Castanea spp. Nut Traceability: A Multivariate Strategy Based on Phytochemical Data
Featured Application
Abstract
1. Introduction
2. Materials and Methods
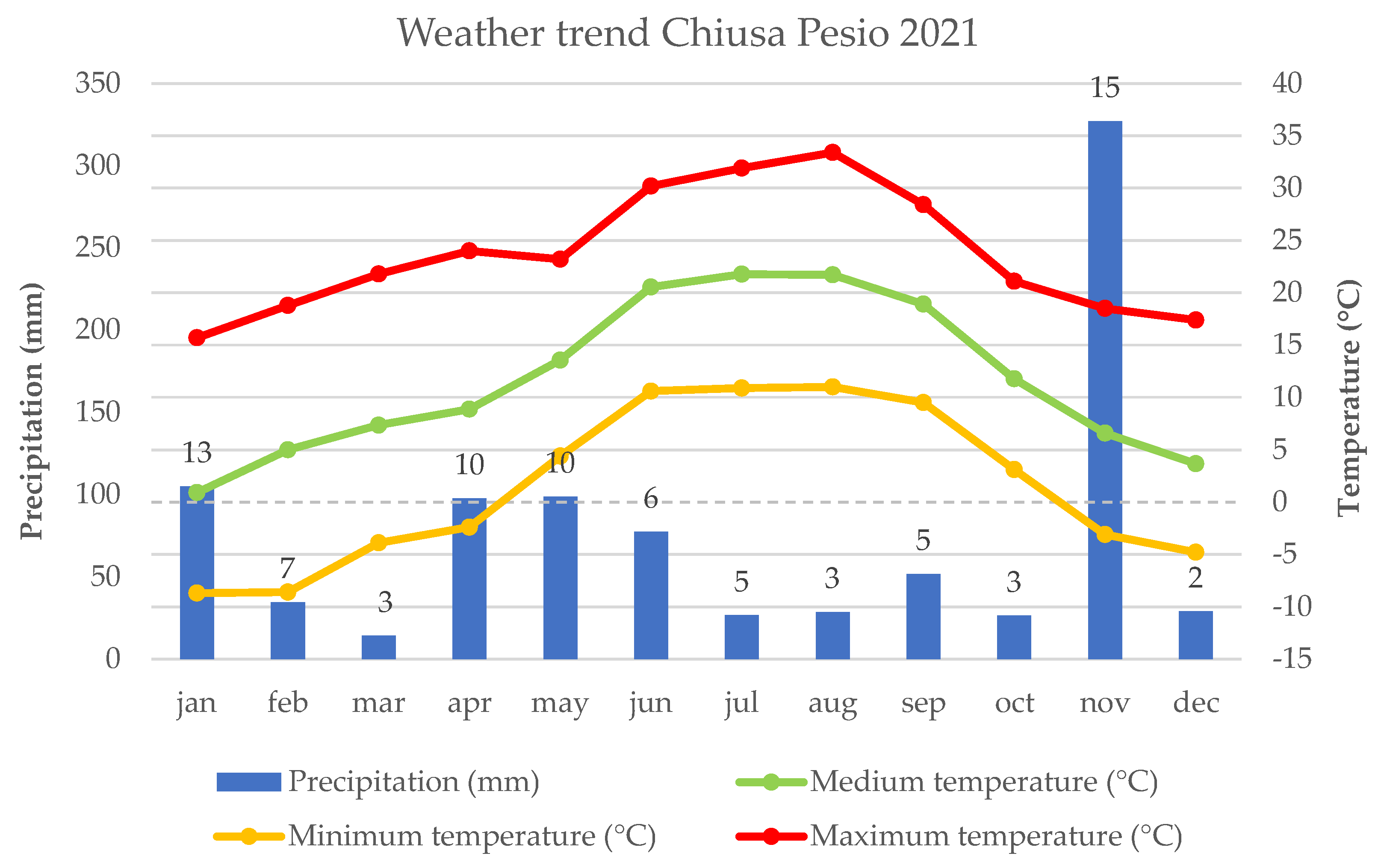
2.1. Plant Material
2.2. Sample Preparation
2.3. Extraction Protocols
2.4. Spectrophotometric Analysis
2.5. Chromatographic Analysis
2.6. Data Analysis
3. Results
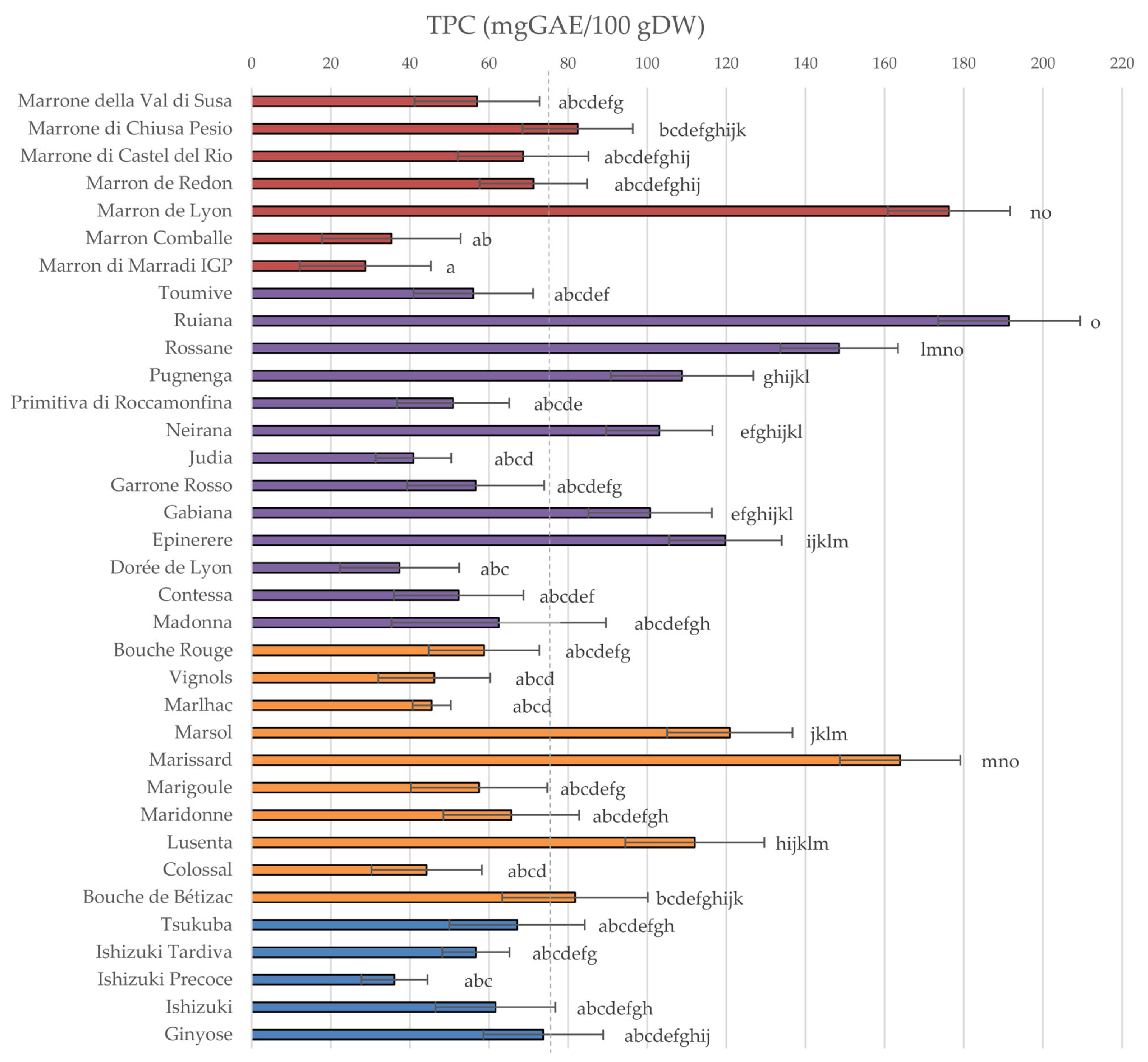
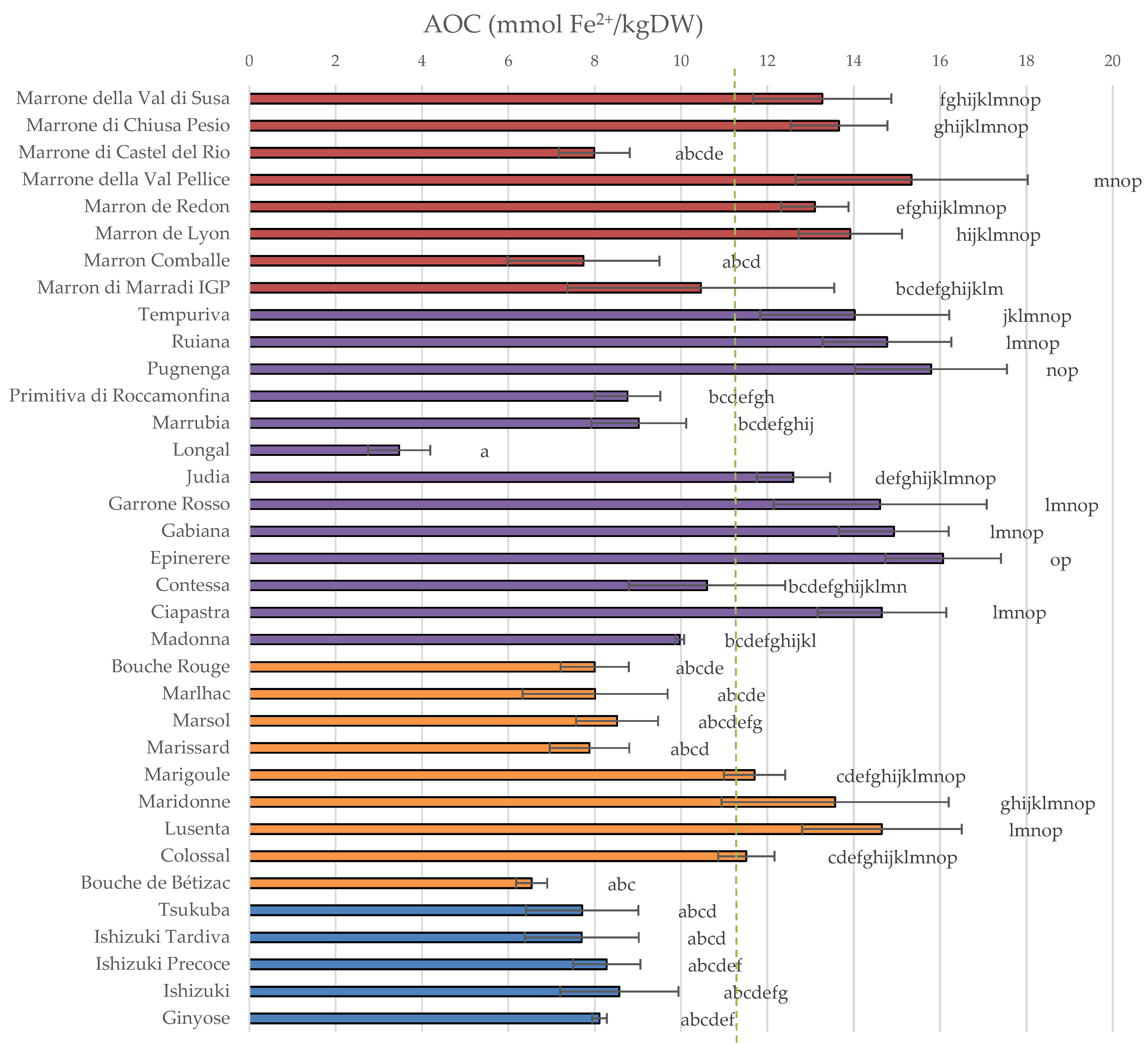
3.1. Total Polyphenol Content and Antioxidant Capacity
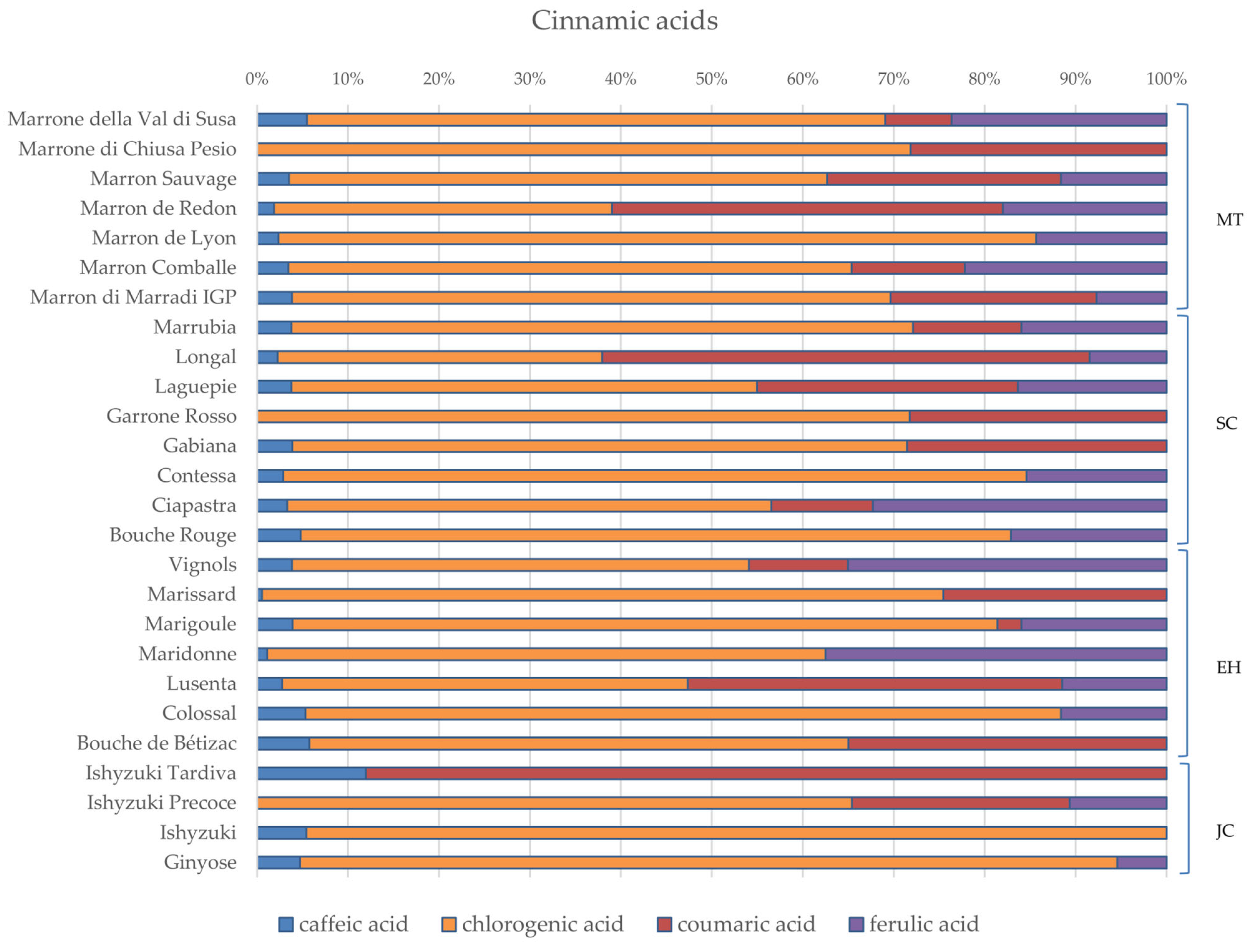
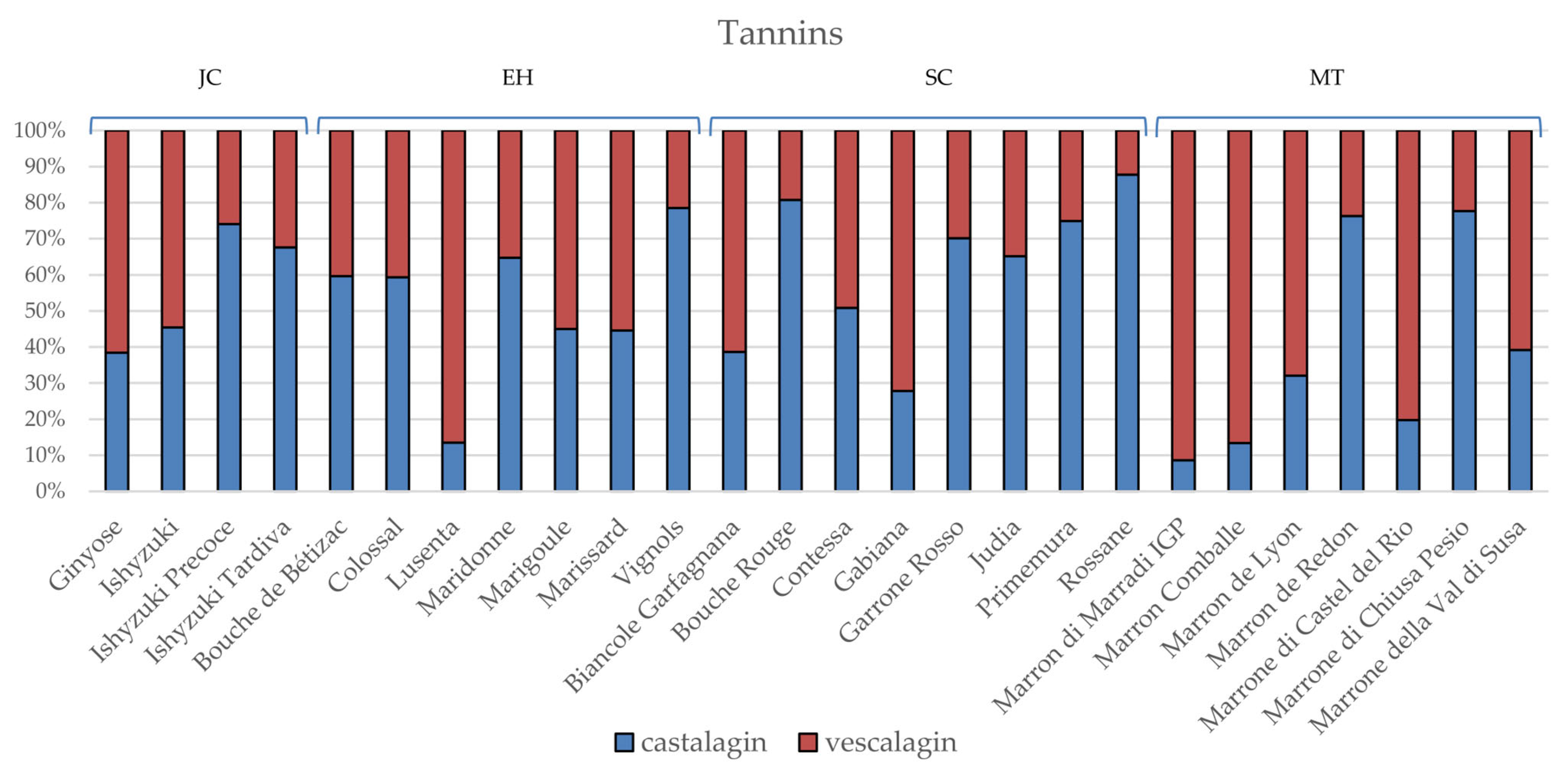
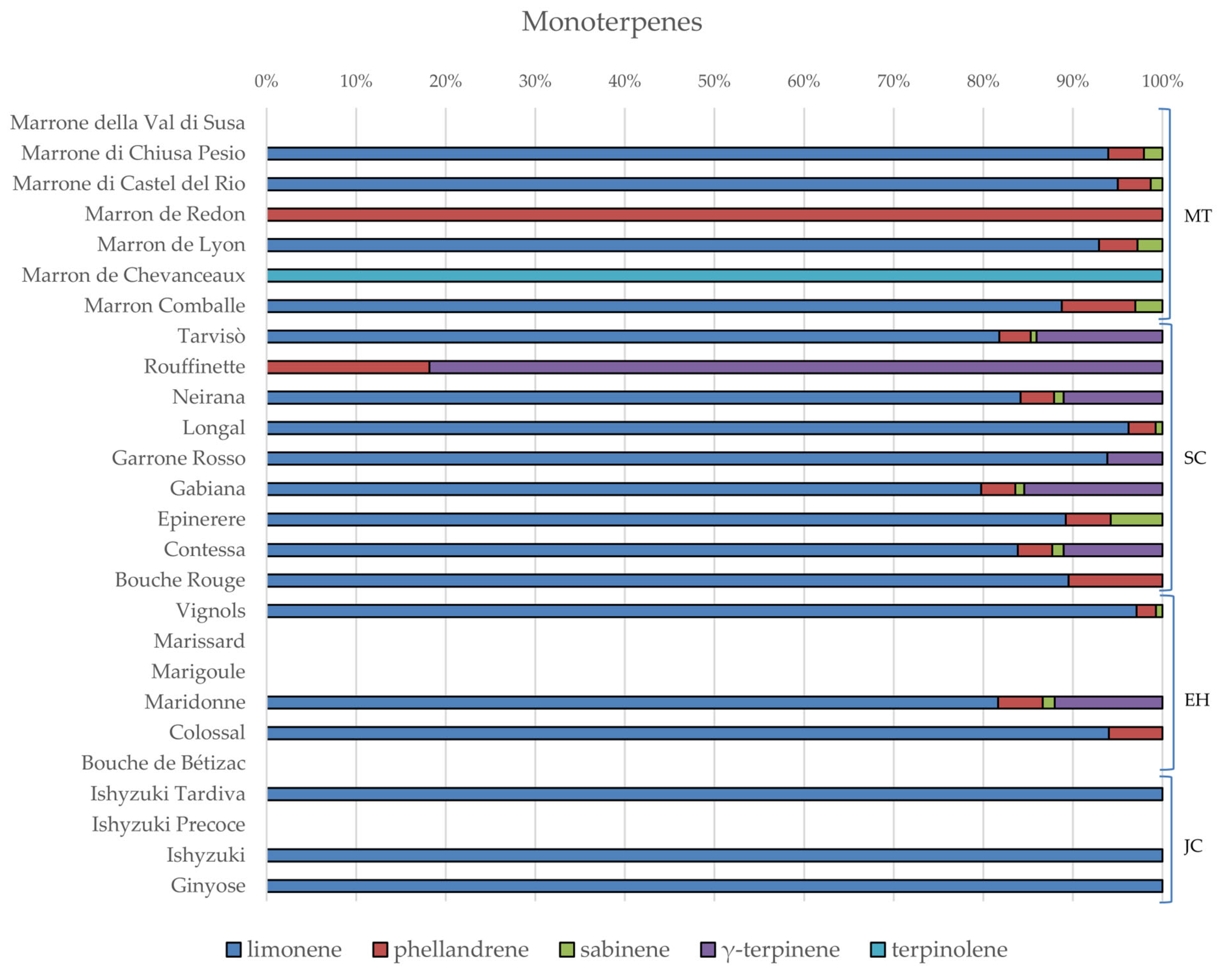
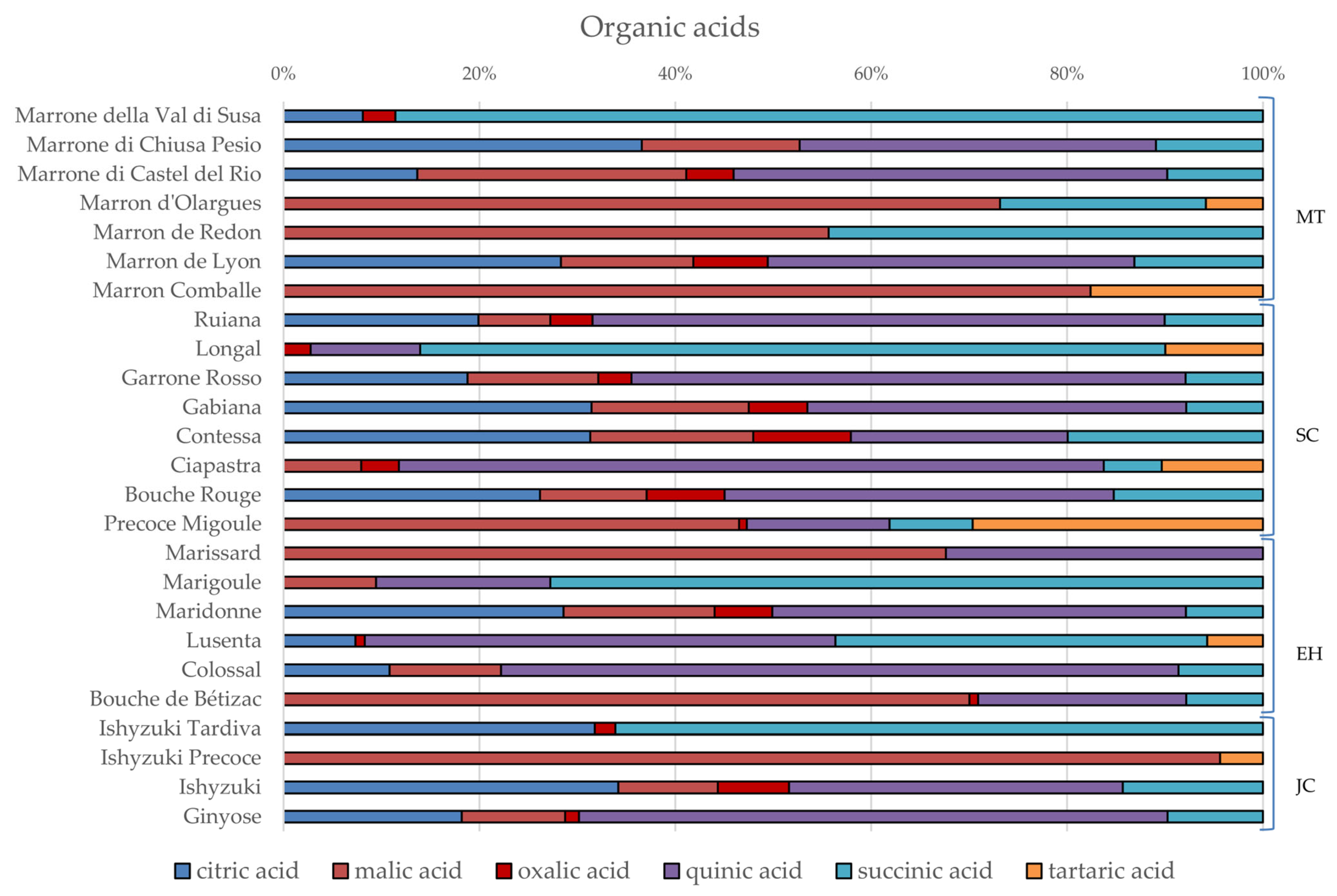
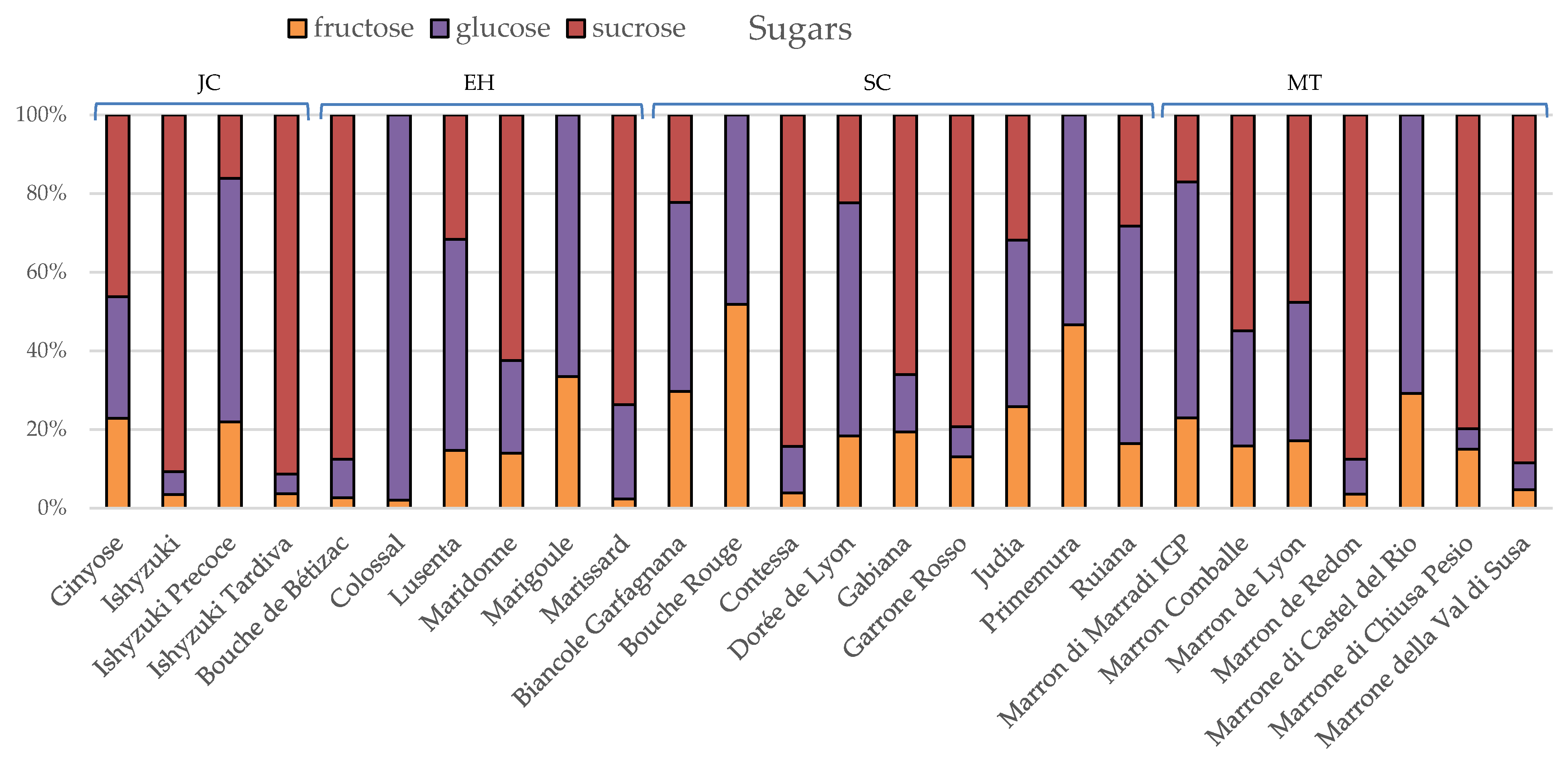
3.2. Phytochemical Composition and Nutritional Properties
3.3. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellano, M.G.; Beccaro, G.L.; Donno, D.; Torello Marinoni, D.; Boccacci, P.; Canterino, S.; Cerutti, A.K.; Bounous, G. Castanea spp. biodiversity conservation: Collection and characterization of the genetic diversity of an endangered species. Genet. Resour. Crop Evol. 2012, 59, 1727–1741. [Google Scholar] [CrossRef]
- Beccaro, G.L.; Alma, A.; Boni, I.; Botta, R.; Bussone, M.; Corgnati, M.; Ebone, A.; Gonthier, P.; Locatelli, G.; Malacarne, E.; et al. Chestnut R&D Centre: The strategic project of Piemonte to support the whole chestnut supply chain. Italia For. Mont. 2018, 75, 39–48. [Google Scholar]
- Poljak, I.; Vahčić, N.; Vidaković, A.; Tumpa, K.; Žarković, I.; Idžojtić, M. Traditional Sweet chestnut and hybrid varieties: Chemical composition, morphometric and qualitative nut characteristics. Agronomy 2021, 11, 516. [Google Scholar] [CrossRef]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. Agrobiodiversity Conservation: Genotype Influence on Chemical and Sensorial Traits of Cultivars Grown on the Same Clonal Rootstock. Foods 2020, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, S.; Krznar, M.; Ajolfi, D.; Ramos Cabrer, A.M.; Pereira-Lorenzo, S.; Dondini, L. Genetic Diversity of Castanea sativa Mill. Accessions from the Tuscan-Emilian Apennines and Emilia Romagna Region (Italy). Agronomy 2020, 10, 1319. [Google Scholar] [CrossRef]
- Nunziata, A.; Ruggieri, V.; Petriccione, M.; De Masi, L. Single Nucleotide Polymorphisms as Practical Molecular Tools to Support European Chestnut Agrobiodiversity Management. Int. J. Mol. Sci. 2020, 21, 4805. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Barreneche, T. Database of European chestnut cultivars and definition of a core collection using simple sequence repeats. Tree Genet. Genomes 2017, 13, 114. [Google Scholar] [CrossRef]
- Nardecchia, A.; Presutto, R.; Bucci, R. Authentication of the Geographical Origin of “Vallerano” Chestnut by Near Infrared Spectroscopy Coupled with Chemometrics. Food Anal. Methods 2020, 13, 1782–1790. [Google Scholar] [CrossRef]
- Míguez-Soto, B.; Fernández-Cruz, J.; Fernández-López, J. Mediterranean and Northern Iberian gene pools of wild Castanea sativa Mill. are two differentiated ecotypes originated under natural divergent selection. PLoS ONE 2019, 14, e0211315. [Google Scholar] [CrossRef]
- Jarman, R.; Mattioni, C.; Russell, K.; Chambers, F.M.; Bartlett, D.; Angela Martin, M.; Cherubini, M.; Villani, F.; Webb, J. DNA analysis of Castanea sativa (sweet chestnut) in Britain and Ireland: Elucidating European origins and genepool diversity. PLoS ONE 2019, 14, e0222936. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Barreneche, T.; Mattioni, C.; Villani, F.; Díaz-Hernández, B.; Martín, L.M.; Robles-Loma, A.; Cáceres, Y.; Martín, A. Instant domestication process of European chestnut cultivars. Ann. Appl. Biol. 2019, 174, 74–85. [Google Scholar] [CrossRef]
- Martín, M.A.; Mattioni, C.; Cherubini, M.; Villani, F.; Martín, L.M. A comparative study of European chestnut varieties in relation to adaptive markers. Agrofor. Syst. 2017, 9, 97–109. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Ciucure, C.T.; Geana, E.I.; Sandru, C.; Tita, O.; Botu, M. Phytochemical and nutritional profile composition in fruits of different sweet chestnut (Castanea sativa Mill.) cultivars grown in Romania. Separations 2022, 9, 66. [Google Scholar] [CrossRef]
- Akbulut, M.; Bozhuyuk, M.R.; Ercisli, S.; Skender, A.; Sorkheh, K. Chemical Composition of Seed Propagated Chestnut Genotypes from Northeastern Turkey. Not. Bot. Horti Agrobot. 2017, 45, 425–430. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Carini, V.; Bergamasco, E.; Gamba, G.; Beccaro, G.L. Application of Traditional Cooking Methods in Chestnut Processing: Effects of Roasting and Boiling on Secondary Metabolites and Antioxidant Capacity in Castanea spp. Fruits. Agriculture 2023, 13, 530. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M.; Admassu, S. Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 38–49. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Donno, D.; Randriamampiona, D.; Andriamaniraka, H.; Torti, V.; Mellano, M.G.; Giacoma, C.; Beccaro, G.L. Biodiversity and traditional medicinal plants from Madagascar: Phytochemical evaluation of Brachylaenaramiflora (DC.) Humbert decoctions and infusions. J. Appl. Bot. Food Qual. 2017, 90, 205–213. [Google Scholar]
- Annunziata, A.; Pascale, P. Consumers’ behaviours and attitudes toward healthy food products: The case of Organic and Functional foods. In Proceedings of the 113th Seminar, Chania, Greece, 3–6 September 2009; European Association of Agricultural Economists: Wageningen, The Netherlands, 2009. [Google Scholar]
- De Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Galdón, B.; Mesa, D.; Romero, C.; Rodríguez, E. Sugars, Organic Acids and Total Phenols in Varieties of Chestnut Fruits from Tenerife (Spain). Food Nutr. Sci. 2012, 3, 705–715. [Google Scholar] [CrossRef]
- Otles, S.; Selek, I. Phenolic compounds and antioxidant activities of chestnut (Castanea sativa Mill.) fruits. Qual. Assur. Saf. Crops Foods 2012, 4, 199–205. [Google Scholar] [CrossRef]
- Xu, Z.; Meenu, M.; Chen, P.; Xu, B. Comparative Study on Phytochemical Profiles and Antioxidant Capacities of Chestnuts Produced in Different Geographic Areas in China. Antioxidants 2020, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Dinis, L.-T.; Oliveira, M.M.; Almeida, J.; Costa, R.; Gomes-Laranjo, J.; Peixoto, F. Antioxidant activities of chestnut nut of Castanea sativa Mill. (cultivar ‘Judia’) as function of origin ecosystem. Food Chem. 2012, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghirardello, D.; Bertolino, M.; Belviso, S.; Dal Bello, B.; Giordano, M.; Rolle, L.; Gerbi, V.; Antonucci, M.; Spigolon, N.; Zeppa, G. Phenolic composition, antioxidant capacity and hexanal content of hazelnuts (Corylus avellana L.) as affected by different storage conditions. Postharvest Biol. Technol. 2016, 112, 95–104. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. HPLC-MSn identification and quantification of phenolic compounds in hazelnut kernels, oil and bagasse pellets. Food Res. Int. 2014, 64, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef]
- Uzun, S. Postharvest Quality Traits of Chestnut (Castanea sativa Mill.) Fruit as Affected by Methyl Jasmonate During Cold Storage. Erwerbs-Obstbau 2023, 65, 1453–1462. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New Findings in Prunus padus L. Fruits as a Source of Natural Compounds: Characterization of Metabolite Profiles and Preliminary Evaluation of Antioxidant Activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Zhou, W.; Lee, W.; Han, M.S.; Na, M.; Bae, J.S. Anti-inflammatory effects of hyperoside in human endothelial cells and in mice. Inflammation 2015, 38, 784–799. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem. 2008, 8, 1179. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Hwang, I.K.; Park, J.B. Analysis of Physicochemical Factors Related to the Automatic Pellicle Removal in Korean Chestnut (Castanea crenata). J. Agric. Food Chem. 2001, 49, 6045–6049. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Mujić, I.; Zivković, J.; Savić, V.; Alibabić, V.; Staver, M.; Jug, T.; Franić, M.; Damijanić, K. Analysis of volatile compounds in chestnut using solid-phase microextraction coupled with GC-MS. Acta Hortic. 2018, 1220, 203–207. [Google Scholar] [CrossRef]
- Grygorieva, O.V.; Klymenko, S.V.; Teslyuk, M.G.; Onyschuk, L.M. Variability of morphological parameters and determination of volatile organic compounds of sweet chestnut (Castanea sativa Mill.) genotypes fruits. Introdukcija Roslin 2018, 2, 74–83. [Google Scholar]
- Santos, M.J.; Pinto, T.; Vilela, A. Sweet chestnut (Castanea sativa Mill.) nutritional and phenolic composition interactions with chestnut flavor physiology. Foods 2022, 11, 4052. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.P. Ascorbic Acid and Total Phenolic Contents of Dried Roasted Chestnut (Castanea sativa) Affected by Drying, Roasting and Preservation. Biosc. Biotech. Res. Comm. 2020, 13, 129–133. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F. Tree Nuts: Composition, Phytochemicals, and Health Effects; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Borges, O.; Costa, H.S.; Bennett, R.N.; Santos, M.; Silva, A.P. Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: Proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010, 122, 154–160. [Google Scholar] [CrossRef]
- Eyduran, S.P.; Ercisli, S.; Akin, M.; Beyhan, O.; Gecer, M.K.; Eyduran, E.; Erturk, Y.E. Organic acids, sugars, vitamin C, antioxidant capacity and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genotypes. J. Appl. Bot. Food Qual. 2015, 88, 134–138. [Google Scholar]
- Spiller, G.A.; Story, J.A.; Furumoto, E.J.; Chezem, J.C.; Spiller, M. Effect of tartaric acid and dietary fibre from sun-dried raisins on colonic function and on bile acid and volatile fatty acid excretion in healthy adults. Br. J. Nutr. 2003, 90, 803–807. [Google Scholar] [CrossRef]
- Sheng, X.; Jung, T.; Wesson, J.A. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc. Natl. Acad. Sci. USA 2005, 102, 267–272. [Google Scholar] [CrossRef]
- Delgado, T.; Pereira, J.A.; Ramalhosa, E.; Casal, S. Comparison of different drying methods on the chemical and sensory properties of chestnut (Castanea sativa M.) slices. Eur. Food Res. Technol. 2017, 243, 1957–1971. [Google Scholar] [CrossRef]
- Neri, L.; Dimitri, G.; Sacchetti, G. Chemical composition and antioxidant activity of cured chestnuts from three sweet chestnut (Castanea sativa Mill.) ecotypes from Italy. J. Food Compos. Anal. 2010, 23, 23–29. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Lê, K.A.; Samra, R.A.; Roger, O.; Green, H.; Macé, K. The scientific basis for healthful carbohydrate profile. Crit. Rev. Food Sci. Nutr. 2019, 59, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Köksal, A.I.; Artik, N.; ¸Simsek, A.; Günes, N. Nutrient composition of hazelnut (Corylus avellana L.) varieties cultivated in Turkey. Food Chem. 2006, 99, 509–515. [Google Scholar] [CrossRef]
- Moodley, R.; Kindness, A.; Jonnalagadda, S.B. Elemental composition and chemical characteristics of five edible nuts (almond, Brazil, pecan, macadamia and walnut) consumed in Southern Africa. J. Environ. Sci. Health Part B 2007, 42, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lorenzo, S.; Costa, R.; Anagnostakis, S.; Serdar, U.; Yamamoto, T.; Saito, T.; Ramos-Cabrer, A.; Ling, Q.; Barreneche, T.; Robin, C.; et al. Interspecific hybridization of chestnut. Polyploidy Hybrid. Crop Improv. 2016, 15, 377–408. [Google Scholar]
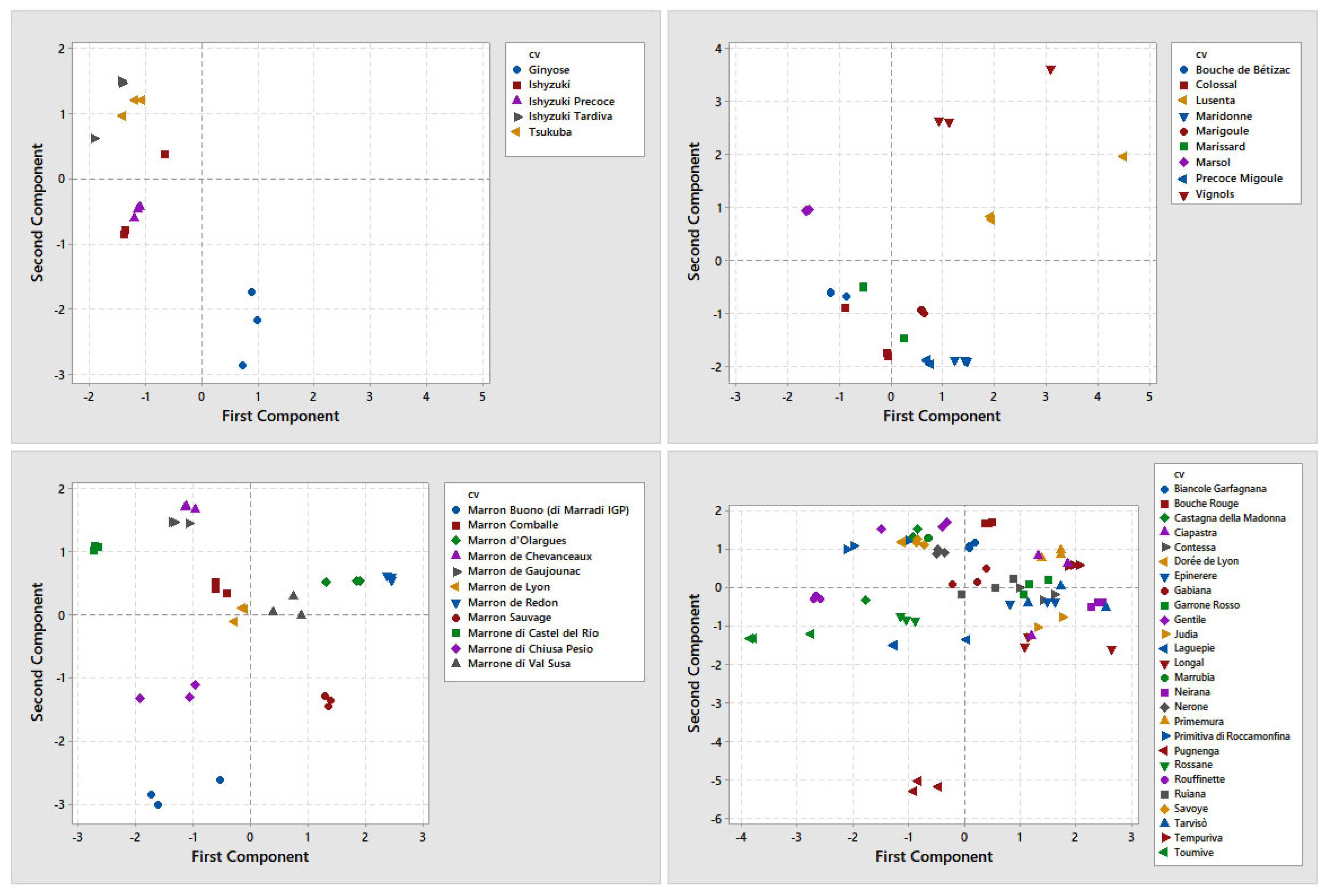
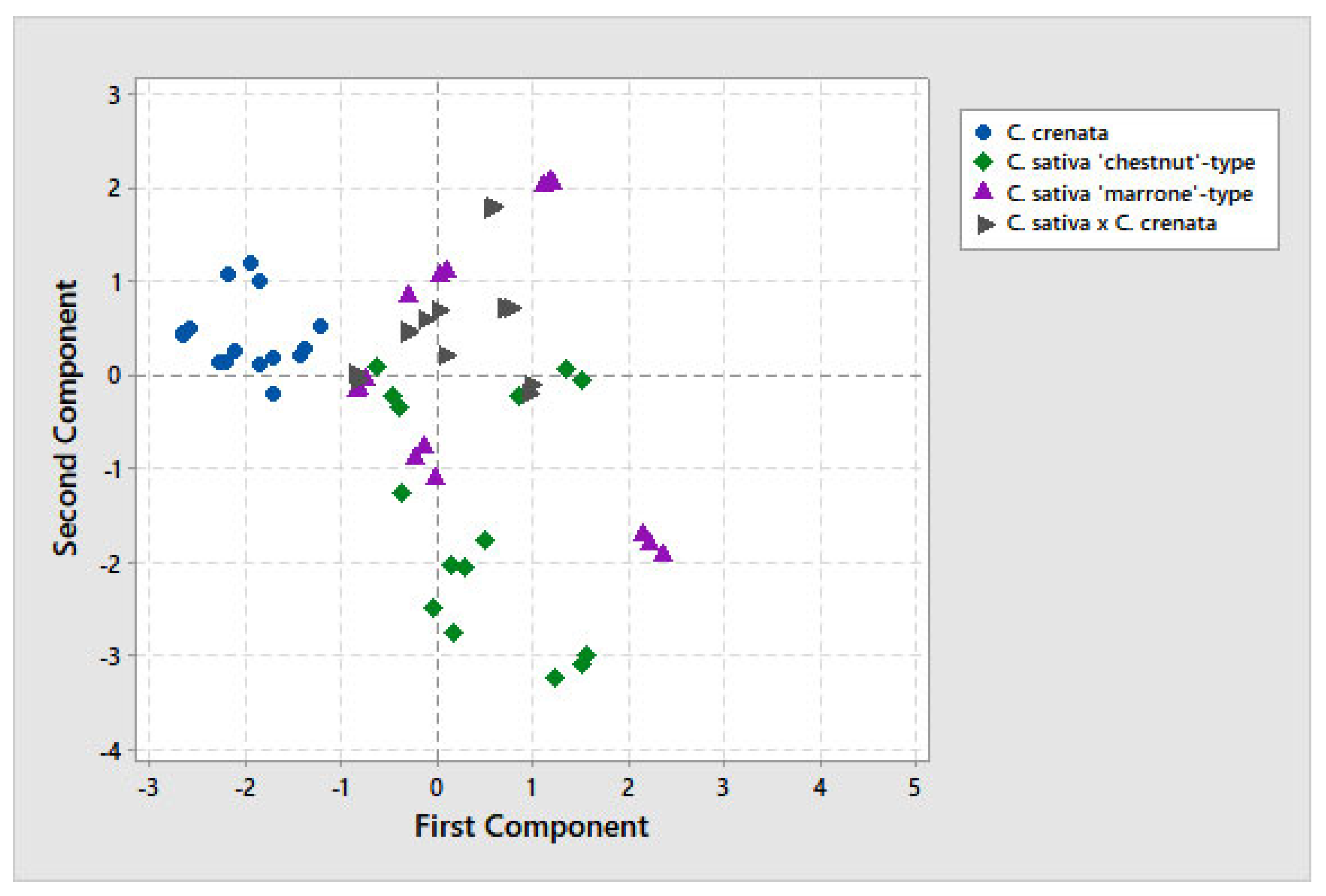
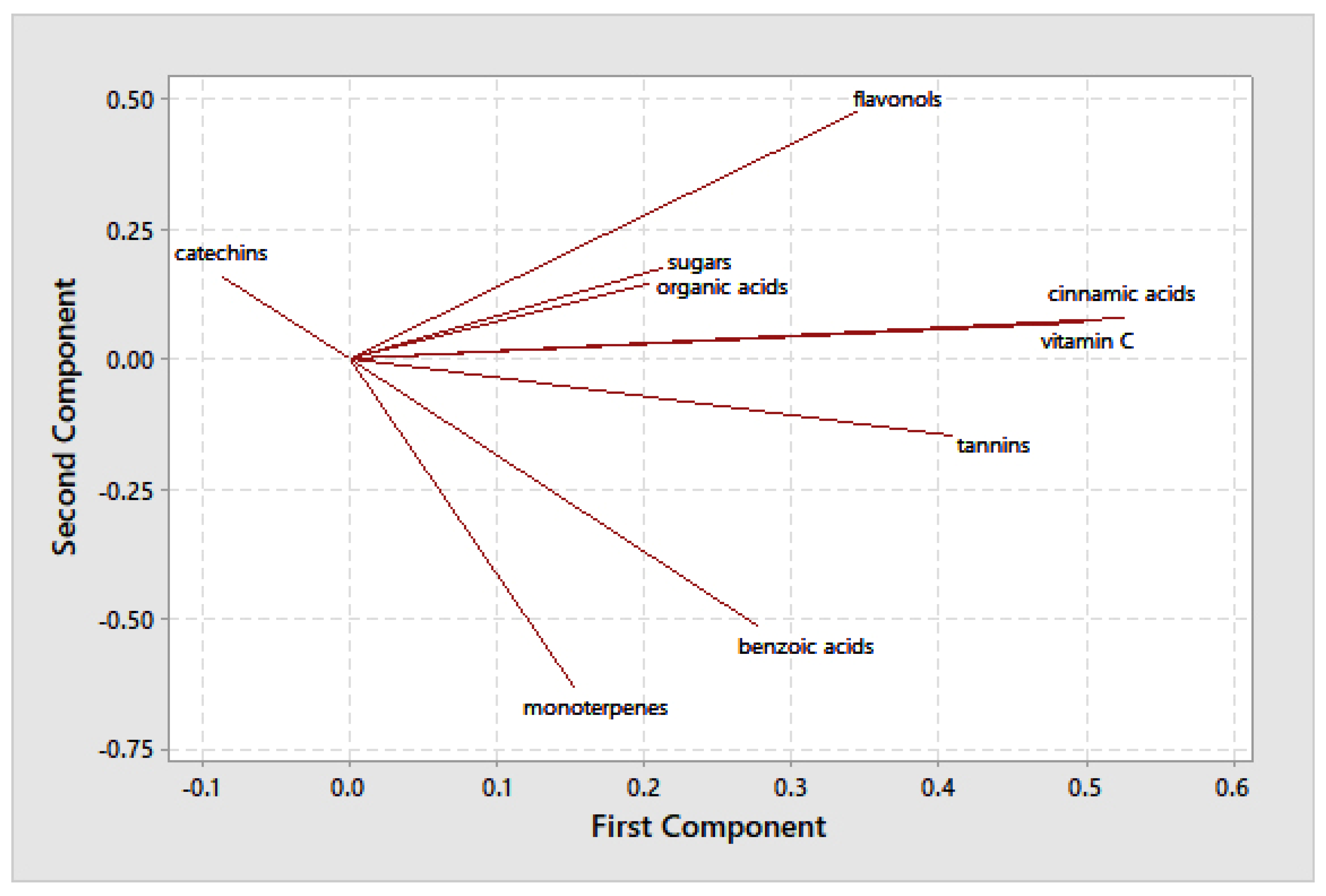
| Species | Cultivar | Origin | ID Sample | |
|---|---|---|---|---|
| 1 | C. crenata | Ginyose | Japan, Korea, North-East China, Taiwan | D18 |
| 2 | Ishizuki | F23 | ||
| 3 | Ishizuki Precoce | O15 | ||
| 4 | Ishizuki Tardiva | E17 | ||
| 5 | Tsukuba | G14 | ||
| 6 | C. sativa × C. crenata | Bouche de Bétizac | France | I10 |
| 7 | Colossal | East USA | M22 | |
| 8 | Lusenta | Italy (Piemonte) | M15 | |
| 9 | Maridonne | France | J14 | |
| 10 | Marigoule | J13 | ||
| 11 | Marissard | N14 | ||
| 12 | Marlhac | K05 | ||
| 13 | Marsol | F06 | ||
| 14 | Precoce Migoule | G18 | ||
| 15 | Vignols | I17 | ||
| 16 | C. sativa (sweet chestnuts) | Biancole della Garfagnana | Italy (Toscana) | B03 |
| 17 | Bouche Rouge | France (Ardèche) | I18 | |
| 18 | Castagna della Madonna | Italy (Piemonte) | G01 | |
| 19 | Ciapastra | T02 | ||
| 20 | Contessa | L02 | ||
| 21 | Dorée de Lyon | Central-West France | H23 | |
| 22 | Epinerere | Italy (Valle d’Aosta) | M16 | |
| 23 | Gabiana | Italy (Piemonte) | O03 | |
| 24 | Garrone Rosso | I03 | ||
| 25 | Gentile | P12 | ||
| 26 | Judia | Portugal (Padrela) | F21 | |
| 27 | Laguepie | France | H24 | |
| 28 | Longal | Portugal (Terra Fria) | E16 | |
| 29 | Marrubia | Italy (Piemonte) | Q13 | |
| 30 | Neirana | Italy (Piemonte) | U09 | |
| 31 | Nerone | Italy (Toscana) | H08 | |
| 32 | Primemura | Italy (Piemonte) | X05 | |
| 33 | Primitiva di Roccamonfina | Italy (Campania) | I07 | |
| 34 | Pugnenga | Italy (Piemonte) | P04 | |
| 35 | Rossane | Italy (Valle d’Aosta) | N13 | |
| 36 | Rouffinette | O11 | ||
| 37 | Ruiana | Italy (Piemonte) | T11 | |
| 38 | Savoye | France (Dordogne, Lot, Cantal) | J16 | |
| 39 | Tarvisò | Italy (Piemonte) | U06 | |
| 40 | Tempuriva | O12 | ||
| 41 | Toumive | France (Cantal-Mourjou) | D11 | |
| 42 | C. sativa (“marrone-type”) | Marron Buono (di Marradi IGP) | Italy (Toscana) | C03 |
| 43 | Marron Comballe | France (Ardèche) | G19 | |
| 44 | Marron de Chevanceaux | France (Charente-Maritime) | L14 | |
| 45 | Marron de Goujounac | France (Ardèche) | F20 | |
| 46 | Marron de Lyon | Central-West France | G23 | |
| 47 | Marrone della Val di Susa | Italy (Piemonte) | P16 | |
| 48 | Marrone della Val Pellice | V07 | ||
| 49 | Marron de Redon | France (Ardèche) | K22 | |
| 50 | Marrone di Castel del Rio | Italy (Emilia-Romagna) | G08 | |
| 51 | Marrone di Chiusa Pesio | Italy (Piemonte) | G02 | |
| 52 | Marron d’Olargues | France (Hérault) | F18 | |
| 53 | Marron Sauvage | France (Ardèche) | K20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prezzi, E.; Donno, D.; Mellano, M.G.; Beccaro, G.L.; Gamba, G. Castanea spp. Nut Traceability: A Multivariate Strategy Based on Phytochemical Data. Appl. Sci. 2023, 13, 12524. https://doi.org/10.3390/app132212524
Prezzi E, Donno D, Mellano MG, Beccaro GL, Gamba G. Castanea spp. Nut Traceability: A Multivariate Strategy Based on Phytochemical Data. Applied Sciences. 2023; 13(22):12524. https://doi.org/10.3390/app132212524
Chicago/Turabian StylePrezzi, Elisabetta, Dario Donno, Maria Gabriella Mellano, Gabriele Loris Beccaro, and Giovanni Gamba. 2023. "Castanea spp. Nut Traceability: A Multivariate Strategy Based on Phytochemical Data" Applied Sciences 13, no. 22: 12524. https://doi.org/10.3390/app132212524
APA StylePrezzi, E., Donno, D., Mellano, M. G., Beccaro, G. L., & Gamba, G. (2023). Castanea spp. Nut Traceability: A Multivariate Strategy Based on Phytochemical Data. Applied Sciences, 13(22), 12524. https://doi.org/10.3390/app132212524









