Abstract
Pallenis spinosa (L.) Cass. is a widespread plant in the Mediterranean region. Traditionally, it is used as a medicinal species to treat several ailments, from inflammation to skin injuries. Although the phytochemical content of this plant has already been investigated, there is currently limited data on Algerian P. spinosa. In this work, we focused on volatile compounds and non-volatile secondary metabolites extracted using HS-SPME and methanol from the aerial parts of P. spinosa collected from Northeast Algeria. Volatile constituents were analyzed by GC-MS, while non-volatile compounds were analyzed by NMR and HPLC-MSn. In total, 48 volatile compounds were identified, including sesquiterpene hydrocarbons (65.8%), monoterpene hydrocarbons (16.9%), and oxygenated monoterpenes and sesquiterpenes (8.3% and 6.5%, respectively). β-Chamigrene (16.2%), α-selinene (12.8%), β-pinene (10.6%), and β-caryophyllene (9.2%) were assessed as the main constituents. Concerning non-volatile metabolites, 23 polyphenols were identified (7.26 mg/g DW), and phenolic acids were predominant (5.83 mg/g DW). Tricaffeoylhexaric acid (1.76 mg/g DW), tetracaffeoylhexaric acid (1.41 mg/g DW), 3,5-dicaffeoylquinic acid (1.04 mg/g DW), caffeoyl dihexoside (0.35 mg/g DW), and chlorogenic acid (0.29 mg/g DW) were the most abundant ones. Several known flavonoids, such as tricin and patuletin glycosides, kaempferol, and apigenin, were also identified, and myricetin hexoside was detected in P. spinosa for the first time. Overall, our work is the first to report an exhaustive characterization of volatile and non-volatile secondary metabolites from Algerian P. spinosa. The results represent a step forward in revealing the chemistry of this widespread plant species. Furthermore, they may contribute to rationalizing its traditional medicinal applications and preserve the biodiversity of Algerian flora.
1. Introduction
The family of Asteraceae contains between 25,000 and 30,000 species belonging to over 1000 genera. Pallenis spinosa (L.) Cass., commonly known as the spiny starwort, is a member of this family, belonging to the tribe of Inuleae, a subtribe of Inulineae, and a group of Inula [1]. The genus Pallenis is monotypic and is widespread in southern Europe, northern Africa, the Canary Islands, the Middle East, and the Mediterranean region, especially in the desert and in the coastal habitats [2]. It is an annual herbaceous plant characterized by large yellow/orange flowers surrounded by long spiny involucre bracts [3]. Traditionally, the flowers and the spiny leaves of the plant have been used as medicine to treat inflammation, gastralgia, mouth infections, skin injuries, and inflammatory contusions [4,5]. Usually, the flowers and the leaves are taken orally or applied topically in the form of infusion or decoction [6].
In the western part of Algeria, P. spinosa is used for the management of skin conditions such as eczema. Furthermore, its flowers hold significant medicinal value in addressing sensitivity issues, as well as aiding in the healing of bruises and wounds. Across the Mediterranean basin, the plant is used in its entirety as an insecticide [7,8].
Some studies have investigated the secondary metabolic products of P. spinosa in order to improve traditional knowledge and find novel medicinal applications. Regarding volatile constituents, the plant is rich in sesquiterpenes and oxygenated sesquiterpenoids, including a ketone with a previously unreported carbon skeleton (dihydroxypallenone) [9,10]. Other compounds such as α-cadinol, shiromool, germacrene D derivatives [11], and germacrene A-type epoxides [12] have been reported. Concerning non-volatile secondary metabolites, P. spinosa tends to accumulate 5-O-glycosyl flavones. Among these, several tricin and patuletin derivatives have been detected in their aerial parts [13].
Natural products have provided many drugs and drug leads and still remain the richest sources of bioactive compounds. In this work, we aimed at moving a step forward in the characterization of secondary metabolites from P. spinosa. Specifically, we investigated volatile compounds and secondary metabolites extracted from the aerial parts of P. spinosa from Northeastern Algeria (Bourj Ghedir). Although the phytochemical content of this plant has already been studied, limited data exists on the species growing in Algeria. Headspace solid-phase micro-extraction coupled with gas chromatography-mass spectrometry (HS-SPME-GC-MS), nuclear magnetic resonance (NMR), and high-performance liquid chromatography-mass spectrometry (HPLC-MSn) were used for the analyses, respectively. Overall, the results are intended to contribute to the valorization of understudied Algerian medicinal and edible plants and the rationalization of their traditional uses.
2. Materials and Methods
2.1. Chemicals and Reagents
HPLC-grade methanol and acetonitrile, n-hexane, dichloromethane, ethyl acetate, butanol, and formic acid were purchased from Sigma-Aldrich (Milan, Italy). Ultrapure water was used, and it was obtained using a Milli-Q® dispenser (Merck, Darmstadt, Germany). Rutin and chlorogenic acid reference standards were purchased from Sigma-Aldrich (Milan, Italy). Individual standard solutions of rutin and chlorogenic acid were prepared at 1 mg/mL in methanol and were stored at −20 °C until use. Sequential dilutions of the stock solutions were prepared in methanol, and the obtained samples were used to build calibration curves, as described in the following paragraphs.
2.2. Plant Material
Aerial parts of P. spinosa were harvested at the full flowering period during the month of May 2020 from the Bordj Ghedir Region, Province of Bordj Bou Arreridj, North-East Algeria, at an altitude of 820 m a.s.l. Botanical identification of the plant was carried out based on morphological features with the help of authentic flora [5], and a voucher specimen was deposited in the Herbarium of M’sila University (MUNIV001220). Before extraction, plant material was washed with tap water to remove dust and dried in the shade in a well-ventilated place for 7 days. A part of the material was used fresh for the extraction of volatiles.
2.3. HS-SPME-GC-MS Analysis of Volatile Compounds
For HS-SPME, the method described by Ascrizzi et al. [14] was applied. Briefly, the fresh plant material (2 g) was placed in a 4 mL glass vial, which was sealed and left to equilibrate at room temperature for 1 h. Afterwards, the headspace was sampled with a Supelco SPME fiber coated with polydimethylsiloxane (PDMS, 100 µm) for 30 min. GC-MS analysis of the headspace extract was performed as described by Bendif et al. [15], using a Varian CP-3800 gas chromatograph coupled to a Varian Saturn 2000 mass spectrometer. An Agilent DB-5 capillary column (30 m × 0.25 mm; coating thickness 0.25 µm) was used as the stationary phase. Chromatographic conditions were as follows: injector and transfer line temperatures, 220 and 240 °C, respectively; oven temperature programmed from 60 to 240 °C at 3 °C/min; helium was used as carrier gas at 1 mL/min; splitless injection. Identification of volatile compounds was performed by comparing retention times with those of reference pure compounds, comparing their linear retention indices (LRI) relative to the C6–C28 series of n-hydrocarbons, and by matching their mass spectra against commercial [16,17] and home-made libraries built up from pure substances.
2.4. Preparation of Extracts for NMR and HPLC-MSn Analyses
Sample preparation was performed using a protocol previously described by Dall’Acqua et al. [18] and Bendif et al. [15]. A total of 20 g of the dried aerial parts were ground with an IKA A11 basic analytical mill to obtain a fine powder. The powder was suspended in 150 mL of methanol and then sonicated for 10 min. After centrifugation, the supernatant was recovered in an Erlenmeyer flask, and the residue was re-extracted with a further 50 mL of the same solvent two more times. Thereafter, supernatants were collected and concentrated under reduced pressure with a rotary evaporator at 35 °C, yielding 2.13 g of crude extract (yield 10.65%, w/w). An amount of the dry extract was stored in dark glass vials at −20 °C until its chemical characterization. Two grams were used for fractionation using solvents at increasing polarity. The extract was suspended in a methanol/water (1:9) mixture (50 mL) and sonicated. The mixture was then partitioned with n-hexane (HEX; 20 mL, 3 times), dichloromethane (DCM; 20 mL, 3 times), ethyl acetate (EA; 20 mL, 3 times), and butanol (BUT; 20 mL, 3 times). The fractions were dried under vacuum and analyzed by NMR.
2.5. NMR Analysis
The crude extract and dried fractions were suspended in deuterated methanol. 1H-NMR spectra were recorded with a Bruker Avance III spectrometer (Bruker, Billerica, MA, USA) operating at 400 MHz, using standard pulse sequences.
2.6. HPLC-MSn Analysis
The HPLC-MSn method was used for the identification of secondary metabolites in the crude methanolic extract of P. spinosa. Before analysis, the dry sample was dissolved in methanol at a concentration of 5 mg/mL and filtered through a 0.45 µm Millipore filter. The HPLC system was composed of a Varian 212 binary pump equipped with a Varian Prostar 430 autosampler coupled to a Varian 500 Ion Trap mass detector (MS). An Agilent Eclipse plus C18 column (2.1 × 150 mm, 3.5 µm) was used as the stationary phase. A gradient of acetonitrile (A) and 0.1% v/v formic acid in water (B) was used as the mobile phase. The gradient was set as follows: 0 min, 10% A; 20 min, 54% A; 23 min, 100% A; isocratic up to 32 min. Re-equilibration time was 8 min. The flow rate was 0.2 mL/min. MS data were acquired in the negative ion mode [ESI (-)], and the operating parameters of the spectrometer were as follows: needle voltage, 4500 V; capillary voltage, 70 V; RF loading, 100%; nebulizing gas pressure, 20 psi (nitrogen); dry in gas pressure, 15 psi; dry in gas temperature, 350 °C. Mass spectra were recorded in the range of m/z 50–2000. The turbo detection data scanning (TDDS) function of the instrument was used to investigate the fragmentation pattern of the eluted compounds, setting n = 3 levels of fragmentation. Fragmentation data, together with information on the molecular weight of the compounds, were used to perform the tentative identification of secondary metabolites from P. spinosa. As a comparison, literature data were evaluated.
Identified phenolic compounds were quantified using linear calibration curves. These latter were calculated by analyzing standard solutions of rutin (for flavonoids) and chlorogenic acid (for phenolic acids) and integrating the corresponding peaks to obtain the area under the curve (AUC) values. Concentration ranges were 12–120 µg/mL and 10.4–104 µg/mL for rutin and chlorogenic acid, respectively. The equations were y = 7342x − 19,707 (R2 = 0.9999) for rutin, and y = 7504.8x − 3890.2 (R2 = 1) for chlorogenic acid. The analysis was performed in triplicate, and the results were expressed as mean ± standard deviation (S.D.).
3. Results and Discussion
3.1. HS-SPME-GC-MS Analysis of Volatile Compounds
The development of quick and sustainable methods for the extraction of volatile compounds from plant materials such as the HS-SPME is important. The rapid sampling shortens the whole length of the analysis, while the avoidance of organic solvents reduces its environmental impact. HS–SPME coupled with the GC–MS analysis of the aerial parts of P. spinosa exhibited 48 volatile compounds, accounting for 98.1% of the whole volatile extract. The identified compounds, their retention indexes, and their relative proportions are presented in Table 1. A representative chromatogram is shown in Figure 1.

Table 1.
Aroma profile (relative percentages of identified compounds) of the aerial parts of P. spinosa obtained using HS-SPME–GC–MS. Compounds are listed in order of their elution from an HP-5MS column.
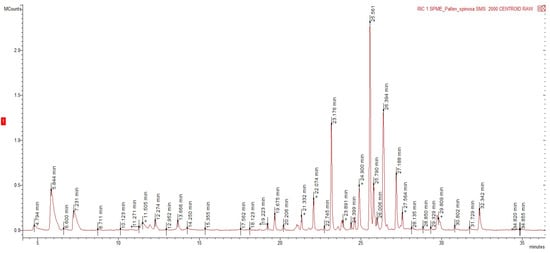
Figure 1.
Exemplificative chromatogram obtained from the GC-MS analysis of the volatile extract obtained from P. spinosa by HS-SPME.
The volatile profile was dominated by sesquiterpene hydrocarbons (65.8%), followed by monoterpene hydrocarbons (16.9%). Oxygenated monoterpenes were less represented, similar to oxygenated sesquiterpenes (8.3% and 6.5%, respectively). Phenylpropanoids (0.5%) and apocarotenes (0.1%) were found as trace components. Considering the single identified compounds, the aerial parts of P. spinosa were characterized mainly by β-chamigrene (16.2%), α-selinene (12.8%), β-pinene (10.6%), and β-caryophyllene (9.2%). Some other compounds, such as trans-γ-cadinene (5.2%), alloaromadendrene (4.4%), and germacrene D (4.3%), were found.
The chemical composition of the volatile compounds extracted from fresh plant material by HS-SPME was different from that reported in a previous study by Al-Qudah et al. [10]. Their chemical investigation demonstrated that the essential oil of flowers was dominated by a mixture of oxygenated sesquiterpenes (61.12%) and sesquiterpene hydrocarbons (34.76%). Moreover, the main constituents were different: α-cadinol (16.48%), germacra-1(10),5-diene-3,4-diol (14.45%), γ-cadinene (12.03%), and α-muurolol (9.89%) were the most abundant ones, while, except for γ-cadinene, they were not detected in our study. Nevertheless, the results from the analysis of HS-SPME fractions and EOs obtained by hydro-distillation are not directly comparable due to the completely different sampling methods. For instance, the low temperature maintained during the HS-SPME sampling avoids the thermal degradation of some constituents, conversely to distillation methods. This aspect can partially justify why many of the major constituents identified by Al-Qudah et al. [10] were not found in our extract or were present in low amounts. For the same reasons, our data differ from older ones published by Senatore & Bruno on P. spinosa harvested in Southern Italy. Their results show a predominance of oxygenated sesquiterpenoids in the EO (60.2% of the oil). Among the 38 components that were identified, the most abundant were germacra-1(10),5-dien-3,4-diol (18.4%), α-cadinol (14.1%), 3-acetoxygermacra-1(10),5-dien-4-ol (13.0%), τ-cadinol (8.2%) and δ-cadinene (5.8%) [19].
Several volatile plant metabolites and their mixtures are known to exert antimicrobial effects. For instance, β- pinene, one of the main volatile compounds from the aerial parts of P. spinosa, is highly toxic to Candida albicans and several pathogenic bacteria, such as the methicillin-resistant Staphylococcus aureus (MRSA), which causes severe infections in humans [20]. β-Caryophyllene, a well-known sesquiterpene, is also toxic to MRSA and exerts anti-inflammatory activity via inhibiting different inflammatory mediators such as interleukin 1β, interleukin-6, and tumor necrosis factor-α. It has also analgesic, myorelaxing, sedative, and antidepressive effects [21]. Other terpenes contributing to the volatile profile of P. spinosa, such as p-cymene and β-selinene, are known to exert antioxidant, analgesic, anti-inflammatory, and antimicrobial activities [22,23]. Overall, these data suggest that these compounds may be involved in the therapeutic effects of P. spinosa, although further studies are needed to verify this hypothesis.
3.2. NMR and HPLC-MSn Analysis of Secondary Metabolites
HEX, EA, and DCM solvents allowed the extraction of the most abundant lipophilic constituents of the plant material. The respective spectra, reported in Figure 2, Figure 3, Figure 4 and Figure 5, support the fact that the most abundant compounds in these fractions are lipids. The assignments of the main signals of these lipids are reported in Table 2.
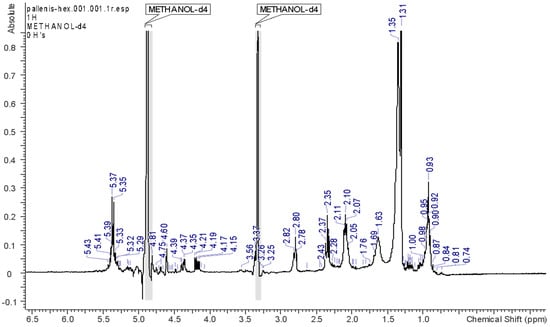
Figure 2.
1H-NMR spectrum of the HEX fraction.
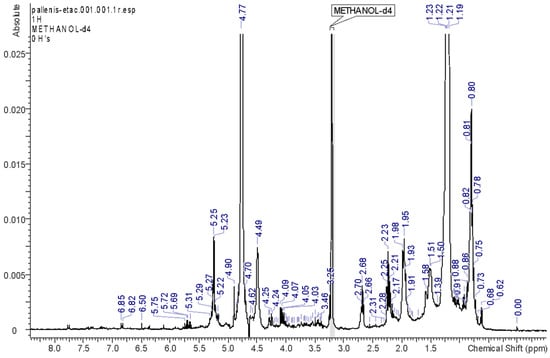
Figure 3.
1H-NMR spectrum of the EA fraction.
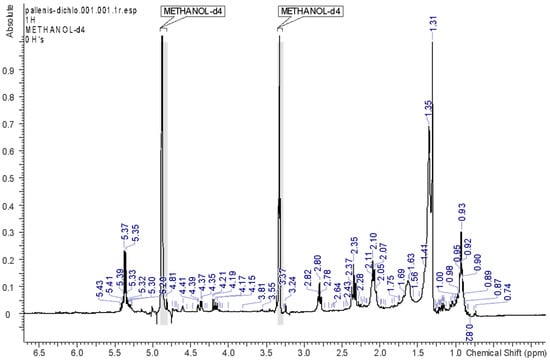
Figure 4.
1H-NMR spectrum of the DCM fraction.
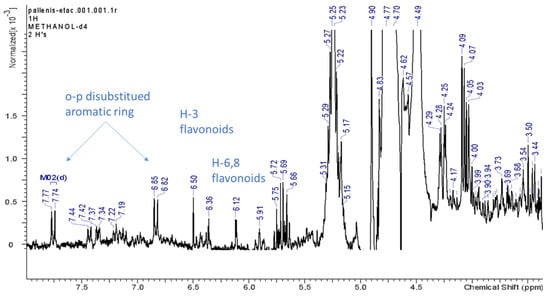
Figure 5.
Magnification of the aromatic area in the 1H-NMR spectrum of the DCM fraction.

Table 2.
Main signals detected in lipophilic fractions attributable to fatty acids.
In the EA fraction, some minor signals could be observed in the aromatic region and suggest the presence of some phenolic derivatives. In particular, two ortho-coupling doublets at δ 7.73 and 6.40 indicate the presence of derivatives bearing an ortho-para disubstituted ring, as well as the singlet at δ 6.50. The meta-coupling doublets at δ 6.13–6.36 could be ascribed to position 3 and positions 6 and 8 of flavonoid derivatives. Moreover, some minor signals at δ 7.74, 7.34, and 7.19 indicate the presence of phenolic compounds.
On the other hand, the spectrum of the BUT fraction (Figure 6) presents enlarged signals suggesting the presence of several polysaccharides. Nevertheless, some resonances can be useful for a screening of the main compounds. Broad signals in the aliphatic part can be ascribed to the non-oxygenated positions of quinic acid moieties, as well as several aromatic signals supporting the presence of phenols.
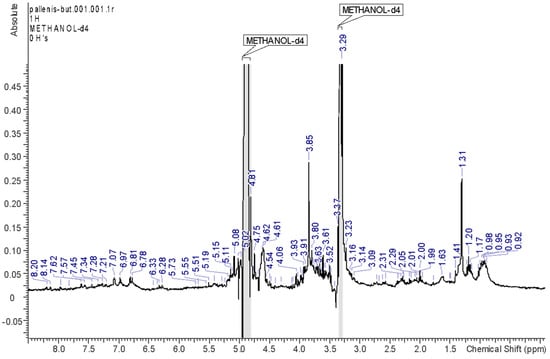
Figure 6.
1H-NMR spectrum of the BUT fraction.
For an exhaustive characterization of phenolic compounds, the chemical composition of the crude methanol extract was explored further by HPLC-MSn. As shown in Table 3 and Figure 7, the analysis allowed us to identify 23 compounds as polyphenols. These constituents accounted for 7.26 mg/g of dried plant material, and phenolic acids were the most representative (5.83 mg/g). Tricaffeoylhexaric acid (1.76 mg/g DW), tetracaffeoylhexaric acid (1.41 mg/g DW), 3,5-dicaffeoylquinic acid (1.04 mg/g DW), caffeoyl dihexoside (0.35 mg/g DW), and chlorogenic acid (0.29 mg/g DW) were identified as the most abundant ones. Their MSn spectra are shown in Figures S1–S5 of the Supplementary Materials. Among the flavonoids, the most abundant ones were tricin (0.24 mg/g DW) and its 7-glucoside (0.26 mg/g DW), rutin (0.22 mg/g DW), apigenin (0.18 mg/g DW), and patuletin galactoside (0.15 mg/g DW).

Table 3.
Results from the HPLC-MSn characterization of secondary metabolites extracted from the aerial parts of P. spinosa. Results are reported as means ± standard deviation (n = 3). The number associated with each metabolite refers to the corresponding peak in the chromatogram shown in Figure 7.
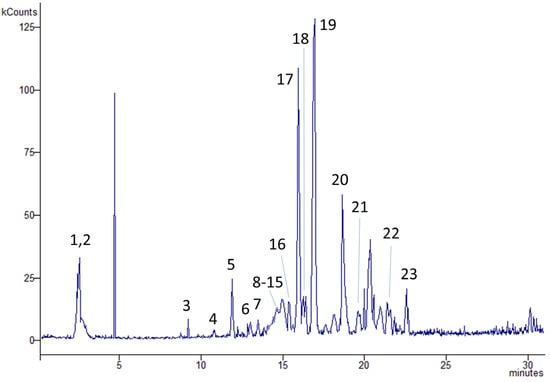
Figure 7.
Exemplificative chromatogram obtained from the HPLC-MS analysis of the P. spinosa methyl extract. Numbers refer to the identified metabolites reported in Table 3. Peaks: (1) caffeoyl dihexoside, (2) ferulic acid, (3) quercetin, (4) caffeoyl hexoside, (5) chlorogenic acid, (6) dicaffeoylhexaric acid, (7) dicaffeoylhexaric acid isomer 1, (8) myricetin hexoside, (9) feruloylquinic acid, (10) dicaffeoylhexaric acid isomer 2, (11) patuletin-rharnnopyranosyl-galactopyranoside, (12) dicaffeoylhexaric acid isomer 3, (13) rutin, (14) dicaffeoylhexaric acid isomer 4, (15) patuletin galactoside, (16) caffeoyl diglucoside, (17) 3,5-dicaffeoylquinic acid, (18) tricin 7-glucoside, (19) tricaffeoylhexaric acid, (20) tetracaffeoylhexaric acid, (21) kaempferol, (22) apigenin, (23) tricin.
In a paper dated 1992, Ahmed et al. reported the identification of 11 flavonoids in the aerial parts of P. spinosa harvested in Egypt. Patuletin 3-O-galactoside and tricin 7-O-glucoside, also detected in our study, were two of these, together with other tricin, patuletin, and quercetin glycosides [13]. More recently, Khettaf & Dridi reported the isolation of tricin 7-O-glucoside from the aerial parts of P. spinosa harvested in Algeria, together with tricin, quercetin, patuletin 7-galactopyranoside, and patuletin-3-O-α-l-rharnnopyranosyl (1–6)-β-d-galactopyranoside [24]. A more exhaustive characterization of phenols from the aerial parts of Algerian P. spinosa has been presented by Amrani-Allalou et al. In their work, the authors reported 13 compounds that, in accordance with our results, were classified into flavonoids and phenolic acids [25]. Considering the single compounds, several were different from those reported in our work. Among the flavonoids, (epi)catechin, epigallocatechin, phlorizin, naringenin, and apigenin glucoside were not detected in our extract. Conversely, the presence of myricetin hexoside in P. spinosa is reported for the first time in our work. Among the phenolic acids, several derivatives of caffeic acid identified in our extract were lacking in the results presented by Amrani-Allalou et al. [25]. These differences can be attributed to several factors. First of all, the different collection sites of the plants generally have a significant influence on the chemical properties of plant specimens. Secondly, the different extraction protocols lead to the enrichment of the extracts in compounds with different physical and chemical properties. Last but not least, the analytical method used for the chemical characterization of extracts.
In Algeria, P. spinosa is used as a medicinal remedy for several health disorders, especially microbial infections and inflammation. Although a bioactivity screening of extracts was not performed in this study, we could assess the presence of some secondary metabolites whose pharmacological properties have already been studied in vitro and in vivo. Among these are some of the most abundant constituents of the methanol extract of P. spinosa, such as chlorogenic acid and 3,5-dicaffeoylquinic acid, whose anti-inflammatory, antioxidant, and antibacterial activities have been reported in vivo. Other bioactive compounds were detected among the flavonoids, such as the well-known apigenin, quercetin, and kaempferol. Moreover, for these, literature data demonstrate their antioxidant, antimicrobial, anti-inflammatory, and antitumor effects in vivo. A summary of these data is reported in Table 4. Overall, these data will be useful as a starting point for further bioactivity assessments on P. spinosa from Algeria.

Table 4.
Main bioactivities of polyphenols detected in the methanol crude extract of P. spinosa. Information is taken from the literature, and the references for each compound are reported in the Table.
4. Conclusions
In this work, we investigated the composition of volatile compounds and non-volatile secondary metabolites of the aerial parts of P. spinosa from Algeria. To the best of our knowledge, this study is the first to report an exhaustive chemical characterization of this plant species, considering that, especially for non-volatile metabolites, previous works have described only a few compounds. The results indicate that the volatile profile of Algerian P. spinosa is dominated by sesquiterpene hydrocarbons and monoterpene hydrocarbons instead of oxygenated terpenes, conversely to what has been previously reported by other authors. This difference can be related to the different techniques used for extraction and analysis of volatile compounds but also to different times, environmental conditions, and geographical locations of plant harvesting. Regarding non-volatile secondary metabolites, phenolic acids are the most abundant and are represented mainly by caffeic acid derivatives. Flavonoids were also detected, although in low amounts. Nevertheless, some of these are already known to exert beneficial effects in humans; hence, they may be at least co-responsible for the bioactivities associated with P. spinosa. In the future, further studies will be required to verify this hypothesis.
Overall, the results of this study represent a contribution to the valorization of understudied plants from Algeria and the preservation of the biodiversity of this region. P. spinosa, which is still used as a remedy for several ailments in Algeria, represents a valuable source of phytochemicals such as polyphenols and volatile terpenes. These data may represent a starting point for further rationalization of the medicinal use of this plant species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app131810113/s1, Figure S1. m/z 695: compound identified as tricaffeoylhexaric acid. Figure S2. m/z 857: compound identified as tetracaffeoylhexaric acid. Figure S3. m/z 515: compound identified as 3,5-dicaffeoylquinic acid. Figure S4. m/z 539: compound identified as caffeoyl dihexoside. Figure S5. m/z 353: compound identified as chlorogenic acid.
Author Contributions
Conceptualization, N.A. and H.B.; methodology, G.F., S.D. and G.P.; software, G.P.; validation, G.P., S.D. and F.M.; formal analysis, G.P.; investigation, H.B., N.A., N.S., S.S. and G.P.; resources, G.F., F.M. and S.D.; data curation, G.P., H.B. and S.D.; writing—original draft preparation, N.A., H.B. and G.P.; writing—review and editing, G.P. and H.B.; visualization, G.P.; supervision, H.B. and G.P.; project administration, H.B. and F.M.; funding acquisition, H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to the corresponding Authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Q.-X.; Shi, Y.-P.; Jia, Z.-J. Eudesmane sesquiterpenoids from the Asteraceae family. Nat. Prod. Rep. 2006, 23, 699–734. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K.; Boulos, L.; Kabiel, H.F.; Sharashy, O.S. Vegetation and species altitudinal distribution in Al-Jabal Al-Akhdar landscape, Libya. Pak. J. Bot 2011, 43, 1885–1898. [Google Scholar]
- Zareh, M.M. Systematic and anatomical studies of Inuleae and Plucheeae in Egypt. Feddes Repert. 2005, 116, 43–53. [Google Scholar] [CrossRef]
- Agelet, A.; Vallès, J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or very rare uses of previously known medicinal plants. J. Ethnopharmacol. 2003, 84, 211–227. [Google Scholar] [CrossRef]
- Chermat, S.; Gharzouli, R.; Quézel, P.; Santa, S. 1962–1963: Nouvelle flore de l’Algérie et des régions désertiques méridionales. Vol. 1, 2. CNRS, Paris, 566, 1170. J. Mater. Sci. Eng. A 2015. [Google Scholar]
- Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Bouabdelli, F.; Djelloul, A.; Kaid-Omar, Z.; Semmoud, A.; Addou, A. Antimicrobial Activity of 22 Plants Used in Urolithiasis Medicine in Western Algeria. Asian Pac. J. Trop. Dis. 2012, 2, S530–S535. [Google Scholar] [CrossRef]
- Karkanis, A.C.; Athanassiou, C.G. Natural insecticides from native plants of the Mediterranean basin and their activity for the control of major insect pests in vegetable crops: Shifting from the past to the future. J. Pest Sci. 2021, 94, 187–202. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Jakupovic, J.; Bohlmann, F. Dihydroxypallenone, a sesquiterpene with a new carbon skeleton from Pallenis spinosa. Phytochemistry 1990, 29, 3355–3358. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Saleh, A.M.; Alhawsawi, N.L.; Al-Jaber, H.I.; Rizvi, S.A.; Afifi, F.U. Composition, Antioxidant, and Cytotoxic Activities of the Essential Oils from Fresh and Air-Dried Aerial Parts of Pallenis spinosa. Chem. Biodivers. 2017, 14, e1700146. [Google Scholar] [CrossRef]
- Appendino, G.; Cravotto, G.; Gariboldi, P.; Claudi, F.; Picci, V. A sesquiterpene alcohol from Pallenis spinosa. Phytochemistry 1989, 28, 849–850. [Google Scholar] [CrossRef]
- Sanz, J.F.; Marco, J.A. A germacrane derivative from Pallenis spinosa. Phytochemistry 1991, 30, 2788–2790. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Spaller, M.; Mabry, T.J. Flavonoids of Pallenis spinosa (Asteraceae). Biochem. Syst. Ecol. 1992, 20, 785–786. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Cioni, P.L.; Amadei, L.; Maccioni, S.; Flamini, G. Geographical patterns of in vivo spontaneously emitted volatile organic compounds in Salvia species. Microchem. J. 2017, 133, 13–21. [Google Scholar] [CrossRef]
- Bendif, H.; Miara, M.D.; Peron, G.; Sut, S.; Dall’Acqua, S.; Flamini, G.; Maggi, F. NMR, HS-SPME-GC/MS, and HPLC/MSn Analyses of Phytoconstituents and Aroma Profile of Rosmarinus eriocalyx. Chem. Biodivers. 2017, 14, e1700248. [Google Scholar] [CrossRef]
- Adams, R.; Sparkman, O. Review of Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 16, 1902–1903. [Google Scholar]
- NIST/EPA/NIH Mass Spectral Library; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2014.
- Dall’Acqua, S.; Maggi, F.; Minesso, P.; Salvagno, M.; Papa, F.; Vittori, S.; Innocenti, G. Identification of non-alkaloid acetylcholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae). Fitoterapia 2010, 81, 1208–1212. [Google Scholar] [CrossRef]
- Senatore, F.; Bruno, M. Composition of the essential oil of Pallenis spinosa (L.) Cass. (Asteraceae). Flavour Fragr. J. 2003, 18, 195–197. [Google Scholar] [CrossRef]
- da Silva Rivas, A.C.; Lopes, P.M.; de Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.L.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.K.; Bhushan, B.; Kumar, M.; Pant, A.K. β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities. Medicines 2017, 4, 52. [Google Scholar] [CrossRef]
- Khettaf, A.; Dridi, S. Chemical Composition Analysis andIn Vivo Anti-diabetic Activity of Aqueous Extract of Aerial Part of Pallenis spinosa in Diabetic Rats. Curr. Bioact. Compd. 2022, 18, e010921191723. [Google Scholar] [CrossRef]
- Amrani-Allalou, H.; Boulekbache-Makhlouf, L.; Izzo, L.; Arkoub-Djermoune, L.; Freidja, M.L.; Mouhoubi, K.; Madani, K.; Tenore, G.C. Phenolic compounds from an Algerian medicinal plant (Pallenis spinosa): Simulated gastrointestinal digestion, characterization, and biological and enzymatic activities. Food Funct. 2021, 12, 1291–1304. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hwang, T.-L.; Yu, H.-P.; Fang, J.-Y.; Chong, K.Y.; Chang, Y.-W.; Chen, C.-Y.; Yang, H.-W.; Chang, W.-Y.; Hsieh, P.-W. Ilex kaushue and Its Bioactive Component 3,5-Dicaffeoylquinic Acid Protected Mice from Lipopolysaccharide-Induced Acute Lung Injury. Sci. Rep. 2016, 6, 34243. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Gao, S.; Lee, J.K.; Chan, Y.-Y.; Wong, E.C.; Zheng, T.; Li, X.-X.; Shaw, P.-C.; Simmonds, M.S.J.; Lau, C.B. A Natural Flavone Tricin from Grains Can Alleviate Tumor Growth and Lung Metastasis in Colorectal Tumor Mice. Molecules 2020, 25, 3730. [Google Scholar] [CrossRef] [PubMed]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular mechanisms of anticancer effect of rutin. Phyther. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).