The Potential for the Implementation of Pea Flower (Clitoria ternatea) Health Properties in Food Matrix
Abstract
1. Introduction
2. Botanical Characteristics of Clitoria ternatea
3. Bioactive Compounds of Clitoria ternatea
4. Medicinal Effects of Clitoria ternatea
4.1. Anti-Arthritic and Anti-Inflammatory Potential
4.2. Anti-Asthmatic Properties
4.3. Analgesic Effects
4.4. Anti-Pyretic Effect
4.5. Anti-Diabetic and Hypolipidemic Activity
4.6. Wound Healing and Blood Platelet Aggregation Inhibition
4.7. Anti-Tumor Activity
4.8. Neuro and Cognitive Enhancing Activity
4.9. Antioxidant Activity
4.10. Anti-Bacterial Activity
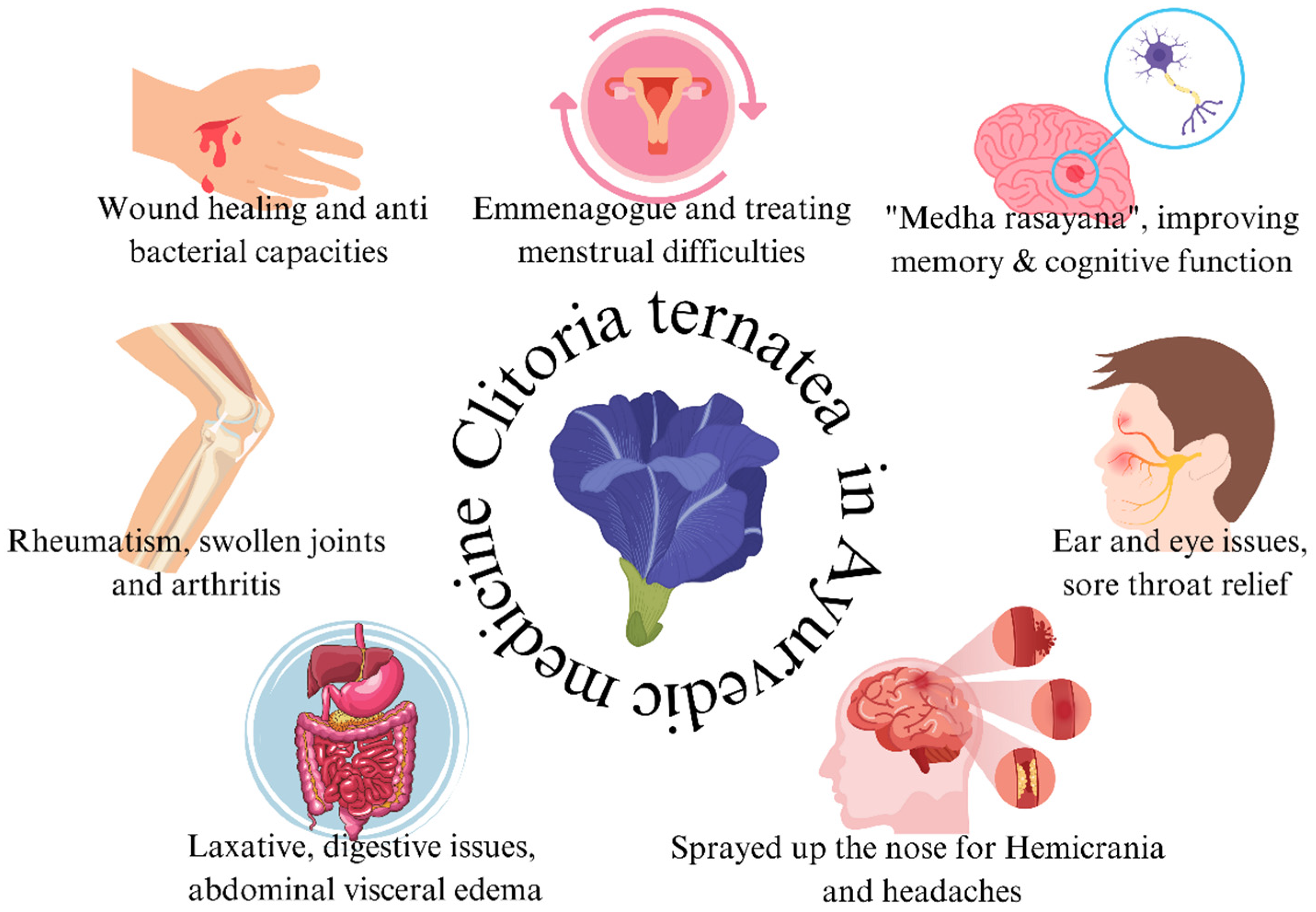
5. Application of Clitoria ternatea in the Indian Ayurvedic System
6. Applications of Clitoria ternatea in South American Medicine
7. Applications of C. ternatea in Experimental and Commercial Food Products
8. Scope of Clitoria ternatea as a Future Food
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oguis, G.; Gilding, E.; Jackson, M.; Craik, D. Butterfly Pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front. Plant Sci. 2019, 10, 645. [Google Scholar] [CrossRef]
- Nhut Pham, T.; Chinh Nguyen, D.; Duc Lam, T.; Van Thinh, P.; Tien Le, X.; Vo Nguyen, D.V.; Quang, H.V.; Duy Nguyen, T.; Bach, L.G. Extraction of anthocyanins from Butterfly pea (Clitoria ternatea L. Flowers) in Southern Vietnam: Response surface modeling for optimization of the operation conditions. IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 12032. [Google Scholar] [CrossRef]
- Suraweera, T.; Jayanath, N.; Abeysekera, K.; Abeysekera, K. A Commercial Potential Blue Pea (Clitoria ternatea L.) flower extract incorporated beverage having functional properties. Evid. Based Complement. Altern. Med. 2019, 2019, 2916914. [Google Scholar] [CrossRef]
- Mukherjee, P.; Kumar, V.; Satheesh Kumar, N.; Heinrich, M. The Ayurvedic medicine Clitoria ternatea-from traditional use to scientific assessment. J. Ethnopharmacol. 2008, 120, 291–301. [Google Scholar] [CrossRef]
- Multisona, R.R.; Shirodkar, S.; Arnold, M.; Gramza-Michalowska, A. Clitoria ternatea flower and its bioactive compounds: Potential use as microencapsulated ingredient for functional foods. Appl. Sci. 2023, 13, 2134. [Google Scholar] [CrossRef]
- Abreu, M.L.C.; Vieira, R.A.; Rocha, N.S.; Araujo, R.P.; Glória, L.S.; Fernandes, A.M.; de Lacerda, P.D.; Júnior, A.G. Clitoria ternatea L. as a potential high quality forage legume. Asian Australas. J. Anim. Sci. 2014, 27, 169–178. [Google Scholar] [CrossRef]
- Gupta, G.; Chahal, J.; Bhatia, M. Clitoria ternatea (L.): Old and new aspects. J. Pharm. Res. 2010, 3, 2610–2614. [Google Scholar]
- Kazuma, K.; Noda, N.; Suzuki, M. Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry 2003, 64, 1133–1139. [Google Scholar] [CrossRef]
- Shen, Y.; Du, L.; Zeng, H.; Zhang, X.; Prinyawiwatkul, W.; Alonso-Marenco, J.R.; Xu, Z. Butterfly pea (Clitoria ternatea) seed and petal extracts decreased HEp-2 carcinoma cell viability. Int. J. Food Sci. Technol. 2016, 51, 1860–1868. [Google Scholar] [CrossRef]
- Crowe-White, K.; Francis, C. Position of the Academy of Nutrition and Dietetics: Functional Foods. J. Acad. Nutr. Diet. 2013, 113, 1096–1103. [Google Scholar] [CrossRef]
- Nystrand, B.; Olsen, S. Relationships between functional food consumption and individual traits and values: A segmentation approach. J. Funct. Foods 2021, 86, 104736. [Google Scholar] [CrossRef]
- Swathi, K.P.; Jayaram, S.; Sugumar, D.; Rymbai, E. Evaluation of anti-inflammatory and anti-arthritic property of ethanolic extract of Clitoria ternatea. Chin. Herb. Med. 2020, 13, 243–249. [Google Scholar] [CrossRef]
- Martirosyan, D.; von Brugger, J.; Bialow, S. Functional food science: Differences and similarities with food science. Funct. Foods Health Dis. 2021, 11, 408. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, Z.; Huang, X. A review of phytochemistry and pharmacology perspectives of Clitoria ternatea L. Asian J. Trad. Med. 2021, 16, 153–160. [Google Scholar]
- Ranaweera, C.; Ranjith, P.; Chandana, A.; Ralalage, J.; Jayakody, A.; Ratnasooriya, D. In vitro antirheumatoid arthritic activity of aqueous root extract of Clitoria ternatea. Int. Res. J. Pharm. 2014, 5, 926–928. [Google Scholar] [CrossRef]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 14, 16–36. [Google Scholar] [CrossRef]
- Chauhan, N.; Rajvaidhya, S.; Dubey, B.K. Pharmacognostical, phytochemical and pharmacological review on Clitoria ternatea for antiasthmatic activity. Int. J. Pharm. Sci. Res. 2012, 3, 398–404. [Google Scholar] [CrossRef]
- Kiran; Singh, A.; Jain, A.K. Biochemical and pharmacological aspects of Clitoria ternatea—A review. Ind. J. Agric. Biochem. 2020, 33, 115–124. [Google Scholar] [CrossRef]
- Bhatia, M.S.; Chahal, J.; Gupta, S. Analgesic and anti-inflammatory activities of Clitoria ternatea Linn. leaves extract on rat model. Int. J. Pharm. Sci. Res. 2014, 41, 600–606. [Google Scholar] [CrossRef]
- Devi, B.P.; Boominathan, R.; Mandal, S.C. Anti-inflammatory, analgesic and antipyretic properties of Clitoria ternatea root. Fitoterapia 2003, 74, 345–349. [Google Scholar] [CrossRef]
- Murugalakshmi, M.; Valli, G.; Mareeswari, P.; Thangapandian, V. Antipyretic and purgative activities of Clitoria ternatea leaves extracts. World J. Pharm. Pharm. Sci. (WJPPS) 2014, 3, 632–637. [Google Scholar]
- Kavitha, R. Effect of ethanolic extracts of leaf and fruit of Trichosanthes dioica and leaf of Clitoria ternatea on serum lipids in streptozotocin-induced diabetic rats. Int. J. Pharma Bio Sci. 2015, 6, P430–P439. [Google Scholar]
- Singh, J.; Tiwari, K.N. High-frequency in vitro multiplication system for commercial propagation of pharmaceutically important Clitoria ternatea L.—A valuable medicinal plant. Ind. Crops Prod. 2010, 32, 534–538. [Google Scholar] [CrossRef]
- Verma, P.R.; Itankar, P.R.; Arora, S.K. Evaluation of antidiabetic antihyperlipidemic and pancreatic regeneration, potential of aerial parts of Clitoria ternatea. Rev. Bras. De Farmacogn. 2013, 23, 819–829. [Google Scholar] [CrossRef]
- Shivaprakash, P.; Balaji, K.S.; Chandrashekara, K.T.; Rangappa, K.S.; Jayarama, S. Induction of apoptosis in mcf-7 cells by methanolic extract of Clitoria ternatea L. Int. J. Appl. Biol. Pharm. Technol. 2015, 6, 80–86. [Google Scholar]
- Rai, K.; Murthy, D.; Karanth, K.; Rao, M. Clitoria ternatea (Linn) root extract treatment during growth spurt period enhances learning and memory in rats. Indian. J. Physiol. Pharmacol. 2001, 45, 305–313. [Google Scholar]
- Taranalli, A.D.; Cheeramkuzhy, T. Influence of Clitoria ternatea extracts on memory and central cholinergic activity in rats. Pharm. Biol. 2000, 38, 51–56. [Google Scholar] [CrossRef]
- Raghu, K.S.; Shamprasad, B.R.; Kabekkodu, S.P.; Paladhi, P.; Joshi, M.B.; Valiathan, M.S.; Guruprasad, K.P.; Satyamoorthy, K. Age dependent neuroprotective effects of medhya rasayana prepared from Clitoria ternatea Linn. in stress induced rat brain. J. Ethnopharmacol. 2017, 197, 173–183. [Google Scholar] [CrossRef]
- Phrueksanan, W.; Yibchok-anun, S.; Adisakwattana, S. Protection of Clitoria ternatea flower petal extract against free radical-induced hemolysis and oxidative damage in canine erythrocytes. Res. Vet. Sci. 2014, 97, 357–363. [Google Scholar] [CrossRef]
- Mahmad, N.; Taha, R.M.; Othman, R.; Abdullah, S.; Anuar, N.; Elias, H.; Rawi, N. Anthocyanin as potential source for antimicrobial activity in Clitoria ternatea L. and Dioscorea alata L. Pigment. Resin. Technol. 2018, 47, 490–495. [Google Scholar] [CrossRef]
- Mukherjee, P.; Rai, S.; Kumar, V.; Hylands, P.; Hider, R. Plants of Indian origin in drug discovery. Expert Opin. Drug Discov. 2007, 2, 633–657. [Google Scholar] [CrossRef]
- Thuy, N.M.; Minh, V.Q.; Ben, T.C.C.; Thi Nguyen, M.T.; Ha, H.T.N.; Van Tai, N. Identification of anthocyanin compounds in butterfly pea flowers (Clitoria ternatea L.) by ultra performance liquid chromatography/ultraviolet coupled to Mass spectrometry. Molecules 2021, 26, 4539. [Google Scholar]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Oxidative stability of cooked pork patties incorporated with Clitoria ternatea extract (Blue Pea Flower Petal) during refrigerated storage: Oxidative stability of cooked pork patties. J. Food Process. Preserv. 2016, 41, e12751. [Google Scholar] [CrossRef]
- Bawar, S.D. Development of Mocktail Drinks with Butterfly Pea Flower Extract; TESDA Women’s Center: Taguig City, Philippines, 2018. Available online: http://twc.tesda.gov.ph/researchanddevelopment/researches/01%20DEVELOPMENT%20OF%20MOCKTAIL%20DRINKS%20WITH%20BUTTERFLY%20PEA%20FLOWER%20EXTRACT.pdf (accessed on 1 May 2023).
- Hutabarat, D.J.C. Chemical and physical characteristics of fermented beverages from plant-based milk with the addition of butterfly pea flower (Clitoria ternatea L.) extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 12140. [Google Scholar] [CrossRef]
- Alfania, U.P. Application of Spray Drying with the Addition of Maltodextrin and Citric Acid to Make Colorant Powder from Butterfly Pea Flower (Clitoria ternatea L.). Bachelor’s Thesis, Faculty of Agricultural Technology, Soegijapranata Catholic University, Semarang, Indonesia, 2018. Available online: https://repository.unika.ac.id/18908/ (accessed on 1 May 2023).
- Thilakarathna, H.I.U.; Jayarathna, G.G.N.; Mudannayake, D.C. Probiotic Ice Cream Incorporated with Blue Pea Flower (Clitoria ternatea) and Dehydrated Banana Flour. In Proceedings of the 5th International Research Conference of Uva Wellassa University, IRCUWU 2021, “Exploring Potentials in Challenging Periods”, Badulla, Sri Lanka, 1–2 July 2021. Paper ID: IRCUWU2021-52. [Google Scholar]
- Shiau, S.; Yu, Y.; Pan, W.; Li, G. Colorful and health improving Chinese steamed bread fortified by anthocyanin-rich extract of butterfly pea flower. J. Food Process. Preserv. 2022, 46, e16925. [Google Scholar] [CrossRef]
- Madukokila, U.A.A.D.; Jemziya, F.; Wijewardana, N.; Rifath, A. Development and quality evaluation of blue Butterfly Pea flower (Clitoria ternatea L.) extract incorporated jelly. In Proceedings of the 1 st International Conference on Science and Technology, Oluvil, Sri Lanka, 7 July 2021; pp. 124–129. [Google Scholar]
- Lestari, P.D.; Kawiji; Yulviatun, A.; Martien, R.; Muhammad, D.R.A. Physical and sensory characteristics of milk and white compound chocolate added with Asian pigeonwings flower (Clitoria ternatea). E3S Web Conf. ICFTNSA 2021, 332, 01001. [Google Scholar] [CrossRef]
- Setiawati, A.E.; Kusnadi, J. Optimization of fermentation time and grain concentration for water kefir production from butterfly pea flower (Clitoria ternatea). IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 12081. [Google Scholar] [CrossRef]
- Sutakwa, A.; Nadia, L.; Suharman, S. Addition of blue pea flower (Clitoria ternatea L.) extract increase antioxidant activity in yogurt from various types of milk. J. Agercolere 2021, 3, 31–37. [Google Scholar] [CrossRef]
- Nguyen, T.; Ben, T.; Ngoc, P.; Ngo, T. Application of butterfly pea flower extract in processing some Vietnamese traditional foods. J. Appl. Biol. Biotechnol. 2022, 10, 143–150. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Pumalee, T.; Sanguansuk, N.; Chumyen, C.; Wongvasu, P.; Adisakwattana, S.; Ngamukote, S. Physicochemical, antioxidant and sensory characteristics of sponge cakes fortified with Clitoria ternatea extract. J. Food Sci. Technol. 2018, 55, 2881–2889. [Google Scholar] [CrossRef]
- Tran, V.; Thanh, V.; Nhi, T.; Linh, N.; Vy, T.; Tran, T.T. Application of anthocyanin natural colors from Butterfly Pea (Clitoria ternatea L.) extracts to cupcake. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 62014. [Google Scholar] [CrossRef]
- Chusak, C.; Henry, C.J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria ternatea flower extract on the in vitro enzymatic digestibility of starch and its application in bread. Foods 2018, 7, 102. [Google Scholar] [CrossRef]
- Ab Rashid, S.; Tong, W.; Leong, C.R.; Ghazali, N.M.A.; Taher, M.A.; Ahmad, N.; Tan, W.-N.; Teo, S.H. Anthocyanin microcapsule from Clitoria ternatea: Potential bio-preservative and blue colorant for baked food products. Arab. J. Sci. Eng. 2020, 46, 65–72. [Google Scholar] [CrossRef]
- Chusak, C.; Ying, J.A.Y.; Zhien, J.L.; Pasukamonset, P.; Henry, C.J.; Ngamukote, S.; Adisakwattana, S. Impact of Clitoria ternatea (butterfly pea) flower on in vitro starch digestibility, texture and sensory attributes of cooked rice using domestic cooking methods. Food Chem. 2019, 295, 646–652. [Google Scholar] [CrossRef]
- Gaytos, C.E.; Lumagbas, N. Acceptability of asian Blue Pea flower (Clitoria ternatea) ice cream. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Appelhagen, I.; Wulff-Vester, A.K.; Wendell, M.; Hvoslef-Eide, A.K.; Russell, J.; Oertel, A.; Martens, S.; Mock, H.P.; Martin, C.; Matros, A. Colour bio-factories: Towards scale-up production of anthocyanins in plant cell cultures. Metab. Eng. 2018, 48, 218–232. [Google Scholar] [CrossRef]
| Name of Compound | Double Blue Flowers [8] (pmol/mg fw *) | Blue Flowers (wild) [8] (pmol/mg fw) | Mauve Flowers [8] (pmol/mg fw) | White Flowers Tissue [8] (pmol/mg fw) | Unknown Tissue [9] (mg g−1 fw) |
|---|---|---|---|---|---|
| Anthocyanins | |||||
| Delphinidin3-(2″-rhamnosyl-6″-malonyl)glucoside) | n.d. ** | n.d. | 142.7 ± 23.4 | n.d. | |
| Delphinidin3-(6″-malonyl)glucoside | n.d. | n.d. | 1691.6 ± 704.6 | n.d. | |
| Delphinidin3-neohesperidoside | n.d. | n.d. | 5.2 ± 2.4 | n.d. | |
| Delphinidin3-glucoside | n.d. | n.d. | 51.5 ± 24.5 | n.d. | |
| Delphinidin derivative | 0.28 ± 0.01 | ||||
| Delphinidin derivative | 2.13 ± 0.16 | ||||
| TernatinA1 | 425.4 ± 87.7 | 289.7 ± 39.5 | n.d. | n.d. | |
| TernatinA2 | 539.6 ± 124.2 | 416.8 ± 6.3 | n.d. | n.d. | |
| TernatinA3 | 100.2 ± 25.6 | 311.3 ± 39.3 | n.d. | n.d. | |
| TernatinB1 | 1544.5 ± 281.0 | 769.5 ± 27.2 | n.d. | n.d. | |
| TernatinB2 | 999.4 ± 254.4 | 928.8 ± 51.7 | n.d. | n.d. | |
| TernatinB3 | 444.8 ± 105.6 | 295.5 ± 16.4 | n.d. | n.d. | |
| TernatinB4 | 111.9 ± 36.3 | 302.1 ± 46.3 | n.d. | n.d. | |
| TernatinC1 | 160.7 ± 65.0 | 157.3 ± 9.2 | n.d. | n.d. | |
| TernatinC2 | 81.7 ± 29.6 | 71.6 ± 4.1 | n.d. | n.d. | |
| TernatinC3 | 14.4 ± 25.2 | 104.4 ± 4.3 | n.d. | n.d. | |
| TernatinC4 | 41.4 ± 16.7 | 186.0 ± 11.1 | n.d. | n.d. | |
| TernatinC5 | 16.5 ± 13.5 | 71.1 ± 24.3 | n.d. | n.d. | |
| TernatinD1 | 1630.6 ± 338.8 | 750.7 ± 31.3 | n.d. | n.d. | |
| TernatinD2 | 622.7 ± 172.2 | 540.0 ± 65.2 | n.d. | n.d. | |
| TernatinD3 | 127.7 ± 41.4 | 206.6 ± 27.5 | n.d. | n.d. | |
| Flavonol glycosides | |||||
| Kaempferol3-(2G-rhamnosylrutinoside) | 1738.2 ± 280.3 | 3571.7 ± 61.0 | 2418.8 ± 181.3 | 1342.1 ± 123.3 | |
| Kaempferol3-neohesperidoside | 6454.9 ± 1125.0 | 8225.1 ± 386.6 | 8547.4 ± 811.5 | 4218.4 ± 464.7 | |
| Kaempferol3-(2″-rhamnosyl-6″-malonyl)glucoside | 567.3 ± 93.6 | 841.5 ± 17.9 | 712.4 ± 66.6 | 417.8 ± 46.2 | |
| Kaempferol3-rutinoside | 23.5 ± 9.6 | 72.4 ± 5.0 | 42.8 ± 1.5 | 81.5 ± 19.0 | |
| Kaempferol3-glucoside | n.q. *** | n.q. | 86.1 ± 2.1 | 71.0 ± 5.8 | |
| Quercetin3-(2G″-rhamnosylrutinoside) | 388.3 ± 61.0 | 501.0 ± 13.0 | 289.2 ± 15.4 | 291.6 ± 30.5 | |
| Quercetin3-neohesperidoside | 1012.7 ± 199.9 | 631.0 ± 37.3 | 764.3 ± 46.6 | 613.7 ± 63.4 | |
| Quercetin3-(2″-rhamnosyl-6″-malonyl)glucoside | n.q. | n.q. | n.q. | n.q. | |
| Quercetin3-rutinoside | 231.9 ± 63.4 | 449.0 ± 78.8 | 94.2 ± 8.2 | 321.2 ± 105.4 | |
| Quercetin3-glucoside | 249.9 ± 119.2 | 335.9 ± 66.0 | 55.2 ± 7.1 | 325.3 ± 147.2 | |
| Myricetin3-(2″-rhamnosylrutinoside) | 56.8 ± 10.0 | 37.4 ± 7.1 | 28.1 ± 2.3 | 246.0 ± 26.2 | |
| Myricetin3-neohesperidoside | n.d. | n.d. | 41.5 ± 4.0 | 328.0 ± 42.8 | |
| Myricetin3-(2″-rhamnosyl-6″-malonyl)glucoside | n.d. | n.d. | n.d. | 38.3 ± 4.1 | |
| Myricetin3-rutinoside | n.d. | n.d. | n.d. | 384.5 ± 45.3 | |
| Myricetin3-glucoside | n.d. | n.d. | n.d. | 391.3 ± 56.5 | |
| The total amount (nmol/mg fw petal) | |||||
| Flavonoids | 17.59 ± 3.30 | 20.07 ± 0.55 | 14.97 ± 0.54 | 9.07 ± 0.83 | |
| Anthocyanins | 6.86 ± 1.61 | 5.40 ± 0.23 | 1.89 ± 0.75 | n.d. | |
| Flavonol glycosides | 10.72 ± 1.69 | 14.6 ± 60.33 | 13.08 ± 1.13 | 9.07 ± 0.83 | |
| Kaempferol glycosides | 8.78 ± 1.49 | 12.71 ± 0.46 | 11.81 ± 1.06 | 6.13 ± 0.65 | |
| Quercetin glycosides | 1.88 ± 0.26 | 1.92 ± 0.12 | 1.20 ± 0.07 | 1.55 ± 0.28 | |
| Myricetin glycosides | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.01 | 1.39 ± 0.17 | |
| Fatty acid | |||||
| Palmitic acid | 2.13 ± 0.18 | ||||
| Stearic acid | 1.99 ± 0.16 | ||||
| Petroselinic acid | 1.01 ± 0.04 | ||||
| Linoleic acid | 4.72 ± 0.51 | ||||
| Arachidic acid | 0.36 ± 0.01 | ||||
| Behenic acid | 0.30 ± 0.03 | ||||
| Phytanic acid | 0.81 ± 0.06 | ||||
| Phytosterols | |||||
| Campesterol | 1.24 ± 0.02 | ||||
| Stigmasterol | 6.70 ± 0.83 | ||||
| β-Sitosterol | 6.77 ± 0.19 | ||||
| Sitostanol | 1.20 ± 0.03 | ||||
| Tocols | |||||
| α-tocopherol | 0.20 ± 0.01 | ||||
| γ-tocopherol | 0.24 ± 0.02 | ||||
| Phenolic acid | |||||
| Ellagic acid | 0.21 ± 0.01 |
| Type of Product | Testing | Outcome | References |
|---|---|---|---|
| Mocktails | Sensory evaluation of C. ternatea mocktails on a nine-point hedonic scale of three alternatives with apple, lemon, and orange. | C. ternatea, lemon-based mocktail drinks are preferred over other alternatives. | [34] |
| Fermented plant milk beverage | Assessment of chemical and physical properties of fermented drinks made from almond, soy, and almond-soy with Clitoria ternatea flower extracts (10%) and control sample (0%). Study method: CRD (complete randomization). | ↑ antioxidant activity (74,665%) and ↑ protein content (342,695 mg/mL) for fermented almond milk beverages. ↓ viscosity of all C. ternatea fermented beverages. Hedonic sensory test: C. ternatea almond-soy > other beverages. For color: C. ternatea almond > other beverages. | [35] |
| Colorant powder | Identification of properties of spray-dried C. ternatea powder with maltodextrin and citric acid. Tested for yield, bulk density, moisture content, solubility, wetting ability, color intensity, total anthocyanin, and antioxidant activity. Extraction by 1:10 (distilled water: a combination of components). | ↑ yield, bulk density, brightness, wetting ability, and solubility. ↓ water content, anthocyanin total, and antioxidant activity due to maltodextrin. Citric acid changes: ∆ Hue of the powder (Blue→ Purple). ↑ anthocyanin content and ↓ antioxidant efficacy. | [36] |
| Ice cream (With banana flour) | C. ternatea powder as a natural colorant in prebiotic banana flour ice cream with single strain Bifidobacterium animalis (Bb-12) and banana flour (0%, 10%, 20%, 30% w/w) with a constant level of (0.67% w/v) C. ternatea powder. | C. ternatea total anthocyanin content: 1168.92 mg L−1. Probiotic viability ↑ during storage (109 CFU mL−1). Sensory assessment: 20% banana flour > other samples. | [37] |
| Chinese steamed bread | To test the effect of C. ternatea (aqueous extract of dried flowers) (0–30%) on the phytochemical, textural, and sensory properties of the steamed bread. | ↑ total anthocyanins, antioxidant activities, and free polyphenols. ↓ springiness, cohesiveness, and elasticity of the 30% bread. Ideal concentration: 20–30% C. ternatea ↓ anthocyanin content and blueness of bread due to steaming. | [38] |
| Jelly | Creation and assessment of commercially viable jelly with C. ternatea (aqueous extract). Different jelly compositions using fruit pulp and C. ternatea extract were created. Its color, pH, moisture, titratable acidity, fiber, ash, and calorie value were assessed. A nine-point hedonic scale test was used to assess the sensory evaluation for taste, texture, color, smell, appearance, and general acceptability. | ∆ in the sensory evaluation for taste, texture, color, smell, appearance, and general acceptristics for all jellies. Moisture content range: 13.1% to 20%. ↑ ash content for C. ternatea jelly. ↑ fiber content of banana pulp-infused jelly. Titratable acidity range: 0.02% to 1.023%. Energy value: 357.03 to 428.66 J/100g. Sensory analysis: C. ternatea watermelon jelly > other samples. | [39] |
| Milk and white chocolate | Evaluation of C. ternatea powder (0%, 5%, 10%, and 15%) in milk and white chocolate for color, hardness, and sensory aspects (color, scent, taste, texture, and overall). | ∆ evaluation of C. Ternatea powder (0%, 5% Clitoria ternatea powder to milk compound chocolate and white compound chocolate (p = 0.05). Sensory analysis results: 0% C. ternatea > other samples. | [40] |
| Water kefir | To optimize fermentation time (24–48 h) and concentration grain (5–15%) for C. ternatea water kefir with a good antioxidant activity using response surface methodology. | ↑ antioxidant activity (58 ppm) and ↑ TPC Ideal conditions: 36 h 2 min fermentation time and 15% grain concentration. | [41] |
| Yogurt | To check the antioxidant activity and color values of C. ternatea yogurt made from liquid skim milk, ultra-high temperature (UHT) milk, pasteurized milk, UHT milk with skim powder, and pasteurized milk with skim powder. Aqueous extract at 3:1 (g L−1) ratio under 45 min at 60 °C. Yogurt incubated for 24 h at 37 °C. | ↑ antioxidant activity after the addition of C. ternatea. ↑ antioxidant activity from skim milk. Maximum antioxidant activity: skim milk Clitoria ternatea yogurt (437.04 ppm), with L*, a*, and b* values: 37.35, 2.02, and −1.32. | [42] |
| Juice | Analyzed for total anthocyanin content remaining after processing (5% BPF extract (v/v), sterilized at 125 °C for 3 min with an extended storage time of 4+ months). | Slow degradation of anthocyanin content during storage. | [43] |
| Beverage (tea) | To check the ideal ratio for a C. ternatea beverage with natural sweetener (Stevia extract) and a flavor (lime). Response surface methodology and Box-Behnken design used for extraction formulations. Antioxidant activity was assessed by ORAC, DPPH, and ABTS. Antiamylase and antiglucosidase activities of glycaemic regulatory properties (GCP) were also assessed. Checked over the course of 28 days at various intervals. | Ideal extraction conditions: 3 g of powdered Clitoria ternatea/L of water for 37 min at 59.6 °C. ↑ antioxidant activity. No glycaemic regulatory properties. A 28-day shelf life without preservatives. | [3] |
| Gin | C. ternatea flower extracts are utilized to make a blue alcoholic gin that changes color in response to pH, e.g., with tonic water or lime. | This is a commercial product. | [1] |
| Sponge cakes | Assessment of sponge cake with C. ternatea extracts (0, 5, 10, 15, and 20%, w/w) for color, volume, water activity, total phenolic content, antioxidant properties, texture, and consumer acceptability. | ↑ polyphenol content and antioxidant activity, ↓ lipid peroxidation. ↑ C. ternatea extract led to ↑ hardness, adhesiveness, gumminess, and chewiness. ↓ cohesiveness, springiness, and resilience. Color analysis: ↓ lightness, redness, and yellowness. | [44] |
| Cupcake | Differences in lipid, moisture, pH, protein, and ash. Sensory tests for control sample vs C. ternatea cupcake (50 g diluted flower, water, or ethanolic mixture extract at a 1:80 ratio). | ↑ moisture (22.12 ± 1.87), protein (3.69 ± 0.51) ↓ pH (7.08 ± 0.54), ash (1.01 ± 0.21), and lipid (12.10 ± 2.77) Sensory: C. ternatea sample > control sample. | [45] |
| Cooked pork patties with incorporated Clitoria ternatea | Checking the effectiveness of C. ternatea extract at preventing protein and lipid oxidation in cooked pork patties stored in the refrigerator. The cooked pork meats were kept in a refrigerator (4 °C ) for 12 days after being mixed with C. ternatea extract (0.02–0.16% w/w). | ↑ radical scavenging activity. ↑ protein oxidation for C. ternatea patties, ↓carbonyl concentration, and ↑ protein thiol levels. C. ternatea extract (0.08–0.16%) has the same ability as synthetic antioxidants to prevent oxidative rancidity (0.02 percent BHT). | [33] |
| Fried meat-stuffed rice ball (viên thịt) | Analyzed for total anthocyanin content remaining after processing (10% extract (v/w)—frying time 4 min). | ↑ anthocyanin content and stability during frying. | [43] |
| Bread and bread flour | Impact of C. ternatea extract on inhibiting pancreatic-amylase, in vitro starch hydrolysis, and forecasting the glycaemic index of potato, cassava, rice, corn, wheat, and glutinous rice flour. Sponge cakes were infused with five different levels of CTE (0, 5, 10, 15, and 20%, w/w) and were assessed for color, volume, water activity, total phenolic content, antioxidant capabilities, and consumer approval. | ↓ pancreatic-amylase activity for 1% and 2% (w/v) C. ternatea extract in all flours. ↓ release of glucose, the hydrolysis index (HI), and the projected glycemic index (PGI) for 0.5%, 1%, and 2% (w/v) C. ternatea in wheat. ↓ quickly digestible starch (RDS) and slowly digestible starch (SDS), ↑ undigested starch in glutinous rice flour with 1% and 2% (w/v) C. ternatea extract. Statistical results: positive associations between C. ternatea extract and undigested starch in wheat and cassava. ↓ speed for starch digestion with the addition of 5%, 10%, and 20% (w/w) C. ternatea extract. ↓ PGI for 5% C. ternatea bread. | [46] |
| Muffins | Application of C. ternatea anthocyanin microcapsule on muffins as a bio-preservative (5 g spray-dried flower acetic water extract: muffin dough). | ↑ inhibitory activity for gram +ve and gram -ve bacteria. ↑ shelf life. | [47] |
| Rice ball sweet soup (chè ỉ) | Analyzed for total anthocyanin content remaining after processing. (15% extract, boiling time of 6 min). | Stable anthocyanin content for up to 9 min of boiling. | [43] |
| Cooked rice | C. ternatea flower (CTE) extracts of 1.25 and 2.5% (w/v) were added to cooked rice using conventional cooking techniques to study the impact on starch digestibility. | ↓ readily digestible starch and ↑ undigested starch. ↑ stickiness and unchanged hardness. Sensory evaluation results: “mostly acceptable”. Overall findings: C. ternatea is an effective component for reducing starch digestibility when combined with cooked rice. | [48] |
| Ice cream | Sensory evaluation via flavor, appearance, texture, aroma, and color. General acceptability for consumers for C. ternatea ice cream. | Overall acceptability by consumers for ice cream. | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirodkar, S.M.; Multisona, R.R.; Gramza-Michalowska, A. The Potential for the Implementation of Pea Flower (Clitoria ternatea) Health Properties in Food Matrix. Appl. Sci. 2023, 13, 7141. https://doi.org/10.3390/app13127141
Shirodkar SM, Multisona RR, Gramza-Michalowska A. The Potential for the Implementation of Pea Flower (Clitoria ternatea) Health Properties in Food Matrix. Applied Sciences. 2023; 13(12):7141. https://doi.org/10.3390/app13127141
Chicago/Turabian StyleShirodkar, Shwetali Mahesh, Ribi Ramadanti Multisona, and Anna Gramza-Michalowska. 2023. "The Potential for the Implementation of Pea Flower (Clitoria ternatea) Health Properties in Food Matrix" Applied Sciences 13, no. 12: 7141. https://doi.org/10.3390/app13127141
APA StyleShirodkar, S. M., Multisona, R. R., & Gramza-Michalowska, A. (2023). The Potential for the Implementation of Pea Flower (Clitoria ternatea) Health Properties in Food Matrix. Applied Sciences, 13(12), 7141. https://doi.org/10.3390/app13127141








