Abstract
The results of studies of marine sponge carbonization processes during thermal treatment in an argon atmosphere in the temperature range from room temperature to 1200 °C are presented. The spatial structure, atomic composition of native and carbonized sponges, and their changes during pyrolysis were characterized using a set of methods that are informative at the macro- (thermogravimetric analysis, derivative thermogravimetric analysis, differential scanning calorimetry), micro- (Raman spectroscopy, scanning electron microscopy, energy dispersive spectroscopy), and nanoscales (X-ray absorption and photoelectron spectroscopy using synchrotron radiation and a sample charge compensation system). Preservation of the 3D architecture at the macro- and microlevels and graphitization of the interfibril medium with the formation of turbostratic graphite at the nanolevel were demonstrated. It was shown that the atomic contents of nitrogen, carbon, and oxygen in the spongin were ~2–3 at.%, ~5 at.%, and ~4 at.%, respectively. The matter concentrated in the space between the spongin fibrils included ~70 at.% carbon and ~11 at.% oxygen, with a large proportion of carbon (~63 at.%) involved in the formation of aromatic and C–C bonds and the remainder in carbon monoxide compounds. After the decomposition of spongin at 400 °C, this substance transformed into turbostratic graphite, preserving the 3D architecture of the original marine sponge as the temperature rose.
1. Introduction
The current work was devoted to the study of the carbonization process of marine sponges belonging exclusively to the Keratosa subclass, which has a mineral-free skeleton that consists of “keratinized” spongin fibers [1,2]. This sponge subclass is characterized by well-developed, anastomosing spongin skeletons that are hierarchically organized into primary, secondary, and sometimes tertiary fiber structures and constitute a significant part of the body volume [3]. The skeletal structures cleaned from mineralized components, waste products, and “contaminants” via depigmentation and demineralization have been called “bath sponges” since ancient times, and in the scientific literature they are defined as “commercial sponges” [4]. Commercial sponges include a number of different members of the phylum Porifera, which differ in fiber morphology, porosity, and size. Their structure includes networks of spongin fibers, which can range in diameter from 5 to 100 microns, and the cells range in size from 100 microns to a millimeter, depending on the type of sponge. Unfortunately, the spongin biosynthesis pathways in bath sponges and the protein sequences of this unique biopolymer are still unknown and, to date, spongin has no clear chemical definition [5]. However, according to [6], spongin from sponges of the Demosponges class has an amino acid composition similar to that of collagen type XIII (COL13) found in vertebrates. Studies [7,8,9] showed that spongin contains the classic collagen motif Gly-Xaa-Yaa, where hydroxyproline (Hyp) occupies any position in the triplet motif except for the glycine (Gly) position. This biopolymer with an unknown chemical structure contains sulfur, bromine, and iodine [10,11,12,13,14]. Due to their biocompatibility and their unique physicochemical, structural, and mechanical properties [15], spongin-based bath sponge scaffolds have been widely used in medicine [16] and tissue engineering [17,18]. Spongin-containing marine sponges are examples of renewable resources due to their ability to be grown under marine breeding conditions in their natural habitat [19,20]. This property makes marine sponges an attractive biological material with great biomimetic potential for various applications. Recently, it was shown [21,22,23,24] that the use of 3D spongin-based scaffolds from natural marine sponges is promising regarding carbonization. Specifically, spongin carbonization in argon at temperatures up to 1200 °C leads to the formation of 3D nanoporous structure of turbostratic graphite, which can be applied to obtain new composite materials and, in particular, catalysts for seawater purification [21]. Many researchers previously attempted to manufacture carbon materials with a controlled microstructure and morphology from renewable and biodegradable natural sources. Although heat treatment of a variety of proteins usually causes their decomposition without the formation of carbonaceous materials [25], fiber-based structural proteins, such as keratin [26], collagen [27], and silk [28,29], appear to be suitable for carbonization at temperatures between 200 °C and 800 °C, and in some cases, even up to 2800 °C [30]. However, to date, there are no published data on the formation of ready-to-use carbon frameworks with a hierarchical structure. For example, the fibers of dried Luffe fruit, which has a spongy network of xylem fibers similar to a sponge, are completely destroyed after carbonization and only the carbonized material powder can be used for further applications [31]. Similar results were obtained for silk carbon fibers, which were too brittle for further processing [32]. In our previous works, we studied the physical and chemical properties of carbonized spongin, such as its structure and elemental composition; characterized the outer surface; and determined the presence of water and carbon oxides on it [21,33]. However, the question about the process of spongin carbonization itself remains open. The aim of this work was to investigate the modification of marine sponges during thermal treatment in an inert medium with control of sample parameters during temperature changes using a complementary set of physical methods that characterized the substance at the macro-, micro-, and nanolevels using thermogravimetric analysis (TGA), Raman spectroscopy, energy dispersive X-ray analysis (EDX), ultrasoft X-ray absorption (NEXAFS—near-edge X-ray absorption fine structure), photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM).
2. Materials and Methods
2.1. Materials
Samples of marine sponges species Hippospongia communis (H. communis) of class Demospongiae, order Dictyoceratida, and family Spongiidae with an organic skeleton were collected from the Mediterranean Sea off the coast of Tunisia in May 2017 and purified using standard methods. The process of preparation and carbonization of spongin is described in [21]. Carbonization of selected spongin scaffolds isolated from H. communis was performed at the NRC “Kurchatov Institute”—IREA Shared Knowledge Center (Moscow, Russia) using an SDTQ600 derivatograph (“TA Instruments”, New Castle, DE, USA) in an argon flow up to 1200 °C.
2.2. Characterization
The samples were studied using a set of complementary methods: thermogravimetric analysis (TGA), derivative thermogravimetric analysis (DTGA) and differential scanning calorimetry (DSC), Raman spectroscopy, scanning electron microscopy (SEM), near-edge X-ray absorption fine structure (NEXAFS), and photoelectron (XPS) spectroscopy.
Thermal analysis is the primary method for determining the thermal properties of chemicals. The TGA–DTGA–DSC measurements provide information on the thermal stability of a substance and its modification during thermal exposure to increasing temperature. Simultaneous differential thermal TGA–DTGA–DSC analyses were performed on SDTQ600 simultaneous differential technics (“TA Instruments”, USA). The marine sponge samples with approximately m ≈ 2.5–3 mg were heated at a heating rate of β = 5 °C min−1 in an Ar atmosphere (argon flow φ = 100 mL·min−1) in the temperature range of ΔT = 20–1200 °C. Raman spectroscopy research was conducted at room temperature in the 80–3500 cm−1 interval using a Horiba-Yvon Jobin LabRam HR800 spectrometer equipped with a 600 g/mm grating and an Ar laser with a 1 mW and 488 nm wavelength. Lenses of ×50 and ×100 were used for analysis. The spectrometer was fitted with neutral filters to limit the laser radiation power. The spectral and spatial resolution was about 1 cm−1 and 1 μm, respectively.
The surface morphology of the sponge samples was analyzed using a couple of scanning electron microscopes (XL 30 ESEM, Philips; ULTRA55, Carl Zeiss, Jena, Germany). The elements were analyzed using energy-dispersive X-ray spectroscopy in an EDX analysis system from EDX (Mahwah, NJ, USA).
The NEXAFS spectroscopy studies were carried out using the synchrotron radiation of the Russian-German dipole beamline at the BESSY II (Berlin, Germany). All NEXAFS spectra were measured in total electron yield (TEY) mode. The energy calibrations of the C 1s NEXAFS spectra were performed using the well-resolved π*-resonance at 285.38 eV in the C 1s spectrum of HOPG [34]. The photon flux spectral dependence was monitored using the TEY signal from a clean Au photocathode. The photon energy resolution was below 0.05 eV. The samples for absorption measurements were prepared ex situ in air via pressing sample powders into the clean surface of a copper or indium plate.
XPS studies were carried out at the resource center “Physical methods of surface investigation” of the Saint Petersburg University Research Park (St. Petersburg, Russia). XPS analysis was performed on a Thermo Fisher Scientific ESCALAB 250Xi X-ray photoelectron spectrometer. The X-ray tube with Al Kα radiation (1486.6 eV) was used as a source of ionizing radiation. The survey spectra and high-resolution core-level spectra were measured with pass energies of 100 eV and 50 eV, respectively. To neutralize the sample charging during the experiments, an electron–ion charge compensation system was used. The experimental data were processed using the ESCALAB 250 Xi spectrometer software.
3. Results and Discussion
The results of a preliminary study of the native sponge after the cleaning procedure are shown in Figure 1a–d, which contains its photo (a) and the scanning electron microscopy data. The microphotographs clearly demonstrated the characteristic multilevel 3D structure of the spongin matrix of individual fibers up to 100 µm thick, consisting of nanoscale collagen filaments, which together formed a complex three-dimensional structure with high microporosity. To elucidate the elemental composition of the sample, EDX was used, which, in addition to the presence of carbon (~74%), nitrogen (~3%), and oxygen (~20%), also showed traces of calcium, magnesium, sodium, aluminum, sulfur, and silicon. Quantitative elemental analysis of the native sponge was also carried out using XPS.
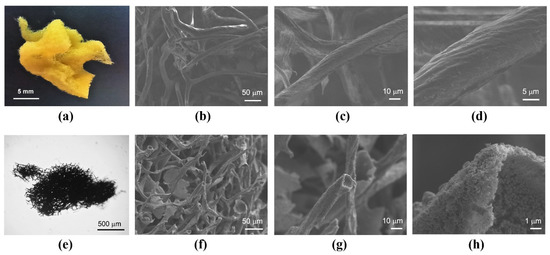
Figure 1.
Photos of the native (a) and carbonized (e) marine sponges, along with the SEM images of the native marine sponge before (b–d) and after carbonization (f–h).
Figure 2a shows the survey XPS spectrum of the native sponge, which contained two intense C 1s and O 1s peaks, as well as a set of low-intensity N 1s, Na 1s, Si 2p, and Ca 2p peaks.

Figure 2.
XPS spectra of pristine and carbonized sponge: survey (a), N 1s (b), C 1s (c), and O 1s (d) spectra.
The XPS data agreed well with the EDX results on the elemental composition of the native sponge, which showed 76.8 at.% carbon, 1.9 at.% nitrogen, 16.0 at.% oxygen, 2.6 at.% silicon, 1.1 at.% sodium, and 1.6 at.% calcium. The observed discrepancy in the content and atomic composition was caused by the different sensitivities of the methods to the atoms of the elements, as well as by the distribution of nitrogen, carbon, and oxygen atoms in the volume and on the surface of the sponge structural elements. It should be noted that EDX gives information on the relative concentration of atoms at a depth of several tenths to several micrometers, and for the XPS this depth is 1–2 nm. Therefore, the agreement of data on carbon, oxygen, and nitrogen indicates an equal distribution in the volume and on the surface of the studied sample. It should be noted that the ratio of atomic concentrations of carbon, nitrogen, and oxygen atoms in the sponge agrees with the data of other authors [35].
Despite the fact that mankind has been actively using sponges for more than a thousand years, data on the chemical properties of spongin are incomplete. To date, there is no clear definition of the chemical composition of this unique biological material. The pathways of spongin biosynthesis in sponges, as well as the genomics, proteomics, and protein sequences of this biopolymer, are still unknown. However, earlier studies [8] showed that spongin is built from amino acids similar to collagen, which consists of a Gly-Xaa-Yaa triplet motif, where the Xaa or Yaa position is usually occupied by hydroxyproline and the remaining position by any of the amino acids present [36]. Hydroxyproline was found in large amounts in several sponges of Dictyoceratida (Demospongiae) [9]. According to [37], the spongin H. communis marine sponges contain 19 different amino acids, with the highest concentration (percentage per 100 g of spongin) of glycine C2H5NO2 (31.9%), followed by aspartic acid C4H7NO4 (9.4%), hydroxyproline C5H9NO3 (8.7%), alanine C3H7NO3 (8.4%), and glutamic acid C5H9NO4 (7.9%).
These amino acids are characterized by the presence of -COOH, -NH2, and -OH functional groups, which are responsible for the effective interaction with other substances in the sponge. Taking into account the data on the relative composition of carbon, oxygen, and nitrogen atoms in the native sponge, one can make a preliminary conclusion about the content of spongin in it. Assuming that the Gly-Xaa-Yaa triplet contains hydroxyproline in the Yaa position and any of the amino acids listed in [37] in the Xaa position, all the nitrogen atoms will be in the peptide bond. Then, each peptide bond will also involve one carbon atom and one oxygen atom, i.e., 2 at.% carbon and 2 at.% oxygen. Assuming the maximum possible content of carbon and oxygen atoms in the side members of amino acids in positions Xaa and Yaa to be 3 and 2 atoms, respectively, one can estimate the upper limit of atoms in the spongin as 2 at.% (nitrogen) + 5 at.% (carbon) + 4 at.% (oxygen). Atoms in the NH2 and COOH groups at the beginning and end of the spongin fibers were not taken into account. As a result, it was found that at least ~70 at.% of carbon and ~11 at.% of oxygen are located in the sponge volume between the spongin fibrils. The sponge C 1s NEXAFS spectrum (Figure 3) was measured to elucidate the chemical composition of the sponge substance. Due to the high oscillator strength of C 1s → π* electron transitions in linear and planar atomic groups, which appear in absorption spectra as narrow and intense peaks with characteristic photon energies, these groups were identified in the studied sample.
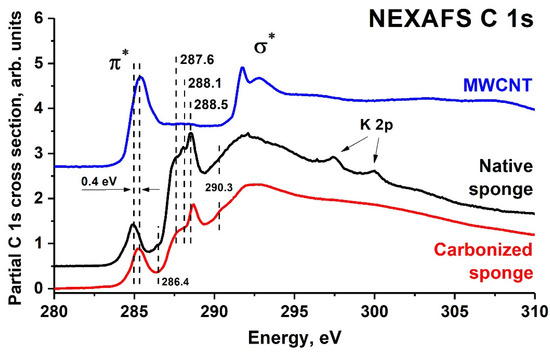
Figure 3.
C 1s NEXAFS spectra of native sponge, carbonized sponge, and MWCNT.
Figure 3 shows the partial C 1s NEXAFS spectra of the native sponge obtained by subtracting the contributions from the overlying electron levels extrapolated by the power dependence of the absorption cross-section from the low energy region before the C 1s absorption edge. In the fine structure region of the C 1s spectrum, there was an intense peak with a photon energy of 285.0 eV related to C 1s → π* transitions in aromatic structures. At the same time, the spectrum also contained four additional less intense narrow peaks and a broad band, which were due to C 1s → π* and C 1s → σ* electron transitions in corresponding atomic groups, respectively. The energy positions of these narrow peaks agreed well with the energies of 1s → π* transitions in the C–O (286.7 eV), C–O–C (287.6 eV), and C=O (288.6 eV) planar oxygen-containing groups (carboxyl and carbonyl), which was confirmed by numerous studies of carbon oxides [38,39,40,41] and various oxygen–carbon molecular groups [42,43,44,45,46]. However, the 287.6 eV peak can also be attributed to transitions in amide (N–C=O) or imide (C=N) groups, while the 288.1 eV peak can be attributed to transitions in the CON peptide group [47]. The broad absorption band at the photon energy of 291.9 eV can be attributed to C 1s → σ* electron transitions in various linear and planar atomic groups. As for the structures at the energies of 297.5 eV and 300.0 eV, they referred to the 2p3/2, 1/2 → 3d electron transitions in potassium compounds, which were present in the form of impurities in the sponge. Normally, the C 1s spectra of proteins are dominated by a narrow and intense π* peak of the peptide group, and thus, the low intensity of this transition in the sponge spectrum confirmed the low spongin content in the native sponge. The apparent dominance in the sponge C 1s NEXAFS spectrum of peaks associated with electron transitions in the aromatic and C=O atomic groups indicated the high content of aromatic compounds and carbon oxides in the substance filling the space between the spongin fibrils in the sponge.
N 1s, C 1s, and O 1s XPS spectra (Figure 2b–d) were measured to quantify the atomic concentration of nitrogen, carbon, and oxygen atoms within atomic groups. The photoelectron (PE) spectrum of nitrogen contained a symmetric peak at a binding energy (BE) of 399.7 eV, which corresponded to the BE of N 1s electrons in the peptide group. The peak in the O 1s PE spectrum was asymmetric and could be decomposed into two components at the BEs of 532.9 eV and 531.45 eV, which corresponded to the BEs of O 1s electrons in the C–OH group and atomic groups containing the double C=O bond, which also include peptide and carboxyl groups. It should be noted that the possible presence of water adsorbed on the sponge surface, for which the O 1s BE lies in the range of 532–534 eV, may have contributed to the formation of the peak at the BE of 532.9 eV [40,48,49,50]. The atomic content of oxygen in these groups was 13.7% (C=O) and 2.3% (C–OH) of the total number of atoms in the sponge. In the carbon spectrum, the peak consisted of three components with BEs of 284.6 eV (C–C, C=C), 286.1 eV (C–OH), and 288.2 eV (C=O). The percentages of carbon atoms in these groups, as determined by the peak areas, were 63.2% (C–C and C=C), 6.3% (C–OH), and 7.3% (C=O).
Taking into account the earlier assessment of the percentage of nitrogen, carbon, and oxygen atoms in the marine sponge spongin, the content of atomic groups in the sponge around the spongin fibrils was estimated. The carbon atom content was obtained for the following groups: C–C and C=C ~61%, C=O ~5%, and C–OH ~5% (taking into account the presence of such a group in the spongin). Similar estimates for the oxygen atom content were as follows: C=O ~12% and C–OH ~2%. The fact that there was twice as much oxygen as carbon per double bond suggested the dominance of carboxyl O=C–OH groups. In turn, about 2.5-fold higher oxygen content relative to carbon in the C–OH groups suggested a significant number of epoxy C–O–C bonds in the sponge.
To study the carbonization process of the native sponge, TGA, DTGA, and DSC were performed by heating the sample in an argon flow in the temperature range of 24–1200 °C.
The thermographic dependencies in Figure 4 show that the thermal modification of the sponge proceeded in three stages. The first stage was accompanied by approximately 10% mass loss in the temperature range of 80–140 °C, which is characteristic of collagen, spongin, and other biomaterials [51,52], and is caused by the evaporation of water adsorbed on the surface and involved in hydrogen bonding in the volume of the sponge. The second stage was observed in the temperature range of 210–450 °C and was accompanied by a significant (more than 40%) mass loss. The DTGA minimum was observed at a temperature of 302 °C. According to previous studies, spongin decomposes in this temperature range and the peptide bond is broken [21,51]. This process is accompanied by the release of nitrogen-rich compounds and partial burnout of carbon with the release of CO2 [24]. The third stage occurred at high temperatures of 800–1100 °C with a loss of about 20% mass and was accompanied by two broad peaks in the DTGA at temperatures of 880 °C and 1014 °C, as well as a sequence of alternating exothermic and endothermic narrow peaks in the DSC curve in the temperature range from 920 °C to 1080 °C. Taking into account the presence of a small (~2 at.%) content of calcium in the native sponge in such probable compounds as calcium carbonate (limestone CaCO3), calcium oxide (CaO), and calcium hydroxide (Ca(OH)2), the following interpretation of the observed changes in the thermographic dependences may be given. If there is Ca(OH)2 in a sponge, then in a range of temperatures from 370 to 440 °C, there will be an endothermic decomposition [53] according to the equation
Ca(OH)2 → CaO + H2O

Figure 4.
TG (1), DTGA (2), and DCS (3) curves of thermal modification of marine sponge.
Due to the above reactions, the appearance of a wide endothermic band in the DTGA dependence at ~400 °C can be associated with this decomposition. If the sponge contains carbonate, then in the temperature range of 550–830 °C should produce endothermic decomposition into carbon dioxide and calcium oxide according to the following equation [53,54,55,56]:
CaCO3 → CaO + CO2
Therefore, the partial mass loss in this interval and the partial contribution to the first endothermic band at a temperature of 880 °C observed in the DTGA dependence can be attributed to reaction (2). In this case, the high-temperature shift of the maximum in the DTGA may be due to the presence of carbon dioxide since the decomposition of CaCO3 shifts to the region of higher temperatures when the partial pressure of CO2 increases [57,58]. As a result of the decomposition reactions (1) and (2) at temperatures above 900 °C, only CaO oxide will remain from the calcium compounds, and carbon dioxide CO2 and H2O will be formed. It is essential to note that according to reference [24], the qualitative analysis by means of quadrupole mass spectrometry of the evolved gases during heat treatment clearly showed the evolution of H2O and CO2 in this temperature range. It is known that under these conditions there is a high-temperature reversible decomposition reaction associated with the release of carbon dioxide in accordance with the reaction
CaCO3 ↔ CaO + CO2
The carbonate decomposition reaction is endothermic and the reverse reaction is exothermic [58,59]; thus, they should appear as minima and maxima in the DSC dependence, respectively. It is obvious that this process must proceed in cycles and, as the carbon dioxide evaporates, it ends in an exothermic reaction with the formation of calcium oxide. Exactly such an alternating sequence of peaks of different polarity is observed in the DSC experimental dependence. When the temperature reaches ~1100 °C, the carbon dioxide volatilizes from the material. As a result, carbon, carbon oxides, and calcium oxide remain in the carbonized sponge after pyrolysis. The EDX analysis of carbonized sponge showed that carbon dominated in the carbonized sponge volume (~97 at.%). In addition, it contained oxygen (~2 at.%) and traces of calcium and silicon. However, according to the XPS data, the oxygen content in the surface layer of carbonized sponge was slightly higher than in the volume (~8–10 at.%), the carbon content was ~90–98 at.%, the calcium content was ~1–2 at.%, and there was an insignificant amount of silicon atoms.
Figure 1e–1h show photos and SEM images of carbonized sponge, from which it is clear that its original 3D structure was preserved. It should be noted that the 3D structure of the native sponge existed solely due to the spongin scaffold, which had already completely decomposed at 450 °C. Therefore, from the end of thermal treatment stage II, the first steps of graphitization of the substance surrounding the sponge fibrils had already taken place. This was convincingly demonstrated in our previous work [21] by the characteristic changes in the C 1s NEXAFS spectra and in the Raman spectra with successive increases in pyrolysis temperature at 400, 600, 800, 1000, and 1200 °C. This process proceeded uniformly up to thermal treatment stage III and continued further after the decomposition of calcium compounds.
Figure 3 shows the C 1s NEXAFS spectra of the sponge after pyrolysis in an argon flow at 1200 °C in comparison with the data for the native sponge and multiwalled carbon nanotube (MWCNT) [38,39]. The spectra of the carbonized sponge clearly showed the absence of the structure associated with the peptide group (peak 288.1 eV) and nitrogen compounds (peak 287.6 eV). At the same time, passing from the spectrum of the native sponge to the one of carbonized sponge, a short-wave shift by 0.4 eV of the main π* absorption peak at energy ~285.0 eV was observed. In the case of native sponge spectra, this peak corresponded to a C 1s → π* electron transition in an aromatic ring, and for carbonized sponge, the peak position coincided with the characteristic energy of this transition for hexagons in the graphene planes of the multi-wall carbon nanotube and highly oriented pyrolytic graphite (285.4 eV) [34]. The NEXAFS data showed the carbon oxide structures on the carbonized sponge surface: a broad band at ~287.2 eV (C–O and C–O–C groups), a peak at 288.5 eV (carbonyl group C=O), and a small peak at 290.3 eV, which corresponded to the anion [CO3]2− or carboxylic group HO–C=O. It follows from the XPS data that carbonized sponge as a whole contained no more than 10% carbide oxides.
The C 1s and O 1s XPS spectra of the carbonized sponge are shown in Figure 2a,c,d. The C 1s XPS spectrum was fitted using three bands. The first at a BE of 284.5 eV corresponded to carbon atoms in turbostratic graphite, the second one at 286.4 eV was associated with the C–O bond of carbon atoms, and the third band at 289.6 eV was associated with the carboxyl C=O bond. This identification was well correlated with the C 1s NEXAFS data. The O 1s XPS spectrum was fitted using three peaks with BEs of 530.6 eV, 531.8 eV, and 533.0 eV. These peaks can be identified as corresponding to OH, H2O, and C–O/C=O groups, respectively [33].
On the carbonized sponge surface, the concentration of oxygen atoms in the form of carbon oxides, as well as in the form of adsorbed water molecules and hydroxyl groups, was rather high and equal to 4–5% and 5–6%, respectively. The high content of adsorbed water on the carbonized sponge surface was due to the strong roughness and high porosity of the surface [33], which can be clearly seen in the SEM image in Figure 1h. Figure 5 shows the Raman spectra of a native sponge before and after pyrolysis at 1200 °C in argon. The sharp changes in the structure of the spectra clearly demonstrated the phenomena of graphitization during pyrolysis. The dramatic changes in the structure of the spectra in the transition from the sponge before and after pyrolysis can be clearly seen in Figure 5. The spectrum of the native sponge was distorted due to the strong fluorescence of the sample and the vibrational structure was extremely low contrast. However, the D, G, and 2D bands appeared in the spectrum of the carbonized sponge. The wavenumber positions of these bands and the ratio of the band areas (D/G) were analyzed by us in [21] using the Ferrari and Robertson model [60]. It was shown that pyrolysis of the sponge involves clustering and formation of sp2 bonds; the material transforms from amorphous carbon to nanocrystalline graphite with increasing temperature and contains mixed sp2 and sp3 bond centers. In the case of marine sponge carbonized at 1200 °C, the average size of graphite nanocrystallites is about 3 nm.
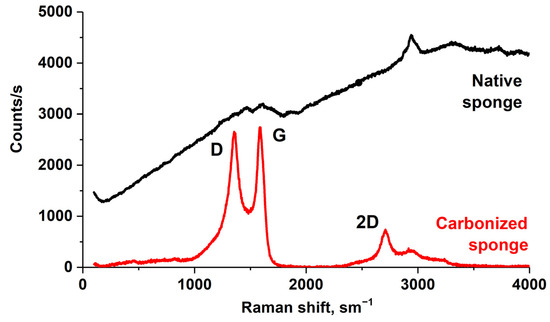
Figure 5.
Raman spectra of the marine sponge before (Native sponge) and after (Carbonized sponge) thermal modification.
4. Conclusions
Studies using a complementary set of methods demonstrated that during pyrolysis of the marine sponge, its three-dimensional architecture at the macro- and microlevels was preserved, and at the nanolevel, the graphitization of the interfibril medium occurred with the formation of turbostratic graphite. The current research for the first time demonstrated that the graphitization process and turgostratic graphite formation directly depended on the composition and properties of the matrix containing a large number of aromatic compounds that were located around the spongin fibers.
EDX, XPS, and NEXAFS studies of the atomic and molecular composition showed that the native sponge composition included carbon (74–77 at.%); oxygen (16–20 at.%); nitrogen (2–3 at.%); calcium (~2 at.%); silicon (~2 at.%); and small amounts of potassium, sodium, magnesium, and sulfur. From a comparative analysis of the spectral data, the contents of carbon atoms in the atomic groups were estimated at 63.2% (C–C and C=C), 6.3% (C–ON), and 7.3% (C=O), and for oxygen at 13.7% (C=O) and 2.3% (C–OH).
Based on the literature data on the contributions of amino acids in the spongin and assuming that all nitrogen was concentrated in the peptide groups, the atomic contents of carbon and oxygen in the spongin were ~5 at.% and ~4 at.%, respectively. The matter concentrated in the space between the spongin fibrils included ~70 at.% carbon and ~11 at.% oxygen, of which ~63 at.% carbon was involved in the formation of aromatic and C–C bonds, while the rest was in carbonyl, carboxyl, phenolic, and epoxy (C–O–C) atomic groups.
The analysis of TG, DTGA, and DSC dependences during the marine sponge pyrolysis in an argon flow at temperatures of 24–1200 °C showed that the process of sample modification proceeded in three stages: stage I involved a mass loss of ~10% (80–140 °C) due to water evaporation, stage II involved a 40% mass loss (210–450 °C) due to peptide bond decomposition and carbon combustion with a release of nitrogen-containing gases and carbon dioxide, and stage III involved a ~20% mass loss (800–1100 °C) and was characterized by a sequence of alternating exothermic and endothermic narrow peaks in the DSC curve due to the auto-oscillatory course of the transformation reaction (3) in the presence of CO2.
It was shown that by the end of stage II at 450 °C after the complete decomposition of spongin, the process of graphitization with the formation of turbostratic graphite had already proceeded with the release of nitrogen-containing gases, carbon dioxide, and water. This led to the preservation of the 3D structure of the native sponge and the transformation of the aromatic bond into an sp2 bond with the formation of graphite nanocrystallites that were ≈3 nm in size.
The results of the studies showed extreme informativity of spectral research methods at macro- (TGA, DTGA, DSC), micro- (SEM, EDX, Raman spectroscopy), and nanolevels (XPS, NEXAFS) when analyzing the graphitization process of biomaterials and also demonstrated the prospects of further studies of marine sponges modification. The obtained data and developed techniques can stimulate the search for other promising biological materials for carbonization that have a complex 3D structure and contain a large number of aromatic atomic groups in their composition.
Author Contributions
Conceptualization, O.V.P. and D.V.S.; research supervision, O.V.P., D.V.S., V.N.S. and A.S.V.; writing—original draft, O.V.P., D.V.S., A.S.V. and V.N.S.; TGA, DTGA and DSC interpretation, R.N.S., K.A.B. and V.N.S.; Raman spectroscopy measurements, S.I.I.; XPS and NEXAFS spectroscopy measurements and its data interpretation, O.V.P., S.V.N., D.V.S., A.S.V., P.M.K., K.A.B., R.N.S. and V.N.S. All the authors were actively involved in discussing the results and writing and drafting the manuscript. All authors read and agreed to the published version of the manuscript.
Funding
This research was supported by the Grant of the President of the Russian Federation MK-3796.2021.1.2 and by the Ministry of Science and Higher Education of Russia under Agreement N075-15-2021-1351 for the part of the research on NEXAFS spectroscopy. The reported study was funded by RFBR, project number 19-32-60018; the Komi Republic, project number 20-42-110002 r-a; and the bilateral program of the RGBL at BESSY II.
Institutional Review Board Statement
Not applicable.
Acknowledgments
Analytical research was undertaken using equipment of the NRC “Kurchatov Institute”—IREA Shared Knowledge Center, as well as the resource center “Physical methods of surface investigation” at the Saint Petersburg University Research Park. The work was carried out using the equipment of the center for collective use “Analytical Center of the IOMC RAS”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Grant, R.E. Tabular View of the Primary Divisions of the Animal Kingdom, Intended to Serve as an Outline of an Elementary Course of Recent Zoology (Caino-Zoology); Walton and Maberly: London, UK, 1861. [Google Scholar]
- Minchin, E. Sponges: The Porifera and Coelenterata. In A Treatise on Zoology, Part II; Lankaster, E., Ed.; Adam & Charles Black: London, UK, 1900; pp. 1–178. [Google Scholar]
- Cook, S.; Bergquist, P. Order Dictyoceratida Minchin, 1900. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J., van Soest, R., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; p. 1021. [Google Scholar]
- DeLaubenfels, M.; Storr, J. The taxonomy of American commercial sponges. Bull. Mar. Sci. 1958, 8, 99–117. [Google Scholar]
- Kim, M.M.; Mendis, E.; Rajapakse, N.; Lee, S.H.; Kim, S.K. Effect of spongin derived from Hymeniacidon sinapium on bone mineralization. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.L. The Cell Biology of Sponges; Springer-Verlag: New York, NY, USA, 1984. [Google Scholar]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural marine sponge fiber skeleton: A biomimetic scaffold. Tissue Eng. 2003, 9, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; Garrone, R. Characterization of a fibrillar collagen gene in sponges reveals the early evolutionary appearance of two collagen gene families. Proc. Natl. Acad. Sci. USA 1990, 87, 6669–6673. [Google Scholar] [CrossRef]
- Pallela, R.; Janapala, V.R. Comparative ultrastructural and biochemical studies of four demosponges from Gulf of Mannar, India. Int. J. Marine Sci. 2013, 3, 295–305. [Google Scholar] [CrossRef]
- Aouacheria, A.; Geourjon, C.; Aghajari, N.; Navratil, V.; Deléage, G.; Lethias, C.; Esposito, J.Y. Insights into early extracellular matrix evolution: Spongin short chain collagen-related proteins are homologous to basement membrane type IV collagens and form a novel family widely distributed in invertebrates. Mol. Biol. Evol. 2006, 23, 2288–2302. [Google Scholar] [CrossRef]
- Exposito, J.Y.; le Guellec, D.; Lu, Q.; Garrone, R. Short chain collagens in sponges are encoded by a family of closely related genes. J. Biol. Chem. 1991, 266, 21923–21928. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Hanke, T.; Meissner, H.; Born, R. Spongins: Nanostructural investigations and development of biomimetic material model. VDI Berichte 2003, 1803, 287–292. [Google Scholar]
- Gross, J.; Soka, Z.; Rougvie, M. Structural and chemical studies on the connective tissue of marine sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef]
- Garrone, R. The collagen of the Porifera. In Biology of Invertebrate and Lower Vertebrate Collagens; Bairati, A., Garrone, R., Eds.; Plenum Press: London, UK, 1985; pp. 157–175. [Google Scholar]
- Louden, D.; Inderbitzin, S.; Peng, Z.; de Nys, R. Development of a new protocol for testing bath sponge quality. Aquaculture 2007, 271, 275–285. [Google Scholar] [CrossRef]
- Von Lendenfeld, R. A Monograph of the Horny Sponges; Published for the Royal Society by Trübner and Co.: London, UK, 1889. [Google Scholar]
- Green, D. Tissue bionics: Examples in biomimetic tissue engineering. Biomed. Mater. 2008, 3, 034010. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Wing-Fu, L.; Han-Sung, J. Evolving marine biomimetics for regenerative dentistry. Mar. Drugs 2014, 12, 2877–2912. [Google Scholar] [CrossRef] [PubMed]
- Pronzato, R. Sponge-fishing, disease and farming in the Mediterranean Sea. Aquat. Conserv. 1999, 9, 485–493. [Google Scholar] [CrossRef]
- Van Treeck, P.; Eisinger, M.; Müller, J.; Paster, M.; Schuhmacher, H. Mariculture trials with Mediterranean sponge species: The exploitation of an old natural resource with sustainable and novel methods. Aquaculture 2003, 218, 439–455. [Google Scholar] [CrossRef]
- Petrenko, I.; Summers, A.P.; Simon, P.; Żółtowska-Aksamitowska, S.; Motylenko, M.; Schimpf, C.; Rafaja, D.; Roth, F.; Kummer, K.; Brendler, E.; et al. Extreme Biomimetics: Preservation of molecular detail in centimeter scale samples of biological meshes laid down by sponge. Sci. Adv. 2019, 5, eaax2805. [Google Scholar] [CrossRef]
- Jesionowski, T.; Norman, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine Spongin: Naturally Prefabricated 3D Scaffold-Based Biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef]
- Szatkowski, T.; Kopczyński, K.; Motylenko, M.; Borrmann, H.; Mania, B.; Graś, M.; Lota, G.; Bazhenov, V.V.; Rafaja, D.; Roth, F.; et al. Extreme biomimetics: A carbonized 3D spongin scaffold as a novel support for nanostructured manganese oxide(IV) and its electrochemical applications. Nano Res. 2018, 11, 4199. [Google Scholar] [CrossRef]
- Żółtowska, S.; Koltsov, I.; Alejski, K.; Ehrlich, H.; Ciałkowski, M.; Jesionowski, T. Thermal decomposition behaviour and numerical fitting for the pyrolysis kinetics of 3D spongin-based scaffolds. The classic approach. Polym. Test. 2021, 97, 107148. [Google Scholar] [CrossRef]
- Ma, H.; Li, C.; Zhang, M.; Hong, J.D.; Shi, G. Graphene oxide induced hydrothermal carbonization of egg proteins for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 17040–17047. [Google Scholar] [CrossRef]
- Kawahara, Y.; Ishibashi, N.; Yamamoto, K.; Wakizaka, H.; Iwashita, N.; Kenjo, S.; Nishikawa, G. Activated carbon production by co-carbonization of feathers using water-soluble phenolic resin under controlled graphitization. Sustain. Mater. Technol. 2015, 4, 18–23. [Google Scholar] [CrossRef][Green Version]
- Luo, D.; Zhu, B.; Li, Z.; Qin, X.; Wen, Y.; Shi, D.; Lu, Q.; Yang, M.; Zhou, H.; Liu, Y. Biomimetic organization of a ruthenium-doped collagen-based carbon scaffold for hydrogen evolution. J. Mater. Chem. A 2018, 6, 2311–2317. [Google Scholar] [CrossRef]
- Wang, Q.; Jian, M.; Wang, C.; Zhang, Y. Carbonized silk nanofiber membrane for transparent and sensitive electronic skin. Adv. Funct. Mater. 2017, 27, 1605657. [Google Scholar] [CrossRef]
- Lu, M.; Qian, Y.; Yang, C.; Huang, X.; Li, H.; Xie, X.; Huang, L.; Huang, W. Nitrogen-enriched pseudographitic anode derived from silk cocoon with tunable flexibility for microbial fuel cells. Nano Energy 2017, 32, 382–388. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yun, Y.S.; Lee, S.; Jang, D.; Park, K.Y.; Kim, J.K.; Kim, B.H.; Kang, K.; Kaplan, D.L.; Jin, H.-J. Carbonization of a stable b-sheet-rich silk protein into a pseudographitic pyroprotein. Nat. Commun. 2015, 6, 7145. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, Z.; Ren, Y.; Zhao, L.; Wang, S.; Yu, J. Activated carbon with micrometer-scale channels prepared from luffa sponge fibers and their application for supercapacitors. RSC Adv. 2014, 4, 35789–35796. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Gotoh, Y.; Morikawa, H.; Miura, M.; Fujimori, Y.; Nagura, M. Carbon fiber from natural biopolymer Bombyx mori silk fibroin with iodine treatment. Carbon 2007, 45, 1035–1042. [Google Scholar] [CrossRef]
- Sivkov, D.V.; Petrova, O.V.; Nekipelov, S.V.; Vinogradov, A.S.; Skandakov, R.N.; Bakina, K.A.; Isaenko, S.I.; Ob’edkov, A.M.; Kaverin, B.S.; Vilkov, I.V.; et al. Quantitative Characterization of Oxygen-Containing Groups on the Surface of Carbon Materials: XPS and NEXAFS Study. Appl. Sci. 2022, 12, 7744. [Google Scholar] [CrossRef]
- Batson, P.E. Carbon 1s near-edge-absorption fine structure in graphite. Phys. Rev. B 1993, 48, 2608–2610. [Google Scholar] [CrossRef]
- Norman, M.; Bartczak, P.; Zdarta, J.; Tylus, W.; Szatkowski, T.; Stelling, A.L.; Ehrlich, H.; Jesionowski, T. Adsorption of C.I. Natural Red 4 onto Spongin Skeleton of Marine Demosponge. Materials 2015, 8, 96–116. [Google Scholar] [CrossRef]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, M.H. Crystal and molecular structure of a collagen-like peptide at 1.9 A resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef]
- Junqua, S.; Robert, L.; Vacelet, J.; Garrone, R.; De Ceccatty, M.P.; Vacelet, J. Biochemical and morphological studies on collagens of horny sponges. Ircinia filaments compared to spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Sivkov, D.; Petrova, O.; Mingaleva, A.; Ob’edkov, A.; Kaverin, B.; Gusev, S.; Vilkov, I.; Isaenko, S.; Bogachuk, D.; Skandakov, R.; et al. The Structure and Chemical Composition of the Cr and Fe Pyrolytic Coatings on the MWCNTs’ Surface According to NEXAFS and XPS Spectroscopy. Nanomaterials 2020, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Sivkov, D.; Nekipelov, S.; Petrova, O.; Vinogradov, A.; Mingaleva, A.; Isaenko, S.; Makarov, P.; Obedkov, A.; Kaverin, B.; Gusev, S.; et al. Studies of Buried Layers and Interfaces of Tungsten Carbide Coatings on the MWCNT Surface by XPS and NEXAFS Spectroscopy. Appl. Sci. 2020, 10, 4736. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Noh, H.-J.; Kim, J.-Y.; Jin, M.H.; Park, C.Y.; Lee, Y.H. X-ray Absorption Spectroscopy of Graphite Oxide. Europhys. Lett. 2008, 82, 67004. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Popova, I.; Yates, J.T.; Bronikowski, M.J.; Huffman, C.B.; Liu, J.; Smalley, R.E.; Hwu, H.H.; Chen, J.G. Oxygen-Containing Functional Groups on Single-Wall Carbon Nanotubes: NEXAFS and Vibrational Spectroscopic Studies. J. Am. Chem. Soc. 2001, 123, 10699–10704. [Google Scholar] [CrossRef]
- Kaznacheyev, K.; Osanna, A.; Jacobsen, C.; Plashkevych, O.; Vahtras, O.; Ågren, H.; Carravetta, V.; Hitchcock, A.P. Innershell Absorption Spectroscopy of Amino Acids. J. Phys. Chem. A 2002, 106, 3153–3168. [Google Scholar] [CrossRef]
- Madix, R.J.; Solomon, J.L.; Stöhr, J. The Orientation of the Carbonate Anion on Ag(110). Surf. Sci. 1988, 197, L253–L259. [Google Scholar] [CrossRef]
- Ikeura-Sekiguchi, H.; Sekiguchi, T. Adsorption Structure of Formic Acid on Si(100) Studied by Surface NEXAFS. Surf. Sci. 1999, 433, 549–553. [Google Scholar] [CrossRef]
- Lehmann, J.; Solomon, D.; Brandes, J.; Fleckenstein, H.; Jacobson, C.; Thieme, J. Synchrotron-Based Near-Edge X-ray Spectroscopy of Natural Organic Matter in Soils and Sediments. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 729–781. [Google Scholar]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution in Situ X-ray-Based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Kummer, K.; Sivkov, V.N.; Vyalikh, D.V.; Maslyuk, V.V.; Blüher, A.; Nekipelov, S.V.; Bredow, T.; Mertig, I.; Mertig, M.; Molodtsov, S.L. Oscillator strength of the peptide bond p* resonances at all relevant x-ray absorption edges. Phys. Rev. B 2009, 80, 155433. [Google Scholar] [CrossRef]
- Erbahar, D.; Susi, T.; Rocquefelte, X.; Bittencourt, C.; Scardamaglia, M.; Blaha, P.; Guttmann, P.; Rotas, G.; Tagmatarchis, N.; Zhu, X.; et al. Spectromicroscopy of C60 and azafullerene C59N: Identifying surface adsorbed water. Sci. Rep. 2016, 6, 35605. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Schedel-Niedrig, T.; Wohlers, M.; Herein, D.; Herzog, B.; Schlögl, R.; Keil, M.; Bradshaw, A.M.; Kirschner, J. Reaction of molecular oxygen with C60: Spectroscopic studies. J. Chem. Soc. Faraday Trans. 1994, 90, 403–409. [Google Scholar] [CrossRef]
- Banerjee, S.; Hemraj-Benny, T.; Balasubramanian, M.; Fischer, D.A.; Misewich, J.A.; Wong, S.S. Surface Chemistry and Structure of Purified, Ozonized, Multiwalled Carbon Nanotubes Probed by NEXAFS and Vibrational Spectroscopies. ChemPhysChem 2004, 5, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Senoz, E.; Wool, R.P.; McChalicher, C.W.; Hong, C.K. Physical and chemical changes in feather keratin during pyrolysis. Polym. Degrad. Stabil. 2012, 97, 297–307. [Google Scholar] [CrossRef]
- Brebu, M.; Spiridon, I. Thermal degradation of keratin waste. J. Anal. Appl. Pyrolysis 2011, 91, 288–295. [Google Scholar] [CrossRef]
- Kezuka, Y.; Kawai, K.; Eguchi, K.; Tajika, M. Article Fabrication of Single-Crystalline Calcite Needle-Like Particles Using the Aragonite–Calcite Phase Transition. Minerals 2017, 7, 133. [Google Scholar] [CrossRef]
- Georgieva, V.; Vlaev, L.; Gyurova, K. Non-Isothermal Degradation Kinetics of CaCO3 from Different Origin. J. Chem. 2013, 2013, 872981. [Google Scholar] [CrossRef]
- Li, X.G.; Lv, Y.; Ma, B.G.; Wang, W.Q.; Jian, S.W. Decomposition kinetic characteristics of calcium carbonate containing organic acids by TGA. Arab. J. Chem. 2017, 10, S2534–S2538. [Google Scholar] [CrossRef]
- Vinnichenko, V.; Riazanov, A.; Riazanov, A. Influence of Organic Matters on the Calcium Carbonate Decarbonization Process. Mater. Sci. Forum 2019, 968, 35–43. [Google Scholar] [CrossRef]
- L’vov, B.V. Mechanism and kinetics of thermal decomposition of carbonates. Thermochim. Acta 2002, 386, 1–16. [Google Scholar] [CrossRef]
- Lin, S.; Kiga, T.; Wang, Y.; Nakayama, K. Energy analysis of CaCO3 calcination with CO2 capture. Energy Procedia 2011, 4, 356–361. [Google Scholar] [CrossRef]
- Gupta, H.; Fan, L.S. Carbonation-Calcination Cycle Using High Reactivity Calcium Oxide for Carbon Dioxide Separation from Flue Gas. Ind. Eng. Chem. Res. 2002, 41, 4035–4042. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).