Effect of Subject-Specific Region of Interest on Motor Imagery Brain–Computer Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant and Sample Size Calculation
- S = sample size for infinite population
- Z = Z score
- P = population proportion (assumed as 50% or 0.5)
- M = margin of error
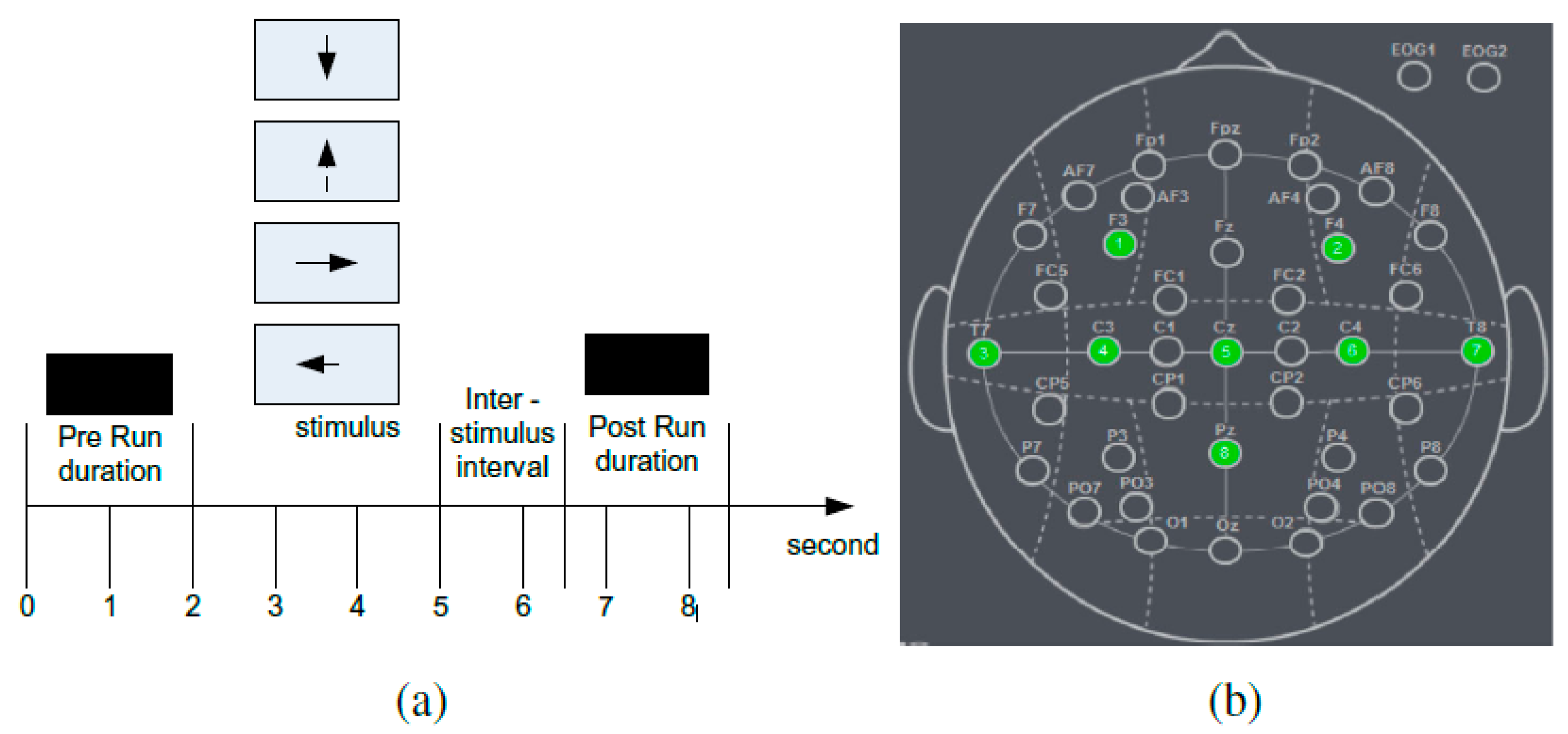
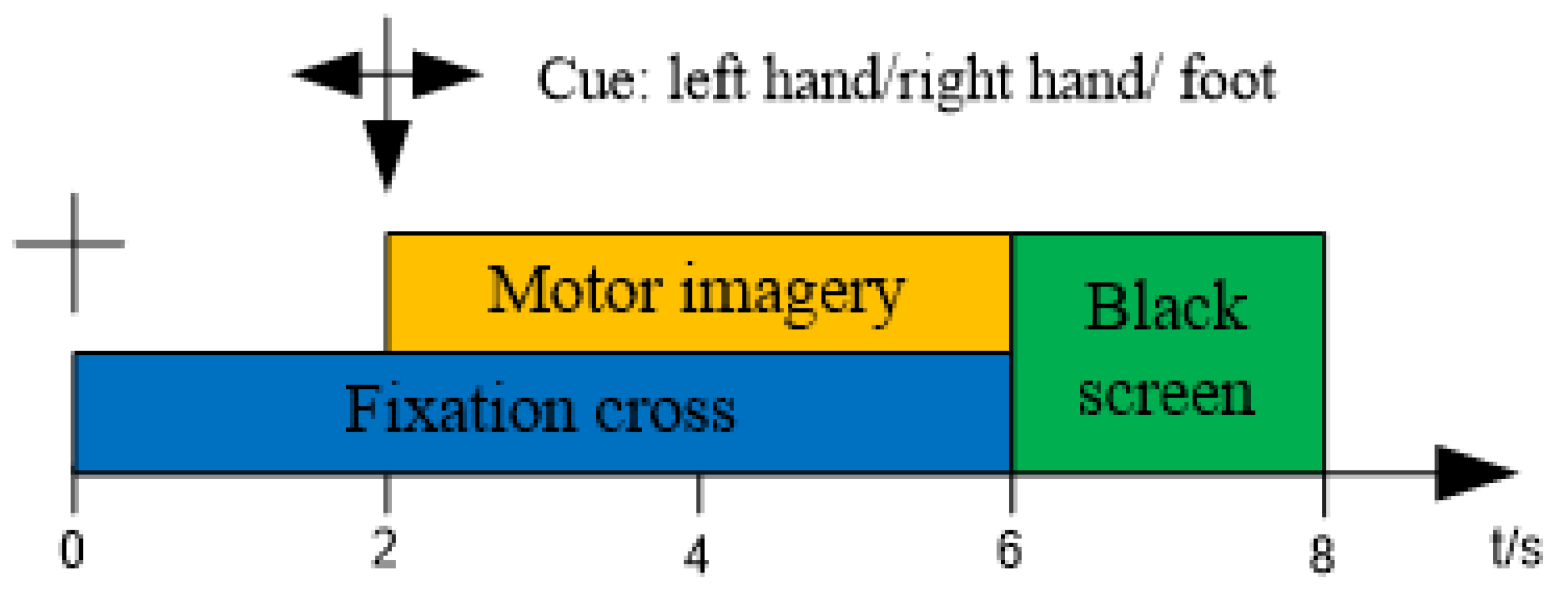
2.2. Experimental Setup
2.3. Preprocessing
2.4. Feature Extraction
2.5. Optimization of Intrinsic Time-Scale Decomposition
- (a)
- Perform ITD, which decomposes the signal into some PRCs.
- (b)
- Calculate the instantaneous amplitude of each PRC based on the half-wave analysis.
- (c)
- Choose the first two PRCs with instantaneous amplitudes fluctuating around the mu rhythm for further analysis.
- (d)
- Calculate the ERD of the instantaneous amplitude as follows:
2.6. Feature Classification
2.7. Validation of Intrinsic Time-Scale Decomposition Method
3. Results and Analysis
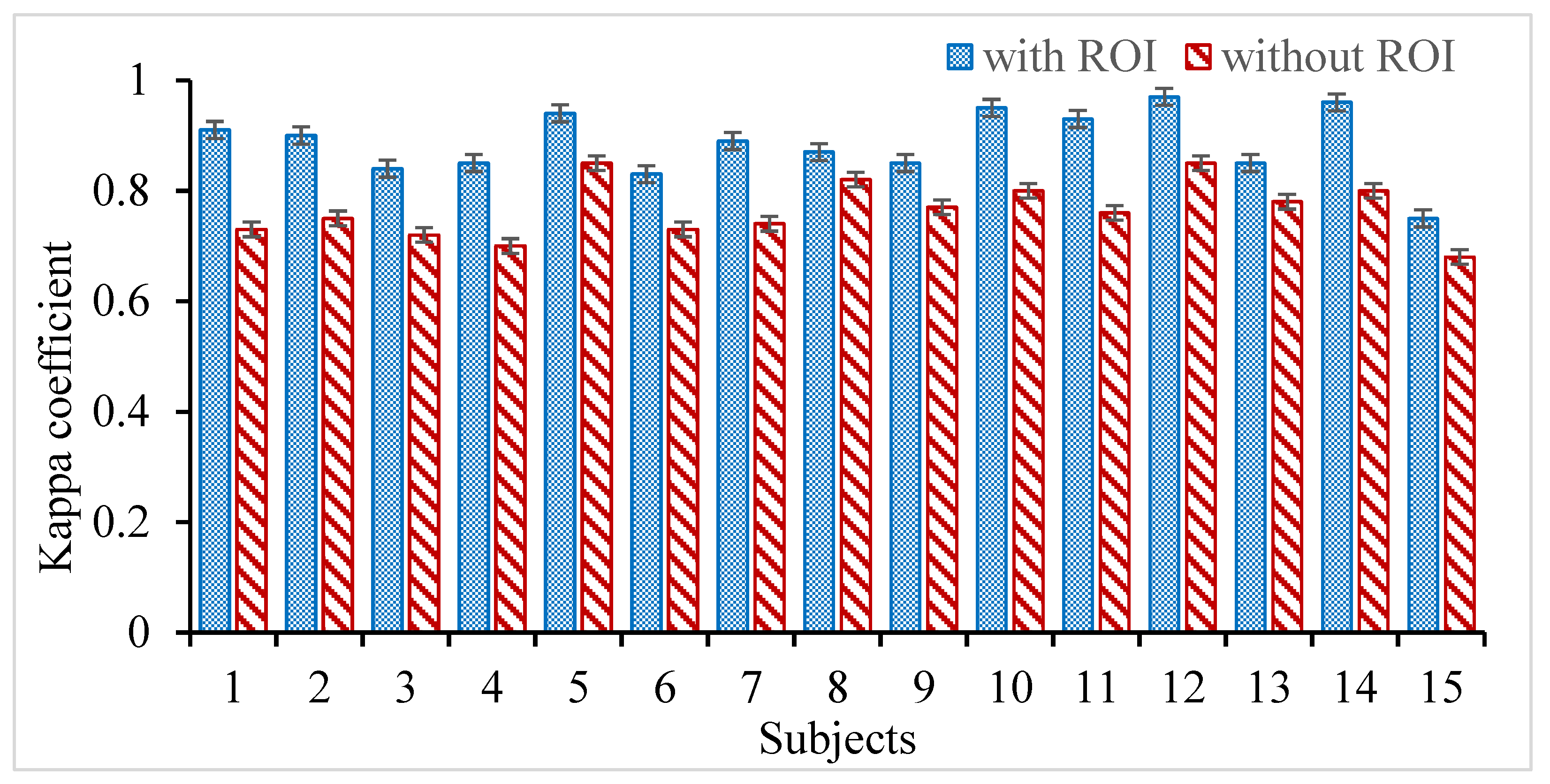
3.1. Effect of ROI on Intrinsic Time-Scale Decomposition
3.2. Neurophysiological Properties of Motor Imagery Movements
3.3. Confusion Matrix
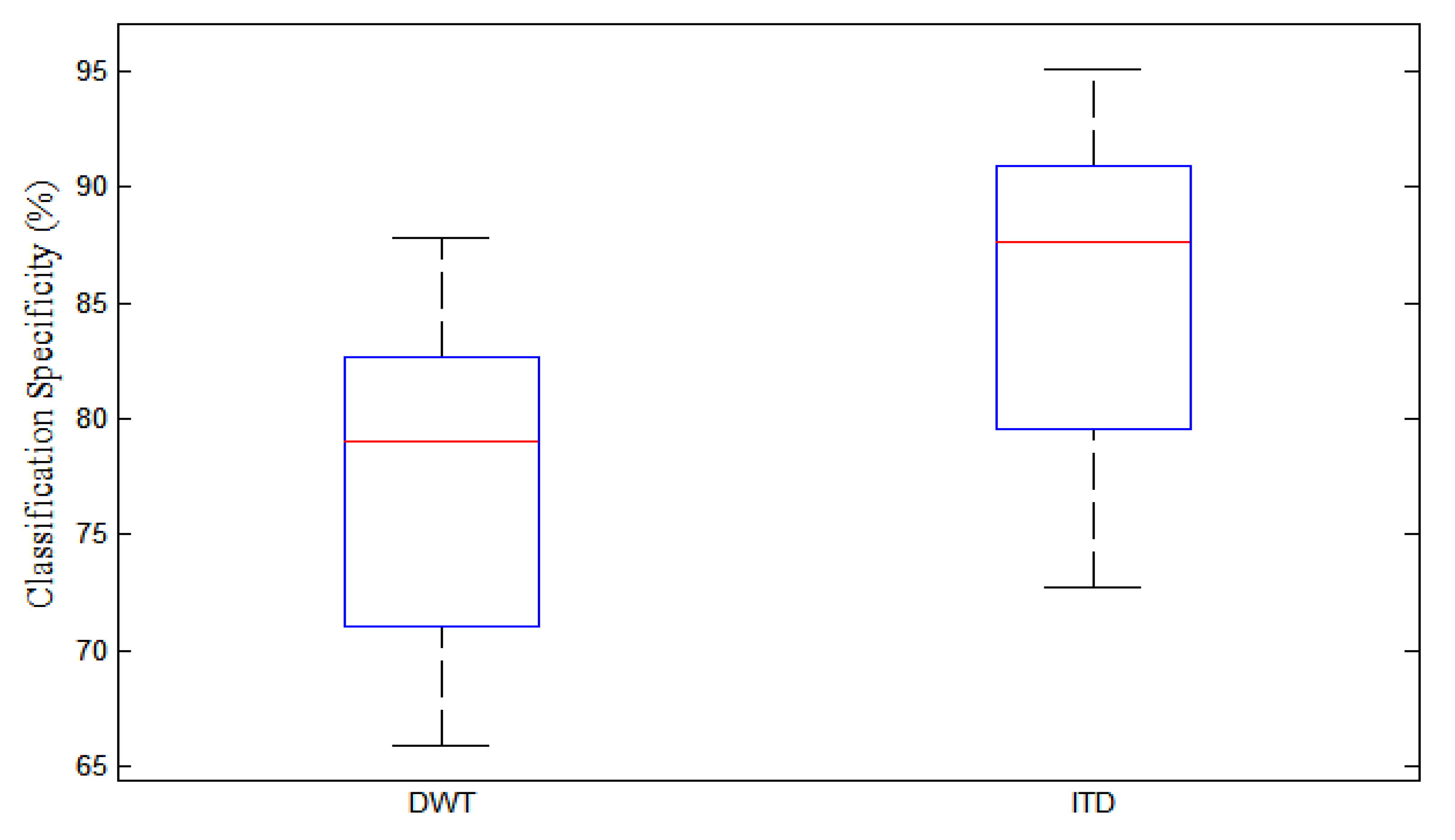
3.4. Comparison of ITD Technique with DWT without ROI Estimation
3.5. Comparison of ITD Technique and DWT with ROI Estimation
3.6. Validation and Comparison with the Existing Methods from the Literature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; He, B. Brain–computer interfaces using sensorimotor rhythms: Current state and future perspectives. IEEE Trans. Biomed. Eng. 2014, 61, 1425–1435. [Google Scholar] [CrossRef]
- Li, J.; Liang, J.; Zhao, Q.; Li, J.; Hong, K.; Zhang, L. Design of assistive wheelchair system directly steered by human thoughts. Int. J. Neural Syst. 2013, 23, 1350013. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Matsunaga, K.; Wang, H.O. Electroencephalogram-based control of an electric wheelchair. IEEE Trans. Robot. 2005, 21, 762–766. [Google Scholar] [CrossRef]
- Galán, F.; Nuttin, M.; Lew, E.; Ferrez, P.W.; Vanacker, G.; Philips, J.; Millán, J.D.R. A brain-actuated wheelchair: Asynchronous and non-invasive brain–computer interfaces for continuous control of robots. Clin. Neurophysiol. 2008, 119, 2159–2169. [Google Scholar] [CrossRef]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain computer interfaces, a review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A comprehensive review of EEG-based brain–computer interface paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef]
- Yang, J.; Singh, H.; Hines, E.L.; Schlaghecken, F.; Iliescu, D.D.; Leeson, M.S.; Stocks, N.G. Channel selection and classification of electroencephalogram signals: An artificial neural network and genetic algorithm-based approach. Artif. Intell. Med. 2012, 55, 117–126. [Google Scholar] [CrossRef]
- Pfurtscheller, G. Functional brain imaging based on ERD/ERS. Vis. Res. 2001, 41, 1257–1260. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Scherer, R.; Muller-Putz, G.R.; Lopes da Silva, F.H. Short-lived brain state after cued motor imagery in naive subjects. Eur. J. Neurosci. 2008, 28, 1419–1426. [Google Scholar] [CrossRef]
- Alonso-Valerdi, L.M.; Salido-Ruiz, R.A.; Ramirez-Mendoza, R.A. Motor imagery based brain-computer interfaces: An emerging technology to rehabilitate motor deficits. Neuropsychologia 2015, 79, 354–363. [Google Scholar] [CrossRef]
- Jeon, Y.; Nam, C.S.; Kim, Y.-J.; Whang, M.C. Event-related (De)synchronization (ERD/ERS) during motor imagery tasks: Implications for brain–computer interfaces. Int. J. Ind. Ergon. 2011, 41, 428–436. [Google Scholar] [CrossRef]
- Abdalsalam, E.; Yusoff, M.Z.; Malik, A.; Kamel, N.S.; Mahmoud, D. Modulation of sensorimotor rhythms for brain-computer interface using motor imagery with online feedback. Signal Image Video Process. 2018, 12, 557–564. [Google Scholar] [CrossRef]
- Padfield, N.; Zabalza, J.; Zhao, H.; Masero, V.; Ren, J. EEG-based brain-computer interfaces using motor-imagery: Techniques and challenges. Sensors 2019, 19, 1423. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, K.; Cassady, K.; Doud, A.; Shades, K.; Rogin, E.; He, B. Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain–computer interface. J. Neural Eng. 2013, 10, 046003. [Google Scholar] [CrossRef]
- Hari, R.; Forss, N.; Avikainen, S.; Kirveskari, E.; Salenius, S.; Rizzolatti, G. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc. Natl. Acad. Sci. USA 1998, 95, 15061–15065. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; McFarland, D.J. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 17849–17854. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.J. The advantages of the surface Laplacian in brain–computer interface research. Int. J. Psychophysiol. 2015, 97, 271–276. [Google Scholar] [CrossRef]
- Li, Y.; Long, J.; Yu, T.; Yu, Z.; Wang, C.; Zhang, H.; Guan, C. An EEG-based BCI system for 2-D cursor control by combining Mu/Beta rhythm and P300 potential. IEEE Trans. Biomed. Eng. 2010, 57, 2495–2505. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Flotzinger, D.; Kalcher, J. Brain-computer interface—A new communication device for handicapped persons. J. Microcomput. Appl. 1993, 16, 293–299. [Google Scholar] [CrossRef]
- Pregenzer, M.; Pfurtscheller, G. Frequency component selection for an EEG-based brain to computer interface. IEEE Trans. Rehabil. Eng. 1999, 7, 413–419. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, W. Local temporal common spatial patterns for robust single-trial EEG classification. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Martis, R.J.; Acharya, U.R.; Tan, J.H.; Petznick, A.; Tong, L.; Chua, C.K.; Ng, E.Y.K. Application of intrinsic time-scale decomposition (ITD) to EEG signals for automated seizure prediction. Int. J. Neural Syst. 2013, 23, 1350023. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xie, L.; Ye, T.; Horch, A. Online detection of time-variant oscillations based on improved ITD. Control Eng. Pract. 2014, 32, 64–72. [Google Scholar] [CrossRef]
- Ince, N.F.; Tewfik, A.H.; Arica, S. Extraction subject-specific motor imagery time–frequency patterns for single trial EEG classification. Comput. Biol. Med. 2007, 37, 499–508. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, C.; Wu, J.; Qin, W.; Xu, M.; Yin, E. Temporal combination pattern optimization based on feature selection method for motor imagery BCIs. Front. Hum. Neurosci. 2020, 14, 231. [Google Scholar] [CrossRef]
- Abdalsalam, E.; Yusoff, M.Z.; Malik, A.S.; Bahloul, M.R.; Adam, D.M.; Adam, I.K. Comparison of EEG signal decomposition methods in classification of motor-imagery BCI. Multimed. Tools Appl. 2018, 77, 21305–21327. [Google Scholar]
- Abdalsalam, E.; Yusoff, M.Z.; Mahmoud, D.; Malik, A.S.; Bahloul, M.R. Discrimination of four class simple limb motor imagery movements for brain–computer interface. Biomed. Signal Process. Control 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; McFarland, D.J.; Neat, G.W.; Forneris, C.A. An EEG-based brain-computer interface for cursor control. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 252–259. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; McFarland, D.J. Multichannel EEG-based brain-computer communication. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 444–449. [Google Scholar] [CrossRef]
- Suzuki, K. Artificial Neural Networks: Methodological Advances and Biomedical Applications; InTech: London, UK, 2011. [Google Scholar]
- Phothisonothai, M.; Watanabe, K. Optimal fractal feature and neural network: EEG based BCI applications. In Brain-Computer Interface Systems-Recent Progress and Future Prospects; InTech: London, UK, 2013. [Google Scholar]
- Sonkin, K.M.; Stankevich, L.A.; Khomenko, J.G.; Nagornova, Z.V.; Shemyakina, N.V. Development of electroencephalographic pattern classifiers for real and imaginary thumb and index finger movements of one hand. Artif. Intell. Med. 2015, 63, 107–117. [Google Scholar] [CrossRef]
- Chen, C.-W.; Lin, C.-C.K.; Ju, M.-S. Detecting movement-related EEG change by wavelet decomposition-based neural networks trained with single thumb movement. Clin. Neurophysiol. 2007, 118, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Chin, Z.Y.; Zhang, H.; Guan, C. Mutual information-based selection of optimal spatial–temporal patterns for single-trial EEG-based BCIs. Pattern Recognit. 2012, 45, 2137–2144. [Google Scholar] [CrossRef]
- Frei, M.G.; Osorio, I. Intrinsic time-scale decomposition: Time–frequency–energy analysis and real-time filtering of non-stationary signals. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 2007, 463, 321–342. [Google Scholar] [CrossRef]









| Subject | Left Hand | Right Hand | Both Hands | Both Feet | ||
|---|---|---|---|---|---|---|
| Subject 1 | ITD with ROI | Accuracy % | 86.25% | 91.46% | 98.12% | 91.45% |
| Sensitivity % | 84.58% | 91.66% | 98.33% | 90.00% | ||
| Specificity % | 87.91% | 91.45% | 97.91% | 92.91% | ||
| AUC % | 92.99% | 97.19% | 99.64% | 97.20% | ||
| ITD without ROI | Accuracy % | 75.00% | 79.79% | 80.21% | 78.12% | |
| Sensitivity % | 75.00% | 81.66% | 79.58% | 74.16% | ||
| Specificity % | 75.00% | 77.91% | 80.83% | 82.08% | ||
| AUC % | 81.91% | 87.03% | 88.08% | 83.87% | ||
| Subject 2 | ITD with ROI | Accuracy % | 96.66% | 87.29% | 90.83% | 90.00% |
| Sensitivity % | 96.66% | 90.41% | 91.66% | 91.66% | ||
| Specificity % | 96.66% | 84.16% | 90.00% | 88.33% | ||
| AUC % | 99.16% | 94.01% | 96.80% | 96.02% | ||
| ITD without ROI | Accuracy % | 78.75% | 73.33% | 72.50% | 71.66% | |
| Sensitivity % | 79.58% | 73.33% | 71.25% | 71.66% | ||
| Specificity % | 77.91% | 73.33% | 73.75% | 71.66% | ||
| AUC % | 87.53% | 79.29% | 80.37% | 80.63% | ||
| True class | Predicted Class | ||||
| Right Hand | Left Hand | Both Hands | Both Feet | ||
| Right hand | 94.40% | 4.03% | 0.75% | 0.82% | |
| Left hand | 6.43% | 91.70% | 1.54% | 0.33% | |
| Both hands | 6.03% | 3.93% | 89.63% | 0.41% | |
| Both feet | 3.01% | 1.07% | 0.84% | 95.08% | |
| True class | Predicted Class | ||||
| Right Hand | Left Hand | Both Hands | Both Feet | ||
| Right hand | 82.94% | 7.53% | 5.71% | 3.82% | |
| Left hand | 13.65% | 79.01% | 4.82% | 2.53% | |
| Both hands | 11.53% | 9.83% | 76.03% | 2.61% | |
| Both feet | 10.01% | 5.07% | 4.84% | 80.08% | |
| Reference and Year | Method | Accuracy (%) |
|---|---|---|
| Jiang et al. [26] | CSP | 88.58 |
| This study | DWT with ROI BCI Competition IV dataset | 89.77 |
| ITD with ROI BCI Competition IV dataset | 96.87 | |
| ITD with ROI Own recorded dataset | 92.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, E.A.; Adam, I.K.; Yusoff, M.Z. Effect of Subject-Specific Region of Interest on Motor Imagery Brain–Computer Interface. Appl. Sci. 2023, 13, 6364. https://doi.org/10.3390/app13116364
Mohamed EA, Adam IK, Yusoff MZ. Effect of Subject-Specific Region of Interest on Motor Imagery Brain–Computer Interface. Applied Sciences. 2023; 13(11):6364. https://doi.org/10.3390/app13116364
Chicago/Turabian StyleMohamed, Eltaf Abdalsalam, Ibrahim Khalil Adam, and Mohd Zuki Yusoff. 2023. "Effect of Subject-Specific Region of Interest on Motor Imagery Brain–Computer Interface" Applied Sciences 13, no. 11: 6364. https://doi.org/10.3390/app13116364
APA StyleMohamed, E. A., Adam, I. K., & Yusoff, M. Z. (2023). Effect of Subject-Specific Region of Interest on Motor Imagery Brain–Computer Interface. Applied Sciences, 13(11), 6364. https://doi.org/10.3390/app13116364





