Preservation of Food Sugar Beet via the Control of Rhizoctonia solani AG 2-2IIIB by Extreme Factors
Abstract
1. Introduction
2. Materials and Methods
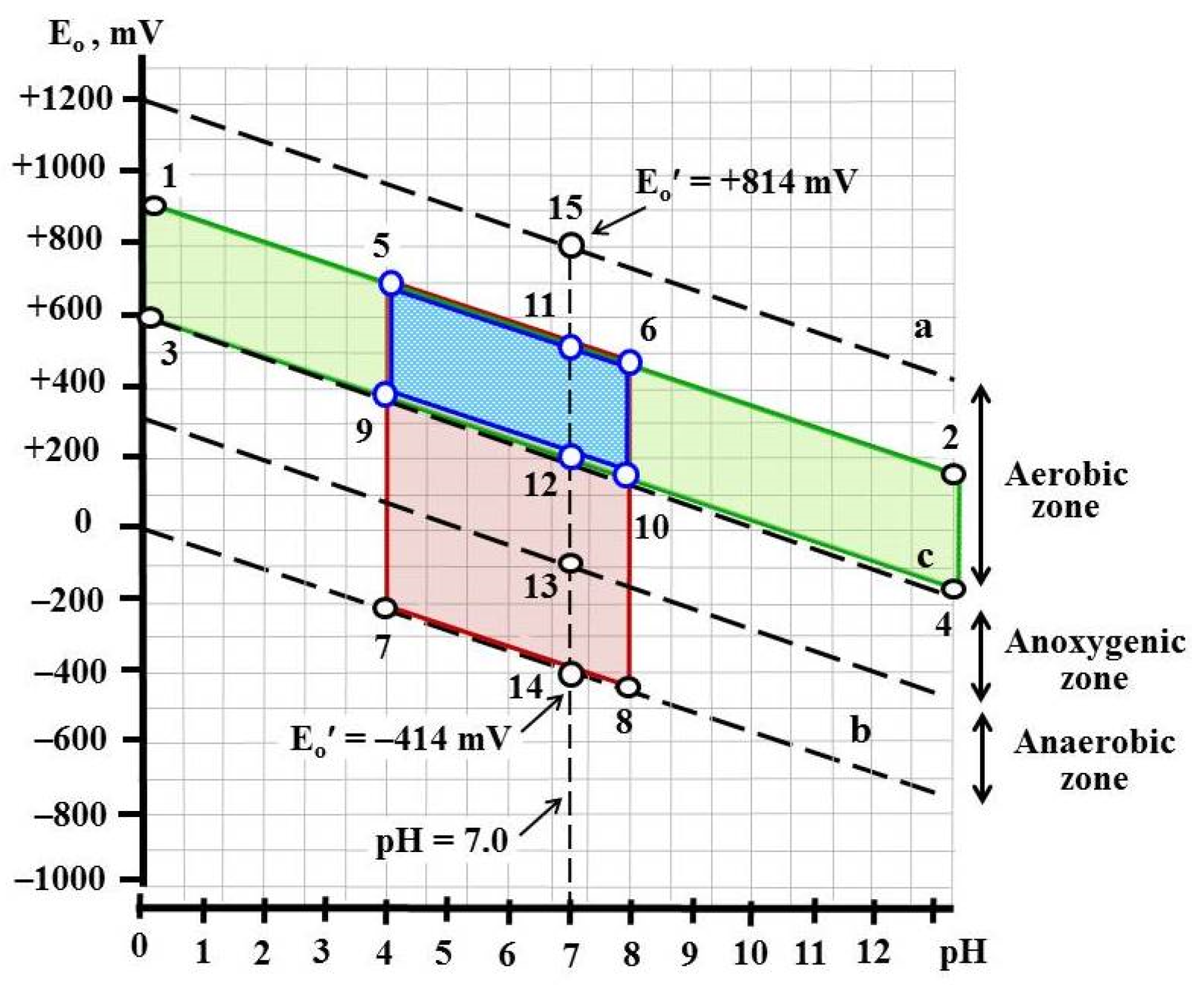
2.1. Thermodynamic Background of the Growth Conditions of Rhizoctonia solani AG 2-2IIIB
2.2. Factors Studied to Control Rhizoctonia solani AG 2-2IIIB
2.3. pH Maintenance via Abiotic Factors to Control Rhizoctonia solani AG 2-2IIIB
2.4. Eh Maintenance via Abiotic Factors to Control Rhizoctonia solani AG 2-2IIIB
2.5. Verification of Rhizoctonia solani AG 2-2IIIB Growth after Unfavorable Anoxygenic and Anaerobic Cultivation Conditions
2.6. Maintenance of Unfavorable Growth Conditions for Rhizoctonia solani AG 2-2IIIB via Biotic Factors
2.7. Isolation of the Best Performing Antagonistic Strains against Rhizoctonia solani AG 2-2IIIB
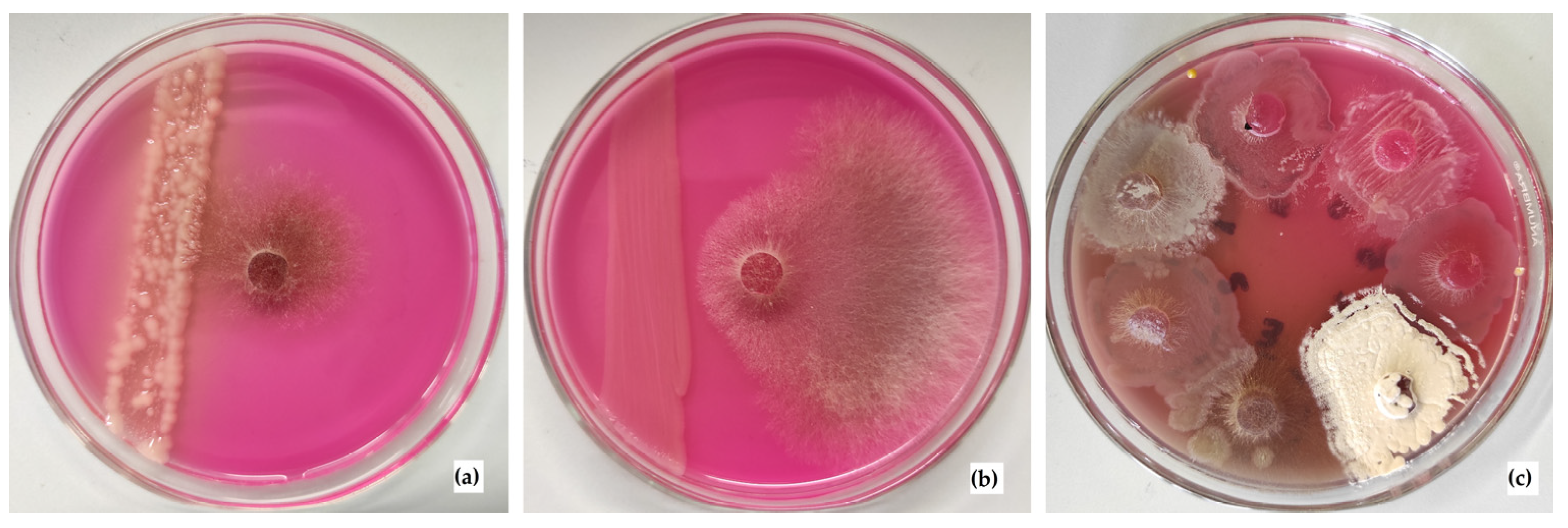
2.8. Study of the Antagonistic Properties of the Isolated Strains against Rhizoctonia solani AG 2-2IIIB
2.9. Data Analysis
3. Results
3.1. Thermodynamic Background for Control of Rhizoctonia solani AG 2-2IIIB
3.2. Application of Abiotic Factors for Control of Rhizoctonia solani AG 2-2IIIB
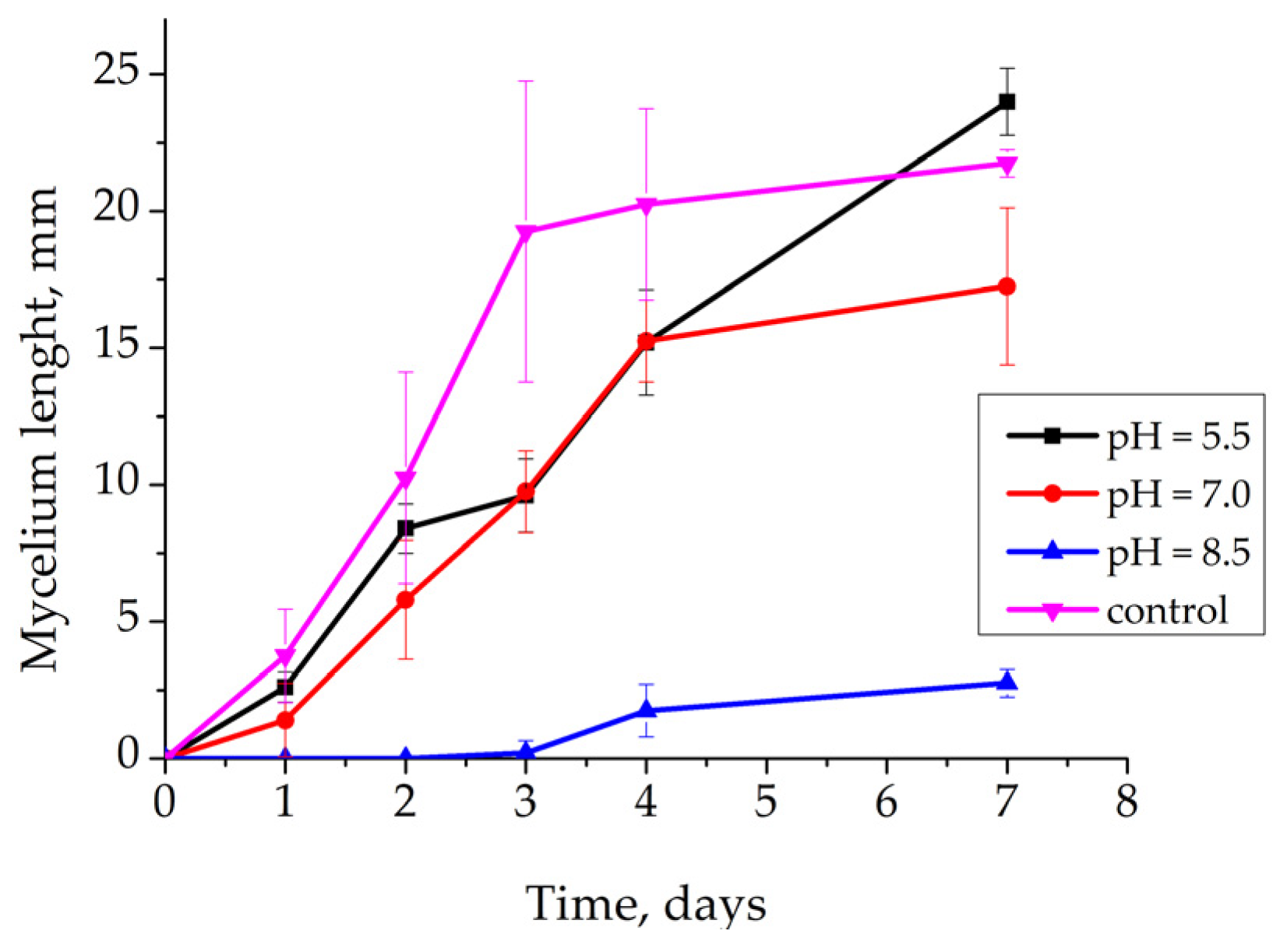
3.2.1. Effect of pH on Rhizoctonia solani AG 2-2IIIB Growth
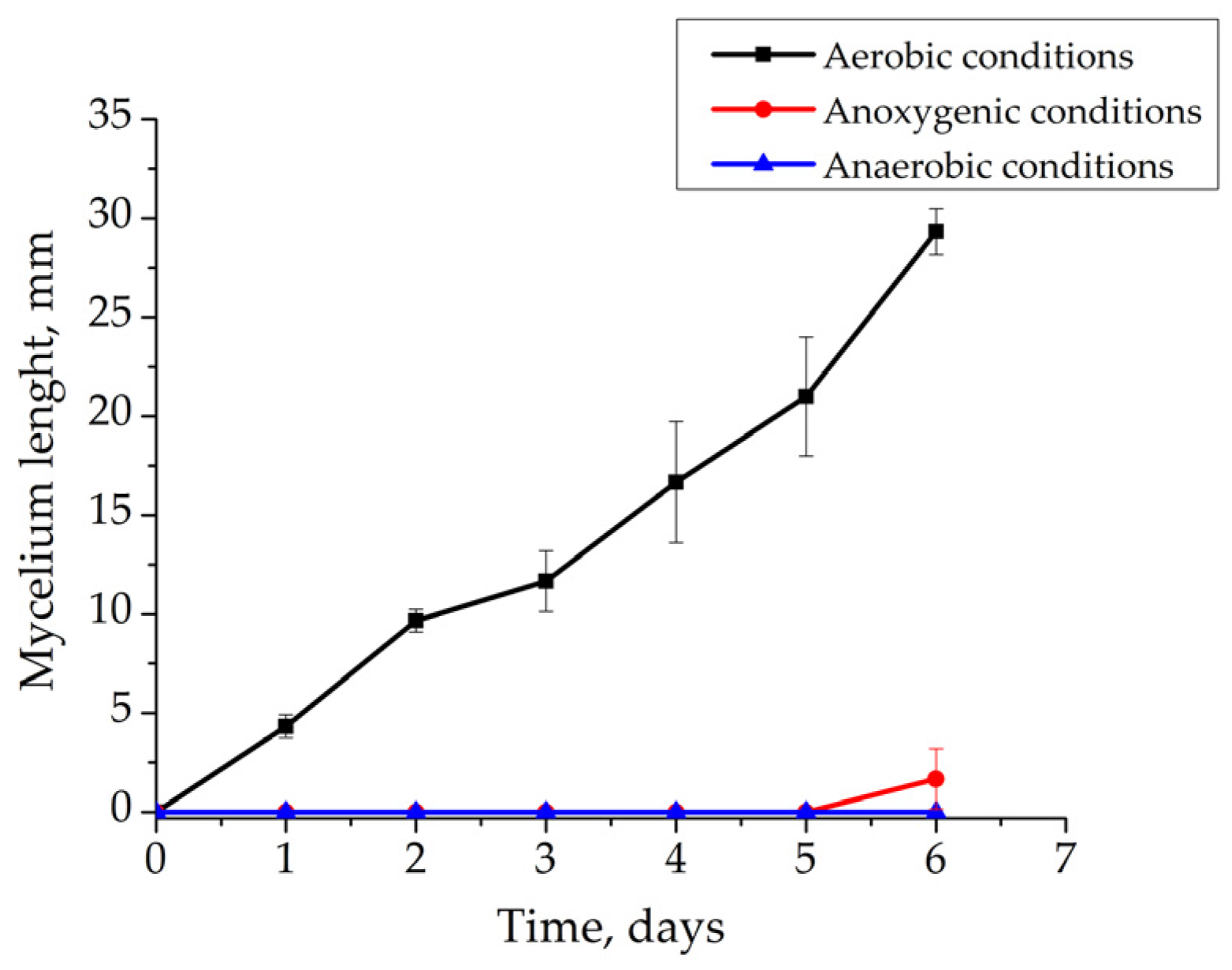
3.2.2. Effect of Eh and Presence of O2 on the Growth of Rhizoctonia solani AG 2-2IIIB
3.2.3. Recovery of Rhizoctonia solani AG 2-2IIIB Growth after Unfavorable Anoxygenic and Anaerobic Cultivation Conditions
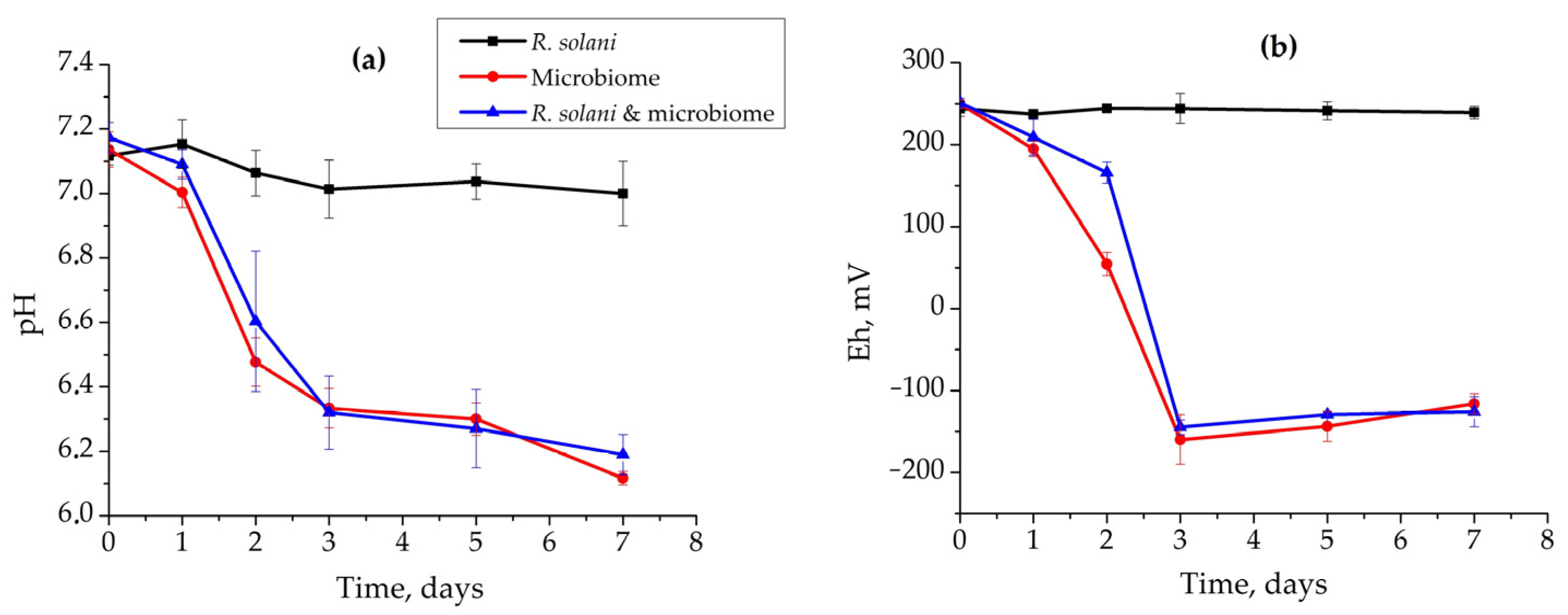
3.3. Application of Biotic Factors for Control of Rhizoctonia solani AG 2-2IIIB
3.4. Antagonism of the Isolated Microbial Strains against Rhizoctonia solani AG 2-2IIIB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Kumar, S.P.J.; Chintagunta, A.D.; Agarwal, D.K.; Pal, G.; Singh, A.N.; Simal-Gandara, J. Biocontrol Potential of Pseudomonas stutzeri Endophyte from Withania somnifera (Ashwagandha) Seed Extract against Pathogenic Fusarium oxysporum and Rhizoctonia solani. Arch. Phytopathol. Plant Prot. 2022, 55, 1–18. [Google Scholar] [CrossRef]
- Luo, G.; Karakashev, D.; Xie, L.; Zhou, Q.; Angelidaki, I. Long-Term Effect of Inoculum Pretreatment on Fermentative Hydrogen Production by Repeated Batch Cultivations: Homoacetogenesis and Methanogenesis as Competitors to Hydrogen Production. Biotechnol. Bioeng. 2011, 108, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, L.; Wang, Y.; Chen, Y.; Hu, K.; Yang, W.; Zuo, S.; Xu, J.; Kang, Z.; Xiao, X.; et al. A High-Quality Genome of Rhizoctonia solani, a Devastating Fungal Pathogen with a Wide Host Range. Mol. Plant-Microbe Interact. 2022, 35, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Strausbaugh, C.A.; Galewski, P.J.; Minocha, R.; Rogers, C.W. Cell-Wall-Degrading Enzymes-Related Genes Originating from Rhizoctonia solani Increase Sugar Beet Root Damage in the Presence of Leuconostoc mesenteroides. Int. J. Mol. Sci. 2022, 23, 1366. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, A.I.; Pérez-Sánchez, R.; Plaza, J.; Morales-Corts, M.R. Compost Tea as a Sustainable Alternative to Promote Plant Growth and Resistance against Rhizoctonia solani in Potato Plants. Sci. Hortic. 2022, 300, 111090–111099. [Google Scholar] [CrossRef]
- Büttner, G.; Pfähler, B.; Märländer, B. Greenhouse and Field Techniques for Testing Sugar Beet for Resistance to Rhizoctonia Root and Crown Rot. Plant Breed. 2004, 123, 158–166. [Google Scholar] [CrossRef]
- Barreto, A.; Paulus, S.; Varrelmann, M.; Mahlein, A.-K. Hyperspectral Imaging of Symptoms Induced by Rhizoctonia solani in Sugar Beet: Comparison of Input Data and Different Machine Learning Algorithms. J. Plant Dis. Prot. 2020, 127, 441–451. [Google Scholar] [CrossRef]
- Postma, J.; Schilder, M.T. Enhancement of Soil Suppressiveness against Rhizoctonia solani in Sugar Beet by Organic Amendments. Appl. Soil Ecol. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Me, H.; Ms, P. Sugar Beet, It ‘disease Rhizoctonia Root Rot, and Potential Biological Agents. Agric. Biol. Res. 2021, 37, 96–101. [Google Scholar]
- Ravi, S.; Hassani, M.; Heidari, B.; Deb, S.; Orsini, E.; Li, J.; Richards, C.M.; Panella, L.W.; Srinivasan, S.; Campagna, G.; et al. Development of an SNP Assay for Marker-Assisted Selection of Soil-Borne Rhizoctonia solani AG-2-2-IIIB Resistance in Sugar Beet. Biology 2022, 11, 49. [Google Scholar] [CrossRef]
- Hassani, M.; Heidari, B.; Mahmoudi, S.B.; Taleghani, D.F.; Stevanato, P. Identification of Owen-Type Male Sterility Maintainers Carrying Resistance Against Rhizoctonia Crown and Root Rot (Rcrr) Disease in Sugar Beet Germplasm. Sugar Tech 2019, 21, 959–965. [Google Scholar] [CrossRef]
- Akhter, W.; Bhuiyan, M.K.A.; Sultana, F.; Hossain, M.M. Integrated Effect of Microbial Antagonist, Organic Amendment and Fungicide in Controlling Seedling Mortality (Rhizoctonia solani) and Improving Yield in Pea (Pisum sativum L.). C. R. Biol. 2015, 338, 21–28. [Google Scholar] [CrossRef]
- Al-Baldawy, M.S.M.; Matloob, A.A.A.H.; Almammory, M.K.N. Effect of Plant Extracts and Biological Control Agents on Rhizoctonia solani Kuhn. IOP Conf. Ser. Earth Environ. Sci. 2021, 735, 012079–012087. [Google Scholar] [CrossRef]
- Díaz-Díaz, M.; Bernal-Cabrera, A.; Trapero, A.; Medina-Marrero, R.; Sifontes-Rodríguez, S.; Cupull-Santana, R.D.; García-Bernal, M.; Agustí-Brisach, C. Characterization of Actinobacterial Strains as Potential Biocontrol Agents against Macrophomina phaseolina and Rhizoctonia solani, the Main Soil-Borne Pathogens of Phaseolus Vulgaris in Cuba. Plants 2022, 11, 645. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Mixtures of Organic Amendments and Biochar Promote Beneficial Soil Microbiota and Affect Fusarium oxysporum f. sp. Lactucae, Rhizoctonia solani and Sclerotinia minor Disease Suppression. Plant Pathol. 2022, 71, 818–829. [Google Scholar] [CrossRef]
- Tashyrev, O.; Hovorukha, V.; Havryliuk, O.; Sioma, I.; Gladka, G.; Kalinichenko, O.; Włodarczyk, P.; Suszanowicz, D.; Zhuk, H.; Ivanov, Y. Spatial Succession for Degradation of Solid Multicomponent Food Waste and Purification of Toxic Leachate with the Obtaining of Biohydrogen and Biomethane. Energies 2022, 15, 911. [Google Scholar] [CrossRef]
- Kucharska, K.; Katulski, B.; Goriewa, K.; Duba, A.; Wachowska, U. Pathogenicity and Fungicide Sensitivity of Rhizoctonia solani and R. cerealis Isolates. Gesunde Pflanz. 2018, 70, 13–19. [Google Scholar] [CrossRef]
- Moliszewska, E.B.; Schneider, J.H.M. Some Pathogenic Properties of Rhizoctonia solani to Sugar Beet Seedlings. Plant Prot. Sci. 2002, 38, 322–324. [Google Scholar] [CrossRef]
- Bolton, M.D.; Panella, L.; Campbell, L.; Khan, M.F.R. Temperature, Moisture, and Fungicide Effects in Managing Rhizoctonia Root and Crown Rot of Sugar Beet. Phytopathology 2010, 100, 689–697. [Google Scholar] [CrossRef]
- Moliszewska, E.; Nabrdalik, M.; Ziembik, Z. Rhizoctonia solani AG 11 Isolated for the First Time from Sugar Beet in Poland. Saudi J. Biol. Sci. 2020, 27, 1863–1870. [Google Scholar] [CrossRef]
- Nabrdalik, M.; Moliszewska, E.; Wierzba, S. Importance of Endophytic Strains Pantoea agglomerans in the Biological Control of Rhizoctonia solani. Ecol. Chem. Eng. S 2018, 25, 331–342. [Google Scholar] [CrossRef]
- Jacobsen, B.J. Root Rot Diseases of Sugar Beet. Zb. Matice Srp. Za Prir. Nauk. 2006, 110, 9–19. [Google Scholar]
- Bartholomäus, A.; Mittler, S.; Märländer, B.; Varrelmann, M. Control of Rhizoctonia solani in Sugar Beet and Effect of Fungicide Application and Plant Cultivar on Inoculum Potential in the Soil. Plant Dis. 2017, 101, 941–947. [Google Scholar] [CrossRef]
- Ayala, G.; Buettner, G.; Guitierrez, H.; Heijbroek, W.; Ioannides, P.; Nihlgaard, M.; Molard, R.; Panella, L.; Rossi, V.; Roesner, H.; et al. Integrated Control of Rhizoctonia Root Rot. First Results of an International Institute for Beet Research Trial Series. In Proceedings of the Comptes-Rendus des Congres de l’Institut International de Recherches Betteravieres, Belgium, 2001; Available online: https://scholar.google.com/scholar_lookup?title=Integrated+control+of+rhizoctonia+root+rot.+First+results+of+an+International+institute+for+beet+research+trial+series&author=Ayala%2C+G.&publication_year=2001 (accessed on 22 May 2023).
- Moliszewska, E.B. Etiologia Wybranych Chorób Korzeni Buraka Cukrowego; Wydawnictwo Uniwersytetu Opolskiego: Opole, Poland, 2009. [Google Scholar]
- Pourbaix, M. Thermodynamics of Dilute Aqueous Solutions: Graphical Representation of the Role of pH and Potential. Ph.D. Thesis, Delft Institute of Technology, Delft, The Netherlands, 1945. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solution. NACE 1974, 648. [Google Scholar]
- Hovorukha, V.; Havryliuk, O.; Gladka, G.; Tashyrev, O.; Kalinichenko, A.; Sporek, M.; Dołhańczuk-Śródka, A. Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste. Energies 2021, 14, 1831. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Amran, M.A.; Norhashim, N.A.; Mohamad-Fauzi, N.; Peng-Hui, F.; Hui-Wen, L.; Kai-Lin, Y.; Jiale, L.; Chian-Yee, M.G.; Jing-Yi, L.; et al. Food Waste Composting and Microbial Community Structure Profiling. Processes 2020, 8, 723. [Google Scholar] [CrossRef]
- Nikolsky, B.P. Chemistry Handbook, Chemical Equilibrium and Kinetics. Properties of Solutions. In Electrode Processes; Chemistry: Leningrad, Russia, 1965; Volume 3, p. 1008. [Google Scholar]
- Lehninger, A.; Nelson, D.; Cox, M. Principles of Biochemistry, 2nd ed.; Worth: New York, NY, USA, 1993; p. 1090. [Google Scholar]
- Herr, L.J. Sugar Beet Diseases Incited by Rhizoctonia spp. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 341–349. ISBN 978-94-017-2901-7. [Google Scholar]
- Kiewnick, S.; Jacobsen, B.J.; Braun-Kiewnick, A.; Eckhoff, J.L.A.; Bergman, J.W. Integrated Control of Rhizoctonia Crown and Root Rot of Sugar Beet with Fungicides and Antagonistic Bacteria. Plant Dis. 2001, 85, 718–722. [Google Scholar] [CrossRef]
- Ritchie, F.; Bain, R.A.; McQuilken, M.P. Effects of Nutrient Status, Temperature and pH on Mycelial Growth, Sclerotial Production and Germination of Rhizoctonia solani from Potato. J. Plant Pathol. 2009, 91, 589–596. [Google Scholar]
- Nuri, T.; Biswas, M.K. Impact of Different Culture Media, Temperature and pH on Growth of Rhizoctonia solani i Kühn Causes Black Scurf of Potato. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 27–33. [Google Scholar]
- Goswami, B.K.; Rahaman, M.M.; Hoque, A.; Bhuiyan, K.; Mian, I.H. Variations In Different Isolates Of Rhizoctonia solani Based On Temperature And pH. Bangladesh J. Agric. Res. 2011, 36, 389–396. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Mazzola, M. Carbon Source-Dependent Effects of Anaerobic Soil Disinfestation on Soil Microbiome and Suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani Diseases of Sugar Beet by Antagonistic and Plant Growth-promoting Yeasts. J. Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef]
- Karimi, E.; Safaie, N.; Shams-Baksh, M.; Mahmoudi, B. Bacillus amyloliquefaciens SB14 from Rhizosphere Alleviates Rhizoctonia Damping-off Disease on Sugar Beet. Microbiol. Res. 2016, 192, 221–230. [Google Scholar] [CrossRef]
- Zachow, C.; Fatehi, J.; Cardinale, M.; Tilcher, R.; Berg, G. Strain-Specific Colonization Pattern of Rhizoctonia Antagonists in the Root System of Sugar Beet. FEMS Microbiol. Ecol. 2010, 74, 124–135. [Google Scholar] [CrossRef]
- Masih, E.I.; Paul, B. Secretion of β-1,3-Glucanases by the Yeast Pichia membranifaciens s and Its Possible Role in the Biocontrol of Botrytis cinerea Causing Grey Mold Disease of the Grapevine. Curr. Microbiol. 2002, 44, 391–395. [Google Scholar] [CrossRef]
- Mao, W.; Lewis, J.A.; Lumsden, R.D.; Hebbar, K.P. Biocontrol of Selected Soilborne Diseases of Tomato and Pepper Plants. Crop Prot. 1998, 17, 535–542. [Google Scholar] [CrossRef]

| Conditions | Factors | ||
|---|---|---|---|
| O2 | Argon | Fe(II) | |
| Aerobic | + 1 | − 2 | − |
| Anoxygenic | − | + | − |
| Anaerobic | − | + | + |
| Strains | Indirect Interaction | Direct Interaction |
|---|---|---|
| A.1 | − 1 | − |
| A.2 | + 2 | + |
| A.3 | − | − |
| A.4 | +++ 4 | +++ |
| A.5 | +++ | ++ |
| A.6 | ++ 3 | ++ |
| A.7 | ++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hovorukha, V.; Tashyrev, O.; Kalinichenko, A.; Moliszewska, E. Preservation of Food Sugar Beet via the Control of Rhizoctonia solani AG 2-2IIIB by Extreme Factors. Appl. Sci. 2023, 13, 6362. https://doi.org/10.3390/app13116362
Hovorukha V, Tashyrev O, Kalinichenko A, Moliszewska E. Preservation of Food Sugar Beet via the Control of Rhizoctonia solani AG 2-2IIIB by Extreme Factors. Applied Sciences. 2023; 13(11):6362. https://doi.org/10.3390/app13116362
Chicago/Turabian StyleHovorukha, Vira, Oleksandr Tashyrev, Antonina Kalinichenko, and Ewa Moliszewska. 2023. "Preservation of Food Sugar Beet via the Control of Rhizoctonia solani AG 2-2IIIB by Extreme Factors" Applied Sciences 13, no. 11: 6362. https://doi.org/10.3390/app13116362
APA StyleHovorukha, V., Tashyrev, O., Kalinichenko, A., & Moliszewska, E. (2023). Preservation of Food Sugar Beet via the Control of Rhizoctonia solani AG 2-2IIIB by Extreme Factors. Applied Sciences, 13(11), 6362. https://doi.org/10.3390/app13116362










