Different Drying Techniques Can Affect the Adsorption Properties of Agarose-Based Gels for Crystal Violet Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
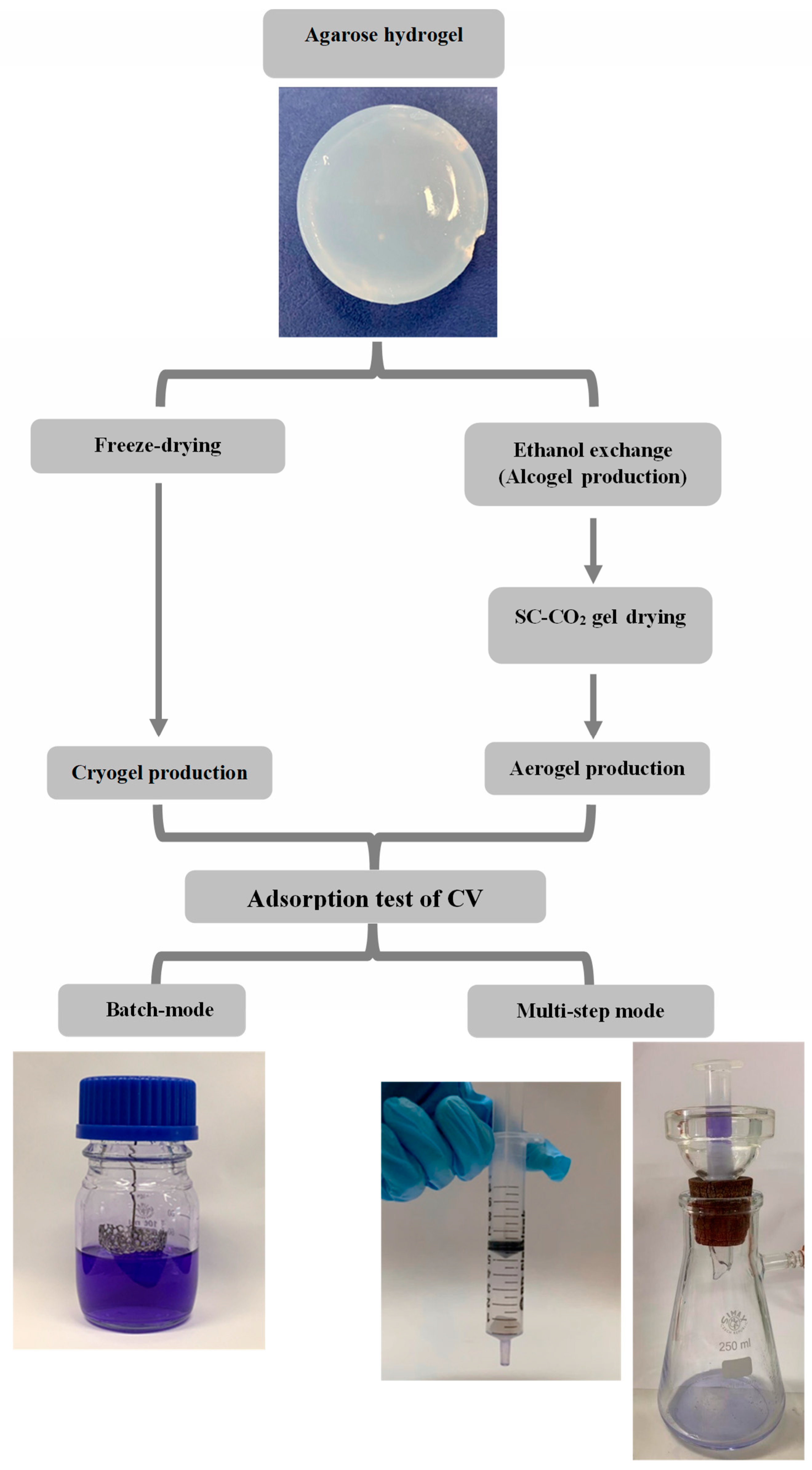
2.2. Hydrogel Preparation
2.3. Cryogel Preparation
2.4. Aerogel Preparation
2.5. Morphology and Physical Properties Analysis
2.6. Adsorption Tests
2.6.1. Adsorption Tests in Batch-Mode
2.6.2. Adsorption Tests in Multi-Step Mode
2.7. Desorption Test
3. Results and Discussion
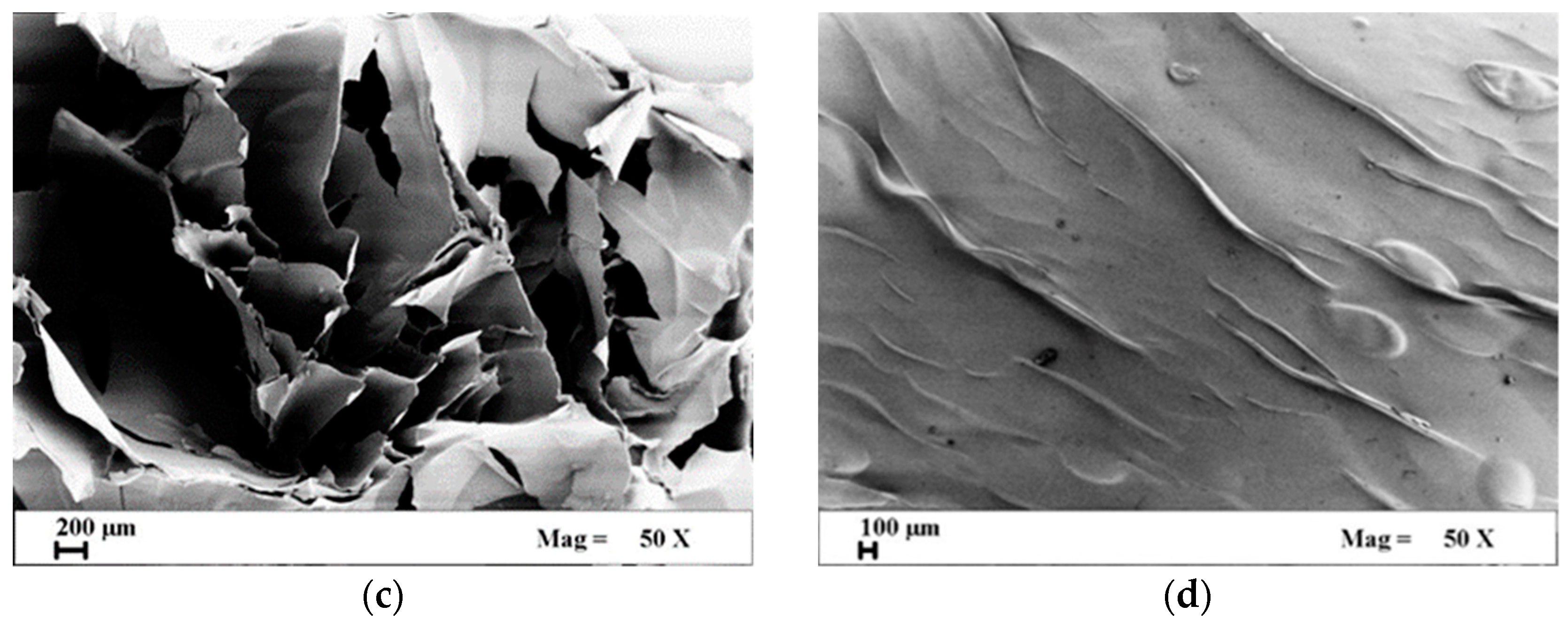
3.1. Porosity and Surface Area Measurement
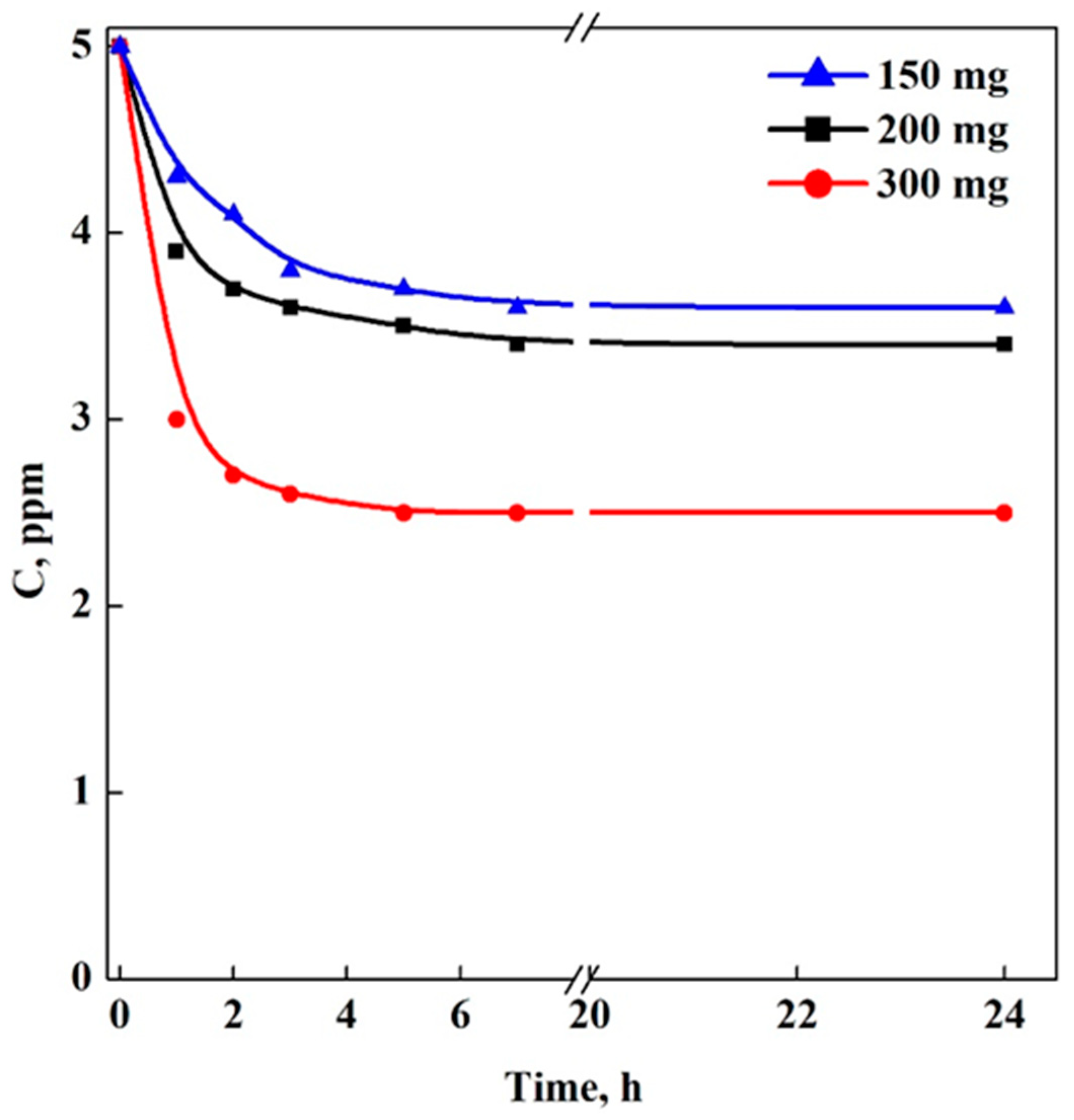
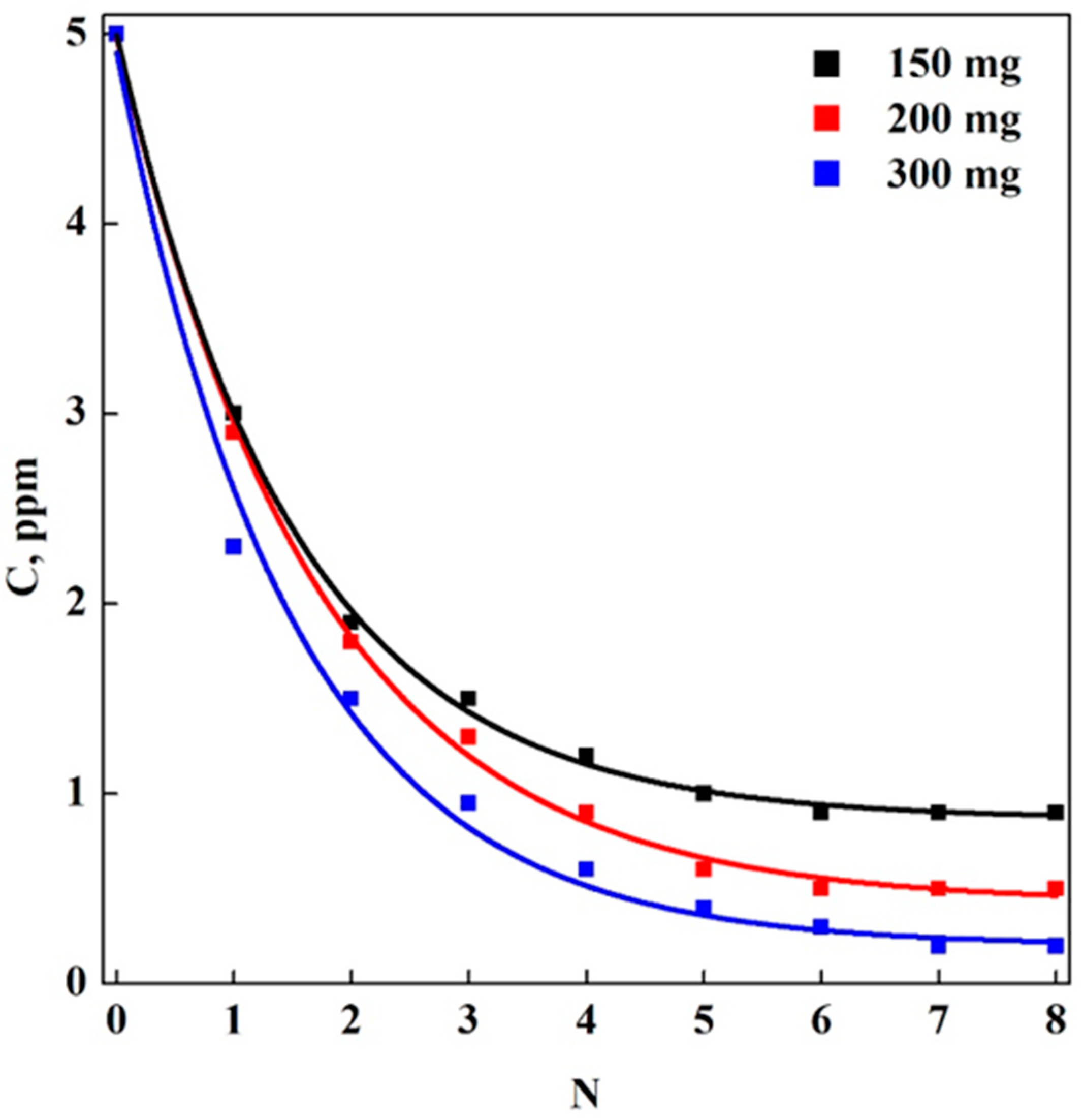
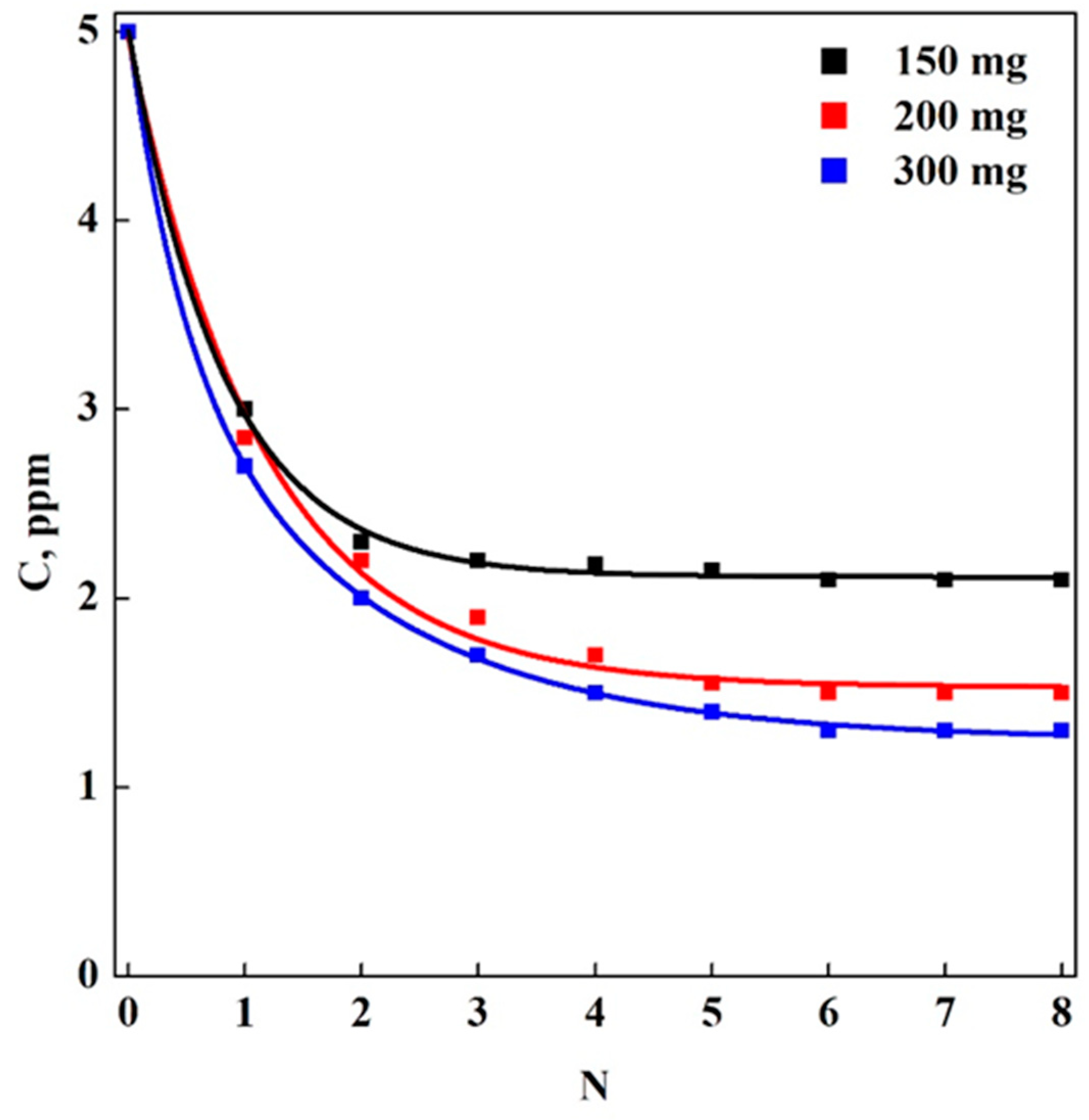
3.2. Adsorption Tests in Batch-Mode: Aerogels (A) vs. Cryogels (C)
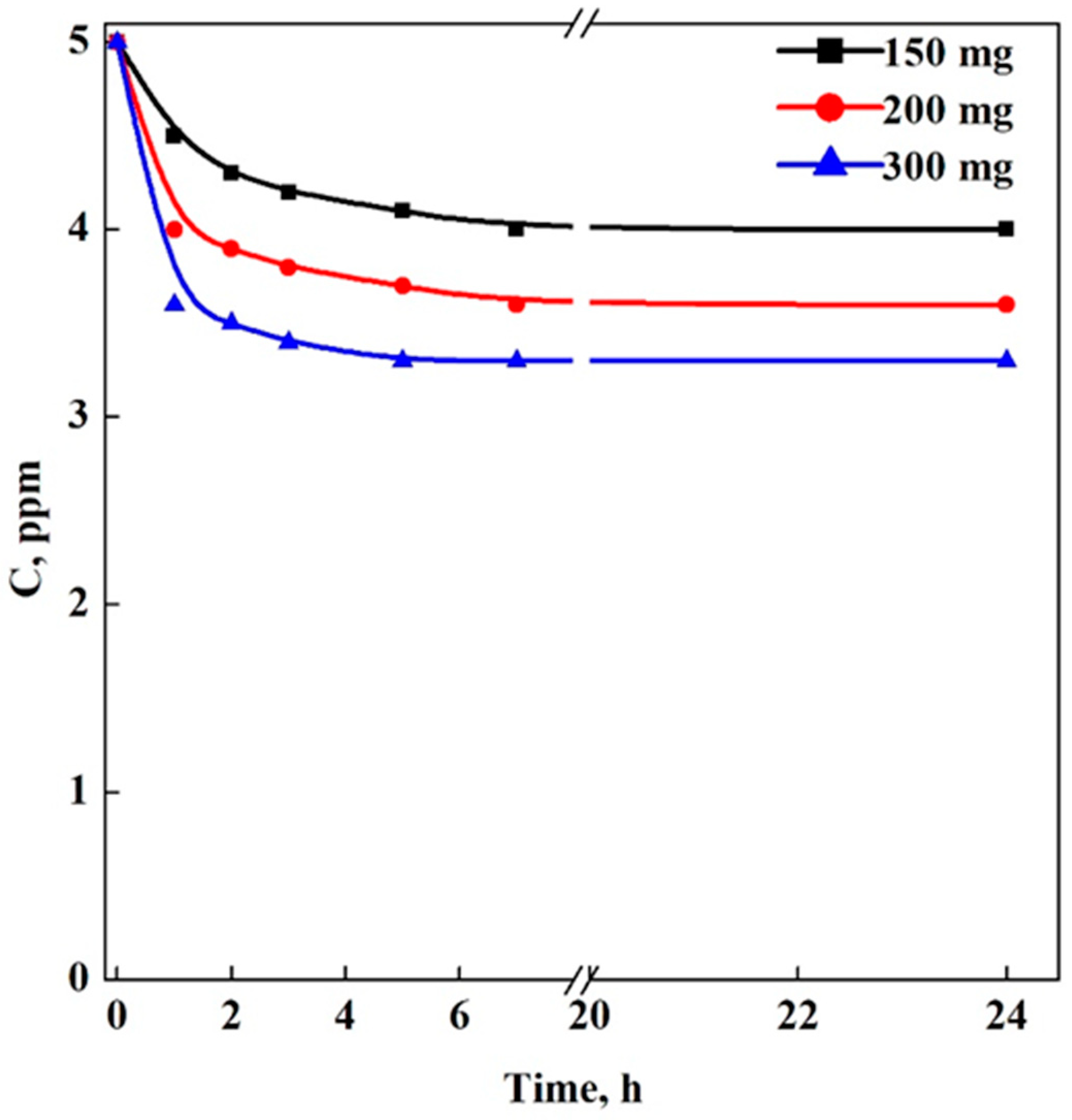
3.3. Adsorption Tests in Multi-Step Mode: Aerogels (A) vs. Cryogels (C)
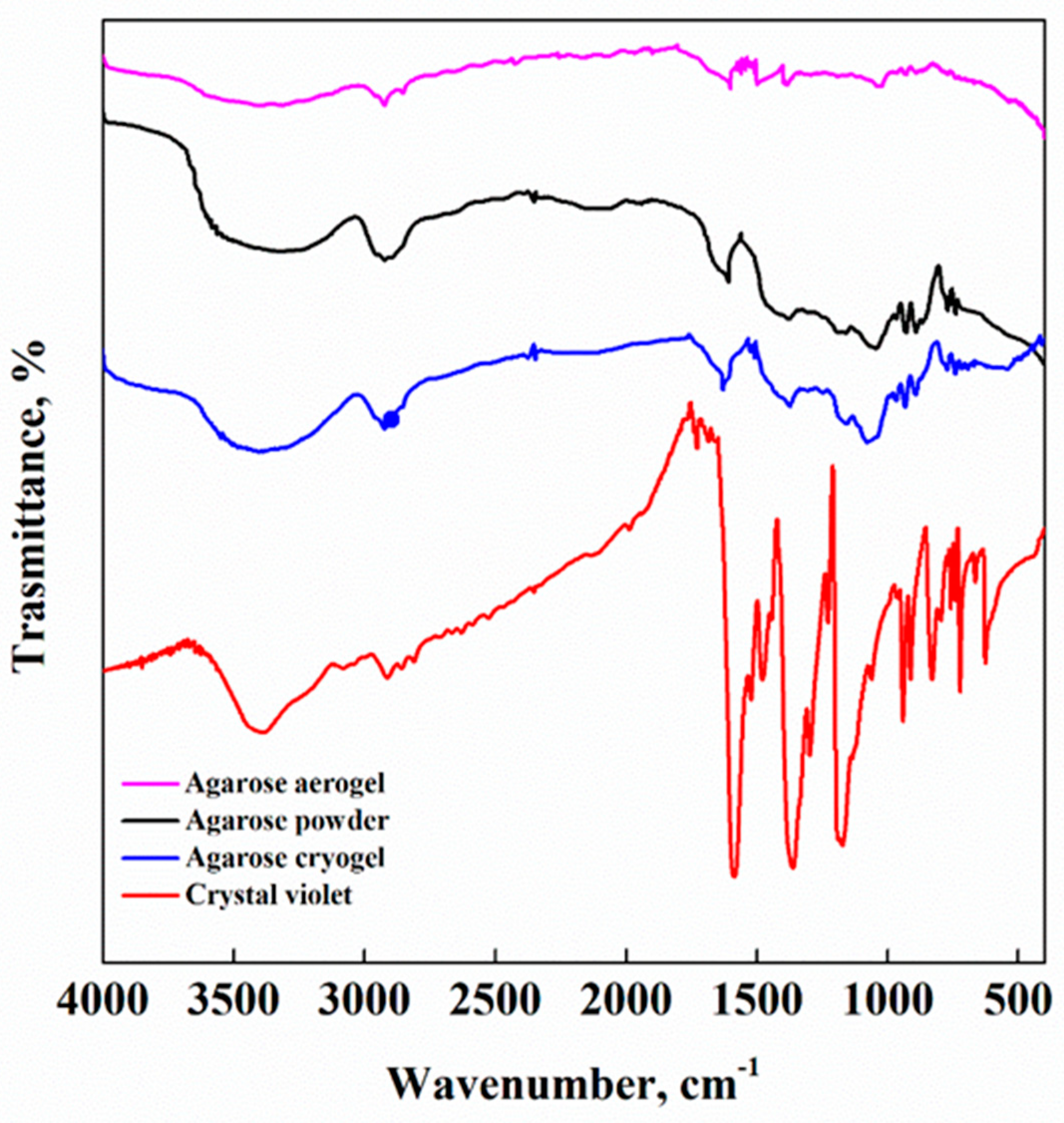
3.4. FT-IR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Guo, Y.; Yang, L.; Han, M.; Zhao, J.; Cheng, X. Nanomaterials as Sorbents to Remove Heavy Metal Ions in Wastewater Treatment. J. Environ. Anal. Toxicol. 2012, 2, 1000154. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Chaukura, N.; Gwenzi, W.; Tavengwa, N.; Manyuchi, M.M. Biosorbents for the Removal of Synthetic Organics and Emerging Pollutants: Opportunities and Challenges for Developing Countries. Environ. Dev. 2016, 19, 84–89. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Khattri, S.D.; Singh, M.K. Colour Removal from Synthetic Dye Wastewater Using a Bioadsorbent. Water Air Soil. Pollut. 2000, 120, 283–294. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Mirza, A.; Ahmad, R. An efficient sequestration of toxic crystal violet dye from aqueous solution by Alginate/Pectin nanocomposite: A novel and ecofriendly adsorbent. Groundw. Sustain. Dev. 2020, 11, 100373. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Assassi, M.; Madjene, F.; Harchouche, S.; Boulfiza, H. Photocatalytic treatment of Crystal Violet in aqueous solution: Box–Behnken optimization and degradation mechanism. Environ. Prog. Sustain. Energy 2021, 40, e13702. [Google Scholar] [CrossRef]
- Yu, k.; Yang, S.; Liu, C.; Chen, H.; Li, H.; Sun, C.; Boyd, S.A. Degradation of Organic Dyes via Bismuth Silver Oxide Initiated Direct Oxidation Coupled with Sodium Bismuthate Based Visible Light Photocatalysis. Environ. Sci Technol. 2012, 46, 7318–7326. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Honarmand, M.; Ghanbari, S. A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 229, 117961. [Google Scholar] [CrossRef]
- Shi, X.; Tian, A.; You, J.; Yang, H.; Wang, Y.; Xue, X. Degradation of organic dyes by a new heterogeneous Fenton reagent—Fe2GeS4 nanoparticle. J. Hazard Mater. 2018, 353, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Hamdani, M. Removal of Persistent Organic Pollutants (POPs) from water and wastewater by adsorption and electrocoagulation process. Groundw. Sustain. Dev. 2021, 13, 100575. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef]
- El-Sayed, G.O. Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination 2011, 272, 225–232. [Google Scholar] [CrossRef]
- Wang, X.S.; Chen, J.P. Biosorption of congo red from aqueous solution using wheat bran and rice bran: Batch studies. Sep. Sci. Technol. 2009, 44, 1452–1466. [Google Scholar] [CrossRef]
- Ramdani, A.; Taleb, S.; Benghalem, A.; Ghaffour, N. Removal of excess fluoride ions from Saharan brackish water by adsorption on natural materials. Desalination 2010, 250, 408–413. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of heavy metals-a review. Mater Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Ip, A.W.M.; Barford, J.P.; McKay, G. Reactive black dye adsorption/desorption onto different adsorbents: Effect of salt, surface chemistry, pore size and surface area. J. Colloid Interface Sci. 2009, 337, 32–38. [Google Scholar] [CrossRef]
- Siahkamari, M.; Jamali, A.; Sabzevari, A.; Shakeri, A. Removal of Lead(II) ions from aqueous solutions using biocompatible polymeric nano-adsorbents: A comparative study. Carbohydr. Polym. 2017, 157, 1180–1189. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Reverchon, E.; Cardea, S. Production of porous agarose-based structures: Freeze-drying vs. supercritical CO2 drying. Gels 2021, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, V.; Singh, J. An analytical study of coupled heat and mass transfer freeze-drying with convection in a porous half body: A moving boundary problem. J. Energy Storage 2022, 55, 105394. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Agarose, alginate and chitosan nanostructured aerogels for pharmaceutical applications: A short review. Front. Bioeng. Biotechnol. 2021, 9, 688477. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Hosseini, S.H.; Seidi, F.; Soleyman, R. Magnetic removal of crystal violet from aqueous solutions using polysaccharide-based magnetic nanocomposite hydrogels. Polym. Int. 2012, 62, 1038–1044. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; He, T.; Chen, L. Removal of crystal violet by clay/PNIPAm nanocomposite hydrogels with various clay contents. Appl. Clay Sci. 2014, 90, 1–5. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Patil, A.P.; Bhanvase, B.A.; Sonawane, S.H. Ultrasonically prepared poly(acrylamide)-kaolin composite hydrogel for removal of crystal violet dye from wastewater. J. Environ. Chem. Eng. 2015, 3, 1152–1162. [Google Scholar] [CrossRef]
- Nasab, S.G.; Semnani, A.; Teimouri, A.; Yazd, M.J.; Isfahani, T.M.; Habibollahi, S. Decolorization of crystal violet from aqueous solutions by a novel adsorbent chitosan/nanodiopside using response surface methodology and artificial neural network-genetic algorithm. Int. J. Biol. Macromol. 2019, 124, 429–443. [Google Scholar] [CrossRef]
- Martins, L.R.; Rodrigues, J.A.V.; Adarme, O.F.H.; Melo, T.M.S.; Gurgel, L.V.A.; Gil, L.F. Optimization of cellulose and sugarcane bagasse oxidation: Application for adsorptive removal of crystal violet and auramine-O from aqueous solution. J. Colloid Interface Sci. 2017, 494, 223–241. [Google Scholar] [CrossRef]
- Druzian, S.P.; Zanatta, N.P.; Borchardt, R.K.; Côrtes, L.N.; Streit, A.F.M.; Severo, E.C.; Gonçalves, J.O.; Foletto, E.L.; Lima, E.C.; Dotto, G.L. Chitin-psyllium based aerogel for the efficient removal of crystal violet from aqueous solutions. Int. J. Biol. Macromol. 2021, 179, 366–376. [Google Scholar] [CrossRef]
- Gong, X.L.; Lu, H.Q.; Li, K.; Li, W. Effective adsorption of crystal violet dye on sugarcane bagasse–bentonite/sodium alginate composite aerogel: Characterisation, experiments, and advanced modelling. Sep. Purif. Technol. 2022, 286, 120478. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.-Y.; Kim, Y.H. Agarose Gel Electrophoresis for the Separation of DNA Fragments. J. Vis. Exp. 2012, 20, e3923. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Y.; Du, Q.; Wang, Z.; Xia, Y.; Yedinak, E.; Lou, J.; Ci, L. High performance agar/graphene oxide composite aerogel for methylene blue removal. Carbohydr. Polym. 2017, 155, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, C.; Wang, M.; Zhang, S.; Sun, J.; Dong, S.; Sun, J. Synergistic effect of adsorption and photocatalysis of 3D g-C3N4-agar hybrid aerogels. Appl. Surf. Sci. 2019, 467–468, 286–292. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, S.; Zhu, W.; Huang, L.; Yang, C.; Li, S.; Liu, X.; Wang, R.; Hu, N.; Suo, Y.; et al. Agar aerogel containing small-sized zeolitic imidazolate framework loaded carbon nitride: A solar-triggered regenerable decontaminant for convenient and enhanced water purification. ACS Sustain. Chem. Eng. 2017, 5, 9347–9354. [Google Scholar] [CrossRef]
- Huang, D.; Quan, Q.; Zheng, Y.; Tang, W.; Zhang, Z.; Qiang, X. Dual-network design to enhance the properties of agar aerogel adsorbent by incorporating in situ ion cross-linked alginate. Environ. Chem. Lett. 2020, 18, 251–255. [Google Scholar] [CrossRef]
- Baldino, L.; Zuppolini, S.; Cardea, S.; Diodato, L.; Borriello, A.; Reverchon, E.; Nicolais, L. Production of biodegradable superabsorbent aerogels using a supercritical CO2 assisted drying. J. Sup. Fluids 2020, 156, 104681. [Google Scholar] [CrossRef]
- Date, P.; Tanwar, A.; Ladage, P.; Kodam, K.M.; Ottoor, D. Biodegradable and biocompatible agarose–poly (vinyl alcohol) hydrogel for the in vitro investigation of ibuprofen release. Chem. Pap. 2020, 74, 1965–1978. [Google Scholar] [CrossRef]
- Subrahmanya, T.M.; Widakdo, J.; Mani, S.; Austria, H.F.M.; Hung, W.S.; Makari, H.K.; Nagar, J.K.; Hu, C.C.; Lai, J.Y. An eco-friendly and reusable syringe filter membrane for the efficient removal of dyes from water via low pressure filtration assisted self-assembling of graphene oxide and SBA-15/PDA. J. Clean Prod. 2022, 349, 131425. [Google Scholar] [CrossRef]
- Haounati, R.; Alakhras, F.; Ouachtak, H.; Saleh, T.A.; Al-Mazaideh, G.; Alhajri, E.; Jada, A.; Hafid, N.; Addi, A.A. Synthesized of Zeolite@Ag2O Nanocomposite as Superb Stability Photocatalysis Toward Hazardous Rhodamine B Dye from Water. Arab. J. Sci. Eng. 2022, 10, 1. [Google Scholar] [CrossRef]
- Ouachtak, H.; El Guerdaoui, A.; Haounati, R.; Akhouairi, S.; El Haouti, R.; Hafid, N.; Addi, A.A.; Šljukić, B.; Santos, D.M.F.; Taha, M.L. Highly efficient and fast batch adsorption of orange G dye from polluted water using superb organo-montmorillonite: Experimental study and molecular dynamics investigation. J. Mol. Liq. 2021, 335, 116560. [Google Scholar] [CrossRef]
- Canizo, B.V.; Agostini, E.; Wevar Oller, A.L.; Dotto, G.L.; Vega, I.A.; Escudero, L.B. Removal of crystal violet from natural water and effluents through biosorption on bacterial biomass isolated from rhizospheric soil. Water Air Soil. Pollut. 2019, 230, 210. [Google Scholar] [CrossRef]

| Adsorbent Material | Adsorption Process | CV Removal Efficiency | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Magnetic starch-graft-poly(acrylic acid) hydrogels | The functionalized hydrogel (10–50 mg) was immersed in a 50 mg/L of CV solution and magnetically stirred | 85% | Easy feasibility of industrial scale-up for adsorption process | Time-consuming process for adsorbent preparation; Hydrogels are not stable over time; Materials not completely biodegradable | Pourjavadi et al. [25] |
| Magnesium silicate hydrate/poly(N-isopropylacrylamide) hydrogels | The hydrogel was immersed in 50 mL of a 10 mg/L CV solution | 50% | Easy feasibility of adsorption process | Time-consuming process for adsorbent preparation (almost 4 days); Hydrogels are not stable over time | Zhang et al. [26] |
| Poly(acrylamide)-kaolin (PAAm-K) hydrogel | 0.5–2 g of hydrogels were stirred in a solution of 10–50 mg/L of CV | 80% | Easy preparation techniques for hydrogels production | Hydrogels are used after water removal and the drying is carried out at high temperature (nanoporous structure is not preserved) | Shrisath et al. [27] |
| Chitosan/nanodiopside based particles | The particles were immersed in a solution of CV having a concentration ranging between 15 and 35 mg/L | 99% | Easy preparation processes (particles production and adsorption test) | Time-consuming process for particles production (almost 3 days); Post-treatments are required to remove particles from aqueous solutions | Nasab et al. [28] |
| Oxidise cellulose and sugarcane bagasse particles | 20 mg of particles are stirred in a solution of 200 mg/L of CV | 95% | Particles allow a larger exposed surface to the liquid medium, providing a higher adsorption capacity | Post-treatments are required to separate particles from liquid medium and these methods are not easily scalable to industrial process | Martins et al. [29] |
| Solid Amount | Aerogel (Set A) | Cryogel (Set C) |
|---|---|---|
| 150 mg | A1 | C1 |
| 200 mg | A2 | C2 |
| 300 mg | A3 | C3 |
| Before | Diameter [cm] | Height [cm] | Volume [cm3] |
|---|---|---|---|
| Aerogel | 1.7 | 1.0 | 2.268 |
| Cryogel | 2.0 | 0.8 | 2.512 |
| After | Diameter [cm] | Height [cm] | Volume [cm3] |
| Aerogel | 1.7 | 0.8 | 1.815 |
| Cryogel | 1.6 | 0.8 | 1.607 |
| Aerogel | Cryogel | |
|---|---|---|
| Porosity [%] | 91 ± 4 | 71 ± 3 |
| BET specific surface area [m2/g] | 154 ± 12 | 11 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Different Drying Techniques Can Affect the Adsorption Properties of Agarose-Based Gels for Crystal Violet Removal. Appl. Sci. 2023, 13, 463. https://doi.org/10.3390/app13010463
Guastaferro M, Baldino L, Cardea S, Reverchon E. Different Drying Techniques Can Affect the Adsorption Properties of Agarose-Based Gels for Crystal Violet Removal. Applied Sciences. 2023; 13(1):463. https://doi.org/10.3390/app13010463
Chicago/Turabian StyleGuastaferro, Mariangela, Lucia Baldino, Stefano Cardea, and Ernesto Reverchon. 2023. "Different Drying Techniques Can Affect the Adsorption Properties of Agarose-Based Gels for Crystal Violet Removal" Applied Sciences 13, no. 1: 463. https://doi.org/10.3390/app13010463
APA StyleGuastaferro, M., Baldino, L., Cardea, S., & Reverchon, E. (2023). Different Drying Techniques Can Affect the Adsorption Properties of Agarose-Based Gels for Crystal Violet Removal. Applied Sciences, 13(1), 463. https://doi.org/10.3390/app13010463










