Abstract
The structure and electrochemical characteristics of composites based on multi-walled carbon nanotubes (MWCNTs) and manganese oxide with the addition of rhenium oxide has been studied. It has shown that the decorating of the MWCNT surface with layers or nanoparticles of manganese oxide (Mn(III) + Mn(IV)) provides more than a twofold increase in the value of the specific capacitance at low potential scan rates. However, composites based only on manganese oxide exhibit poor electrochemical behavior and the value of the specific capacitance decreases rapidly with increasing potential scan rate due to the limitation of diffusion processes. The addition of rhenium oxide to composites significantly increases their electrochemical properties due to changes in the chemical composition and morphology of composites. Studies of the structure and chemical state have shown that an improvement in the specific capacitance is provided by increasing in the proportion of Mn(IV) oxide in such composites, which has the ability to rapidly and completely reverse redox reactions and has lower electrical resistance values, compared to Mn(III) oxide. A detailed analysis of the voltammetric data showed that an increase in the rate capability in composites with the addition of rhenium oxide can also be provided by increasing the availability of the electrode surface for electrolyte ions and increasing the amount of charge stored due to the formation of a double electric layer.
1. Introduction
Currently, electrochemical devices, such as supercapacitors, are increasingly used in various fields, where high pulse power is required for fairly short time intervals and high cyclic stability [1,2,3]. Examples of their application are hybrid electric vehicles, wind power structures or uninterruptible power supplies (UPS). In accordance with the charge accumulation mechanism, supercapacitors are usually divided into electric double layer capacitors (EDLC) and pseudocapacitors [4,5]. The accumulation and conversion of energy into EDLC is carried out due to the separation of static charge in the Helmholtz layer. Various carbon materials (such as carbon black, activated carbon, few-layer graphene, etc.) are often used as the basis for the production of electrodes of this type of supercapacitors, providing a high speed of the charging and discharging process, as well as sufficient cyclic stability [5,6,7,8,9,10,11]. At the same time, their specific capacitance and energy density (<10 Wh/kg) are low [12]. Another type of supercapacitor is pseudocapacitors. The specific capacitance and energy density (20 Wh/kg and higher) of pseudocapacitors compared to EDLC can be significantly improved by reversible redox reactions (Faraday processes) on the surface or in the bulk of the electrode material [13]. Transition metal oxides [14,15,16] are commonly used as electrode materials for pseudocapacitors. Among transition metal oxides, manganese oxides are a very promising option, which have a high theoretical specific capacitance up to 1370 F/g, as well as low toxicity and production cost [17,18]. There are several kinds of stable manganese oxide in nature: MnO (Mn(II) oxide), Mn3O4 (Mn(II, III) oxide), Mn2O3 (Mn(III) oxide), MnO2 (Mn(IV) oxide) and Mn2O7 (Mn(VII) oxide) [19,20]. However, these materials have significant disadvantages when used as supercapacitor electrodes, namely: they are characterized by poor structural stability and low conductivity due to a significant change in volume during the charge–discharge process [21]. Therefore, different approaches are used to improve the stability of electrodes containing manganese oxide. One of these approaches is to apply MnxOy (where x = 1, 2 or 3, and y = 1, 3 or 4) nanostructures to a carbon matrix, which has high conductivity, on the one hand, and high specific surface area and flexibility, on the other [22]. In this regard, it is advisable to use composite nanomaterials based on promising carbon materials (carbon nanotubes (CNTs) or graphene) and manganese oxide as the basis of pseudocapacitor electrodes, demonstrating their unique characteristics during energy storage and conversion [23,24,25,26,27,28]. An analysis of the literature has shown that many works have been devoted to nanostructured materials based on MnO2, while some electrode materials based on MnxOy have great potential for use as supercapacitor materials, in particular, Mn3O4-based [21,29,30]. Moreover, as shown in [30,31,32,33], annealing to 600 °C allows the transformation of amorphous or aqueous MnOx into Mn2O3 or Mn3O4 with a crystalline structure. It should be noted that in terms of the crystallinity of manganese oxide, the phase in which it is located, its distribution over the surface of the carbon matrix and interaction with it depend on the final capacitive characteristics of the electrode.
Another interesting metal for supercapacitors is rhenium oxide, a metal belonging to the manganese group that has several oxidation states as manganese. In particular, it was shown in [34] that rhenium-modified activated carbon (AC) showed high values of specific capacitance (57 F/g at a discharge current of 0.2 A/g) and cyclic stability (more than 800 charge/discharge cycles). Nevertheless, it was shown in [35] that the co-deposition of manganese and rhenium oxide on the AC surface leads to a significant improvement in the catalytic properties of such a composite material. According to the authors, the observed improvement in characteristics is associated, on the one hand, with an increase in the dispersion of manganese, and, on the other hand, with the diversity of the chemical state of manganese in the presence of rhenium. Unfortunately, research in this area is mostly limited and does not contain detailed information about the structure of the material and its electrochemical properties when using other carbon materials, such as carbon nanotubes, carbon nanofibers, graphene or fullerene with co-deposited manganese and rhenium oxides.
In our previous work [36], we began to study the structure and electrochemical properties of the freshly prepared composite, based on manganese oxide and nitrogen-doped MWCNTs (N-MWCNTs). We have shown that the formed composite contains a sufficiently large number of oxygen-containing functional groups of various types on the outer walls of carbon nanotubes, which ensure the fixation of manganese oxide particles on their surface. However, despite the increase from 20 to 34 F/g of the specific capacitance of an electrode based on a composite in comparison with N-MWCNTs, the electrochemical characteristics of such a material are low.
In this work, to improve the electrochemical properties of a composite based on manganese oxide and MWCNTs, we use a combined approach based on the thermal post-treatment of the synthesized materials and the introduction of rhenium as an additional component to change the chemical state of manganese oxide and increase the proportion of Mn(IV). To characterize composites, we used scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and the cyclic voltammetry (CV) method.
2. Materials and Methods
2.1. Composite Synthesis
In this work, commercial MWCNTs as a basis for composites were used [37]. These CNTs were synthesized by chemical vapor deposition (CVD) decomposition of ethylene in the presence of a Fe-Co bimetallic catalyst at a temperature of 670 °C [37,38]. The average diameter of MWCNTs was about 20 nm, and the number of graphene layers was about 12–14 pieces. The concentration of carbon atoms in MWCNTs sample was more than 99.4 at.%. The MWCNTs before composite synthesis were treated in a 56% aqueous solution of nitric acid for 1 min, followed by washing in distilled water and drying in air at room temperature for 24 h. This procedure was carried out to increase the wettability of the MWCNTs surface during subsequent exposure in a solution of potassium permanganate. Next, the MWCNTs were mixed with KMnO4 powder in a mass ratio of 7:3, followed by dissolution in distilled water and exposure of the solution for 24 h under normal conditions. Then, the composites were washed with distilled water and dried in air at 60 °C for 24 h. To change the structure and phase composition of manganese oxide, the composites were annealed in a vacuum furnace at a pressure of about 1 Pa and temperatures of 580 and 700 °C for 30 min. To add rhenium, annealed samples of composites with manganese oxide were used, which were placed for 24 h in a 3% NH4ReO4 solution, followed by washing in distilled water. Next, the composites were annealed in vacuum at a pressure of 1 Pa and a temperature of 360 °C for the decomposition of ammonium perrhenate and the formation of rhenium oxide [39]. For convenience, all samples are presented in Table 1 with their designation.

Table 1.
Designation of samples.
2.2. Characterization of the Samples
2.2.1. SEM
The surface topography of the samples was studied using a Carl Zeiss AURIGA Laser microscope (Carl Zeiss Microscopy, Jena, Germany), operating in Dual-Beam SEM-Focused Ion Beam (FIB) mode. Registration of SEM images was performed at 10 kV and a magnification of 200,000 times. Obtaining information on the chemical composition of the samples by energy dispersive X-ray analysis (EDX) was carried out using the INCAx-act attachment (Oxford Instruments, UK, Abingdon) to the microscope.
2.2.2. XRD
X-ray diffraction measurements were performed to determine the structure and presence of various phases in the materials under study using a Bruker D2 Phaser X-ray diffractometer (Bruker Corporation, Billerica, MA, USA) and Cu-Kα radiation with an accelerating voltage of 30 kV. Registration of XRD patterns was carried out in the range of 2θ = 5.0017−80.0067° with a step of 0.0202°. To process XRD patterns, we used the DIFFRAC.SUITE software package with ICDD RDB Databases.
2.2.3. XPS
The overview and core-level (C1s, O1s, Mn2p, Re4f) photoemission (PE) spectra were registered by a double-focusing full 180° spherical sector electron analyzer of the ESCALAB 250 Xi (Thermo Fisher Scientific, Waltham, MA, USA) spectrometer at an exciting photon energy of 1487 eV (Al-anticathode). The binding energy scale was calibrated to the Au 4f7/2 peak and the Fermi level recorded on pure gold foil. When registering the overview and core-level spectra, the analyzer operated in the constant analyzer energy (CAE) mode with a pass energy of 50 and 10 eV, respectively. A detailed core-level peak analysis was performed by peak fitting using the Gaussian/Lorentzian product formula by Casa XPS 2.3.16 software [40].
2.2.4. Electrochemical Measurements
Electrochemical characteristics of composite samples were evaluated by cyclic voltammetry (CV) using a potentiostat-galvanostat P-40X (Elins, Zelenograd, Russia). CV curves were recorded using a three-electrode scheme. A platinum plate 10 × 15 mm2 in size served as a counter electrode. A standard electrode Ag|AgCl with a KCl was the reference electrode. A one molar aqueous solution of Na2SO4 was used as the electrolyte. The working electrodes based on MWCNTs or composites were produced with the addition of polyvinylidene fluoride (PVDF) as a binder (10 wt.%) and N-Methyl-2-Pyrrolidone (NMP) as a solvent. The resulting substance was applied to glass and dried for two hours at a temperature of 80 °C and a pressure of about 1 Pa and then rolled into flat plates with a thickness of less than 50 μm using rollers. After that, the electrodes were dried under the same conditions for 12 h to remove NMP residues. The total specific capacitance Cs of the electrode was calculated based on the formula Cs = A/(∆U V m), where A is the area under the CV curve with a positive potential course, ∆U is the potential window, V is the scan rate and m is the mass of MWCNTs or composites.
3. Results
3.1. SEM
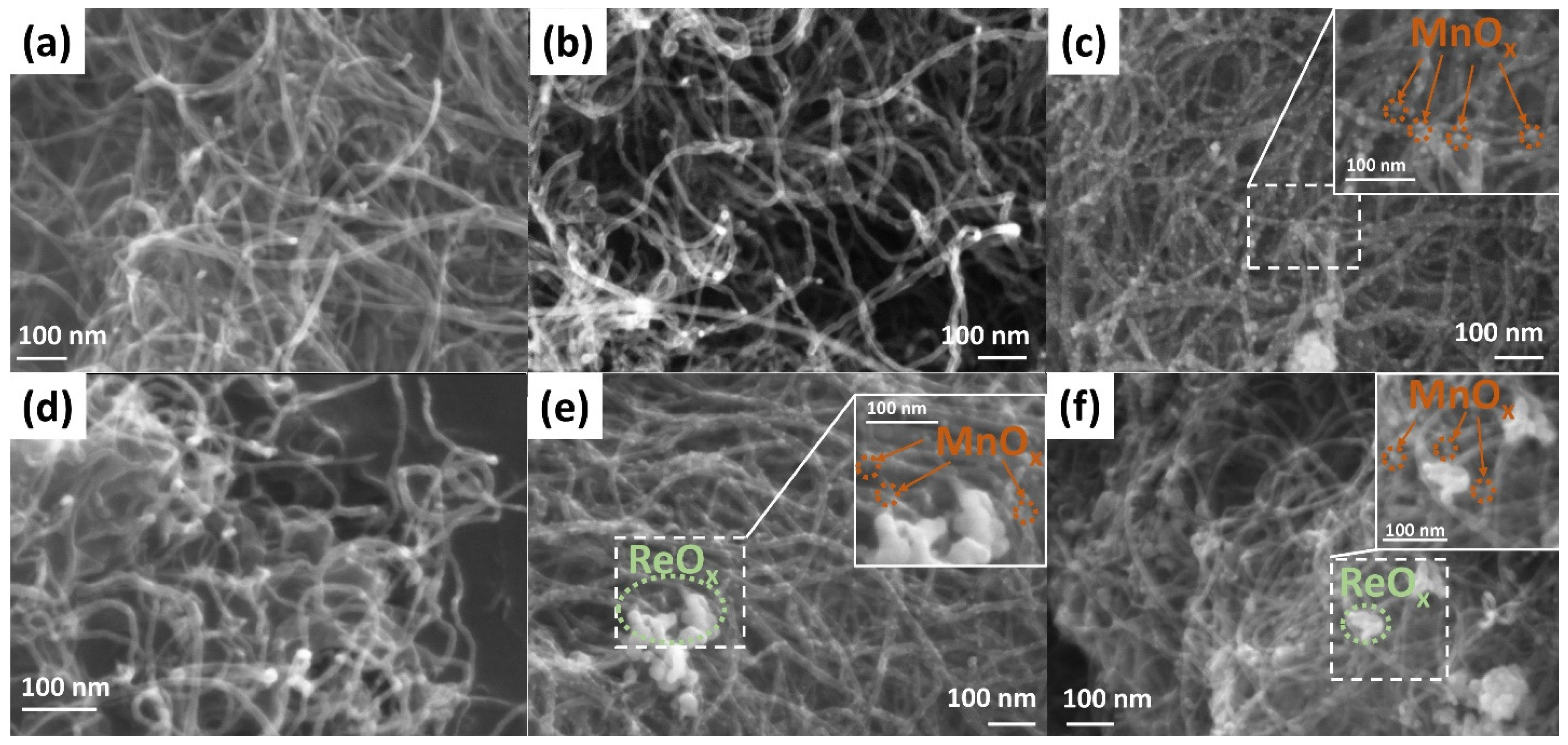
Figure 1a shows the SEM image of MWCNTs. The average outer diameter of nanotubes ranges from 9 to 16 nm. The Mn-0 composite looks similar to MWCNTs (Figure 1b), however, an irregular increase in the diameter of individual MWCNTs is observed, apparently due to the presence of a manganese oxide layer with different thicknesses on their surface. The presence of manganese on the MWCNTs surface is also confirmed by the EDX results (Figure S1). A comparative analysis of the average outer diameter, carried out on individual nanotubes (MWCNTs and Mn-0 samples), showed that the thickness of the manganese oxide layer in the Mn-0 composite is about 10–20 nm. The Mn-580 sample looks strikingly different (Figure 1c), namely: on the CNT surface, there are spherical particles with a characteristic size of 5–20 nm, fairly evenly distributed over the entire surface of the MWCNTs. Apparently, the formation of nanoparticles is due to the processes of the thermally initiated crystallization of manganese oxide. Rhenium (Re-360) formed on the surface of MWCNTs, just like manganese oxide in the Mn-0 sample, covers individual sections of nanotubes with a layer that has a different thickness in different areas (Figure 1d). The insertion of rhenium into the Mn-580 composite (Mn-580 + Re sample) leads to a decrease in the characteristic particle size of manganese oxide below 15 nm (Figure 1e). Herewith, rhenium is distributed in the form of agglomerates with a characteristic size of 50–100 nm. The presence of manganese and rhenium in this sample is confirmed by the EDX results (Figure S2). In the Mn-700 + Re sample, an insignificant amount of manganese oxide particles is observed on the CNT surface, and rhenium particles are combined into larger agglomerates than in the Mn-580 + Re sample (Figure 1e,f). We believe that part of the manganese oxide may be present in the agglomerates separately or together with rhenium, since a number of processes occur as a result of annealing in a vacuum at a temperature of 700 °C: (i) on the one hand, the restoration of the graphene structure of MWCNTs results in any functional surface oxygen-containing groups being removed [41,42], thereby increasing the hydrophobicity of the MWCNTs surface, (ii) on the other hand, the association of separate particles of manganese oxide and their crystallization.
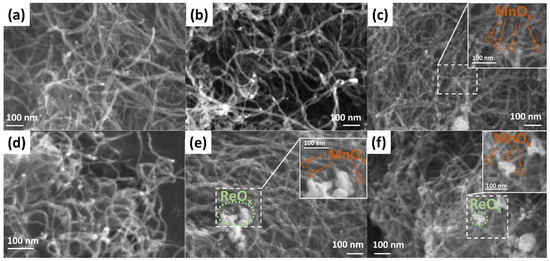
Figure 1.
SEM images for (a) MWCNTs and composites: (b) Mn-0; (c) Mn-580; (d) Re-360; (e) Mn-580 + Re; (f) Mn-700 + Re.
3.2. XRD
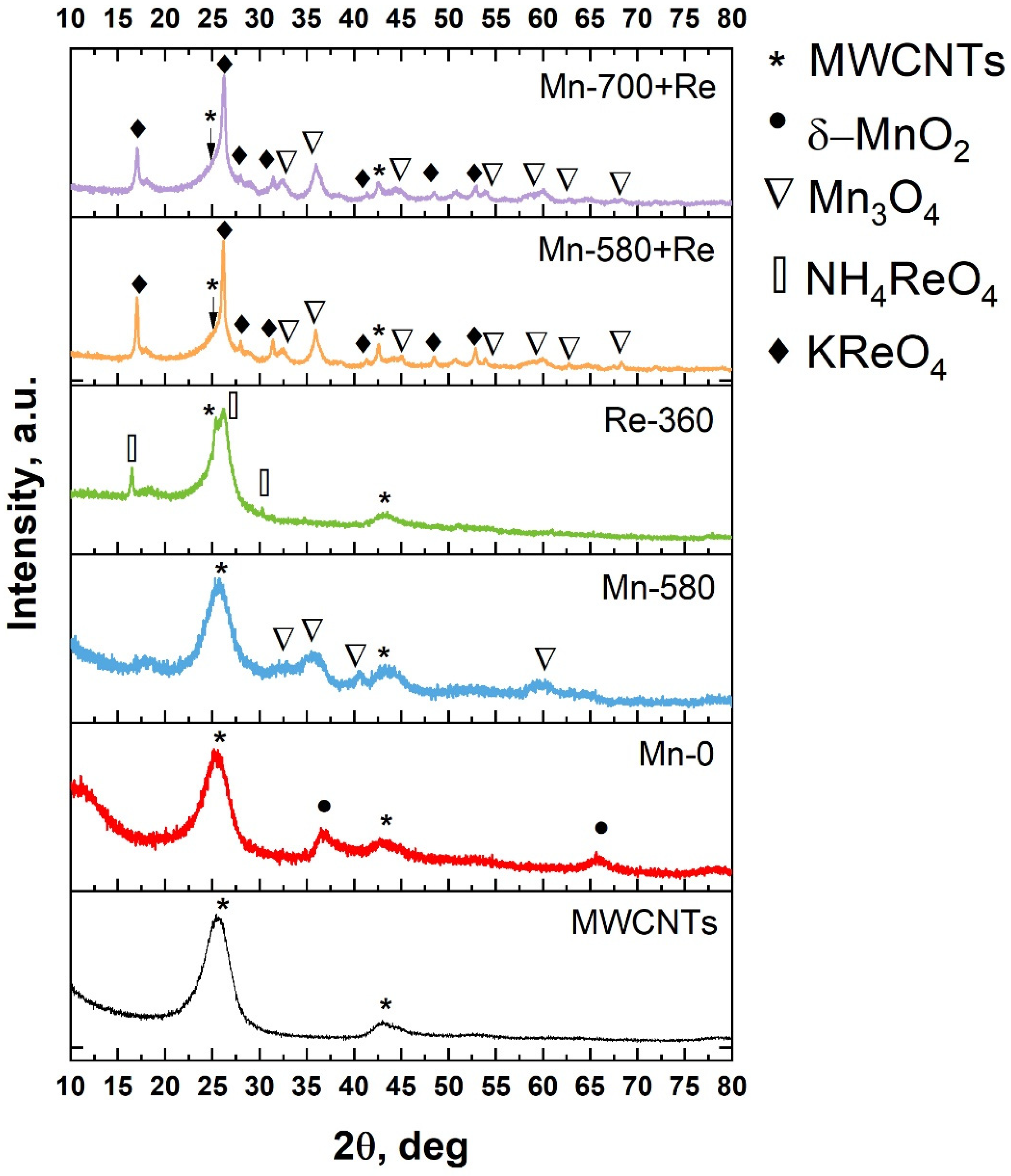
In Figure 2, the XRD pattern for MWCNTs and composites are shown. As can be seen, in the case of MWCNTs, an intense peak is observed at 2θ = 25.5°, which corresponds to the (002) reflection. The shift towards small angles by 1° relative to graphite (26.15°; ICDD (PDF-2/Release 2011 01-073-5918 01-073-5918) and the broadening of the CNT (002) peak indicates an increase in the interplanar spacing (up to 3.5 Å) [43]. This result is associated with the pretreatment of MWCNTs in nitric acid, which damages their outer layers. Another diffraction peak near 43° is associated with reflection from (100) planes. The XRD spectrum of the Mn-0 composite differs from that of the MWCNTs by the presence of two additional peaks at angles of 36.65 and 65.63°, which correspond to (110) and (020) planes, with d = 2.45 Å and d = 1.42 Å of K-birnessite manganese oxide (δ-MnO2), respectively. The existence of the latter may be due to the fact that part of the MnO2 formed as a result of the redox reaction between MWCNTs and KMnO4 (4MnO4 + 3C + H2O→4MnO2 + CO32− + 2HCO3−) being doped with K [44,45]. The presence of low-intensity peaks and their broadening indicates a low concentration of manganese oxide in the sample and, most likely, it is in a nanocrystalline state; this is also evidenced by SEM data (Figure 1). The thermal treatment of Mn-0 composite (sample Mn-580) leads to the appearance of a series of new peaks in the diffraction pattern. According to the ICDD database (PDF-2/Release 2011 DB card number 01-071-6262), the peaks at 32.5, 35.97, 40.53 and 59.4° correspond to dimanganese(III) (Mn3O4). As in the case of Mn-0, manganese in this sample is most likely in the nanocrystalline state, as evidenced by the presence of diffuse and low-intensity diffraction peaks. The analysis of the diffractogram of the Re-360 sample showed the presence of peaks at angles of 16.5, 26.17 and 30.24°. According to [46], these peaks can be attributed to the reflection from the (011), (112) and (020) planes of ammonium tetraoxorhenate (VII). The presence of the latter is due to incomplete thermal decomposition. In this case, the rhenium oxide formed as a result of heating NH4ReO4 is most likely in a nanocrystalline or amorphous state. The XRD patterns of the last two samples (Mn-580 + Re and Mn-700 + Re) contain a large number of peaks that belong to the two types of phases Mn3O4 (dimanganese(III)) and KReO4 (ICDD PDF-2/Release 2011 DB card number 01-071-6262 and PDF-2/Release 2011 DB card number 00-008-0044, respectively). Wherein, the phase composition does not change depending on the treatment temperature.
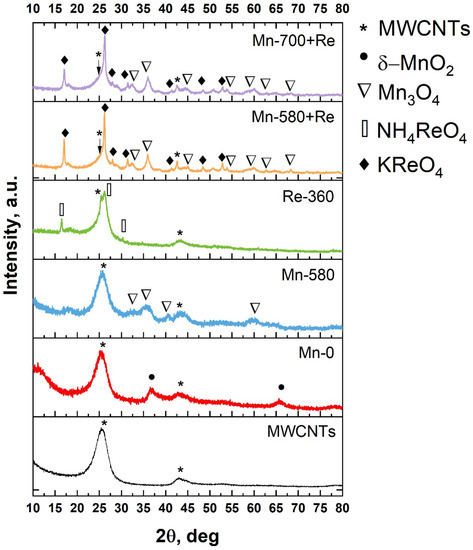
Figure 2.
XRD patterns for MWCNTs and composites based on them.
The presence of the KReO4 phase can be explained by a number of processes: (i) thermal decomposition of KMnO4 with the formation of products containing potassium [47]; (ii) thermal decomposition of NH4ReO4 with the formation of intermediate products, including ReO2 [48]; (iii) the interaction of potassium-containing products with ReO2 to form KReO4.
3.3. XPS
The chemical composition of all samples was determined by XPS using the overview spectra data (Figure S3); the results are summarized in Table 2. This method of analysis is surface-sensitive and makes it possible to analyze thin (up to 5 nm) layers of material. An analysis of overview PE spectra showed that the surface layers of MWCNTs samples and composites do not contain any foreign impurities, except for a trace amount of potassium in the composition of the Mn-0 composite. The intensity of the potassium 2p lines in the overview spectrum of this sample barely exceeded the noise level. Low-intensity K2p peaks were also observed in the high-energy region of the C 1s PE spectrum, measured at lower analyzer pass energy and with higher accumulation statistics (see Figure S4). According to our estimates, the concentration of this element on the surface of the composite was less than 0.05 at.% and was not taken into account in quantitative calculations. The concentration of manganese and rhenium in the composites is approximately 3.3 and 1.5 at.%, respectively. In this case, the high value of the oxygen concentration for the composites indicates the presence of metals in the oxidized state.

Table 2.
Chemical analysis of samples by XPS.
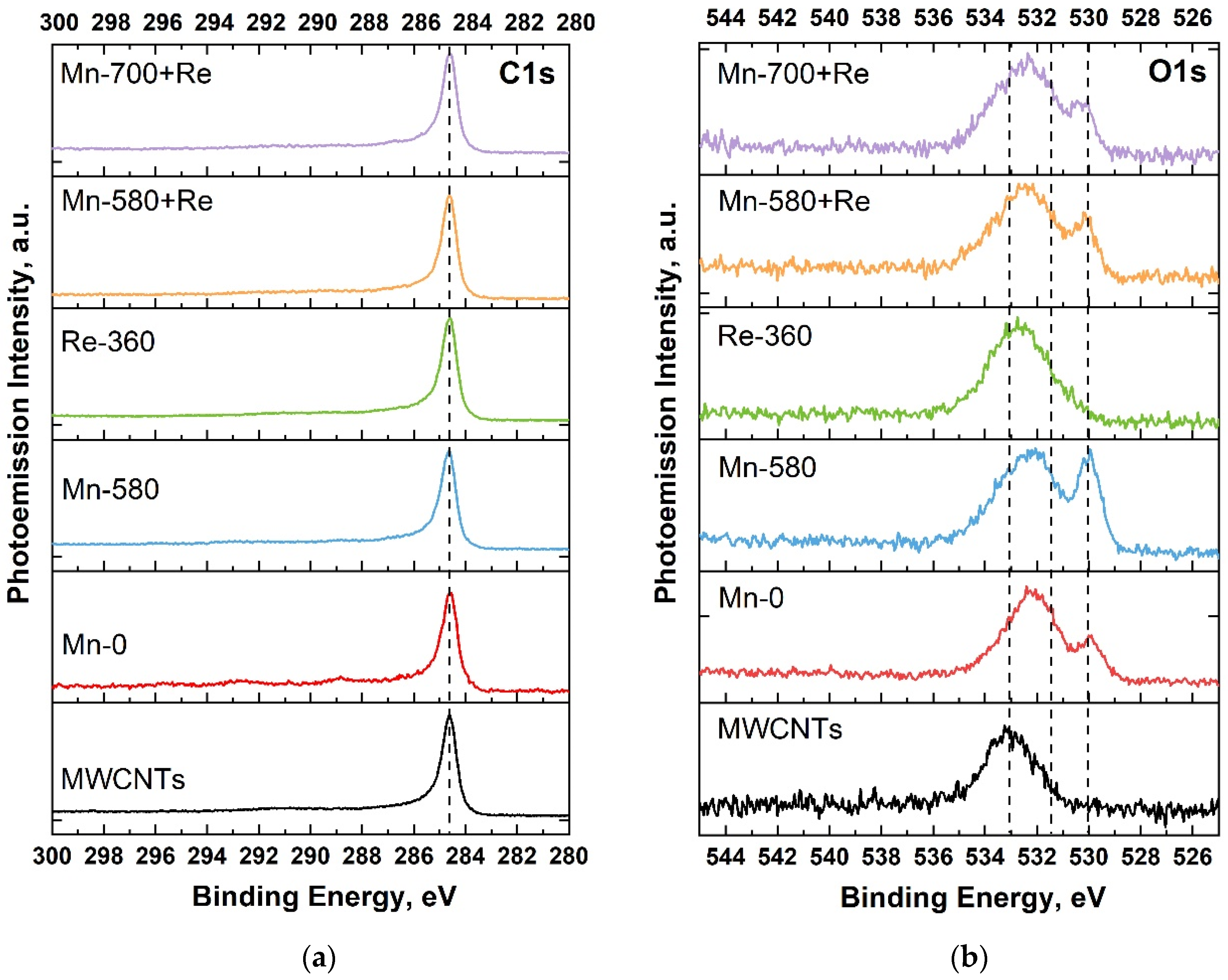
Figure 3a shows the C1s PE spectra for all samples. It can be seen that the position of the maximum of the C1s line practically does not change from sample to sample and is about 284.6 eV (which corresponds to C=C bonds (sp2) [49]), and the FWHM of the lines has a close value of 0.7 eV (Table 2). This result indicates that after the deposition of manganese oxide, heat treatments, and the addition of rhenium, the structure of the nanotubes is not disturbed. For Mn-580 + Re and Mn-700 + Re samples, a slight decrease in the FWHM value is even observed, which may indicate an improvement in the crystal structure of nanotubes due to double heat treatment at the stages of manganese and then rhenium oxide deposition. This leads to the annealing of structural defects and the removal of functional oxygen-containing groups formed as a result of the pretreatment of MWCNTs in nitric acid.
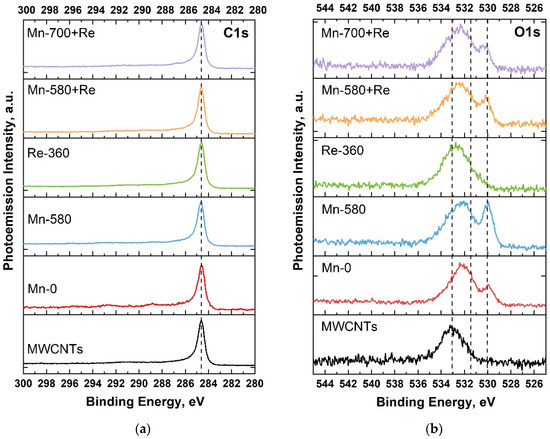
Figure 3.
C1s (a) and O1s (b) PE spectra of MWCNTs and composites.
Figure 3b shows the O1s PE spectra for the systems under study. As can be seen, in all Mn-0, Mn-580, Mn-580 + Re and Mn-700 + Re composites, two peaks are distinguished, which belong to different oxygen-containing bonds. So, manganese oxide (Mn–O bond) is located at 529.5–530.5 eV, whereas C=O bond and water (H–O–H) is at 531.8–532.2 and 532–534 eV, respectively [50,51]. In the case of the Re-360 composite, oxygen is present as a single broad peak. This is due to the fact that oxygen, in bonding with rhenium, is located at 531–532 eV and is masked under the oxygen peak in C=O and water (H–O–H) [50].
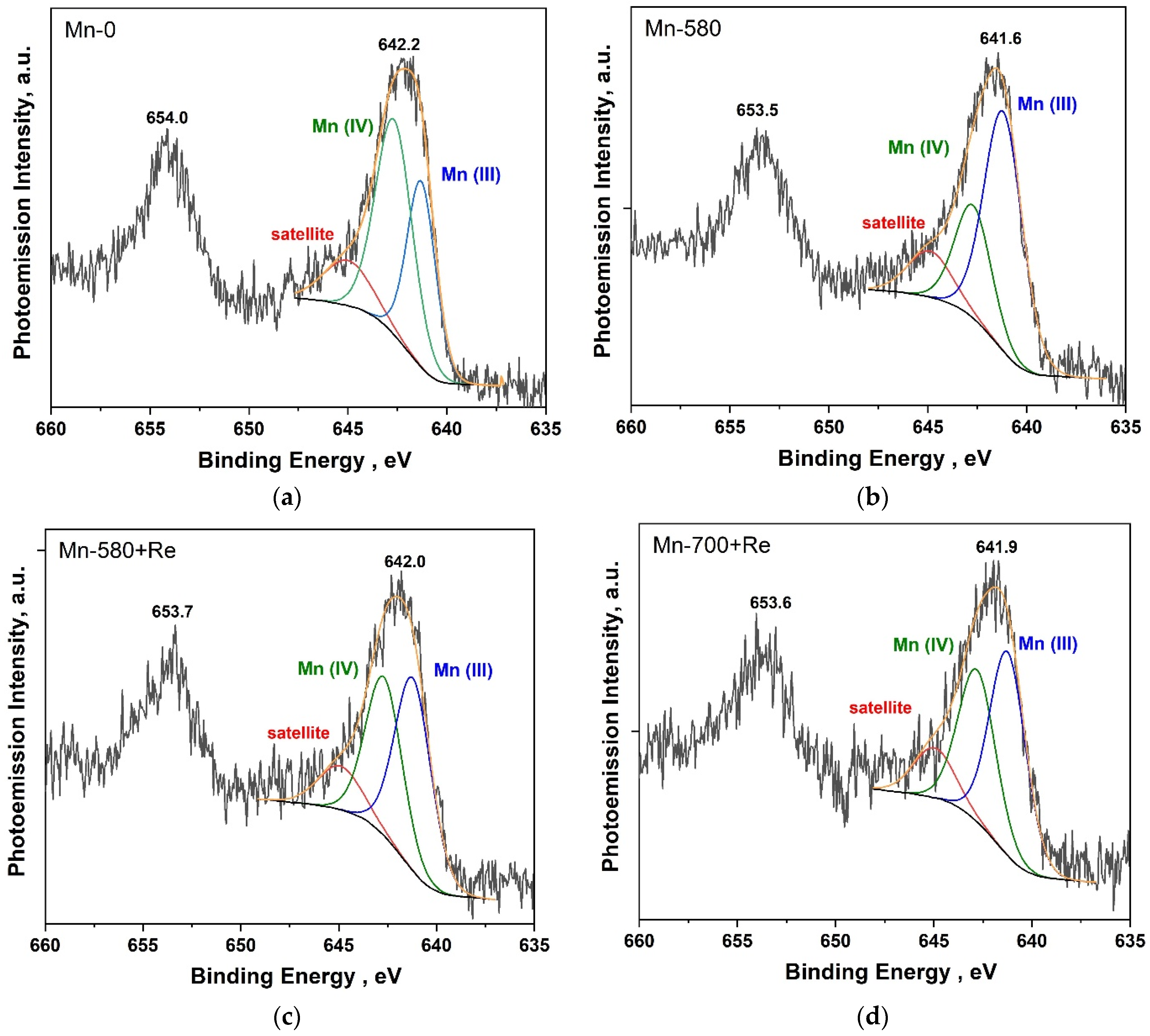
Figure 4 shows the Mn2p PE spectra of Mn-0, Mn-580, Mn-580 + Re and Mn-700 + Re composites. The analysis of the chemical state was carried out using the most intense Mn2p3/2 doublet line (binding energy of about 642 eV). The fitting of the spectra into components was carried out taking into account the position of the states for Mn(III) and Mn(IV) oxides, which are about 641.3 and 642.5 eV, respectively [52]. It was also taken into account that in the high-energy region of the Mn2p3/2 spectrum for manganese oxides, states are observed due to the effect of multiplet splitting and also observed in the PE spectra of other transition metals [52,53]. We approximated these states using a component with a binding energy of about 645 eV (indicated in the figure as satellite). The relative share of this component in all spectra was about 14% and was not taken into account in the final assessment of the content of various manganese oxides (Mn(III) and Mn(IV)) in the samples. The maximum of the Mn 2p3/2 in the PE spectrum of the Mn-0 composite (Figure 4a) is located at a binding energy of 642.2 eV. The approximation result shows that Mn(IV) oxide predominates—the relative proportion of the corresponding component is 59.7% (Table 3). In the Mn2p PE spectrum of the Mn-580 composite (Figure 4b), the maximum is significantly shifted towards low-binding energies (the offset is −0.6 eV), which is most likely due to a significant increase in the proportion of Mn(III) oxide in the composite. Apparently, when the composite was annealed in an inert atmosphere or vacuum at a given temperature, Mn(IV) oxide disproportionated to form Mn(III) oxide [23,54,55]. In the Mn2p PE spectra of the heat-treated composites, after the addition of rhenium oxide (Figure 4c,d), the maximum shifts to high-binding energies (relative to the spectrum of the Mn-580 composite), and its width increases significantly (Table 3). The result of the spectrum approximation showed that after the addition of rhenium in the composite, the proportion of Mn(III) oxide increases. At the same time, the relative areas of the components corresponding to Mn(III) and Mn(IV) oxides in the Mn-580 + Re and Mn-700 + Re composites have fairly close values with a slightly higher Mn(IV) content in the Mn-580 + Re composite.
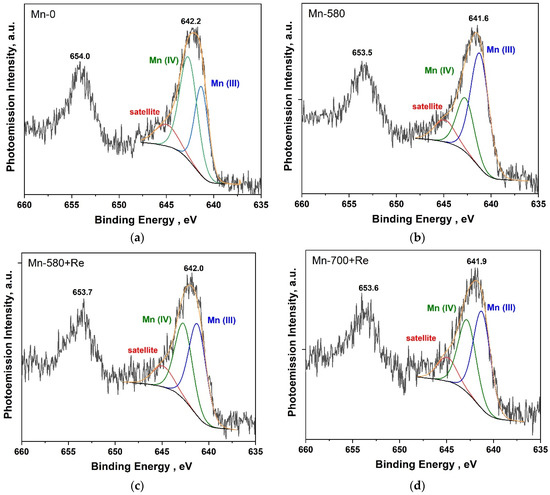
Figure 4.
Mn 2p PE spectra of the composites: Mn-0 (a), Mn-580 (b), Mn-580 + Re (c), Mn-700 + Re (d).

Table 3.
Results of the analysis of Mn 2p PE spectra of composites.
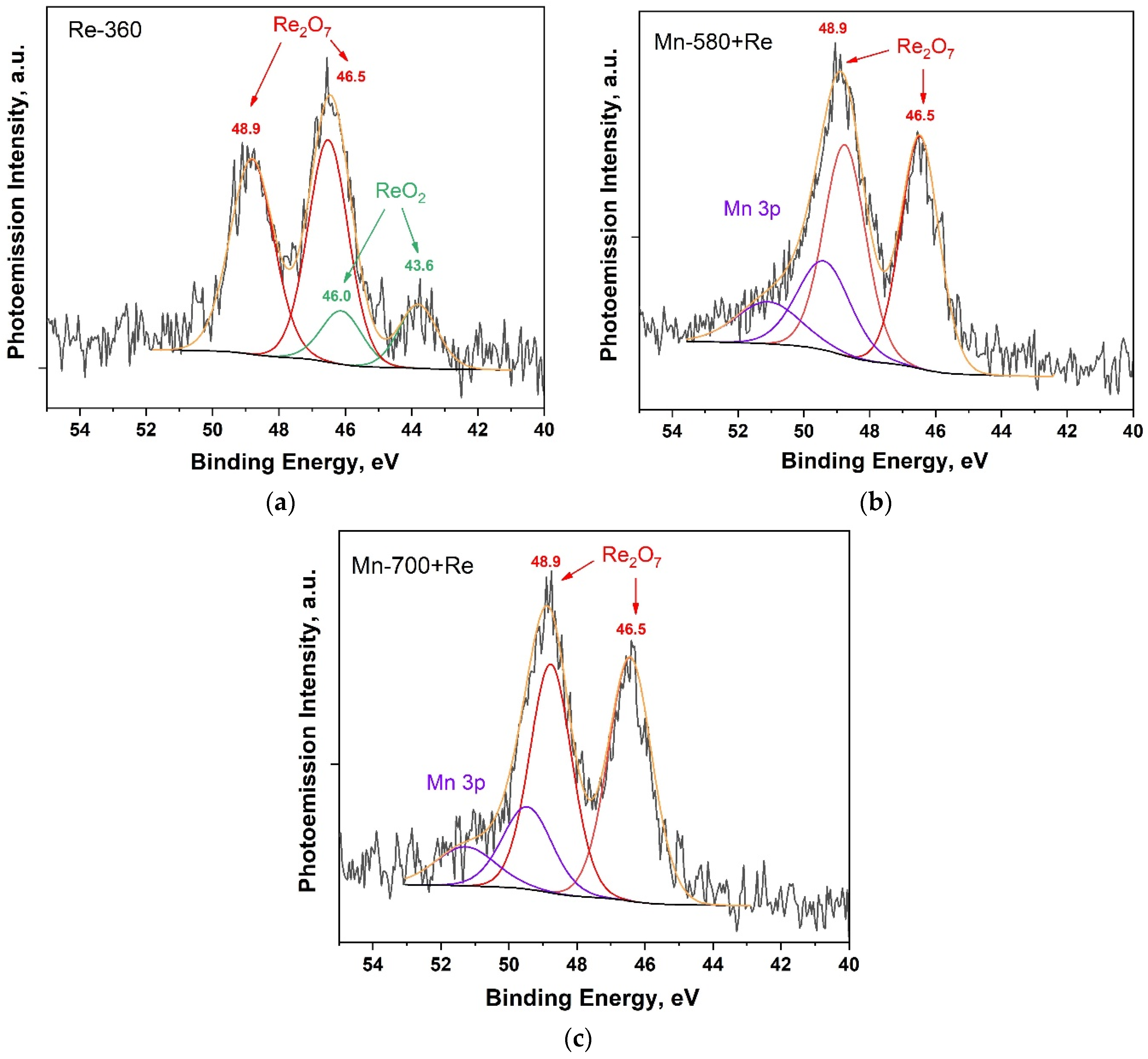
Figure 5 shows Re 4f PE spectra for Re-360, Mn-580 + Re and Mn-700 + Re composites.
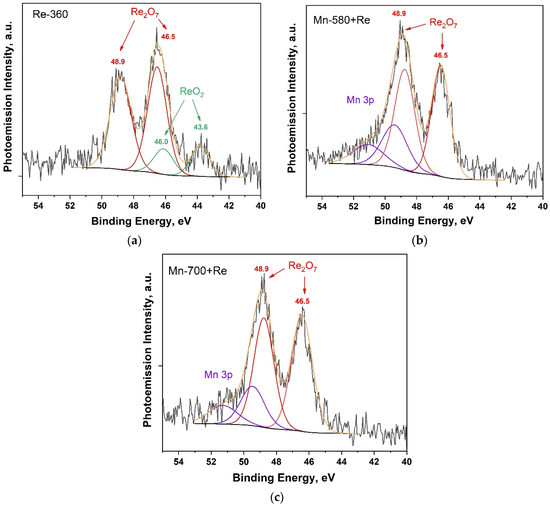
Figure 5.
Re 4f PE spectra of composites: Re-360 (a); Mn-580 + Re (b); and Mn-580 + Re (c).
In the Re4f PE spectrum of the Re-360 composite (Figure 5a), states with maxima at binding energies of 43.6 and 46.0 eV are observed, as well as states with maxima at binding energies of 46.5 and 48.9, which according to [50] correspond to ReO2 and Re2O7 oxides, respectively. This fact is also confirmed by the pieces of work in [39,56,57], where rhenium oxides (IV) and (VII) were obtained under similar conditions. It is important to note that in the temperature range of 300–400 °C, the probability of direct decomposition of ammonium perrhenate into rhenium heptoxide is unlikely due to positive Gibbs free energy [57]. In view of this, we believe that Re2O7 is formed through the formation of an intermediate product of the ReO3 reaction from ammonium perrhenate having negative Gibbs free energy, followed by disproportionation to Re2O7 + ReO2 [39]. In the spectra of Mn-580 + Re and Mn-700 + Re composites (Figure 5b,c), only states corresponding to Re2O7 oxide are observed, and in the high-energy region of the spectrum there is an overlap of the Re4f and Mn3p ranges. The absence of states corresponding to ReO2 in the analyzed spectra may indicate the participation of this oxide in the reaction of the formation of the KReO4 compound, the presence of which was shown by the results of the XRD analysis (Figure 2). At the same time, the absence of KReO4 states in the Re4f PE spectra, which according to [50] are at a binding energy of 46.1 eV, may be due to the overlap of the binding energy ranges for KReO4 and rhenium heptoxide in the PE spectra, as well as the possible heterogeneity of the distribution of these compounds in depth.
The totality of the presented results of XPS analysis suggests that the electrochemical behavior of the composites will be determined by the difference in the chemical state and crystal structure of manganese oxide. Composites Mn-0 and Mn-580, due to the high content of Mn(III) oxide in them, may have poor conductivity and be unstable when interacting with an electrolyte due to the oxidation of Mn(III) to Mn(IV). The first of these composites may also exhibit low electrochemical characteristics due to the high content of the amorphous inclusions of oxidized manganese. Composites with the addition of rhenium (Mn-580 + Re and Mn-780 + Re) should show good electrochemical behavior and stable characteristics due to the predominance of crystalline Mn(IV) oxides. Herewith, the difference in their characteristics will be determined by the number of nanoparticles, their size and their morphology, as well as the distribution of particles over the MWCNT surface.
3.4. Electrochemical Measurements
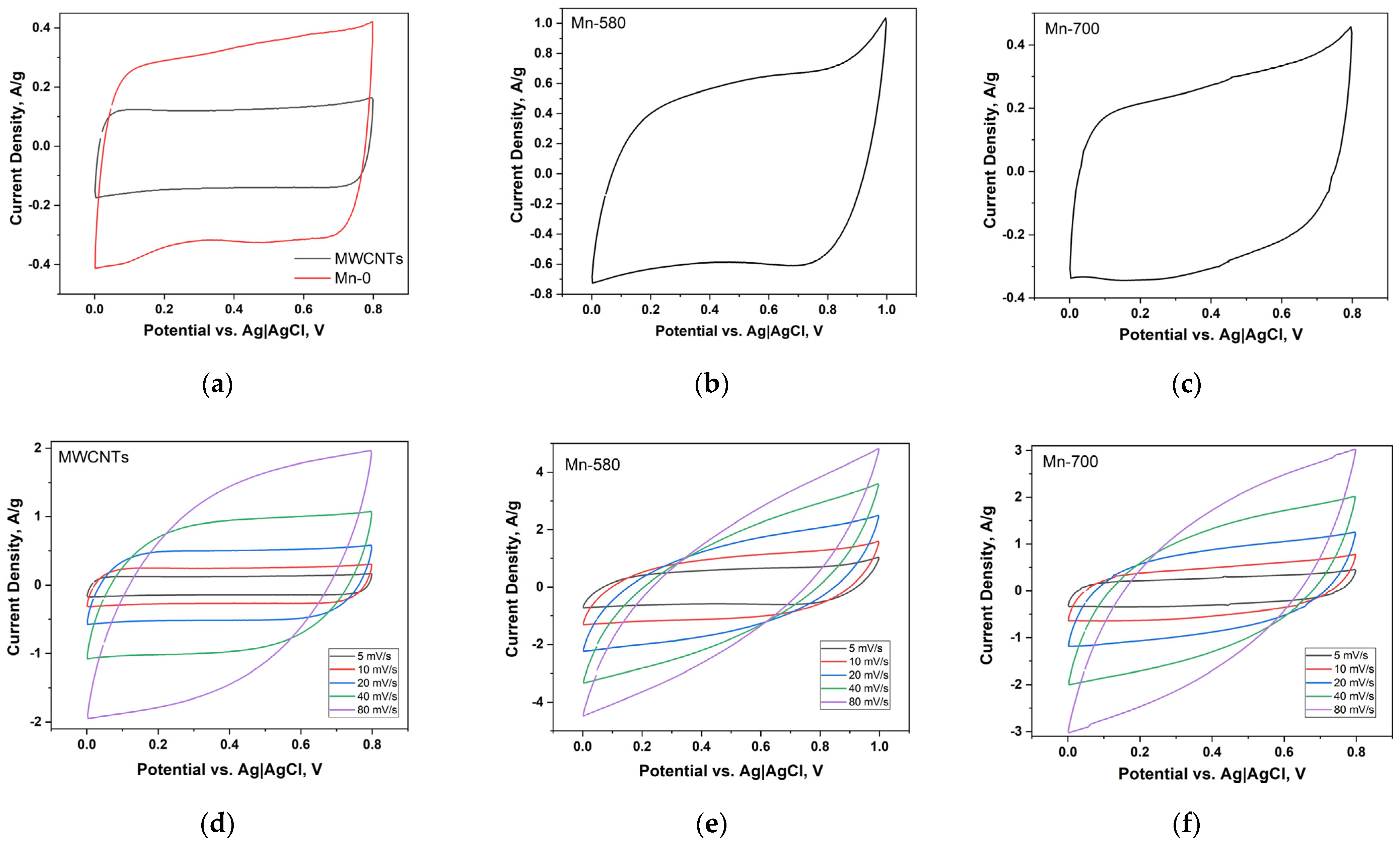
Figure 6a demonstrates the CV curves of electrodes based on MWCNTs and Mn-0 composites, measured at a potential scan rate of 5 mV/s. The CV curve for the MWCNTs-based electrode is symmetrical and has a shape close to rectangular. This designates that the storage of charge in this case is realized in an electric double layer (EDL). Apparently, a small number of functional groups present on the surface of the initial MWCNTs do not significantly affect the mechanism of charge accumulation. The specific capacitance of the MWCNTs-based electrode at 5 mV/s scan rate is 21.1 F/g (Table 4). The CV curve area of the Mn-0 electrode is significantly higher compared to MWCNTs. This indicates that redox reactions involving manganese oxide and electrolyte ions make the main addition to the capacitance of the Mn-0 composite electrode. The specific capacitance of the Mn-0 composite electrode is 49.2 F/g at 5 mV/s scan rate.
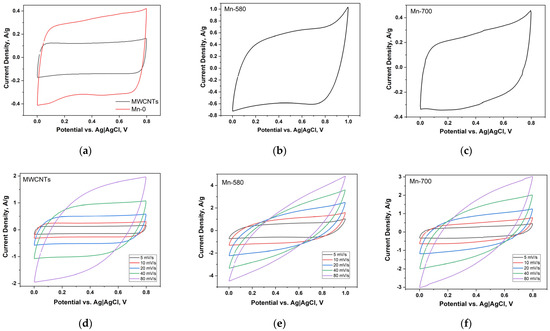
Figure 6.
CV curves of electrodes based on MWCNTs and Mn-0, measured at a potential scan rate of 5 mV/s (a); CV curve of the electrode Mn-580 (b); CV curve of the electrode Mn-700 (c); CV curves measured at different potential scan rates for electrodes based on MWCNTs (d), Mn-580 (e) and Mn-700 (f).

Table 4.
Specific capacitance (Cs) versus potential scan rate for MWCNTs and composites.
The CV curve of the electrode based on Mn-0 composite has no pronounced peaks, which is characteristic of redox reactions involving magnesium oxides when interacting with an electrolyte [58]. The CV curves of the electrodes based on Mn-580 and Mn-700 composite are also without pronounced redox peaks (Figure 6b,c). The peak on the cathode branch near the potential value of 1 V is probably associated with the onset of water decomposition in the electrolyte. The specific capacitance of this electrode is 61.7 F/g at 5 mV/s scan rate (Table 4). The following redox reactions (Equations (1)–(3)) can occur on the surface of the electrodes obtained on the basis of composites [58]:
It is probable that the increase in the specific capacitance of the composite due to heat treatment is due to changes in the morphology of the surface, namely, the formation of individual nanoparticles on the surface of the CNTs, which increases the contact area of manganese oxide with the electrolyte compared to the continuous distribution of oxide over the surface of the MWCNTs. Additionally, the rise in the specific capacitance may be a consequence of the crystallization of manganese oxide. The analysis of the MWCNTs-based electrode’s rate capability indicates good electrochemical behavior—with an increase in the potential scan rate, a noticeable increase in the area of the CV curves is observed (Figure 6d) and the shape of the curves remains quasi-rectangular. The specific capacitance retains 52% (the R value in Table 4) with an increase in the scanning speed from 5 to 80 mV/s. This indicates a sufficiently high availability of the CNT surface for electrolyte ions.
Electrodes based on Mn-0, Mn-580 and Mn-700 composites do not exhibit sufficiently good electrochemical behavior and have a significantly lower rate capability (Figure 6e,f, Table 4). At a potential scan rate of 80 mV/s, the value of the specific capacitance for the Mn-580 composite practically coincides with the value of the specific capacitance MWCNTs and is slightly inferior to the specific capacitance value of the Mn-0 electrode. This indicates that diffusion processes involving manganese oxide do not have time to proceed at high potential scan rates, and the capacitance of composite electrodes is almost completely determined by the formation of EDL. The higher rate capability of the electrode based on the untreated (initial) composite can be interpreted by the higher concentration of the Mn(IV) oxide, which was established on the basis of the XPS analysis. It should be noted that the conductivity of the Mn(IV) oxide exceeds the conductivity of the Mn(III) oxide by several orders of magnitude [30].
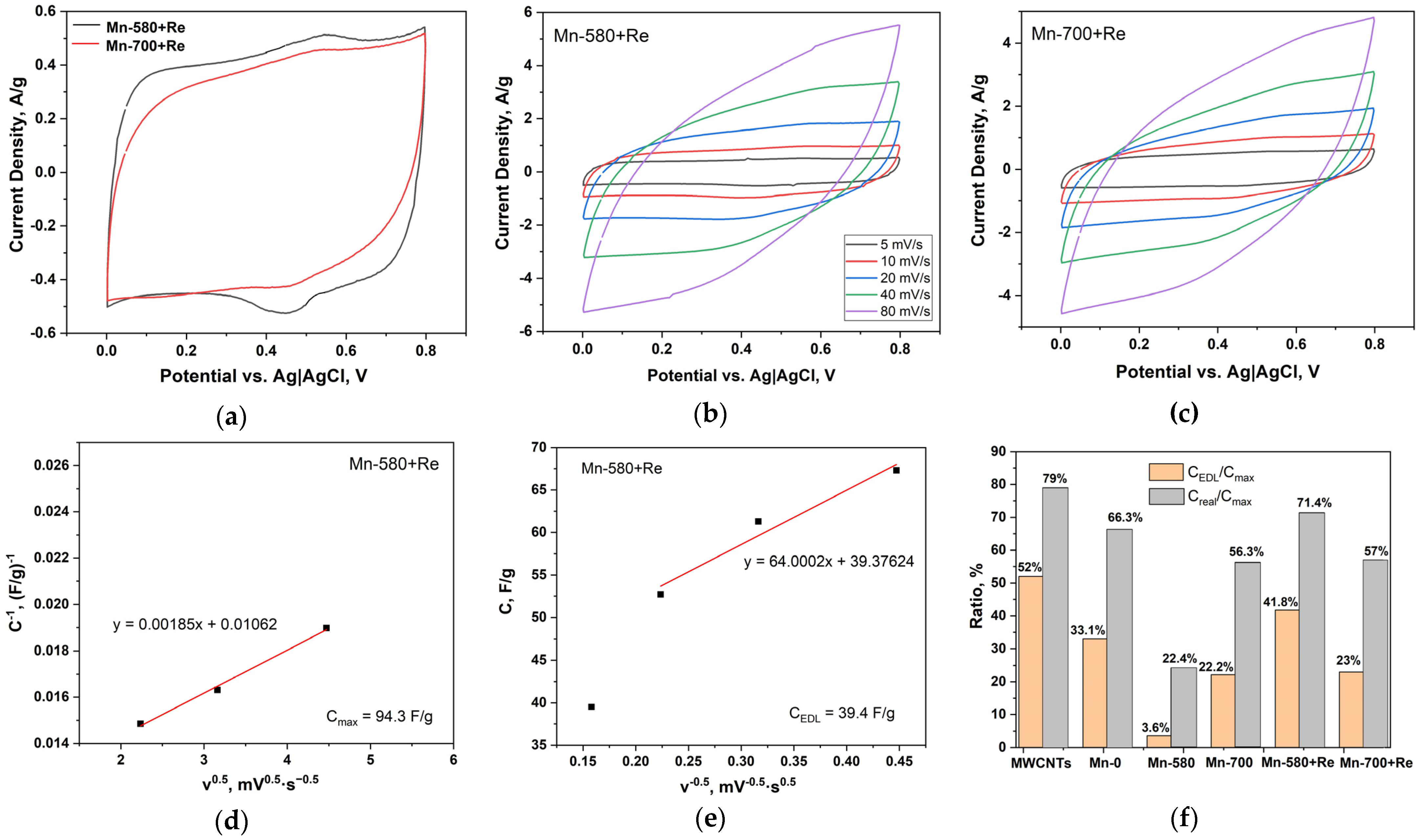
The CV curves of Mn-580 + Re and Mn-700 + Re composites (Figure 7a) have a pair of peaks at potential values of 0.55 and 0.48 V on the cathode and anode branches of the curve, respectively. These peaks are associated with a redox reaction on the surface of the electrodes, apparently associated with changes in the degree of the oxidation of rhenium, since these maxima are not observed on the CV curves of composites that do not contain rhenium oxide. The intensity of these peaks, as well as the area of the CV curve, is noticeably greater in the case of the Mn-580 + Re composite. This composite has a maximum specific capacitance at both high and low potential scan rates (Table 4). The good electrochemical behavior and the shape of the CV curves for composites with rhenium oxide (Figure 7b,c) suggest a higher availability of the surface of these composites for electrolyte ions, as well as the possible occurrence of redox reactions, even at high potential scan rates. In addition, the reason for the increase in rate capacitance may also be an increase in the electrical conductivity of the oxide component of composites due to an increase in the content of Mn(IV) oxide in them (see XPS data).
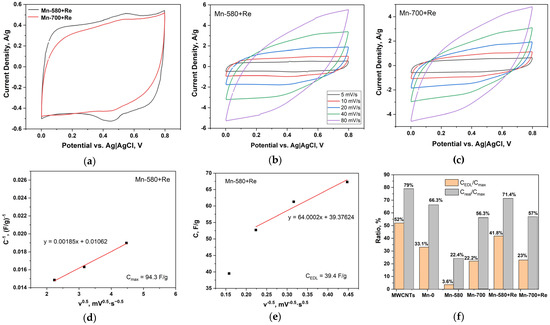
Figure 7.
(a) CV curves of electrodes based on Mn-580 + Re and Mn-700 + Re, measured at a potential scan rate of 5 mV/s; CV curves measured at different potential scan rates for electrodes based on (b) Mn-580 + Re and (c) Mn-700 + Re; (d) the dependence of the reciprocal of the total capacitance (C−1, (F/g)−1) on the square root of the scan rate (v0.5, mV0.5·s−0.5) and (e) the total capacitance (C, F/g) on the reciprocal of the square root of the scan rate (v−0.5, mV−0.5·s0.5) for the Mn-580 + Re electrode; (f) the ratios of the electric double layer capacitance (CEDL) and the experimental capacitance (Creal) to the total theoretical capacitance (Cmax) for electrodes based on MWCNTs and composites.
To assess the contribution of capacitive and pseudocapacitance (e.g., Faraday) processes in the total capacitance of materials, a method is used based on the analysis of the capacitance characteristics of electrodes depending on the potential scan rate, taking into account Equations (4) and (5) [59,60]:
where C is the total specific capacitance, is a specific capacitance of EDL (CEDL), is a maximal specific capacitance (Cmax), and a and b are the constants.
The dependences of C on ν0.5 and C−1 on ν−0.5 for the Mn-580 + Re composites are shown in Figure 7d,e. For electrodes based on other composites under study, these dependencies are presented in Figure S5. The analysis of the data obtained (Figure 7f) showed that for manganese oxide composites, the highest contribution of Faraday processes to the total capacitance is observed for the composite after annealing at 580 °C. The addition of rhenium oxide leads to a noticeable increase in the contribution of EDL during charge accumulation, especially for the Mn-580 + Re composite. This composite also showed the retention of its specific capacitance after 1000 charge/discharge cycles (Figure S6). The obtained results indicate that the increase in electrochemical characteristics for composites with the addition of rhenium oxides, among other things, may also be due to an increase in the specific surface area of metal oxide nanoparticles.
4. Conclusions
A detailed study of the structural morphology and electrochemical characteristics of composites based on MWCNTs and manganese oxide with the addition of rhenium was carried out using a combination of SEM, EDX, XPS and CV methods. It has been established that, as a result of the thermal treatment of the MnOx/MWCNTs composite in vacuum condition at 580 °C, crystalline manganese oxide nanoparticles are formed, well distributed over the CNTs surface. Phase analysis performed on this sample indicates the presence of Mn3O4 (dimanganese(III)).
The addition of rhenium to a composite annealed at 580 °C leads to a decrease in the average size of nanoparticles to 15 nm, as well as additional oxidation of manganese oxide and an increase in the proportion of Mn(IV). It is shown that MWCNTs preserve their crystalline structure after heat treatment and the deposition of manganese and rhenium. In this case, the phase composition of metal oxides is represented mainly by Mn3O4 (dimanganese(III)) and KReO4. The formation of the KReO4 phase is the result of the interaction of the KMnO4 decomposition products and rhenium (IV) oxide, formed due to the decomposition of the NH4ReO4 compound. It has been shown that the addition of rhenium to annealed composites leads to the additional oxidation of manganese oxide and an increase in the proportion of Mn(IV). The electrochemical tests of the composites showed that the best specific capacitance result (in both variants at high and low potential scan rates) is observed for the MnOx/MWCNTs sample annealed at 580 °C with the addition of rhenium oxide. Such a high result is due to the synergistic effect achieved by combining the properties of manganese and rhenium oxides, which, on the one hand, provides an increase in the proportion of Mn(IV) oxides in the composite and leads to an increase in the proportion of pseudocapacitance (reversible RedOx processes), as well as an increase in the electrical conductivity of nanoparticles, and, on the other hand, leads to a noticeable increase in the contribution of the EDL during charge accumulation.
Thus, this composite can be further used as an electrode material in the development of new electrochemical devices for storing and converting energy with good technical characteristics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122412827/s1, Figure S1: SEM image of Mn-0 composite (a) and the EDX spectrum recorded in the selected area (b); Figure S2: SEM image of Mn-580 + Re composite (a) and the EDX spectrum recorded in the selected area (b); Figure S3: Overview spectra of MWCNTs and composites; Figure S4: K2p photoemission spectra for different composites; Figure S5: The dependence of the reciprocal of the total capacitance (C−1, (F/g)−1) on the square root of the scan rate (v0.5, mV0.5·s−0.5) and the total capacitance (C, F/g) on the reciprocal of the square root of the scan rate (v−0.5, mV−0.5·s0.5), respectively for the electrodes: MWCNTs (a) and (b), Mn-0 (c) and (d), Mn-580 (e) and (f), Mn-700 + Re (g) and (h); Figure S6: Change in specific capacitance depending on the number of cycles for electrodes based on MWCNTs (a) Mn-0 (b), Mn-580 (c) and Mn-580 + Re (d).
Author Contributions
Conceptualization, P.M.K. and S.N.N.; methodology, P.M.K. and S.N.N.; formal analysis, P.M.K. and S.N.N.; investigation, P.M.K. and S.N.N.; writing—original draft preparation, P.M.K. and S.N.N.; writing—review and editing, P.M.K. and S.N.N.; visualization, P.M.K. and S.N.N.; supervision, P.M.K. and S.N.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scholarship of the President of the Russian Federation CΠ-58.2022.1 and supported under Governmental order for Omsk Scientific Center SB RAS (project registration number 121021600004-7), in part, for the formation and investigation of composites with rhenium additive, as well as being funded by the Russian Science Foundation (grant No. 21-72-10029), in part, for the investigation of MWCNTs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work was performed using the equipment of the St. Petersburg State University Research Park: Centre for Physical Methods of Surface Investigation, Centre for X-ray Diffraction Studies and Interdisciplinary Resource Centre for Nanotechnology. The authors are thankful to Yu. A. Sten’kin for the synthesis of the composites.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ma, R.; Wei, B.; Xu, C.; Liang, J.; Wu, D. Development of supercapacitors based on carbon nanotubes. Sci. China Ser. E Technol. Sci. 2000, 43, 178–182. [Google Scholar] [CrossRef]
- Guo, F.M.; Xu, R.Q.; Cui, X.; Zang, X.B.; Zhang, L.; Chen, Q.; Wang, K.L.; Wei, J.Q. Highly flexible, tailorable and all-solid-state supercapacitors from carbon nanotube–MnOx composite films. RSC Adv. 2015, 5, 89188–89194. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Jover, E.; Bertran, E. Water Plasma Functionalized CNTs/MnO2 Composites for Supercapacitors. Sci. World J. 2013, 2013, 832581. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, D.; Song, X.; Du, W.; Zhao, X.; Zhang, D. Review on Carbon/Polyaniline Hybrids: Design and Synthesis for Supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, X.; Zhou, K.; Jia, J. Carbon Materials for Supercapacitors. In Nanomaterials in Advanced Batteries and Supercapacitors; Nanostructure Science and Technology; Springer: Cham, Switzerland, 2016; pp. 271–315. [Google Scholar]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Arshid, E.; Arshid, H.; Amir, S.; Mousavi, S.B. Free vibration and buckling analyses of FG porous sandwich curved microbeams in thermal environment under magnetic field based on modified couple stress theory. Arch. Civ. Mech. Eng. 2021, 21, 6. [Google Scholar] [CrossRef]
- Khorasani, M.; Soleimani-Javid, Z.; Arshid, E.; Lampani, L.; Civalek, Ö. Thermo-elastic buckling of honeycomb micro plates integrated with FG-GNPs reinforced Epoxy skins with stretching effect. Compos. Struct. 2021, 258, 113430. [Google Scholar] [CrossRef]
- Arshid, E.; Soleimani-Javid, Z.; Amir, S.; Duc, N.D. Higher-order hygro-magneto-electro-thermomechanical analysis of FG-GNPs-reinforced composite cylindrical shells embedded in PEM layers. Aerosp. Sci. Technol. 2022, 126, 107573. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, M.; Li, X. Carbon nanomaterials and their composites for supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors; Springer Science+Business Media: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Xia, H.; Meng, Z.Y.S.; Yuan, G.; Cui, A.C.; Luc, L. A Symmetric RuO2/RuO2 Supercapacitor Operating at 1.6 V by Using a Neutral Aqueous Electrolyte. Electrochem. Solid-State Lett. 2012, 15, A60–A63. [Google Scholar] [CrossRef]
- Nguyen, T.; Montemor, M.D.F. Metal Oxide and Hydroxide–Based Aqueous Supercapacitors: From Charge Storage Mechanisms and Functional Electrode Engineering to Need-Tailored Devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, W.; Yokoshima, K.; Murakami, Y.; Takasu, Y. Charge storage mechanism of nanostructured anhydrous and hydrous ruthenium-based oxides. Electrochim. Acta 2006, 52, 1742–1748. [Google Scholar] [CrossRef]
- Wei, B.; Wang, L.; Miao, Q.; Yuan, Y.; Dong, P.; Vajtai, R.; Fei, W. Fabrication of manganese oxide/three-dimensional reduced graphene oxide composites as the supercapacitors by a reverse microemulsion method. Carbon 2015, 85, 249–260. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Fan, D.; Yang, P. Introduction to and classification of manganese deposits of China. Ore Geol. Rev. 1999, 15, 1–13. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Uke, S.J.; Akhare, V.P.; Bambole, D.R.; Bodade, A.B.; Chaudhari, G.N. Recent Advancements in the Cobalt Oxides, Manganese Oxides, and Their Composite as an Electrode Material for Supercapacitor: A Review. Front. Mater. 2017, 4, 21. [Google Scholar] [CrossRef]
- Iurchenkova, A.A.; Fedorovskaya, E.O.; Matochkin, P.E.; Sakhapov, S.Z.; Smovzh, D.V. Supercapacitor behavior of carbon-manganese oxides nanocomposites synthesized by carbon arc. Int. J. Energy Res. 2020, 44, 10754–10767. [Google Scholar] [CrossRef]
- Wang, B.; Park, J.; Wang, C.; Ahn, H.; Wang, G. Mn3O4 nanoparticles embedded into graphene nanosheets: Preparation, characterization, and electrochemical properties for supercapacitors. Electrochim. Acta 2010, 55, 6812–6817. [Google Scholar] [CrossRef]
- An, G.; Yu, P.; Xiao, M.; Liu, Z.; Miao, Z.; Ding, K.; Mao, L. Low-temperature synthesis of Mn3O4 nanoparticles loaded on multi-walled carbon nanotubes and their application in electrochemical capacitors. Nanotechnology 2008, 19, 275709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Kucernak, A. Electrochemical supercapacitor material based on manganese oxide: Preparation and characterization. Electrochim. Acta 2002, 47, 2381–2386. [Google Scholar] [CrossRef]
- Makgopa, K.; Ejikeme, P.M.; Jafta, C.J.; Raju, K.; Zeiger, M.; Presser, V.; Ozoemena, K.I. A high-rate aqueous symmetric pseudocapacitor based on highly graphitized onion-like carbon/birnessite-type manganese oxide nanohybrids. J. Mater. Chem. A 2015, 3, 3480–3490. [Google Scholar] [CrossRef]
- Ma, S.B.; Nam, K.-W.; Yoon, W.-S.; Yang, X.-Q.; Ahn, K.-Y.; Oh, K.-H.; Kim, K.-B. Electrochemical properties of manganese oxide coated onto carbon nanotubes for energy-storage applications. J. Power Sources 2008, 178, 483–489. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.; Wei, B. Synthesis and electrochemical characterizations of amorphous manganese oxide and single walled carbon nanotube composites as supercapacitor electrode materials. Electrochem. Commun. 2006, 8, 827–832. [Google Scholar] [CrossRef]
- Makgopa, K.; Ejikeme, P.M.; Ozoemena, K.I. Nanostructured Manganese Oxides in Supercapacitors. In Nanomaterials in Advanced Batteries and Supercapacitors. Nanostructure Science and Technology; Springer: Cham, Switzerland, 2016; pp. 345–376. [Google Scholar]
- Zhang, S.W.; Chen, G. Manganese oxide based materials for supercapacitors. Energy Mater. 2013, 3, 186–200. [Google Scholar] [CrossRef]
- Augustin, M.; Fenske, D.; Bardenhagen, I.; Westphal, A.; Knipper, M.; Plaggenborg, T.; Kolny-Olesiak, J.; Parisi, J. Manganese oxide phases and morphologies: A study on calcination temperature and atmospheric dependence. Beilstein J. Nanotechnol. 2015, 6, 47–59. [Google Scholar] [CrossRef]
- Chang, J.-K.; Tsai, W.-T. Microstructure and Pseudocapacitive Performance of Anodically Deposited Manganese Oxide with Various Heat-Treatments. J. Electrochem. Soc. 2005, 152, A2063–A2068. [Google Scholar] [CrossRef]
- Chang, J.-K.; Chen, Y.-L.; Tsai, W.-T. Effect of heat treatment on material characteristics and pseudo-capacitive properties of manganese oxide prepared by anodic deposition. J. Power Sources 2004, 135, 344–353. [Google Scholar] [CrossRef]
- Ciszewski, M.; Koszorek, A.; Hawełek, Ł.; Osadnik, M.; Szleper, K.; Drzazga, M. Active Carbon Modified by Rhenium Species as a Perspective Supercapacitor Electrode. Electrochem 2020, 1, 278–285. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Vankova, S.; Bozhkov, O.; Mehandjiev, D. Rhenium and manganese modified activated carbon as catalyst for methanol decomposition. Can. J. Chem. 2007, 85, 118–123. [Google Scholar] [CrossRef]
- Nesov, S.N.; Korusenko, P.M. Structure and electrochemical characteristics of composites based on multi-walled carbon nanotubes and manganese oxide. AIP Conf. Proc. 2020, 2310, 020223. [Google Scholar] [CrossRef]
- Romanenko, A.I.; Anikeeva, O.B.; Buryakov, T.I.; Tkachev, E.N.; Zhdanov, K.R.; Kuznetsov, V.L.; Mazov, I.N.; Usoltseva, A.N. Electrophysical properties of multiwalled carbon nanotubes with various diameters. Phys. Status Solidi B 2009, 246, 2641–2644. [Google Scholar] [CrossRef]
- Barabashko, M.S.; Drozd, M.; Szewczyk, D.; Jeżowski, A.; Bagatskii, M.I.; Sumarokov, V.V.; Dolbin, A.V.; Nesov, S.N.; Korusenko, P.M.; Ponomarev, A.N.; et al. Calorimetric, NEXAFS and XPS studies of MWCNTs with low defectiveness. Full. Nanotub. Carbon Nanostruct. 2020, 29, 331–336. [Google Scholar] [CrossRef]
- Louis-Jean, J.; Jang, H.; Swift, A.J.; Poineau, F. Thermal Analysis of Benzotriazolium Perrhenate and Its Implication to Rhenium Metal. ACS Omega 2021, 6, 26672–26679. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Vigolo, B.; Hérold, C. Processing Carbon Nanotubes. In Carbon Nanotubes—Synthesis, Characterization, Applications; Yellampalli, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 3–28. [Google Scholar]
- Liang, X.; Zhong, J.; Zhao, T.; Yao, P.; Chu, W.; Zhao, H.; Ibrahim, K.; Qian, H.; Wu, Z. Removal of oxidative carbonaceous fragments by annealing treatment studied by XANES. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 619, 323–325. [Google Scholar] [CrossRef]
- Gupta, V.; Saleh, T.A. Syntheses of Carbon Nanotube-Metal Oxides Composites; Adsorption and Photo-degradation. In Carbon Nanotubes—From Research to Applications; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, X.; Ji, J.; Duan, X.; Qian, G.; Zhou, X.; Chen, D.; Yuan, W. Modified carbon nanotubes by KMnO4 supported iron Fischer–Tropsch catalyst for the direct conversion of syngas to lower olefins. J. Mater. Chem. A 2015, 3, 4560–4567. [Google Scholar] [CrossRef]
- Chen, X.; Yan, S.; Wang, N.; Peng, S.; Wang, C.; Hong, Q.; Zhang, X.; Dai, S. Facile synthesis and characterization of ultrathin δ-MnO2 nanoflakes. RSC Adv. 2017, 7, 55734–55740. [Google Scholar] [CrossRef]
- Aliaga, J.A.; Alonso-Núñez, G.; Zepeda, T.; Araya, J.F.; Rubio, P.F.; Bedolla-Valdez, Z.; Paraguay-Delgado, F.; Farías, M.; Fuentes, S.; González, G. Synthesis of highly destacked ReS2 layers embedded in amorphous carbon from a metal-organic precursor. J. Non-Cryst. Solids 2016, 447, 29–34. [Google Scholar] [CrossRef]
- L’Vov, B.V.; Ugolkov, V.L. Decomposition of KMnO4 in different gases as a potential kinetics standard in thermal analysis. J. Therm. Anal. Calorim. 2009, 100, 145–153. [Google Scholar] [CrossRef]
- Keilholz, S.; Kohlmann, H.; Uhlenhut, H.; Gabke, A.; García-Schollenbruch, M. In situ X-ray diffraction studies on the production process of rhenium. Z. Anorg. Allg. Chem. 2022, 648, e202200232. [Google Scholar] [CrossRef]
- Nesov, S.; Korusenko, P.; Sachkov, V.; Bolotov, V.; Povoroznyuk, S. Effects of preliminary ion beam treatment of carbon nanotubes on structures of interfaces in MOx/multi-walled carbon nanotube (M = Ti,Sn) composites: Experimental and theoretical study. J. Phys. Chem. Solids 2022, 169, 110831. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. [CrossRef]
- Cestaro, R.; Schweizer, P.; Philippe, L.; Maeder, X.; Serrà, A. Phase and microstructure control of electrodeposited Manganese Oxide with enhanced optical properties. Appl. Surf. Sci. 2022, 580, 152289. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Polonyankin, D.; Fedorov, A.; Blesman, A.; Nesov, S. Structural coloration of AISI 321 steel surfaces textured by ultrasonic impact treatment. Opt. Laser Technol. 2022, 150, 107948. [Google Scholar] [CrossRef]
- Agopsowicz, A.; Hitchcock, J.L.; Tye, F.L. ChemInform Abstract: Thermal Behavior of Partially Reduced Γ-Manganese Dioxide in Argon Atmosphere. Chem. Inf. 1979, 10. [Google Scholar] [CrossRef]
- Deljoo, B.; Tan, H.; Suib, S.L.; Aindow, M. Thermally activated structural transformations in manganese oxide nanoparticles under air and argon atmospheres. J. Mater. Sci. 2020, 55, 7247–7258. [Google Scholar] [CrossRef]
- Bai, M.; Liu, Z.-H.; Zhou, L.-J.; Zhang, C.-F. Preparation of ultrafine rhenium powders by CVD hydrogen reduction of volatile rhenium oxides. Trans. Nonferrous Met. Soc. China 2013, 23, 538–542. [Google Scholar] [CrossRef]
- Javaherian, S.S.; Aghajani, H.; Tavakoli, H. Investigation on ammonium perrhenate behaviour in nitrogen, argon and hydrogen atmosphere as a part of rhenium extraction process. Miner. Process. Extr. Met. 2017, 127, 182–188. [Google Scholar] [CrossRef]
- Han, Z.J.; Seo, D.H.; Yick, S.; Chen, J.H.; Ostrikov, K. MnOx/carbon nanotube/reduced graphene oxide nanohybrids as high-performance supercapacitor electrodes. NPG Asia Mater. 2014, 6, e140. [Google Scholar] [CrossRef]
- Korusenko, P.M.; Nesov, S.N.; Iurchenkova, A.A.; Fedorovskaya, E.O.; Bolotov, V.V.; Povoroznyuk, S.N.; Smirnov, D.A.; Vinogradov, A.S. Comparative Study of the Structural Features and Electrochemical Properties of Nitrogen-Containing Multi-Walled Carbon Nanotubes after Ion-Beam Irradiation and Hydrochloric Acid Treatment. Nanomaterials 2021, 11, 2163. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, I.V.; Bulavskiy, M.O.; Iurchenkova, A.A.; Aguilar-Martinez, Y.; Fedorov, F.S.; Fedorovskaya, E.O.; Mikladal, B.; Kallio, T.; Nasibulin, A.G. Flexible supercapacitors based on free-standing polyaniline/single-walled carbon nanotube films. J. Power Sources 2022, 541, 231691. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).