of biological barriers in a dual-flow membrane bioreactor. Biosens. Bioelectron. 2019, 140, 111340. [Google Scholar] [CrossRef]
- Costa, J.; Almonti, V.; Cacopardo, L.; Poli, D.; Rapposelli, S.; Ahluwalia, A. Investigating curcumin/intestinal epithelium interaction in a millifluidic bioreactor. Bioengineering 2020, 7, 100. [Google Scholar] [CrossRef]
- Colombo, R.; Paolillo, M.; Papetti, A. A new millifluidic-based gastrointestinal platform to evaluate the effect of simulated dietary methylglyoxal intakes. Food Funct. 2019, 10, 4330–4338. [Google Scholar] [CrossRef]
- Colombo, R.; Paolillo, M.; Frosi, I.; Ferron, L.; Papetti, A. Effect of the simulated digestion process on the chlorogenic acid trapping activity against methylglyoxal. Food Funct. 2023, 14, 541–549. [Google Scholar] [CrossRef]
- ICH Guideline Q2(R1), Validation of Analytical Procedures: Text and Methodology Guidance for Industry; 2021. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r1-validation-analytical-procedures-text-and-methodology-guidance-industry (accessed on 15 January 2022).
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Alam, M.; Ashraf, G.M.; Sheikh, K.; Khan, A.; Ali, S.; Ansari, M.M.; Adnan, M.; Pasupuleti, V.R.; Hassan, M.I. Potential therapeutic implications of caffeic acid in cancer signaling: Past, present, and future. Front. Pharmacol. 2022, 13, 845871. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, Y.; Li, B. Dietary polyphenols alleviate autoimmune liver disease by mediating the intestinal microenvironment: Challenges and hopes. J. Agric. Food Chem. 2022, 70, 10708–10737. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Yang, X.W.; Yang, X.; Wang, K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009, 367, 58–64. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Dubiard, C.G.; Cheynier, V.; Meudec, E.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. In vitro digestion of dairy and egg products enriched with grape extracts: Effect of the food matrix on polyphenol bioaccessibility and antioxidant activity. Food Res. Int. 2016, 88, 284–292. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena-Artieda, D. Bioaccessibility of Rutin, Caffeic Acid and Rosmarinic Acid: Influence of the in Vitro Gastrointestinal Digestion Models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Magnani Spagnol, C.; Issac, V.L.; Corrêa, M.A.; Salgado, H.R. Validation of HPLC–UV assay of caffeic acid in emulsions. J. Chromatogr. Sci. 2016, 54, 305–311. [Google Scholar]
- Chaowuttikul, C.; Palanuvej, C.; Ruangrungs, N. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz. J. Pharm. Sci. 2020, 56, e17547. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Carazzone, C.; Stauder, M.; Spratt, D.A.; Wilson, M.; Pratten, J.; Ciric, L.; Lingström, P.; Zaura, E.; et al. Identification of organic acids in Cichorium intybus inhibiting virulence-related properties of oral pathogenic bacteria. Food Chem. 2013, 138, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.P.; Shi, X.F.; Zhang, Y.C.; Li, Z.X.; Zhang, L.; Wang, Z.Y. Quantitative analysis of quercetin in euphorbia helioscopia L by RP-HPLC. Cell Biochem. Biophys. 2011, 61, 59–64. [Google Scholar] [CrossRef]
- Camilleri, M.; Colemont, L.J.; Phillips, S.F.; Brown, M.L.; Thormforde, G.M.; Chapman, N.; Zinsmeister, A.N. Human gastric emptying and colonic filling of solids characterized by a new method. Am. J. Physiol. 1989, 257, G284–G290. [Google Scholar] [CrossRef]
- Mortelè, O.; Jörissen, J.; Spacova, I.; Lebeer, S.; van Nuijs, A.L.N.; Hermans, N. Demonstrating the involvement of an active efflux mechanism in the intestinal absorption of chlorogenic acid and quinic acid using a Caco-2 bidirectional permeability assay. Food Funct. 2021, 12, 417–425. [Google Scholar] [CrossRef]
- Bermejo, E.; Carballo, R.; Castiñeiras, A.; Lago, A.B. Coordination of α-hydroxycarboxylic acids with first-row transition ions. Coord. Chem. Rev. 2013, 257, 2639–2651. [Google Scholar] [CrossRef]
- Barba-Behrens, N.; Salazar-García, F.; Mara Bello-Ramírez, A.; García-Báez, E.; de Jesús Rosales-Hoz, M.; Contreras, R.; Flores-Parra, A. Novel coordination compounds of quinic acid. X-ray diffraction study of copper (II) complexes where the metal ion was a chiral centre. Trans. Metal Chem. 1994, 19, 575–581. [Google Scholar] [CrossRef]
- Wang, S.J.; Zeng, J.; Yang, B.K.; Zhing, Y.M. Bioavailability of caffeic acid in rats and its absorption properties in the Caco-2 cell model. Pharm. Biol. 2014, 52, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Birková, A.; Hubková, B.; Bolerázska, B.; Mareková, M.; Čižmárová, B. Caffeic acid: A brief overview of its presence, metabolism, and bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Veras, K.S.; Fachel, F.N.S.; de Araújo, B.V.; Teixeira, H.F.; Koester, L.S. Oral pharmacokinetics of hydroxycinnamic acids: An updated erview. Pharmaceutics 2022, 14, 2663. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of rosmarinic acid in intestinal Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2005, 69, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Ye, Z.; Hauck, C.; Murphy, P.A.; McCoy, J.A.; Widrlechner, M.P.; Reddy, M.B.; Hendrich, S. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J. Ethnopharmacol. 2011, 137, 1107–1112. [Google Scholar] [CrossRef]
- Amoah, S.K.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic Acid—Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef]
- Zorić, Z.; Markić, J.; Pedisić, S.; Bučević-Popović, V.; Generalić-Mekinić, I.; Grebenar, K.; Kulišić-Bilušić, T. Stability of rosmarinic acid in aqueous extracts from different lamiaceae species after in vitro digestion with human gastrointestinal enzymes. Food Technol. Biotechnol. 2016, 54, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Cai, Q.; Liu, C.; Bao, F.W.; Zhang, Z.Q. Simultaneous determination and pharmacokinetic comparisons of multi-ingredients after oral administration of radix salviae miltiorrhizae extract, hawthorn extract, and a combination of both extracts to rats. J. Anal. Methods Chem. 2014, 2014, 617367. [Google Scholar] [CrossRef]
- Boyer, J.; Brown, D.; Liu, R.H. In vitro digestion and lactase treatment influence uptake of quercetin and quercetin glucoside by the Caco-2 cell monolayer. Nutr. J. 2005, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Walgren, R.A.; Walle, U.K.; Walle, T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem. Pharmacol. 1998, 55, 1721–1727. [Google Scholar] [CrossRef]
- Zhang, X.; Song, J.; Shi, X.; Miao, S.; Li, Y.; Wen, A. Absorption and metabolism characteristics of rutin in Caco-2 cells. Sci. World J. 2013, 2013, 382350. [Google Scholar] [CrossRef] [PubMed]
| Time (h) | |||||
|---|---|---|---|---|---|
| Compound (0.1 mg/mL) | 1 | 2 | 4 | 6 | 24 |
| Quinic acid | 0 | 0 | 0 | 0 | 0 |
| Caffeic acid | 98.48 ± 1.62 | 96.67 ± 2.14 | 91.48 ± 4.92 | 86.14 ± 7.15 | 52.52 ± 2.31 |
| Rosmarinic acid | 93.15 ± 3.58 | 86.23 ± 4.05 | 68.20 ± 3.38 | 59.53 ± 0.98 | 0 |
| Quercetin | 99.55 ± 4.15 | 94.10 ± 5.36 | 49.16 ± 3.73 | 37.15 ± 2.68 | 0 |
| Rutin | 96.67 ± 1.66 | 96.58 ± 1.00 | 90.98 ± 7.08 | 90.74 ± 4.70 | 86.85 ± 8.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, R.; Paolillo, M.; Papetti, A. A Dynamic In Vitro Model for Testing Intestinal Absorption of Different Vegetable Food Secondary Metabolites. Appl. Sci. 2023, 13, 5033. https://doi.org/10.3390/app13085033
Colombo R, Paolillo M, Papetti A. A Dynamic In Vitro Model for Testing Intestinal Absorption of Different Vegetable Food Secondary Metabolites. Applied Sciences. 2023; 13(8):5033. https://doi.org/10.3390/app13085033
Chicago/Turabian StyleColombo, Raffaella, Mayra Paolillo, and Adele Papetti. 2023. "A Dynamic In Vitro Model for Testing Intestinal Absorption of Different Vegetable Food Secondary Metabolites" Applied Sciences 13, no. 8: 5033. https://doi.org/10.3390/app13085033
APA StyleColombo, R., Paolillo, M., & Papetti, A. (2023). A Dynamic In Vitro Model for Testing Intestinal Absorption of Different Vegetable Food Secondary Metabolites. Applied Sciences, 13(8), 5033. https://doi.org/10.3390/app13085033








