Preparation of Nanoparticle-Loaded Microbubbles via an Electrohydrodynamic Atomization Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
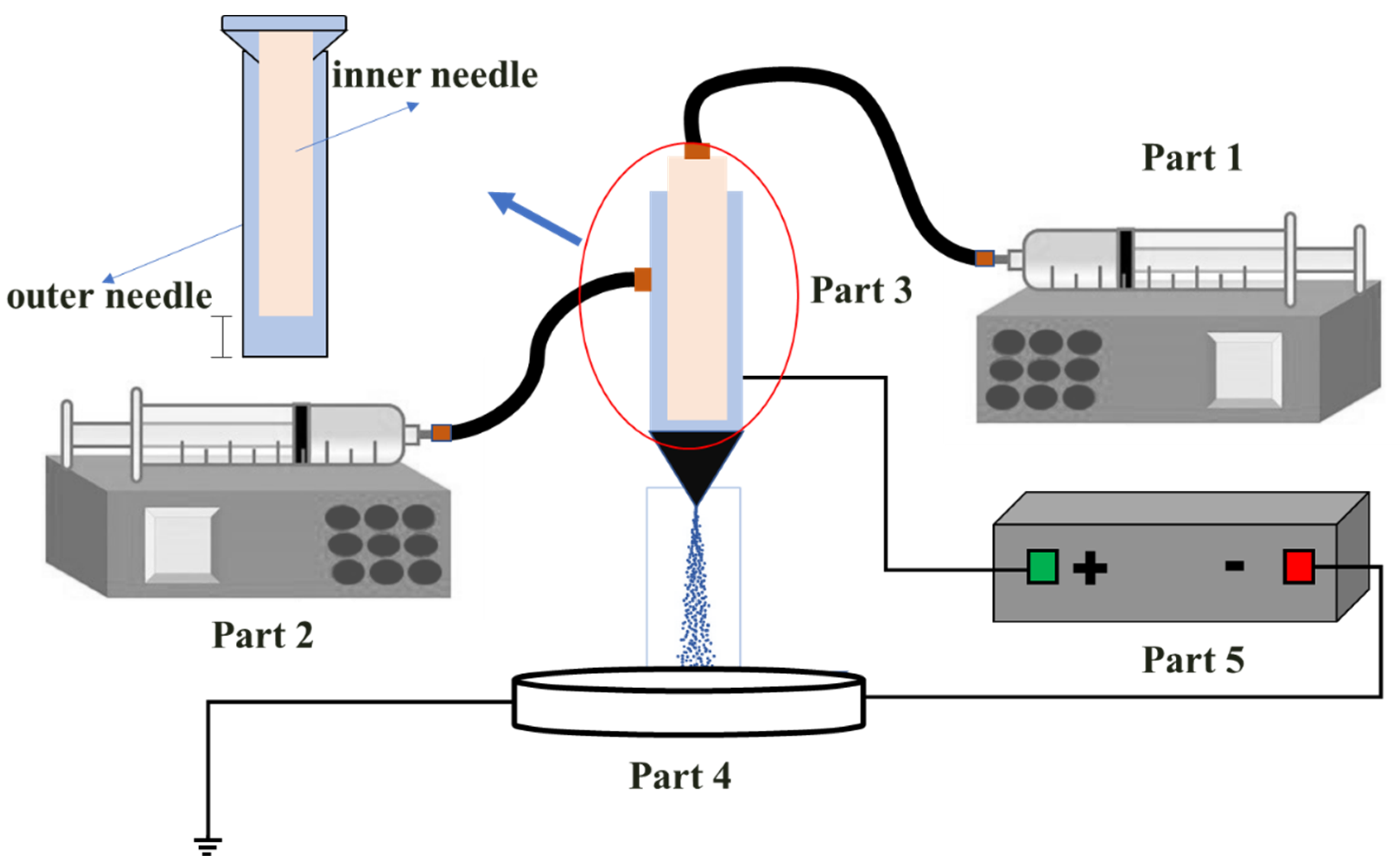
2.2. Experimental Setup
2.3. Preparation of Shell Layer Solutions and Collecting Medium
2.4. Preparation of Microbubbles
2.5. Characterization
3. Results and Discussion
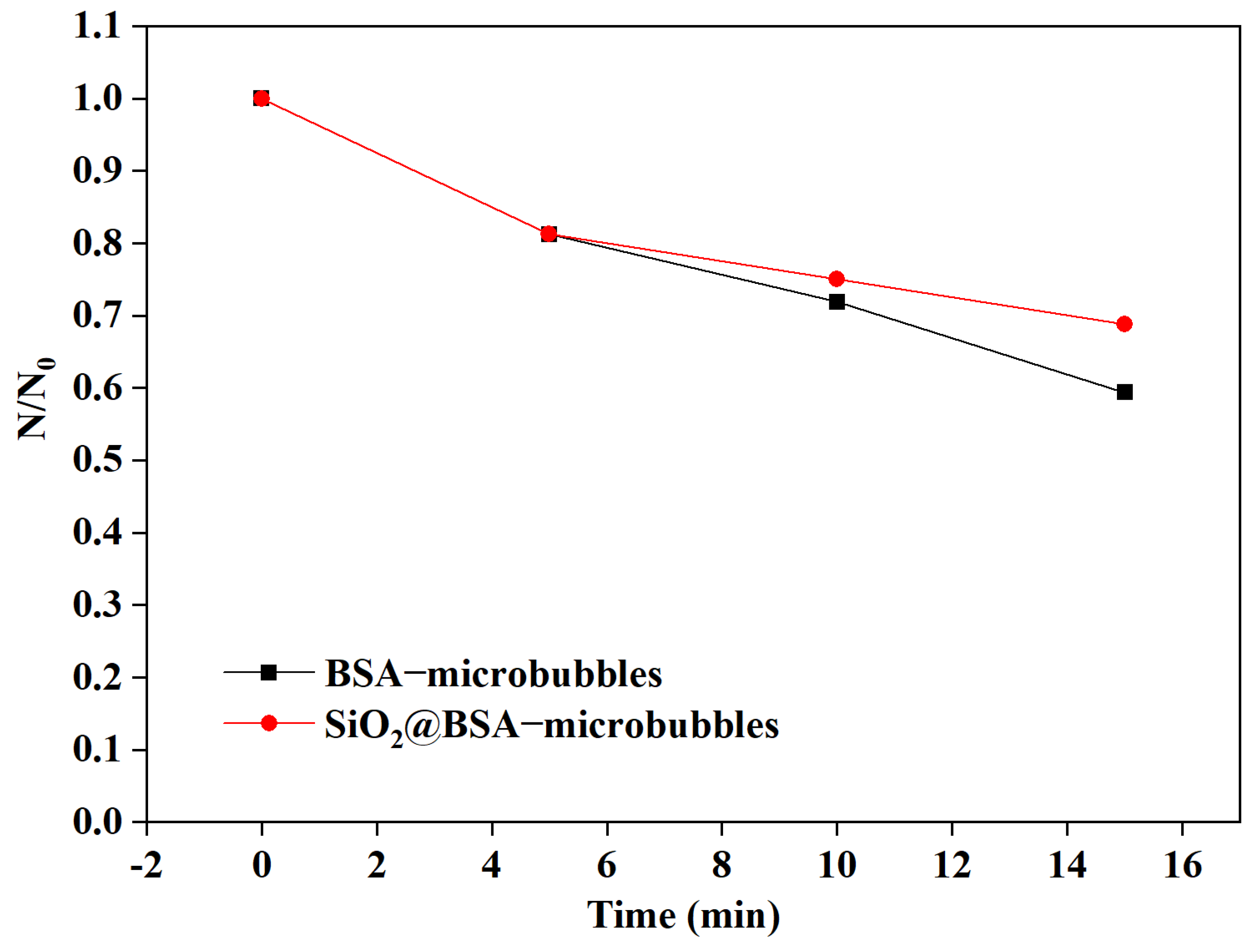
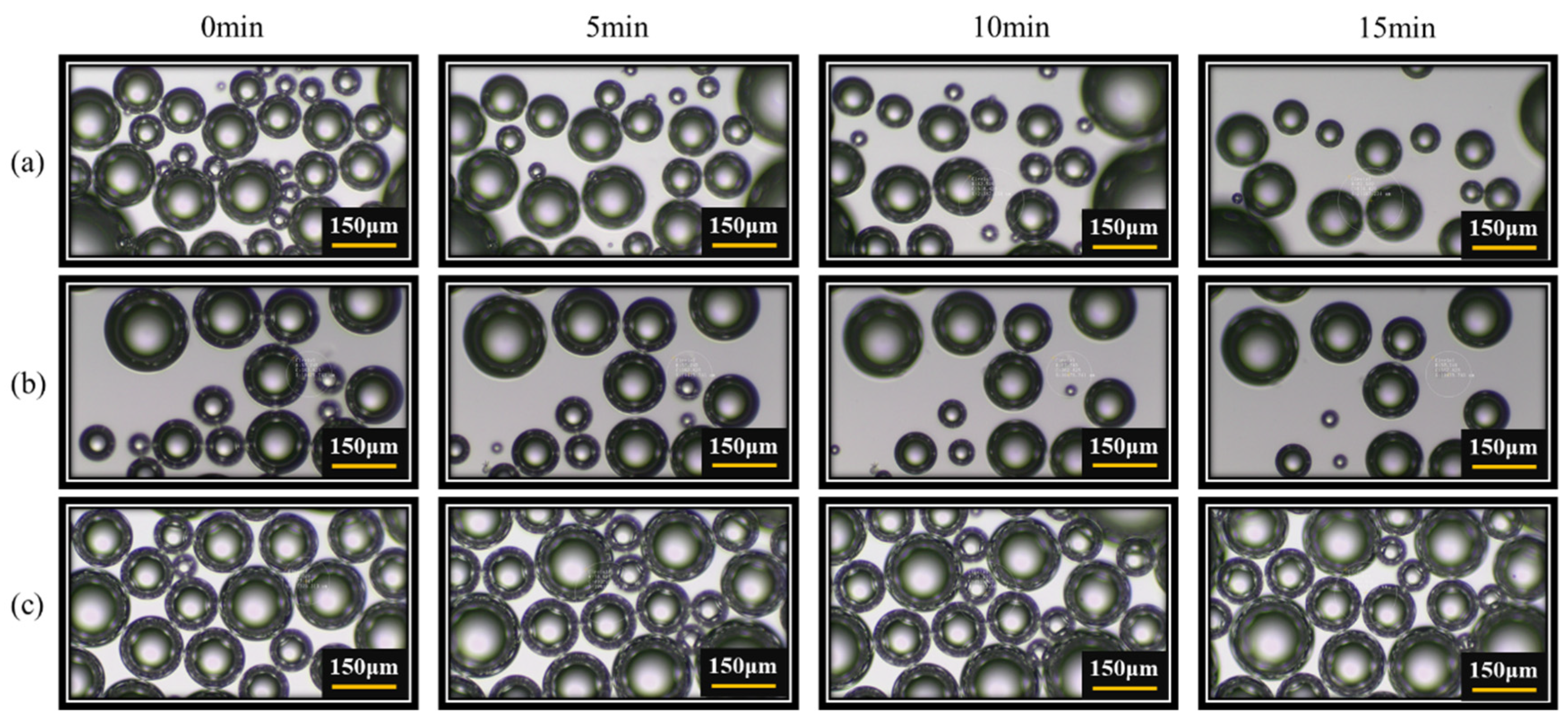
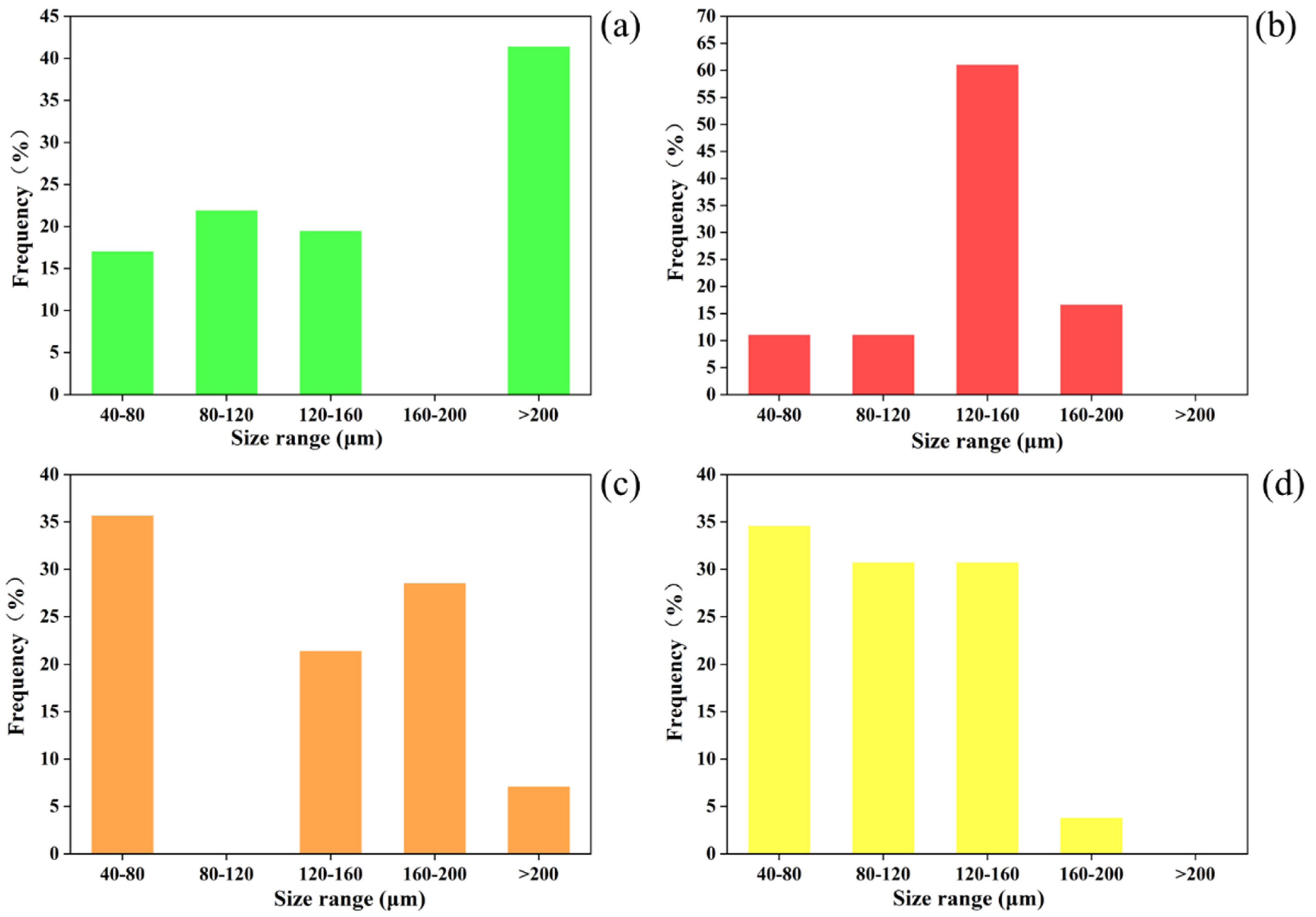
3.1. Microbubbles with and without Nanoparticles Loaded
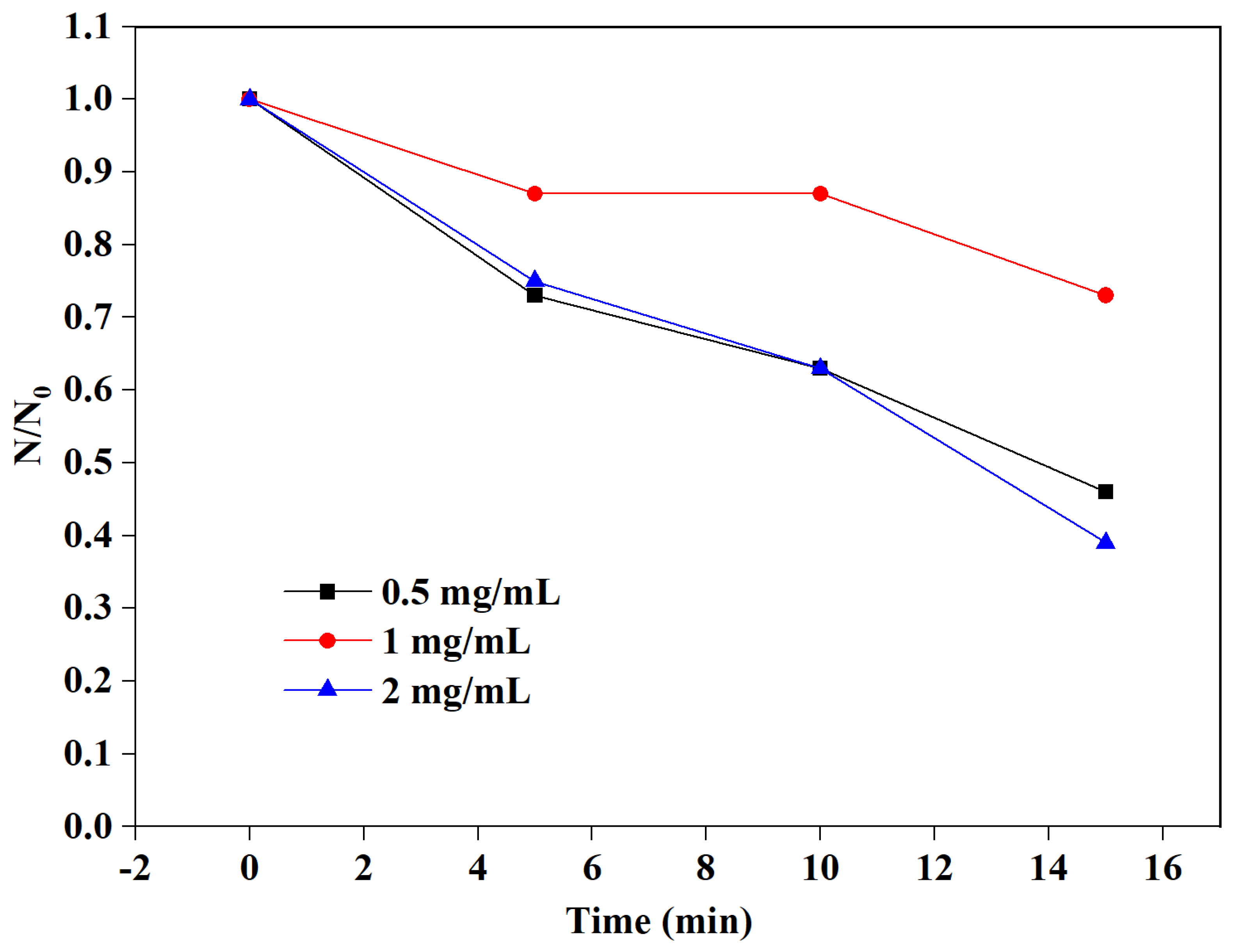
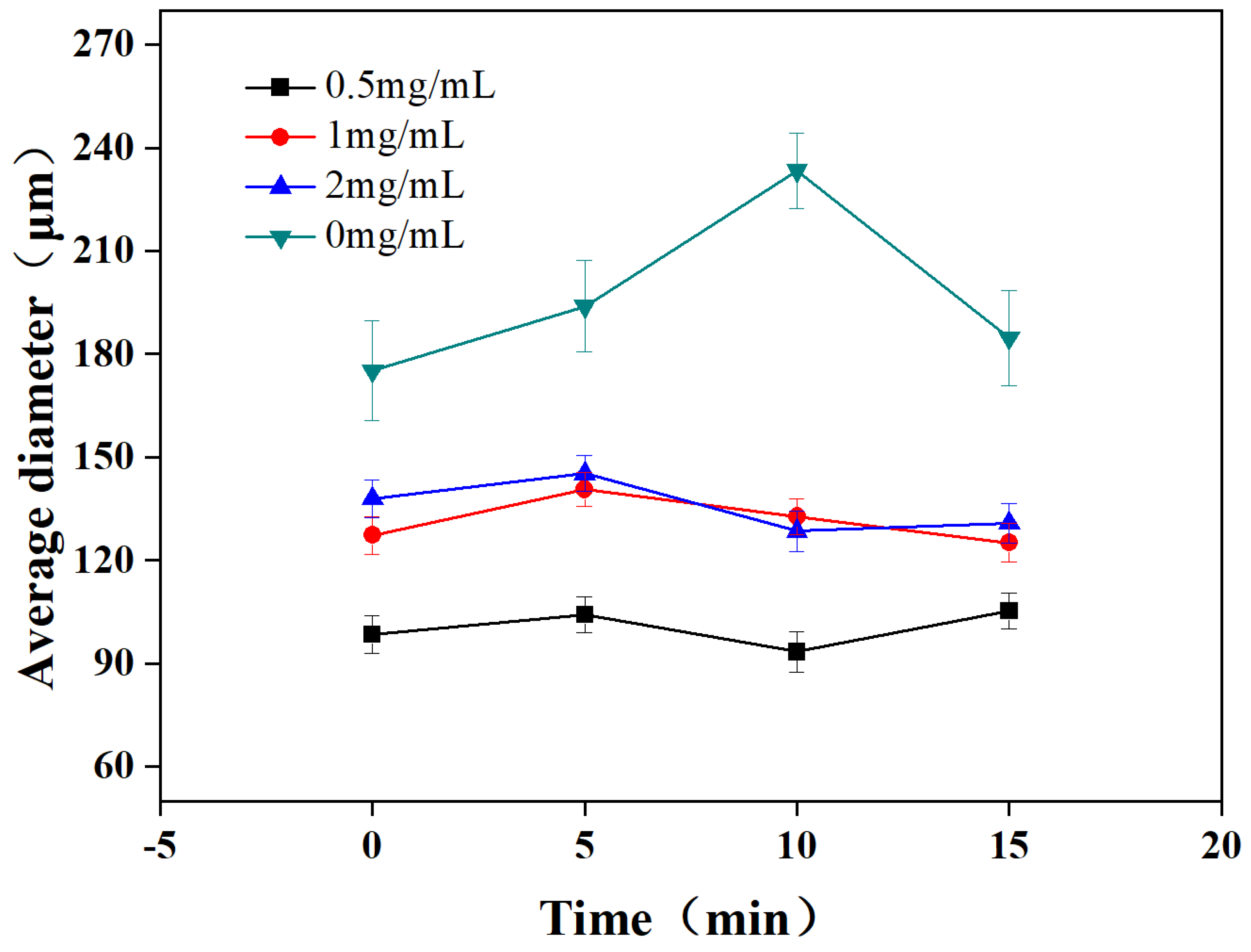
3.2. Effect of Nanoparticle Concentrations on the Microbubbles
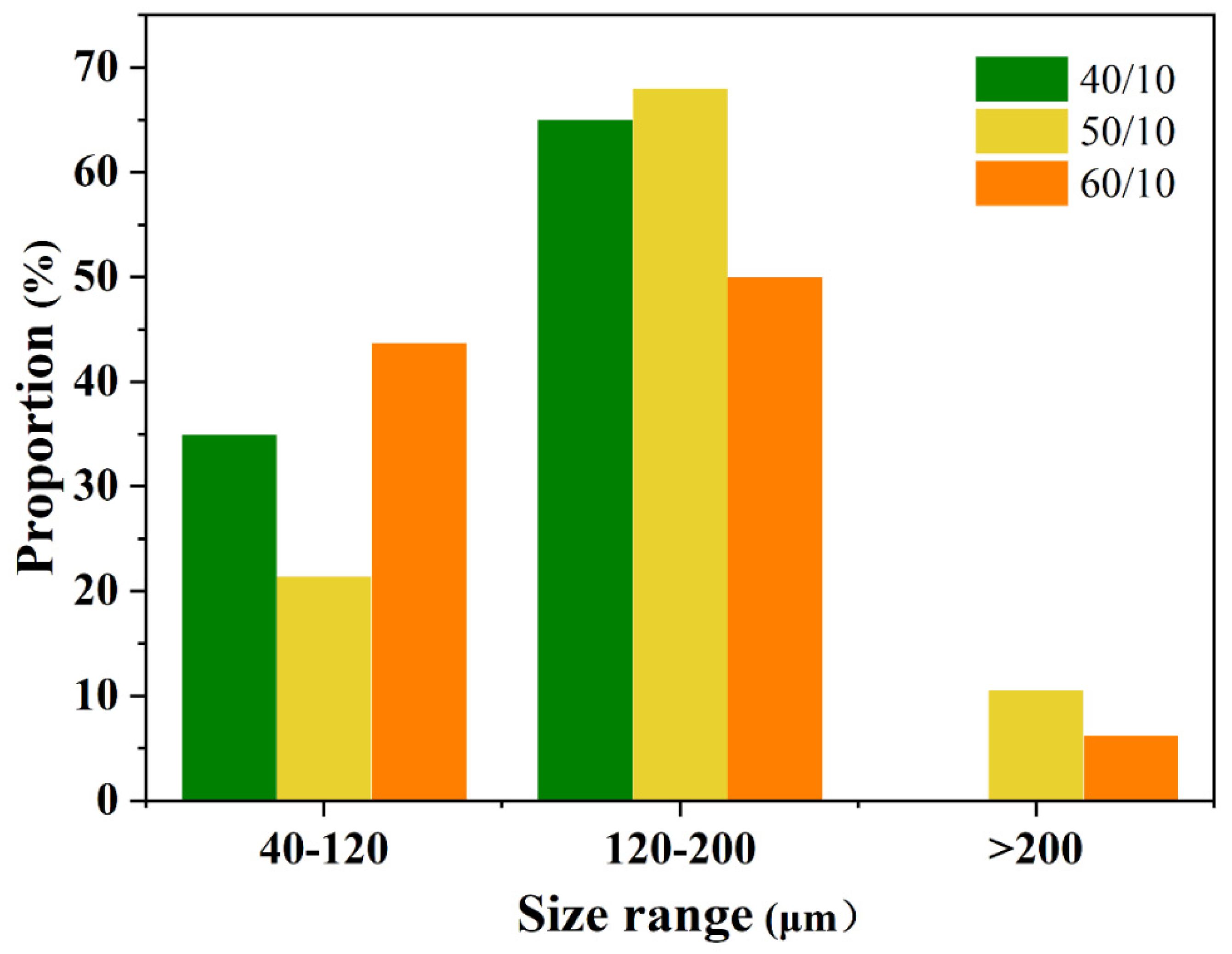
3.3. Effect of Gas to Liquid Flow Rate Ratio on the Microbubbles
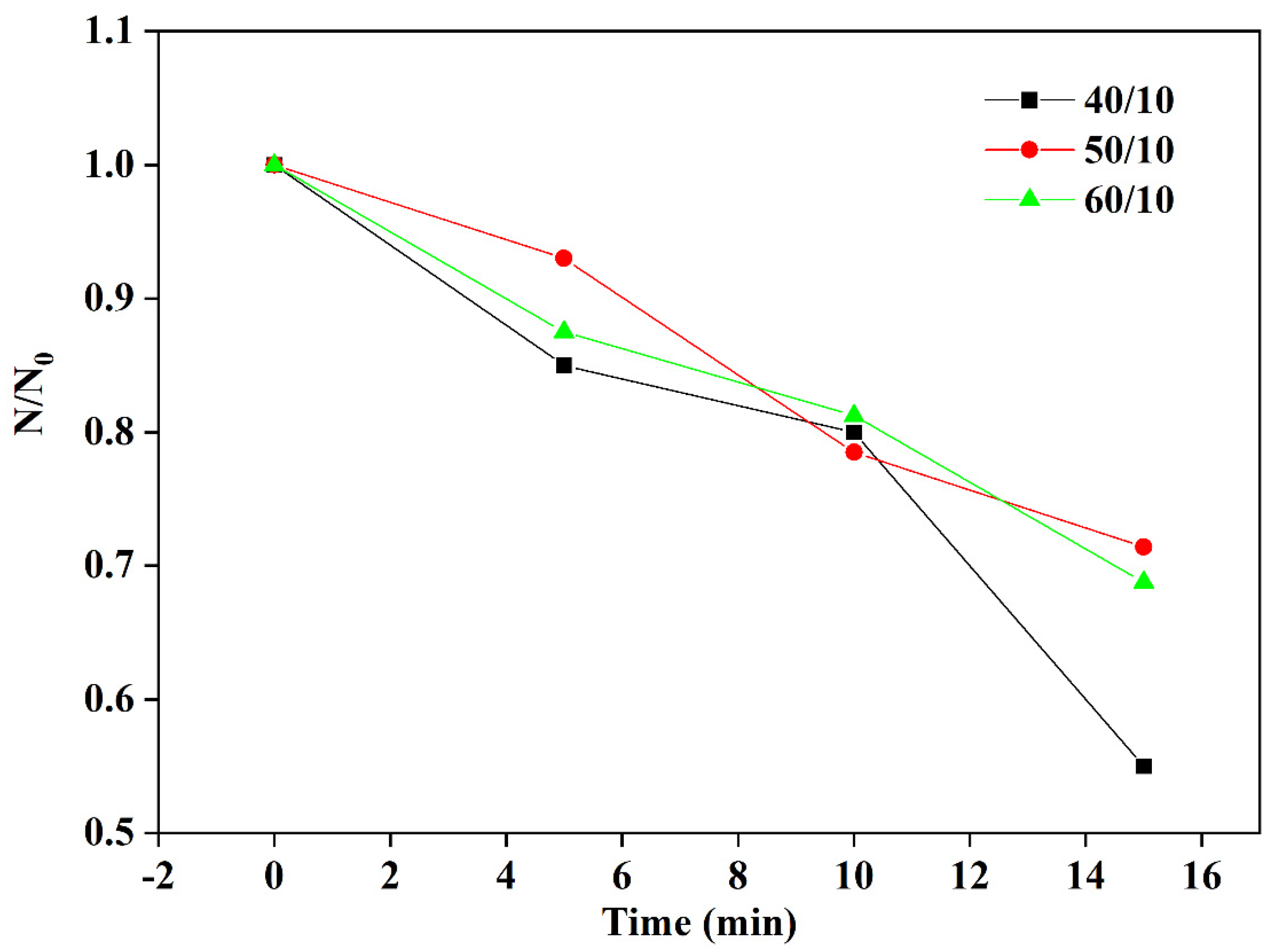
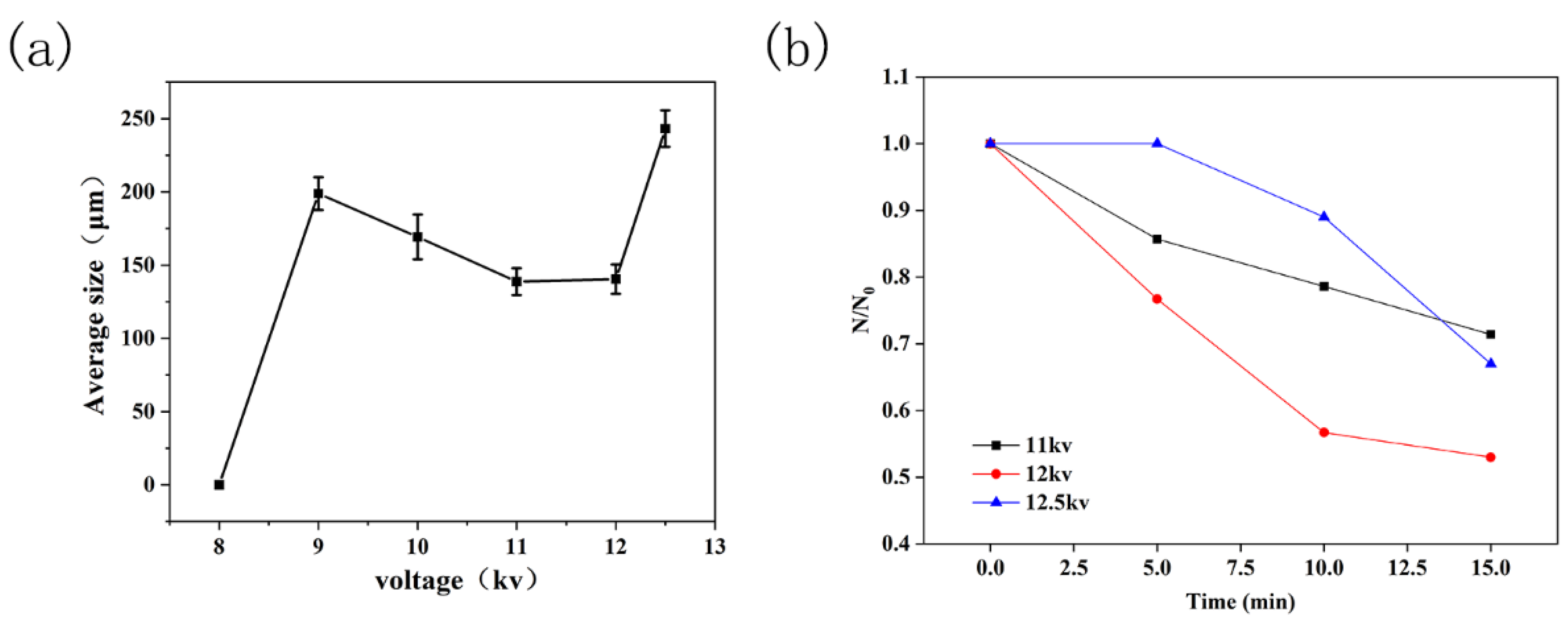
3.4. Effect of Applied Voltage on the Microbubbles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in Microstructured Reactors. Angew. Chem. Int. Ed. 2004, 43, 406–446. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, K.; Hirabayashi, A.; Nishino, T.; Fujioka, S.; Kobayashi, D. Development of microbubble aerator for waste water treatment using aerobic activated sludge. Chem. Eng. Sci. 2011, 66, 3172–3179. [Google Scholar] [CrossRef]
- Shen, Y.; Longo, M.L.; Powell, R.L. Stability and rheological behavior of concentrated monodisperse food emulsifier coated microbubble suspensions. J. Colloid Interface Sci. 2008, 327, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Bjerknes, K.; Sontum, P.C.; Smistad, G.; Agerkvist, I. Preparation of polymeric microbubbles: Formulation studies and product characterisation. Int. J. Appl. Pharm. 1997, 158, 129–136. [Google Scholar] [CrossRef]
- Kwan, J.J.; Borden, M.A. Microbubble Dissolution in a Multigas Environment. Langmuir 2010, 26, 6542–6548. [Google Scholar] [CrossRef]
- Lindner, J.R. Microbubbles in medical imaging: Current applications and future directions. Nat. Rev. Drug Discov. 2004, 3, 527–533. [Google Scholar] [CrossRef]
- Sirsi, S.; Borden, M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci. Eng. Technol. 2009, 1, 3–17. [Google Scholar] [CrossRef]
- Unnikrishnan, S.; Klibanov, A.L. Microbubbles as ultrasound contrast agents for molecular imaging: Preparation and application. AJR Am. J. Roentgenol. 2012, 1992, 292–299. [Google Scholar] [CrossRef]
- Schutt, E.G.; Klein, D.H.; Mattrey, R.M.; Riess, J.G. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: The key role of perfluorochemicals. Angew. Chem. Int. Ed. 2003, 4228, 3218–3235. [Google Scholar] [CrossRef]
- Stride, E.; Saffari, N. Microbubble ultrasound contrast agents: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2003, 217, 429–447. [Google Scholar] [CrossRef]
- Pancholi, K.; Stride, E.; Edirisinghe, M. Generation of microbubbles for diagnostic and therapeutic applications using a novel device. J. Drug Target 2008, 16, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Hao, L.; Hu, W.; Ran, Y.; Bai, Y.; Zhang, L. Novel multifunctional pH-sensitive nanoparticles loaded into microbubbles as drug delivery vehicles for enhanced tumor targeting. Sci. Rep. 2016, 6, 29321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Saint Victor, M.; Crake, C.; Coussios, C.-C.; Stride, E. Properties, characteristics and applications of microbubbles for sonothrombolysis. Expert Opin. Drug Deliv. 2014, 11, 187–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Roovers, S.; Lajoinie, G.; De Cock, I.; Brans, T.; Dewitte, H.; Braeckmans, K.; Versluis, M.; De Smedt, S.C.; Lentacker, I. Sonoprinting of nanoparticle-loaded microbubbles: Unraveling the multi-timescale mechanism. Biomaterials 2019, 217, 119250. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, 1802309. [Google Scholar] [CrossRef]
- Park, J.I.; Jagadeesan, D.; Williams, R.; Oakden, W.; Chung, S.; Stanisz, G.J.; Kumacheva, E. Microbubbles Loaded with Nanoparticles: A Route to Multiple Imaging Modalities. ACS Nano 2010, 4, 6579–6586. [Google Scholar] [CrossRef]
- Mohamedi, G.; Azmin, M.; Pastoriza-Santos, I.; Huang, V.; Pérez-Juste, J.; Liz-Marzán, L.M.; Edirisinghe, M.; Stride, E. Effects of Gold Nanoparticles on the Stability of Microbubbles. Langmuir 2012, 28, 13808–13815. [Google Scholar] [CrossRef]
- Owen, J.; Crake, C.; Lee, J.Y.; Carugo, D.; Beguin, E.; Khrapitchev, A.A.; Browning, R.J.; Sibson, N.; Stride, E. A versatile method for the preparation of particle-loaded microbubbles for multimodality imaging and targeted drug delivery. Drug Deliv. Transl. Res. 2018, 8, 342–356. [Google Scholar] [CrossRef] [Green Version]
- Tay, L.M.; Xu, C. Coating microbubbles with nanoparticles for medical imaging and drug delivery. Nanomedicine 2017, 12, 91–94. [Google Scholar] [CrossRef]
- Seo, M.; Gorelikov, I.; Williams, R.; Matsuura, N. Microfluidic Assembly of Monodisperse, Nanoparticle-Incorporated Perfluorocarbon Microbubbles for Medical Imaging and Therapy. Langmuir 2010, 26, 13855–13860. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Carugo, D.; Crake, C.; Owen, J.; de Saint Victor, M.; Seth, A.; Coussios, C.; Stride, E. Nanoparticle-Loaded Protein–Polymer Nanodroplets for Improved Stability and Conversion Efficiency in Ultrasound Imaging and Drug Delivery. Adv. Mater. 2015, 27, 5484–5492. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-C.; Chua, Q.W.; Ong, X.J.; Sharma, V.K.; Tong, Y.W.; Wang, C.-H. Fabrication of ultrasound-responsive microbubbles via coaxial electrohydrodynamic atomization for triggered release of tPA. J. Colloid Interface Sci. 2017, 501, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Zhou, W.; Pelan, E.G.; Stoyanov, S.D.; Arnaudov, L.N.; Stone, H.A. Sonication–Microfluidics for Fabrication of Nanoparticle-Stabilized Microbubbles. Langmuir 2014, 30, 4262–4266. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Bick, A.; Sullivan, M.; Stone, H.A. Controllable Microfluidic Production of Microbubbles in Water-in-Oil Emulsions and the Formation of Porous Microparticles. Adv. Mater. 2008, 20, 3314–3318. [Google Scholar] [CrossRef]
- Xu, J.H.; Li, S.W.; Chen, G.G.; Luo, G.S. Formation of monodisperse microbubbles in a microfluidic device. AIChE J. 2006, 52, 2254–2259. [Google Scholar] [CrossRef]
- Farook, U.; Zhang, H.B.; Edirisinghe, M.J.; Stride, E.; Saffari, N. Preparation of microbubble suspensions by co-axial electrohydrodynamic atomization. Med. Eng. Phys. 2007, 29, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, W.; Jiang, P.; Hong, T. Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles. Micromachines 2019, 10, 125. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-C.; Ong, X.J.; Pun, K.T.; Tan, D.Y.; Sharma, V.K.; Tong, Y.W.; Wang, C.-H. Preparation of tPA-loaded microbubbles as potential theranostic agents: A novel one-step method via coaxial electrohydrodynamic atomization technique. Chem. Eng. J. 2017, 307, 168–180. [Google Scholar] [CrossRef]
- Yan, W.-C.; Tong, Y.W.; Wang, C.-H. Coaxial electrohydrodynamic atomization toward large scale production of core-shell structured microparticles. AIChE J. 2017, 63, 5303–5319. [Google Scholar] [CrossRef]
- Farook, U.; Stride, E.; Edirisinghe, M.; Moaleji, R. Microbubbling by co-axial electrohydrodynamic atomization. Med. Biol. Eng. Comput. 2007, 45, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar, M.; Stride, E.; Edirisinghe, M. Preparation of monodisperse microbubbles using an integrated embedded capillary T-junction with electrohydrodynamic focusing. Lab Chip 2014, 14, 2437–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, B.S. Stabilization of bubbles and foams. Curr. Opin. Colloid Interface Sci. 2007, 12, 232–241. [Google Scholar] [CrossRef]
- Brugarolas, T.; Gianola, D.S.; Zhang, L.; Campbell, G.M.; Bassani, J.L.; Feng, G.; Lee, D. Tailoring and Understanding the Mechanical Properties of Nanoparticle-Shelled Bubbles. ACS Appl. Mater. Interfaces 2014, 6, 11558–11572. [Google Scholar] [CrossRef]
- Lee, M.; Lee, E.Y.; Lee, D.; Park, B.J. Stabilization and fabrication of microbubbles: Applications for medical purposes and functional materials. Soft Matter 2015, 11, 2067–2079. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Masliyah, J.H. Numerical Simulation of Ostwald Ripening in Emulsions. J. Colloid Interface Sci. 1997, 196, 157–169. [Google Scholar] [CrossRef]
- Jin, Z.; Mantri, Y.; Retout, M.; Cheng, Y.; Zhou, J.; Jorns, A.; Fajtova, P.; Yim, W.; Moore, C.; Xu, M.; et al. A Charge-Switchable Zwitterionic Peptide for Rapid Detection of SARS-CoV-2 Main Protease. Angew. Angew. Chem. Int. Ed. 2022, 61, e202112995. [Google Scholar] [CrossRef]
- Welsh, T.J.; Krainer, G.; Espinosa, J.R.; Joseph, J.A.; Sridhar, A.; Jahnel, M.; Arter, W.E.; Saar, K.L.; Alberti, S.; Collepardo-Guevara, R.; et al. Surface Electrostatics Govern the Emulsion Stability of Biomolecular Condensates. Nano Lett. 2022, 22, 612–621. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.-B.; Wang, Y.; Ran, X.; Wu, J.-C.; Wei, R.; Yan, W.-C. Preparation of Nanoparticle-Loaded Microbubbles via an Electrohydrodynamic Atomization Process. Appl. Sci. 2022, 12, 3621. https://doi.org/10.3390/app12073621
Nie X-B, Wang Y, Ran X, Wu J-C, Wei R, Yan W-C. Preparation of Nanoparticle-Loaded Microbubbles via an Electrohydrodynamic Atomization Process. Applied Sciences. 2022; 12(7):3621. https://doi.org/10.3390/app12073621
Chicago/Turabian StyleNie, Xin-Bin, Yong Wang, Xiong Ran, Ji-Chuan Wu, Ran Wei, and Wei-Cheng Yan. 2022. "Preparation of Nanoparticle-Loaded Microbubbles via an Electrohydrodynamic Atomization Process" Applied Sciences 12, no. 7: 3621. https://doi.org/10.3390/app12073621
APA StyleNie, X.-B., Wang, Y., Ran, X., Wu, J.-C., Wei, R., & Yan, W.-C. (2022). Preparation of Nanoparticle-Loaded Microbubbles via an Electrohydrodynamic Atomization Process. Applied Sciences, 12(7), 3621. https://doi.org/10.3390/app12073621





