Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System
Abstract
1. Introduction
2. Material and Methods
2.1. Drugs and Reagents
2.2. Extract Preparation and Procedures for Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia Insignis Seed Extract
2.2.1. Hexane Extract from Platonia Insignis Seeds
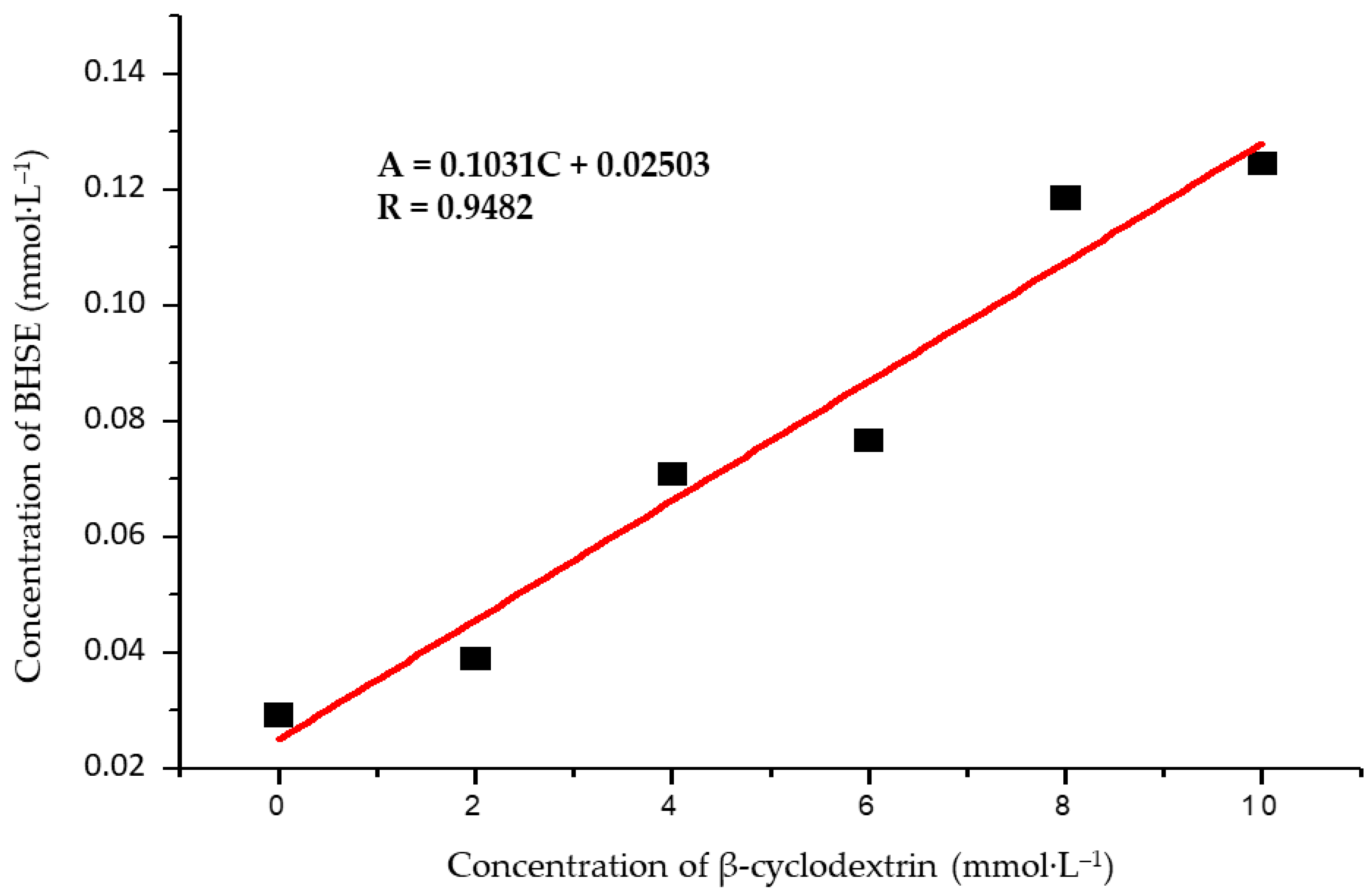
2.2.2. Phase Solubility Studies
2.2.3. Production of BSHE:β-CD Inclusion Complexes
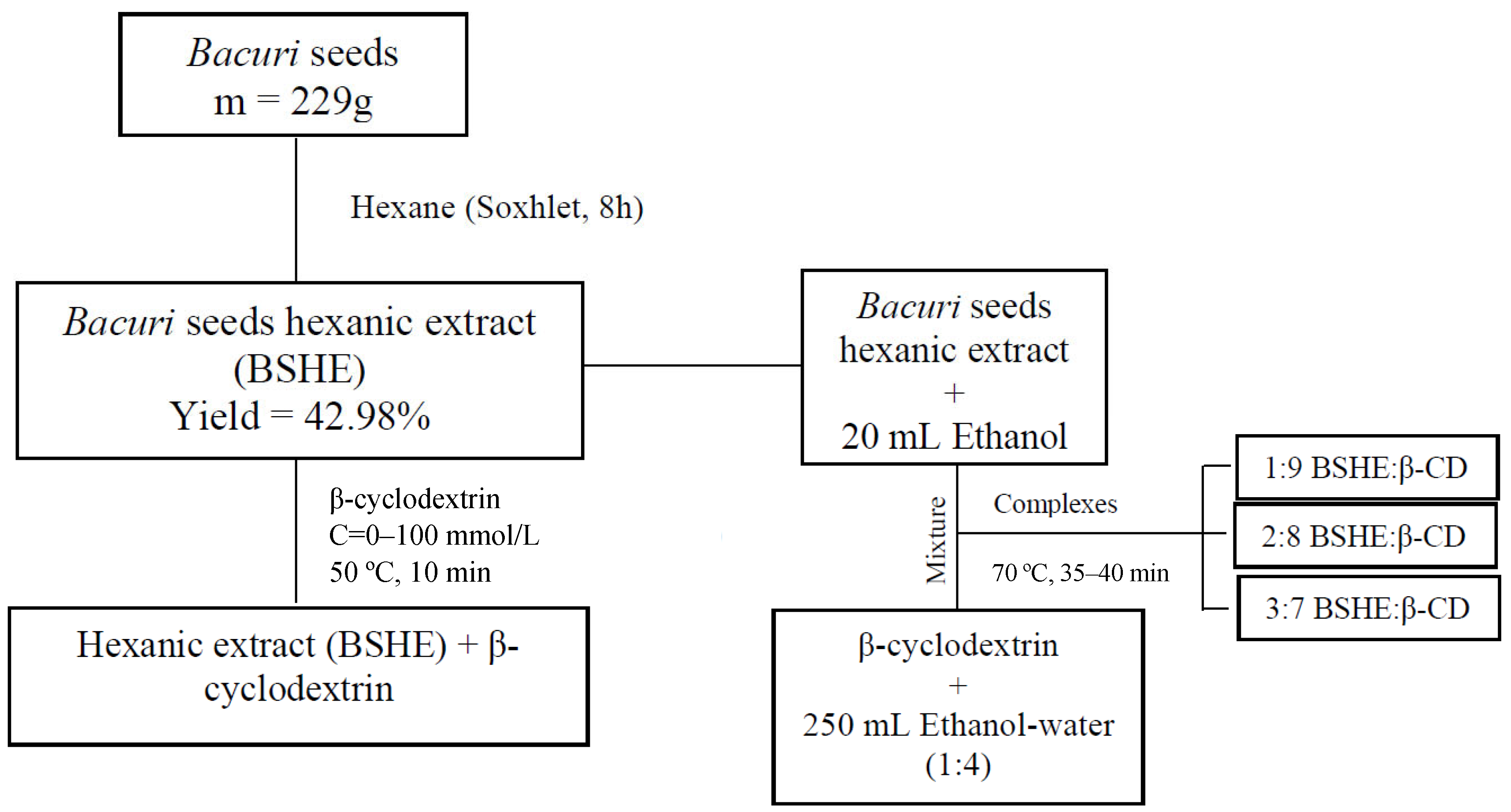
2.2.4. Determination of Garcinielliptone FC by UV-Vis in BSHE and BSHE:β-CD Inclusion Complexes
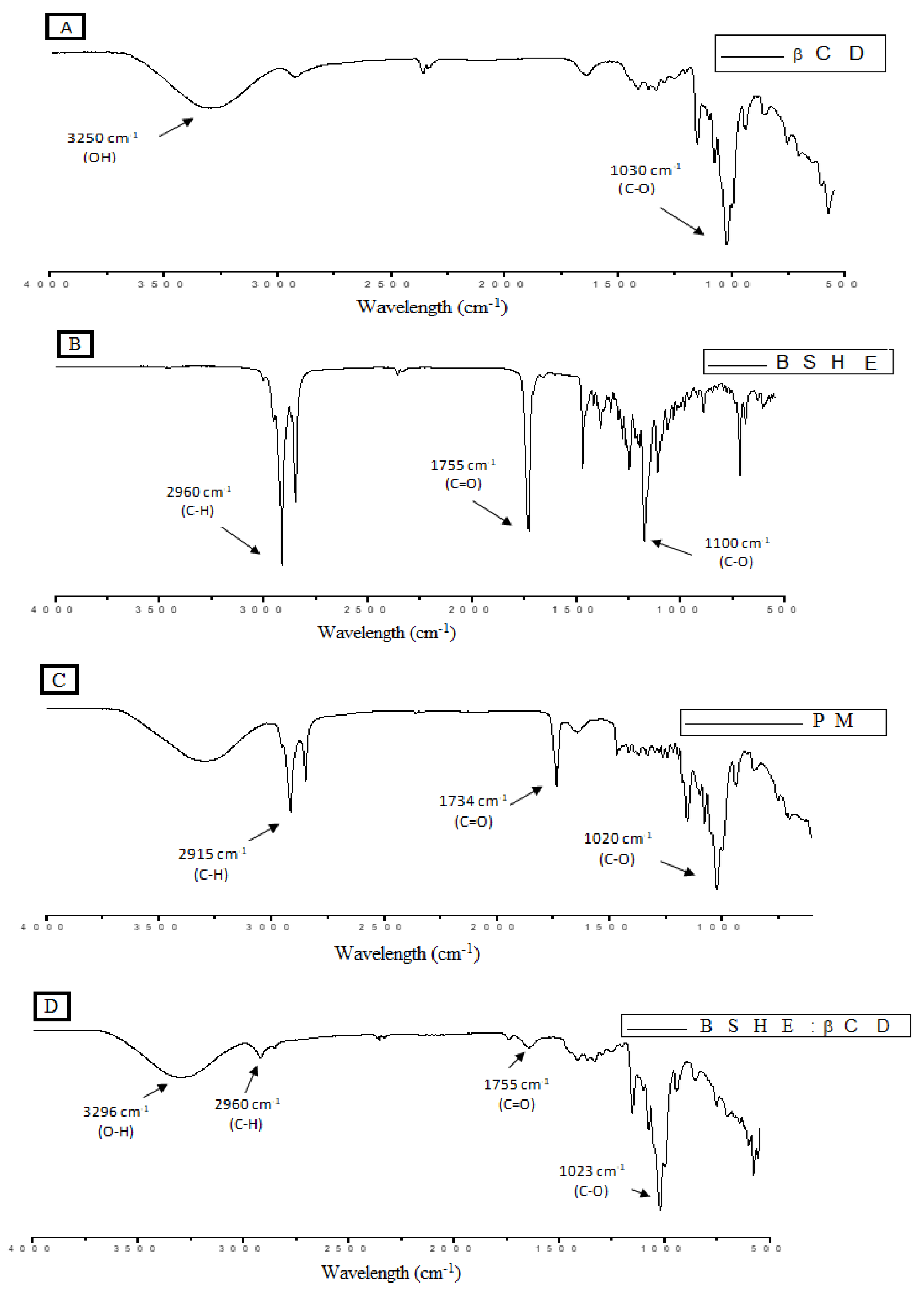
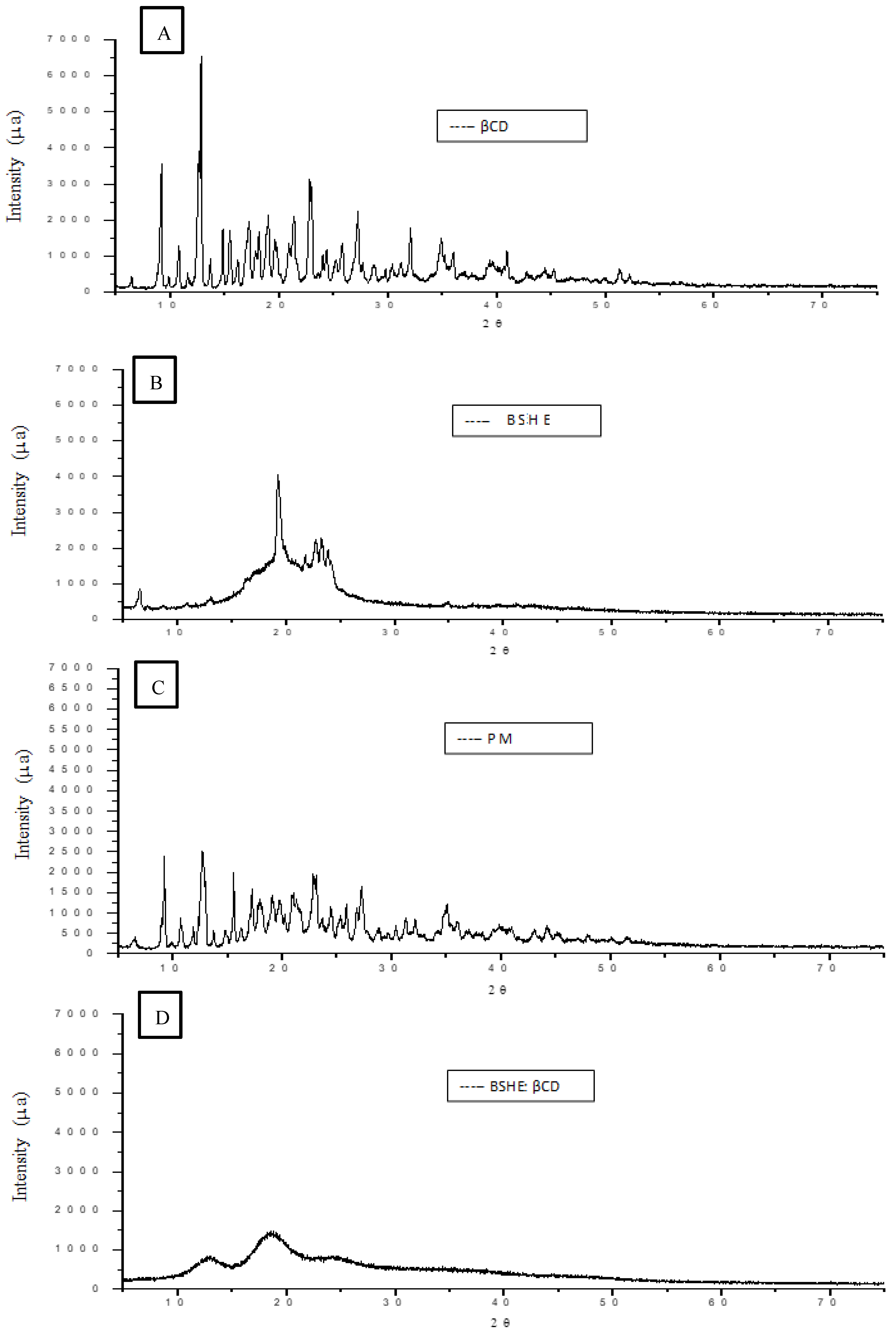
2.2.5. Characterization of BSHE:β-CD Inclusion Complexes
2.3. In Vivo Study by Animal Model: Performance as Gastroprotective System
2.3.1. Animal Models
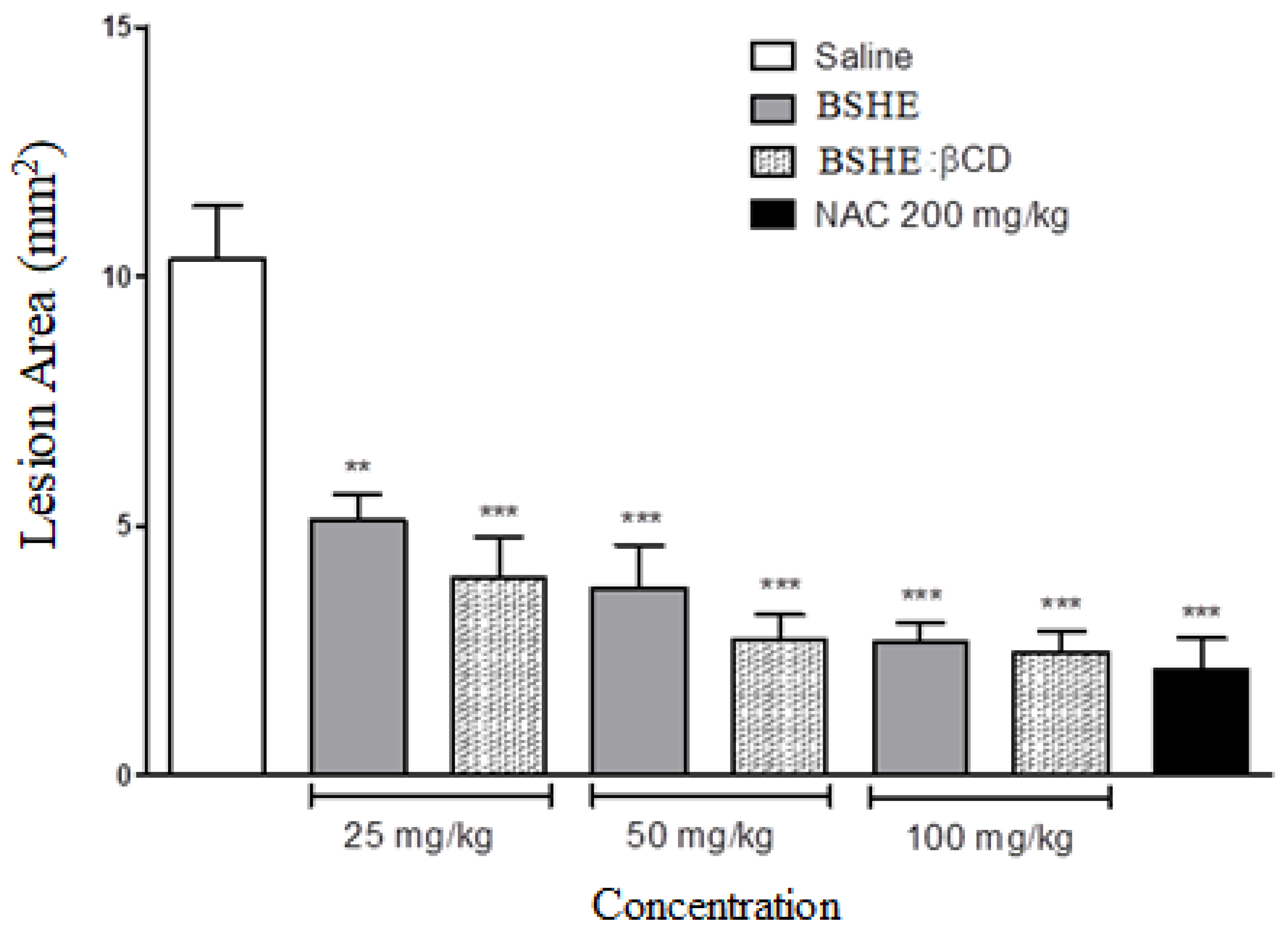
2.3.2. Ethanol-Induced Gastric Ulcer
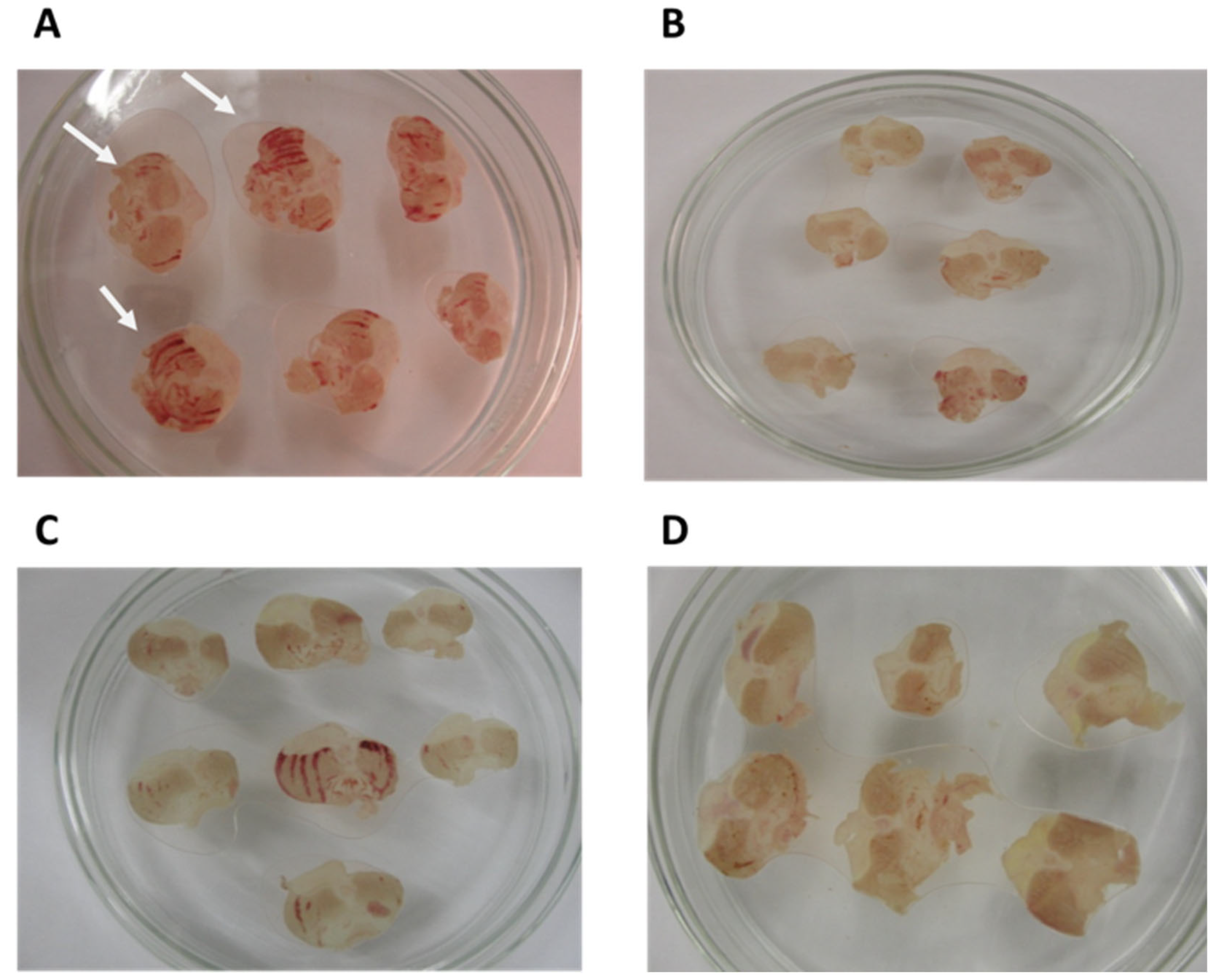
2.3.3. Gastric Lesions Induced by Ischemia and Reperfusion in Rats
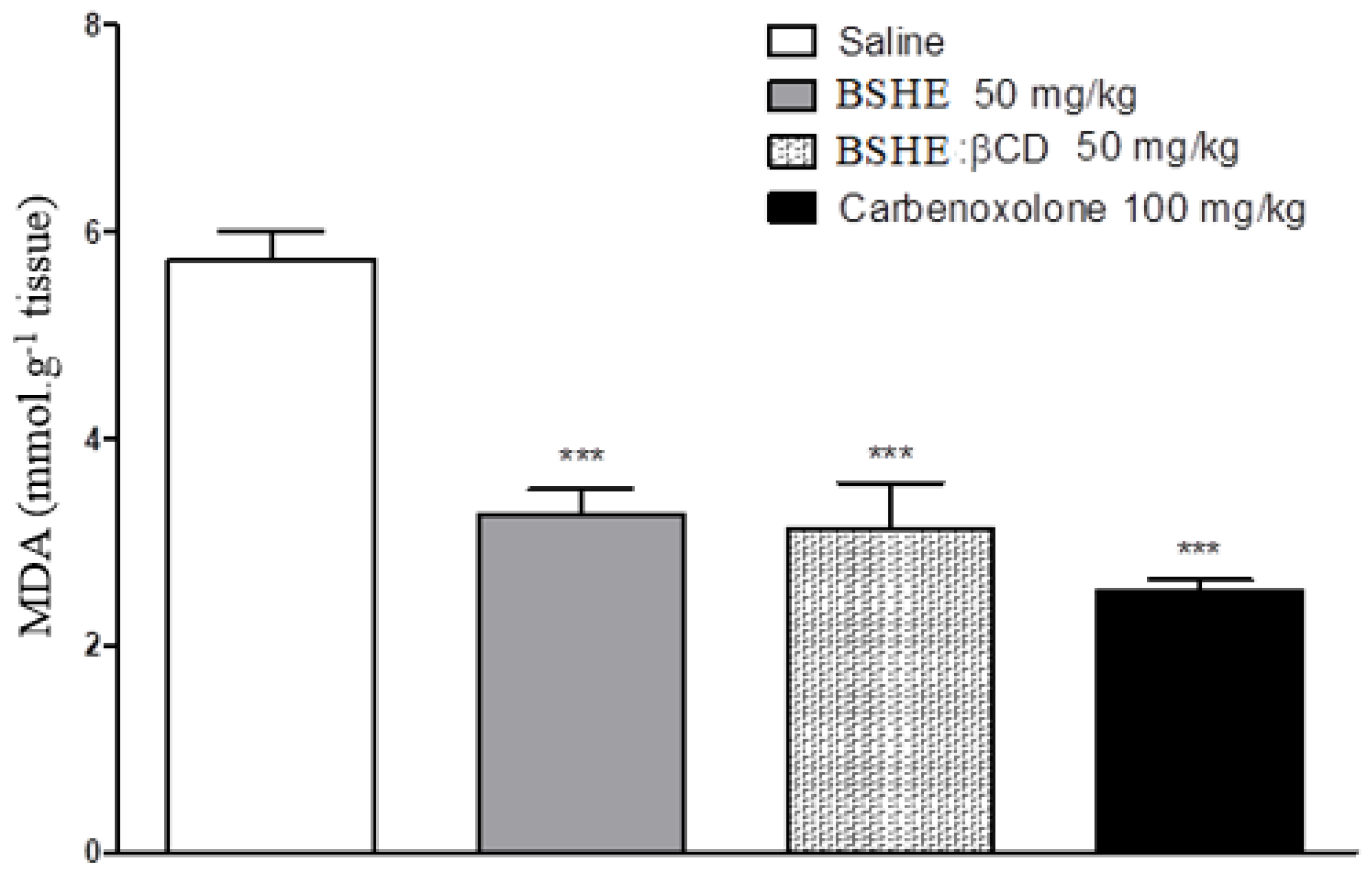
2.3.4. Dosage of Malonaldehyde (MDA)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the BSHE:β-CD Inclusion Complexes
3.2. Gastroprotective effect of BSHE:β-CD Inclusion Complexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilon, A.C.; Valli, M.; Dametto, A.C.; Pinto, M.E.F.; Freire, R.T.; Castro-Gamboa, I.; Andricopulo, A.D.; Bolzani, V.S. NuBBEDB: An updated database to uncover chemical and biological information from Brazilian biodiversity. Sci. Rep. 2017, 7, 7215. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Durazzo, A.; Lucarini, M. Advances in Research on Food Bioactive Molecules and Health. Molecules 2021, 26, 7678. [Google Scholar] [CrossRef]
- Figueiredo, A.; Hugueney, P.; Durazzo, A. Recent Advances in Plant Metabolomics: From Metabolic Pathways to Health Impact. Biology 2022, 11, 238. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M. Environmental, Ecological and Food Resources in the Biodiversity Overview: Health Benefits. Life 2021, 11, 1228. [Google Scholar] [CrossRef] [PubMed]
- Tabajara de Oliveira Martins, D.; Rodrigues, E.; Casu, L.; Benítez, G.; Leonti, M. The historical development of pharmacopoeias and the inclusion of exotic herbal drugs with a focus on Europe and Brazil. J. Ethnopharmacol. 2019, 240, 111891. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Ciências 2019, 91 (Suppl. 3), e20190105. [Google Scholar] [CrossRef] [PubMed]
- Uekane, T.M.; Nicolotti, L.; Griglione, A.; Bizzo, H.R.; Rubiolo, P.; Bicchi, C.; Rocha-Leão, M.H.; Rezende, C.M. Studies on the volatile fraction composition of three native Amazonian-Brazilian fruits: Murici (Byrsonima crassifolia L., Malpighiaceae), bacuri (Platonia insignis M., Clusiaceae), and sapodilla (Manilkara sapota L., Sapotaceae). Food Chem. 2017, 219, 13–22. [Google Scholar] [CrossRef]
- Souza, A.C.; Alves, M.M.D.M.; Brito, L.M.; Oliveira, L.G.C.; Sobrinho-Júnior, E.P.C.; Costa, I.C.G.; Freitas, S.D.L.; Rodrigues, K.A.D.F.; Chaves, M.H.; Arcanjo, D.D.R.; et al. Platonia insignis Mart., a Brazilian Amazonian Plant: The Stem Barks Extract and Its Main Constituent Lupeol Exert Antileishmanial Effects Involving Macrophages Activation. Evid. Based Complement. Altern. Med. 2017, 2017, 3126458. [Google Scholar] [CrossRef]
- Costa Júnior, J.S.; Ferraz, A.B.F.; Sousa, T.O.; Silva, R.A.; De Lima, S.G.; Feitosa, C.M.; Citó, A.M.; Melo Cavalcante, A.A.; Freitas, R.M.; Moura Sperotto, A.R.; et al. Investigation of biological activities of dichloromethane and ethyl acetate fractions of Platonia insignis Mart. seed. Basic Clin. Pharmacol. Toxicol. 2013, 112, 34–41. [Google Scholar] [CrossRef]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- de Freitas, F.A.; Araújo, R.C.; Soares, E.R.; Nunomura, R.C.S.; Silva, F.M.A.; Silva, S.R.S.; Souza, A.Q.L.; Souza, A.D.L.; Franco-Montalbán, F.; Acho, L.D.R.; et al. Biological evaluation and quantitative analysis of antioxidant compounds in pulps of the Amazonian fruits bacuri (Platonia insignis Mart.), ingá (Inga edulis Mart.), and uchi (Sacoglottis uchi Huber) by UHPLC-ESI-MS/MS. J. Food Biochem. 2018, 42, e12455. [Google Scholar] [CrossRef]
- Coêlho, E.D.S.; Lopes, G.L.N.; Pinheiro, I.M.; Holanda, J.N.P.; Alves, M.M.M.; Carvalho Nogueira, N.; Carvalho, F.A.A.; Carvalho, A.L.M. Emulgel based on amphotericin B and bacuri butter (Platonia insignis Mart.) for the treatment of cutaneous leishmaniasis: Characterization and in vitro assays. Drug Dev. Ind. Pharm. 2018, 44, 1713–1723. [Google Scholar] [CrossRef]
- da Silva, A.P.D.S.C.L.; Lopes, J.S.L.; Vieira, P.D.S.; Pinheiro, E.E.; da Silva, M.L.; Silva Filho, J.C.; da Costa, J.S., Jr.; David, J.M.; de Freitas, R. Behavioral and neurochemical studies in mice pretreated with garcinielliptone FC in pilocarpine-induced seizures. Pharmacol. Biochem. Behav. 2014, 124, 305–310. [Google Scholar] [CrossRef]
- Lustosa, A.K.M.F.; Arcanjo, D.D.R.; Ribeiro, R.G.; Rodrigues, K.A.F.; Passos, F.F.B.; Piauilino, C.A.; Silva-Filho, J.C.; Araújo, B.Q.; Lima-Neto, J.S.; Costa-Júnior, J.S.; et al. Immunomodulatory and toxicological evaluation of the fruit seeds from Platonia insignis, a native species from Brazilian Amazon Rainforest. Rev. Bras. Farmacogn. 2016, 26, 77–82. [Google Scholar] [CrossRef]
- Mei, B.; Chen, J.; Yang, N.; Peng, Y. The regulatory mechanism and biological significance of the Snail-miR590-VEGFR-NRP1 axis in the angiogenesis, growth and metastasis of gastric cancer. Cell Death Dis. 2020, 11, 241. [Google Scholar] [CrossRef]
- Kong, H.; You, N.; Chen, H.; Teng, Y.S.; Liu, Y.G.; Lv, Y.P.; Mao, F.Y.; Cheng, P.; Chen, W.; Zhao, Z.; et al. Helicobacter pylori-induced adrenomedullin modulates IFN-γ-producing T-cell responses and contributes to gastritis. Cell Death Dis. 2020, 11, 189. [Google Scholar] [CrossRef]
- Kalange, M.; Nansunga, M.; Kasozi, K.I.; Kasolo, J.; Namulema, J.; Atusiimirwe, J.K.; Ayikobua, E.T.; Ssempijja, F.; Munanura, E.I.; Matama, K.; et al. Antimalarial combination therapies increase gastric ulcers through an imbalance of basic antioxidative-oxidative enzymes in male Wistar rats. BMC Res. Notes 2020, 13, 230. [Google Scholar]
- Ahmed, O.A.A.; Fahmy, U.A.; Bakhaidar, R.; El-Moselhy, M.A.; Alfaleh, M.A.; Ahmed, A.F.; Hammad, A.S.A.; Aldawsari, H.; Alhakamy, N.A. Pumpkin Oil-Based Nanostructured Lipid Carrier System for Antiulcer Effect in NSAID-Induced Gastric Ulcer Model in Rats. Int. J. Nanomed. 2020, 15, 2529–2539. [Google Scholar] [CrossRef]
- Abed, M.N.; Alassaf, F.A.; Jasim, M.H.M.; Alfahad, M.; Qazzaz, M.E. Comparison of Antioxidant Effects of the Proton Pump-Inhibiting Drugs Omeprazole, Esomeprazole, Lansoprazole, Pantoprazole, and Rabeprazole. Pharmacology 2020, 105, 645–651. [Google Scholar] [CrossRef]
- Azmi, L.; Shukla, I.; Goutam, A.; Rao, C.V.; Jawaid, T.; Awaad, A.S.; Alqasoumi, S.I.; AlKhamees, O.A.; Kamal, M. Oxidative free radicals scavenging activity (in vitro and in vivo assay) of standardized fractions from the seeds of Argyreia speciosa (Ghav-patta) a traditional Indian medicine. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2019, 27, 1210–1215. [Google Scholar] [CrossRef]
- Sokolova, O.; Naumann, M. Crosstalk Between DNA Damage and Inflammation in the Multiple Steps of Gastric Carcinogenesis. Curr. Top. Microbiol. Immunol. 2019, 421, 107–137. [Google Scholar]
- Yanaka, A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef]
- Carneiro, J.G.; Holanda, T.D.B.L.; Quinderé, A.L.G.; Frota, A.F.; Soares, V.V.M.; Sousa, R.S.; Carneiro, M.A.; Martins, D.S.; Gomes Duarte, A.S.; Benevides, N.M.B. Gastroprotective Effects of Sulphated Polysaccharides from the Alga Caulerpa mexicana Reducing Ethanol-Induced Gastric Damage. Pharmaceuticals 2018, 11, 6. [Google Scholar] [CrossRef]
- Mileva, M.; Dimitrova, A.; Krastev, D.; Alexandrova, A.; Tsvetanova, E.; Georgieva, A.; Galabov, A. Oseltamivir and S-Adenosyl-L-Methionine Combination as Effective Therapeutic Strategy for Suppression of Oxidative Damage in Lung Caused by Influenza Virus Infection in Mice. Drug Res. 2020, 70, 273–279. [Google Scholar] [CrossRef]
- Queiroz, J.L.C.; Medeiros, I.; Piuvezam, G.; de França Nunes, A.C.; Gomes, C.C.; Maciel, B.L.L.; de Araújo Morais, A.H.; Passos, T.S. Effect of carotenoid encapsulation on antioxidant activities: A protocol for systematic review. Medicine 2020, 99, e19772. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q.; Lv, H.; Chen, W.; Wang, L.; Shi, S.; Yang, J.; Zhao, P.; Li, Y.; Christopher, R.; et al. Toxicity of different forms of antimony to rice plants: Effects on reactive oxidative species production, antioxidative systems, and uptake of essential elements. Environ. Pollut. 2020, 263, 114544. [Google Scholar] [CrossRef]
- Durazzo, A.; Lombardi-Boccia, G.; Santini, A.; Lucarini, M. Dietary Antioxidants and Metabolic Diseases. Int. J. Mol. Sci. 2021, 22, 12558. [Google Scholar] [CrossRef]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell. Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Patlevič, P.; Vašková, J.; Švorc, P., Jr.; Vaško, L.; Švorc, P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr. Med. Res. 2016, 5, 250–258. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef]
- Moorcroft, S.C.T.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Evans, S.D. Nanoparticle loaded hydrogel for the light-activated release and photothermal enhancement of antimicrobial peptides. ACS Appl. Mater. Interfaces 2020, 12, 24544–24554. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; Urru, M.; Chiarugi, A.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced dissolution, permeation and oral bioavailability of aripiprazole mixed micelles: In vitro and in vivo evaluation. Int. J. Pharm. 2020, 583, 119361. [Google Scholar] [CrossRef] [PubMed]
- Alves Batista, F.; Brena Cunha Fontele, S.; Beserra Santos, L.K.; Filgueiras, L.A.; Nascimento, S.Q.; Sousa, J.M.C.; Gonçalves, J.C.R.; Mendes, A.N. Synthesis, characterization of α-terpineol-loaded PMMA nanoparticles as proposed of therapy for melanoma. Mater. Today Commun. 2020, 22, 100762. [Google Scholar] [CrossRef]
- Mendes, A.N.; Filgueiras, L.A.; Siqueira, M.R.P.; Barbosa, G.M.; Holandino, C.; de Lima Moreira, D.; Pinto, J.C.; Nele, M. Encapsulation of Piper cabralanum (Piperaceae) nonpolar extract in poly(methyl methacrylate) by miniemulsion and evaluation of increase in the effectiveness of antileukemic activity in K562 cells. Int. J. Nanomed. 2017, 12, 8363–8373. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I-Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II-Production Scales and Clinically Compliant Production Methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Souto, E.B.; Durazzo, A.; Lucarini, M.; Novellino, E.; Tewari, D.; Wang, D.; Atanasov, A.G.; Santini, A. Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol. 2020, 2, 53–63. [Google Scholar] [CrossRef]
- Arcanjo, D.D.R.; da Costa-Júnior, J.S.; Moura, L.H.P.; Ferraz, A.B.; Rossatto, R.R.; David, J.M.; Quintans-Júnior, L.J.; Oliveira, R.C.M.; Citó, A.M.G.L.; Oliveira, A.P. Garcinielliptone FC, a polyisoprenylated benzophenone from Platonia insignis Mart., promotes vasorelaxant effect on rat mesenteric artery. Nat. Prod. Res. 2014, 28, 923–927. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 207–212. [Google Scholar]
- Coelho, L.N. Estudo da Inclusão de Filtros Solares em Ciclodextrinas e suas Aplicações em Dermocosmética. Master’s Thesis, Faculdade de Farmácia do Rio de Janeiro, Rio de Janeiro, Brazil, 2001. [Google Scholar]
- Costa Júnior, J.S.D.; Ferraz, A.D.B.F.; Feitosa, C.M.; Citó, A.M.D.G.L.; Saffi, J.; Freitas, R.M.D. Evaluation of effects of dichloromethane fraction from Platonia insignis on pilocarpine-induced seizures. Rev. Bras. Farmacogn. 2011, 21, 1104–1110. [Google Scholar] [CrossRef][Green Version]
- Weng, J.-R.; Tsao, L.-T.; Wang, J.-P.; Wu, R.R.; Lin, C.N. Anti-inflammatory phloroglucinols and terpenoids from Garcinia subelliptica. J. Nat. Prod. 2004, 67, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-R.; Lin, C.-N.; Tsao, L.-T.; Wang, J.P. Novel and anti-inflammatory constituents of Garcinia subelliptica. Chemistry 2003, 9, 1958–1963. [Google Scholar] [CrossRef]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Hanchar, A.J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 1979, 77, 433–443. [Google Scholar] [CrossRef]
- Viana, A.F.S.C.; Lopes, M.T.P.; Oliveira, F.T.B.; Nunes, P.I.G.; Santos, V.G.; Braga, A.D.; Silva, A.C.A.; Sousa, D.P.; Viana, D.A.; Rao, V.S.; et al. (−)-Myrtenol accelerates healing of acetic acid-induced gastric ulcers in rats and in human gastric adenocarcinoma cells. Eur. J. Pharmacol. 2019, 854, 139–148. [Google Scholar] [CrossRef]
- Ueda, S.; Okada, Y. Acid secretagogues induce Ca2+ mobilization coupled to K+ conductance activation in rat parietal cells in tissue culture. Biochim. Biophys. Acta 1989, 1012, 254–260. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Geng, Q.; Li, T.; Wang, X.; Chu, W.; Cai, M.; Xie, J.; Ni, H. The mechanism of bensulfuron-methyl complexation with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin and effect on soil adsorption and bio-activity. Sci. Rep. 2019, 9, 1882. [Google Scholar] [CrossRef]
- Chen, X.; Taguchi, T. Injectable inclusion complex composed of α-cyclodextrin and hydrophobically modified poly(vinyl alcohol) as a cerebral aneurysm embolization material. Polym. J. 2020, 52, 793–802. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, É.; Sohajda, T. Cyclodextrin-enabled green environmental biotechnologies. Environ. Sci. Pollut. Res. Int. 2022, 29, 20085–20097. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef] [PubMed]
- Prodea, A.; Mioc, A.; Banciu, C.; Trandafirescu, C.; Milan, A.; Racoviceanu, R.; Ghiulai, R.; Mioc, M.; Soica, C. The Role of Cyclodextrins in the Design and Development of Triterpene-Based Therapeutic Agents. Int. J. Mol. Sci. 2022, 23, 736. [Google Scholar] [CrossRef] [PubMed]
- Bouhadiba, A.; Rahali, S.; Belhocine, Y.; Allal, H.; Nouar, L.; Rahim, M. Structural and energetic investigation on the host/guest inclusion process of benzyl isothiocyanate into β-cyclodextrin using dispersion-corrected DFT calculations. Carbohydr. Res. 2020, 491, 107980. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Takahashi, C.; Yamamoto, H. Physicochemical characterization of cyclodextrin-drug interactions in the solid state and the effect of water on these interactions. J. Pharm. Sci. 2015, 104, 942–954. [Google Scholar] [CrossRef]
- Aytac, Z.; Ipek, S.; Erol, I.; Durgun, E.; Uyar, T. Fast-dissolving electrospun gelatin nanofibers encapsulating ciprofloxacin/cyclodextrin inclusion complex. Colloids Surf. B Biointerfaces 2019, 178, 129–136. [Google Scholar] [CrossRef]
- de Miranda, J.C.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef]
- Banjare, M.K.; Behera, K.; Banjare, R.K.; Pandey, S.; Ghosh, K.K. Inclusion complexation of imidazolium-based ionic liquid and β-cyclodextrin: A detailed spectroscopic investigation. J. Mol. Liq. 2020, 302, 112530. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.-Y.; Liu, Y.-Y.; Fu, Y.; Zhao, L.X.; Li, C.Y.; Ye, F. Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin. Polymers 2019, 11, 1396. [Google Scholar] [CrossRef]
- Jansook, P.; Prajapati, M.; Pruksakorn, P.; Loftsson, T. Antifungal activity of econazole nitrate/cyclodextrin complex: Effect of pH and formation of complex aggregates. Int. J. Pharm. 2020, 574, 118896. [Google Scholar] [CrossRef]
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as solubilizers: Formation of complex aggregates. J. Pharm. Sci. 2010, 99, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.K.; Gupta, S.; Rai, S.; Shrivatava, A.; Tripathi, S.; Singh, S.; Khopade, A.J.; Kesharwani, P. Curcumin loaded poly (amidoamine) dendrimer-plamitic acid core-shell nanoparticles as anti-stress therapeutics. Drug Dev. Ind. Pharm. 2020, 46, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yang, X.; Watson, P.; Yang, F.; Liu, H. A cyclodextrin polymer membrane-based passive sampler for measuring triclocarban, triclosan and methyl triclosan in rivers. Sci. Total Environ. 2019, 648, 109–115. [Google Scholar] [CrossRef]
- Rama, A.C.R.; Veiga, F.; Figueiredo, I.V.; Sousa, A.; Caramona, M. Aspectos biofarmacêuticos da formulação de medicamentos para neonatos: Fundamentos da complexação de indometacina com hidroxipropil-b-ciclodextrina para tratamento oral do fechamento do canal arterial. Rev. Bras. Ciências Farm. 2005, 41, 281–299. [Google Scholar] [CrossRef][Green Version]
- Nascimento, J.L.; Coêlho, A.G.; Barros, Y.S.O.; Silva, O.A.; Freitas, R.M.; Rocha, M.S.; David, J.M.; Costa Júnior, J.S.; Arcanjo, D.D.R.; Oliveira, R.C.M.; et al. Avaliação da atividade antioxidante in vitro do extrato hexânico da semente do bacuri (Platonia insignis Mart.) e de seu complexo de inclusão com β-ciclodextrina. Bol. Inf. Geum 2014, 5, 44–53. [Google Scholar]
- Bezamat, J.M.; Yokaichiya, F.; Dias Franco, M.K.K.; Castro, S.R.; Paula, E.; Cabeça, L.F. Complexation of the local anesthetic pramoxine with hydroxypropyl-beta-cyclodextrin can improve its bioavailability. J. Drug Deliv. Sci. Technol. 2020, 55, 101475. [Google Scholar] [CrossRef]
- Praphanwittaya, P.; Saokham, P.; Jansook, P.; Loftsson, T. Aqueous solubility of kinase inhibitors: II the effect of hexadimethrine bromide on the dovitinib/γ-cyclodextrin complexation. J. Drug Deliv. Sci. Technol. 2020, 55, 101463. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Z.; Han, L.; Li, S.; Zhou, W. Preparation and characterization of phloretin by complexation with cyclodextrins. New J. Chem. 2020, 44, 5218–5223. [Google Scholar] [CrossRef]
- Adeoye, O.; Conceição, J.; Serra, P.A.; Bento da Silva, A.; Duarte, N.; Guedes, R.C.; Corvo, M.C.; Aguiar-Ricardo, A.; Jicsinszky, L.; Casimiro, T.; et al. Cyclodextrin solubilization and complexation of antiretroviral drug lopinavir: In silico prediction; Effects of derivatization, molar ratio and preparation method. Carbohydr. Polym. 2020, 227, 115287. [Google Scholar] [CrossRef]
- Somer, A.; Roik, J.R.; Ribeiro, M.A.; Urban, A.M.; Schoeffel, A.; Urban, V.M.; Farago, F.V.; Castro, L.V.; Sato, F.; Jacinto, C.; et al. Nystatin complexation with β-cyclodextrin: Spectroscopic evaluation of inclusion by FT-Raman, photoacoustic spectroscopy, and 1H NMR. Mater. Chem. Phys. 2020, 239, 122117. [Google Scholar] [CrossRef]
- Ghosh, R.; Roy, K.; Subba, A.; Mandal, P.; Basak, S.; Kundu, M.; Roy, M.N. Case to case study for exploring inclusion complexes of an anti-diabetic alkaloid with α and β cyclodextrin molecules for sustained dischargement. J. Mol. Struct. 2020, 1200, 126988. [Google Scholar] [CrossRef]
- Tom, L.; Nirmal, C.R.; Dusthackeer, A.; Magizhaveni, B.; Kurup, M.R.P. Formulation and evaluation of β-cyclodextrin-mediated inclusion complexes of isoniazid scaffolds: Molecular docking and in vitro assessment of antitubercular properties. New J. Chem. 2020, 44, 4467–4477. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Hydrocortisone/cyclodextrin complex electrospun nanofibers for a fast-dissolving oral drug delivery system. RSC Med. Chem. 2020, 11, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, B.; Guan, J.; Jiang, Z.; Guo, X. Preparation of sulfobutylether β-cyclodextrin-silica hybrid monolithic column, and its application to capillary electrochromatography of chiral compounds. J. Chromatogr. A 2020, 1620, 460932. [Google Scholar] [CrossRef]
- Banjare, M.K.; Banjare, R.K.; Behera, K.; Pandey, S.; Mundeja, P.; Ghosh, K.K. Inclusion complexation of novel synthesis amino acid based ionic liquids with β-cyclodextrin. J. Mol. Liq. 2020, 299, 112204. [Google Scholar] [CrossRef]
- Yap, K.Y.L.; Chan, S.Y.; Lim, C.S. Infrared-based protocol for the identification and categorisation of ginseng and its products. Food Res. Int. 2007, 40, 643–652. [Google Scholar] [CrossRef]
- Singh, G.; Singh, P.K. Complexation of a cationic pyrene derivative with sulfobutylether substituted β-cyclodextrin: Towards a stimulus-responsive supramolecular material. J. Mol. Liq. 2020, 305, 112840. [Google Scholar] [CrossRef]
- Simsek, T.; Simsek, S.; Mayer, C.; Rasulev, B. Combined computational and experimental study on the inclusion complexes of β-cyclodextrin with selected food phenolic compounds. Struct. Chem. 2019, 30, 1395–1406. [Google Scholar] [CrossRef]
- Paulino, P.H.S.; de Sousa, S.M.R.; Da Silva, H.C.; De Almeida, W.B.; Ferrari, J.L.; Guimarães, L.; Nascimento Júnior, C.S. A theoretical investigation on the encapsulation process of mepivacaine into β-cyclodextrin. Chem. Phys. Lett. 2020, 740, 137060. [Google Scholar] [CrossRef]
- Bouchemela, H.; Madi, F.; Nouar, L. DFT investigation of host–guest interactions between α-Terpineol and β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 247–258. [Google Scholar] [CrossRef]
- Yue, L.; Li, J.; Jin, W.; Zhao, M.; Xie, P.; Chi, S.; Lei, Z.; Zhu, H.; Zhao, Y. Host–guest interaction between 20(S)-protopanaxatriol and three polyamine-modified β-cyclodextrins: Preparation, characterization, inclusion modes, and solubilization. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 29–42. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zhang, S.; Jiang, Q.; Shang, J.; Zhou, L.; Li, Q.; Li, S.; Shi, S.; Li, Y.; et al. Maltosyl-β-cyclodextrin mediated Supramolecular Host-Guest inclusion complex used for enhancing baicalin antioxidant activity and bioavailability. J. Drug Deliv. Sci. Technol. 2019, 54, 101346. [Google Scholar] [CrossRef]
- Qiu, N.; Zhao, X.; Liu, Q.; Shen, B.; Liu, J.; Li, X.; An, L. Inclusion complex of emodin with hydroxypropyl-β-cyclodextrin: Preparation, physicochemical and biological properties. J. Mol. Liq. 2019, 289, 111151. [Google Scholar] [CrossRef]
- Osaki, M.; Takashima, Y.; Kamitori, S.; Yamaguchi, H.; Harada, A. X-ray crystal structures of α-cyclodextrin–5-hydroxypentanoic acid, β-cyclodextrin–5-hydroxypentanoic acid, β-cyclodextrin–ε-caprolactone, and β-cyclodextrin–ε-caprolactam inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 93–99. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Ji, Q.; Fu, Y.; Zhao, L.; Li, C.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.; Li, X.; Liu, Y.; Zhao, L.; Fu, Y.; Ye, F. Enhanced physicochemical properties and herbicidal activity of an environment-friendly clathrate formed by β-cyclodextrin and herbicide cyanazine. J. Mol. Liq. 2020, 305, 112858. [Google Scholar] [CrossRef]
- Aree, T. β-Cyclodextrin encapsulation of nortriptyline HCl and amitriptyline HCl: Molecular insights from single-crystal X-ray diffraction and DFT calculation. Int. J. Pharm. 2020, 575, 118899. [Google Scholar] [CrossRef]
- Tambe, A.; Pandita, N.; Kharkar, P.; Niteshkumar, S. Encapsulation of boswellic acid with β- and hydroxypropyl-β-cyclodextrin: Synthesis, characterization, in vitro drug release and molecular modelling studies. J. Mol. Struct. 2018, 1154, 504–510. [Google Scholar] [CrossRef]
- Mura, P.; Bettinetti, G.P.; Cirri, M.; Maestrelli, F.; Sorrenti, M.; Catenacci, L. Solid-state characterization and dissolution properties of Naproxen–Arginine–Hydroxypropyl-β-cyclodextrin ternary system. Eur. J. Pharm. Biopharm. 2005, 59, 99–106. [Google Scholar] [CrossRef]
- Toropainen, T.; Velaga, S.; Heikkilä, T.; Matilainen, L.; Jarho, P.; Carlfors, J.; Lehto, V.P.; Järvinen, T.; Järvinen, K. Preparation of budesonide/γ-cyclodextrin complexes in supercritical fluids with a novel SEDS method. J. Pharm. Sci. 2006, 95, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Corti, G.; Capasso, G.; Maestrelli, F.; Cirri, M.; Mura, P. Physical–chemical characterization of binary systems of metformin hydrochloride with triacetyl-β-cyclodextrin. J. Pharm. Biomed. Anal. 2007, 45, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.M.; Martins, J.L.R.; de Oliveira, D.R.; Florentino, I.F.; da Silva, D.P.B.; Dos Santos, F.C.A.; Costa, E.A. Effect of allantoin on experimentally induced gastric ulcers: Pathways of gastroprotection. Eur. J. Pharmacol. 2018, 821, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; de Freitas, R.B.; de Brum, T.F.; Waczuk, E.P.; Klimaczewski, C.V.; de Ávila, D.S.; Athayde, M.L.; de Freitas Bauermann, L. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm. Sin. B 2014, 4, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; Portella, R.L.; da Silva, M.H.; Lugokenski, T.H.; Dias, G.R.; da Luz, S.C.; Boligon, A.A.; et al. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef]
- de Barros, M.P.; Lemos, M.; Maistro, E.L.; Leite, M.F.; Sousa, J.P.; Bastos, J.K.; Andrade, S.F. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J. Ethnopharmacol. 2008, 120, 372–377. [Google Scholar] [CrossRef]
- Santos, A.C.V.; Fernandes, C.C.; Lopes, L.M.; Sousa, A.H. Use of plant oils from the southwestern Amazon for the control of maize weevil. J Stored Prod Res 2015, 63, 67–70. [Google Scholar] [CrossRef]
- Da Silva, B.J.M.; Hage, A.A.P.; Silva, E.O.; Rodrigues, A.D.P. Medicinal plants from the Brazilian Amazonian region and their antileishmanial activity: A review. J. Integr. Med. 2018, 16, 211–222. [Google Scholar] [CrossRef]
- Costa, J.S.; de Almeida, A.A.C.; Tomé, A.D.R.; Citó, A.M.G.L.; Saffi, J.; Freitas, R.M. Evaluation of possible antioxidant and anticonvulsant effects of the ethyl acetate fraction from Platonia insignis Mart. (Bacuri) on epilepsy models. Epilepsy Behav. 2011, 22, 678–684. [Google Scholar] [CrossRef]
- Moonens, K.; Gideonsson, P.; Subedi, S.; Bugaytsova, J.; Romaõ, E.; Mendez, M.; Nordén, J.; Fallah, M.; Rakhimova, L.; Shevtsova, A.; et al. Structural Insights into Polymorphic ABO Glycan Binding by Helicobacter pylori. Cell Host Microbe 2016, 19, 55–66. [Google Scholar] [CrossRef]
- Jaccob, A.A. Protective effect of N-acetylcysteine against ethanol-induced gastric ulcer: A pharmacological assessment in mice. J. Intercult. Ethnopharmacol. 2015, 4, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.A.; Zineldeen, D.H.; Katary, M.A.; Ali, D.A. N-acetylcysteine a possible protector against indomethacin-induced peptic ulcer: Crosstalk between antioxidant, anti-inflammatory, and antiapoptotic mechanisms. Can. J. Physiol. Pharmacol. 2017, 95, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Hegab, I.I.; Abd-Ellatif, R.N.; Sadek, M.T. The gastroprotective effect of N-acetylcysteine and genistein in indomethacin-induced gastric injury in rats. Can. J. Physiol. Pharmacol. 2018, 96, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, J.; Motilva, V.; Martín, M.J.; de La Lastra, C.A. Mechanisms involved in gastric protection of melatonin against oxidant stress by ischemia-reperfusion in rats. Life Sci. 2001, 68, 1405–1415. [Google Scholar] [CrossRef]
- Cássia Dos Santos, R.; Bonamin, F.; Périco, L.L.; Rodrigues, V.P.; Zanatta, A.C.; Rodrigues, C.M.; Sannomiya, M.; Dos Santos Ramos, M.A.; Bonifácio, B.V.; Bauab, T.M.; et al. Byrsonima intermedia A. Juss partitions promote gastroprotection against peptic ulcers and improve healing through antioxidant and anti-inflammatory activities. Biomed. Pharmacother. 2019, 111, 1112–1123. [Google Scholar] [CrossRef]
- Magierowski, M.; Magierowska, K.; Hubalewska-Mazgaj, M.; Sliwowski, Z.; Pajdo, R.; Ginter, G.; Kwiecien, S.; Brzozowski, T. Exogenous and Endogenous Hydrogen Sulfide Protects Gastric Mucosa against the Formation and Time-Dependent Development of Ischemia/Reperfusion-Induced Acute Lesions Progressing into Deeper Ulcerations. Molecules 2017, 22, 295. [Google Scholar] [CrossRef]
- Fagundes, F.L.; de Morais Piffer, G.; Périco, L.L.; Rodrigues, V.P.; Hiruma-Lima, C.A.; Dos Santos, R.C. Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing. Int. J. Mol. Sci. 2020, 21, 760. [Google Scholar] [CrossRef]
- Milano, S.P.; Boucheix, O.B.; Reinheimer, T.M. Selepressin, a novel selective V(1A) receptor agonist: Effect on mesenteric flow and gastric mucosa perfusion in the endotoxemic rabbit. Peptides 2020, 129, 170318. [Google Scholar] [CrossRef]
- Fanjul, C.; Barrenetxe, J.; De Pablo-Maiso, L.; Lostao, M.P. In vivo regulation of intestinal absorption of amino acids by leptin. J. Endocrinol. 2015, 224, 17–23. [Google Scholar] [CrossRef][Green Version]
- Menguy, R. Role of gastric mucosal energy metabolism in the etiology of stress ulceration. World J. Surg. 1981, 5, 175–180. [Google Scholar] [CrossRef]
- Andrews, F.J.; Malcontenti-Wilson, C.; O’Brien, P.E. Polymorphonuclear leukocyte infiltration into gastric mucosa after ischemia-reperfusion. Am. J. Physiol. 1994, 266, G48–G54. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Kamisaki, Y.; Kitano, M.; Kishimoto, Y.; Nakamoto, K.; Itoh, T. A new gastric ulcer model induced by ischemia-reperfusion in the rat: Role of leukocytes on ulceration in rat stomach. Life Sci. 1996, 59, PL295–PL301. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Bernsand, M.; Kishimoto, Y.; Norlén, P.; Håkanson, R.; Haenuki, Y.; Kudo, M.; Hasegawa, J. Ischemia of rat stomach mobilizes ECL cell histamine. Am. J. Physiol. Gastrointest. Liver. Physiol. 2005, 288, G1084–G1090. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aboul Naser, A.; Younis, E.; El-Feky, A.; Elbatanony, M.; Hamed, M. Management of Citrus sinensis peels for protection and treatment against gastric ulcer induced by ethanol in rats. Biomarkers 2020, 25, 349–359. [Google Scholar] [CrossRef]
- Ahmed, I.; Elkablawy, M.A.; El-Agamy, D.S.; Bazarbay, A.A.; Ahmed, N. Carvedilol safeguards against aspirin-induced gastric damage in rats. Hum. Exp. Toxicol. 2020, 39, 1257–1267. [Google Scholar] [CrossRef]
- Doğan, G.; İpek, H. The protective effect of Ganoderma lucidum on testicular torsion/detorsion-induced ischemia-reperfusion (I/R) injury. Acta Cir. Bras. 2020, 35, e202000103. [Google Scholar] [CrossRef]
- Liu, R.; Hao, Y.-T.; Zhu, N.; Liu, X.R.; Kang, J.W.; Mao, R.X.; Hou, C.; Li, Y. The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients 2020, 12, 1138. [Google Scholar] [CrossRef]


| Peak | Constituents | Tr (min) | Area (%) |
|---|---|---|---|
| 1 | Methyl ester of palmitoleic acid C16:1 cis 9 | 19.131 | 0.44 |
| 2 | Palmitic acid methyl ester C16:0 | 19.934 | 4.66 |
| 3 | Palmitic acid ethyl ester C16:0 | 22.033 | 0.42 |
| 4 | Linolenic acid methyl ester C18:3 cis, cis, cis 9,12,15 | 24.596 | 0.13 |
| 5 | Linoleic acid methyl ester C18:2 cis, cis 9.12 | 24.747 | 0.17 |
| 6 | Oleic acid methyl ester C18:1 cis 9 | 24.93 | 3.57 |
| 8 | Stearic acid methyl ester C18:0 | 25.647 | 0.49 |
| 9 | Gadoleic acid methyl ester C20:1 cis 9 | 26.599 | 0.18 |
| 10 | Squalene | 39.48 | 0.52 |
| 11 | GFC (garcinielliptone FC) | 41.037 | 0.50 |
| 12 | Prenylated benzophenone (γ-mangostine) | 43.754 | 77.35 |
| 13 | Campesterol | 46.122 | 0.74 |
| 14 | Stigmasterol | 46.506 | 1.14 |
| 15 | Sitosterol | 47.447 | 1.06 |
| 16 | Lanosterol | 47.93 | 3.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima Nascimento, J.; Coelho, A.G.; Oliveira Barros, Y.S.; Sousa Oliveira, I.; Vieira da Silva, F.; Custódio Viana, A.F.S.; Araújo, B.Q.; dos Santos Rocha, M.; das Chagas Pereira de Andrade, F.; de Oliveira Barbosa, C.; et al. Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System. Appl. Sci. 2023, 13, 58. https://doi.org/10.3390/app13010058
Lima Nascimento J, Coelho AG, Oliveira Barros YS, Sousa Oliveira I, Vieira da Silva F, Custódio Viana AFS, Araújo BQ, dos Santos Rocha M, das Chagas Pereira de Andrade F, de Oliveira Barbosa C, et al. Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System. Applied Sciences. 2023; 13(1):58. https://doi.org/10.3390/app13010058
Chicago/Turabian StyleLima Nascimento, Juliana, Angélica Gomes Coelho, Ytallo Samuel Oliveira Barros, Irisdalva Sousa Oliveira, Francilene Vieira da Silva, Ana Flávia Seraine Custódio Viana, Bruno Quirino Araújo, Márcio dos Santos Rocha, Francisco das Chagas Pereira de Andrade, Celma de Oliveira Barbosa, and et al. 2023. "Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System" Applied Sciences 13, no. 1: 58. https://doi.org/10.3390/app13010058
APA StyleLima Nascimento, J., Coelho, A. G., Oliveira Barros, Y. S., Sousa Oliveira, I., Vieira da Silva, F., Custódio Viana, A. F. S., Araújo, B. Q., dos Santos Rocha, M., das Chagas Pereira de Andrade, F., de Oliveira Barbosa, C., de Barros Fernandes, H., Mendes, A. N., da Costa-Júnior, J. S., Oliveira, R. d. C. M., Lucarini, M., Durazzo, A., Arcanjo, D. D. R., & Citó, A. M. d. G. L. (2023). Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System. Applied Sciences, 13(1), 58. https://doi.org/10.3390/app13010058









