Abstract
Microcapsules are employed extensively in various applications; however, most are composed of synthetic plastics. Thus, substitution of their component materials is essential to prevent environmental problems associated with primary microplastics. Herein, we report the synthesis of eco-friendly silica core–shell microcapsules for fragrance retention. The silica shell was prepared via oil/water emulsion template synthesis using tetraethyl orthosilicate (TEOS), which was added to the immature silica microcapsules prior to complete formation of primary silica shells to promote seeded growth for further reaction of silica. The thickness of the silica shell increased from 42.29 to 70.03 nm, while the Brunauer–Emmett–Teller surface area and internal pore area decreased from 155.16 and 30.08 m2/g to 92.28 and 5.36 m2/g, respectively. The silica microcapsules with lower surface areas retained fragrance for more than 80 days, even in a harsh environment of 15% sodium dodecyl sulfate at 60 °C, whereas the fragrance compound in those without additional TEOS treatment was completely released within seven days. Practical qualitative evaluation of fragrance was also performed for application in fragrance delivery because of the enhanced long-term fragrance retention ability. Our findings show the widespread potential of microcapsules synthesized from eco-friendly materials in industrial applications.
1. Introduction
The materials of microcapsules, composed of core–shell or matrix-embedded systems, have been extensively studied to control the use of active materials in various fields, including home care, cosmetics, pharmaceuticals, and food technology [1,2,3,4,5]. Synthetic polymers such as melamine [6,7], polymethacrylate [8,9], and polyurethane/urea [10,11] are widely used in the fabrication of microcapsules; however, they are difficult to degrade naturally [12,13,14]. Microcapsules are classified as primary microplastics because their materials are hardly biodegradable [15,16]; hence, most countries have issued fixed-term regulatory legislation recommending alternatives to microplastics [17,18].
Silica, which is widely used in anticaking agents, abrasives, moisture or oil absorbers, additives, etc., is one of the alternative material candidates [19,20,21,22]. Although the encapsulation of active ingredients using silica has been investigated, further work is needed to understand the mesoporous features of silica, so that its applicability can be extended to other areas such as fragrance microcapsule fabrication [23,24,25]. To compensate for the disadvantages of silica, microcapsules have been synthesized by hybridization with ethyl cellulose [26], polyurea [27], melamine [28], or have been hydrophobically modified by adding methyltriethoxysilane (MTES) and dimethyldiethoxysilane (DMDES) [29,30]. However, these microcapsules are not free of microplastics. During water/oil (W/O) emulsion template synthesis, the silica reaction proceeds more slowly in an acidic than in a basic medium, resulting in dense and less porous microcapsules [31,32,33]. Although the addition of tetraethyl orthosilicate (TEOS) can reduce the porosity, in contrast to our study, the phase of the studies was inverted and the encapsulated ingredient exhibited a retention time of only about 100 h in deionized (DI) water [33]. Products such as cosmetics and laundry detergents typically contain emulsifiers and have a shelf life of at least three years [34]. The fragrance in household products should be retained within the capsule for as long as possible; however, the fragrance retention of microcapsules in such products can be negatively impacted by harsh conditions, including high emulsifier concentrations, high temperature washing cycles, and irregular temperatures during distribution.
In this study, we developed fragrance microcapsules with low porosities composed entirely of silica. These microcapsules contain no microplastics and retain the fragrance for extended periods, even under harsh conditions. Using a fragrance O/W emulsion as a template, TEOS in the oil phase was hydrolyzed at the O/W interface to form silica shells under mild conditions (25 °C without pH adjustment). TEOS was subsequently applied to the premature silica microcapsules for additional silica coating owing to seeded growth. After the addition of TEOS, microcapsule thickness was assessed using focused ion beam scanning electron microscopy (FIB-SEM), and the specific surface area was determined by Brunauer–Emmett–Teller (BET) analysis. In addition, the fragrance retention time and practical qualitative fragrance were evaluated in a high-concentration emulsifier solution at a high temperature to mimic a harsh environment.
2. Materials and Methods
2.1. Materials
TEOS was purchased from Wacker (München, Germany), methyltrimethoxysilane (MTMS), DMDES, 3-aminopropyltriethoxysilane (APTES), and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and n-octyltriethoxysilane (n-OTES) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Cetyltrimethylammonium bromide (CTAB), ethanol, and acetone were purchased from Daejeong (Siheung, Korea). Hexyl cinnamaldehyde was obtained from Firmenich (Geneva, Switzerland). Dihydrogenated tallowamidoethyl hydroxyethylmonium methosulfate (DTHM) was obtained from Osung chemical (Incheon, Korea). Syringe filters (0.22 μm, Millex®) were purchased from Merck Millipore (Burlington, MA, USA). Syringes (10 mL) were purchased from the Korea Vaccine Co., Ltd. (Seoul, Korea).
2.2. Synthesis of Silica Microcapsules
TEOS was mixed with hexyl cinnamaldehyde in a weight ratio of 1:1 in the oil phase. CTAB (10 wt%) was dissolved in DI water at 80 °C. After complete dissolution, the concentrated CTAB solution was diluted to 1.5 mM with DI water. The oil phase was gradually added to the aqueous phase and mixed using a homogenizer at 3000 rpm (T-25 Ultra-Turrax, IKA). The mixture was subsequently homogenized at 11,000 rpm for 10 min to form the fragrance O/W emulsion. The emulsion was maintained at 25 °C for two days, during which time microcapsules with premature silica shells formed. The thickness of the silica microcapsules was enhanced by adding varying amounts of TEOS (1, 3, 5 wt%) to the precapsule dispersion and mixing for 10 min with a mechanical overhead stirrer (Eurostar 20, IKA, London, UK) at 200 rpm. The reaction was allowed to proceed at 25 °C for three days, after which the silica microcapsules were centrifuged and rinsed with DI water. The resulting pellets were dried in an oven at 40 °C for 48 h.
2.3. Characterization
The fragrance compound in the silica microcapsules was completely removed prior to analysis of the various silica microcapsules. The dispersion process in ethanol, thorough crushing with a tip sonicator (Qsonica, Newtown, CT, USA), and precipitation by centrifugation was repeated six times. After centrifugation, the silica shell particles were freeze dried for 24 h and then dried at 100 °C for one week to obtain a pure silica shell.
The silica microcapsules were cross-sectioned using a focused ion beam scanning electron microscope (Helios 5UX, ThermoFisher, Waltham, MA, USA). The thickness of the silica shell was randomly measured at 150 spots in the SEM image with an Image J analyzer (IMAGE J 1.53k, Bethesda, MD, USA) to determine the average thickness of the shell.
The nitrogen adsorption isotherm was obtained at 77 K using a surface area analyzer (ASAP-2420, Micromeritics, Norcross, GA, USA), from which the surface area was calculated by BET analysis; the internal pore area was calculated using the t-plot model, and the pore size distribution was calculated using the Barrett–Joyner–Halenda (BJH) method. The samples were stored under vacuum at 200 °C for 24 h to completely eliminate gas and moisture from the surface and pores before any measurement was taken.
Before the microcapsules were dried, as mentioned in Section 2.2, the fragrance encapsulation efficiency was determined by adding 2 g of the reaction product to 98 g of the 1% SDS solution, which was immediately diluted with ethanol using a syringe filter after mixing. Further, the concentration of the fragrance compound was measured using a UV spectrophotometer (Lambda 365, Perkin Elmer, Waltham, MA, USA) at 285 nm, which is the maximum absorption wavelength of hexyl cinnamaldehyde. The fragrance encapsulation efficiency was calculated using Equation (1):
where CS is the concentration of the fragrance compound in the SDS solution (as it is unencapsulated fragrance) and C0 is the concentration of the initial fragrance compound before the synthesis of silica microcapsules. Cs was calculated using the absorbance vs. concentration standard curve of hexyl cinnamaldehyde concentration. Each sample was analyzed in triplicate.
2.4. Perfume Leakage Test
The fragrance silica microcapsules (0.2 g) were dispersed in 99.8 g of the SDS solution (15 wt%, pH 3.5). The samples were stored at room temperature and 60 °C for seven-day time intervals for a total of 113 and 84 days at RT and 60 °C, respectively. After each interval, the samples were filtered using a syringe filter and diluted with ethanol before the concentration of the fragrance compound leaked from the silica microcapsules was measured using a UV spectrophotometer. In addition, residual fragrance in the silica microcapsules remaining on the filter was assessed, those were dispersed in ethanol, and the corresponding shells were crushed by a tip sonicator and filtered using a syringe filter before the residual concentration of the fragrance compound in the microcapsules was measured using a UV spectrophotometer. The total fragrance concentration, which is the sum of the fragrance residual in the microcapsules and the fragrance leaked out from the microcapsules, is 100%. Total fragrance concentration was calculated using Equation (2). Each sample was analyzed in triplicate.
The leaked fragrance concentration was calculated using Equation (3):
where CL is the leaked concentration of the fragrance compound in the SDS solution from the fragrance silica microcapsules and CT is the concentration of the initial fragrance compound in the microcapsules.
CL was calculated using the same method as that used to calculate CS (described in Section 2.3). CR was calculated as follows. First, the silica microcapsules were dispersed in ethanol. Thereafter, the corresponding shells were crushed by a tip sonicator and filtered using a syringe filter, after which the residual concentration of the fragrance compound in the microcapsules was measured using a UV spectrophotometer.
2.5. Qualitative Evaluation of Fragrance
The fragrance silica microcapsules (0.2%) were dispersed in a 10% solution of dihydrogenated tallowamidoethyl hydroxyethylmonium methosulfate (DTHM), the key ingredient in fabric softeners, antistatic agents, and surfactants. The samples were stored at room temperature for specific point-in-time durations for 15 months (1 week, 2 weeks, 4 weeks, 8 weeks, 24 weeks, 40 weeks, and 60 weeks) and at 60 °C for time-point durations for 2 months (1 week, 2 weeks, 4 weeks, and 8 weeks).
To perform the fragrance qualitative evaluation test, we used a B-type washing machine (LG inverter direct drive 10.0 kg, Busan, Korea) that followed the ISO 6330 5.12 standards [35]. We used 20 cotton towels, which are a weight-compensation fabric for type B washing machines according to the ISO 6330 5.3 standards [35]. According to the standard test method, 20 g of the DTHM solutions including the fragrance silica microcapsules was added to 50 L of water, swirled for 15 min, and then drained for 6 min. Subsequently, the evaluation was carried out after air drying for one day.
Subjective fragrance evaluation was used to determine the fragrance retention strength of the finished fabrics. The evaluation test included 20 people aged between 25 and 40 (10 males and 10 females; all had completed at least graduate school). Each cotton towel was folded and rubbed by the panelists, who then sniffed the scent. They conducted a blind test in which they had no prior knowledge of the samples. The intensity of the scent was divided into 6 levels (5 = very strong scent, 4 = strong scent, 3 = medium scent, 2 = weak scent, 1 = very weak scent, and 0 = no scent). The response of each member on fabric fragrance retention was recorded.
3. Results and Discussion
3.1. Suitable Synthesis Conditions for Fragrance Silica Microcapsules
Silica microcapsules can be synthesized using several techniques. High temperature, sonication, and high or low pH can be employed to rapidly synthesize microcapsules by increasing the TEOS hydrolysis rate [33,36,37,38]. Before synthesizing fragrance containing silica microcapsules for our fragrance delivery system, we first examined the feasibility of each synthetic method.
After emulsifying the fragrance compound and TEOS mixture at 11,000 rpm at 25 °C, samples of the emulsion were subjected to the following reactions: (i) a 7-d reaction at room temperature, (ii) a 2-d reaction at 50 °C followed by a 7-d reaction at room temperature, and (iii) 1 h of sonication followed by a 7-d reaction. The silica microcapsules formed under each set of reaction conditions had the following characteristics (Figure 1G): (i) particle size of 1.18 μm and an encapsulation efficiency of 99.61 ± 0.003%, (ii) particle size of 0.87 μm and an encapsulation efficiency of 84.19 ± 0.464%, and (iii) particle size of 0.43 μm and an encapsulation efficiency of 99.13 ± 0.004%. The silica microcapsule (0.2 wt%) was added to a 15% SDS solution to assess the fragrance retention. The fragrance in the capsules synthesized at room temperature was retained for 1000 h (approximately 40 d), whereas the fragrance in capsules synthesized at 50 °C was completely released in 4 h and that in the capsules synthesized by sonolysis was completely released within 2 d (Figure 1H).
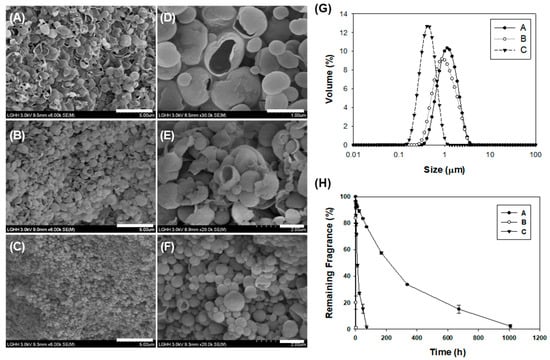
Figure 1.
Scanning electron micrographs of silica microcapsules synthesized (A) at room temperature, (B) at 50 °C, and (C) by sonication, (bar: 5 μm). Magnified image (D) of (A), (E) of (B), and (F) of (C) (bar: 1 μm). (G) Size distribution of silica microcapsules and (H) fragrance retention in a 15% SDS solution at room temperature.
The fragrance retention of the silica microcapsules was also analyzed by measuring the concentration of hexyl cinnamaldehyde that leaked into the aqueous phase of the SDS solution from the silica microcapsules at room temperature by measuring the absorbance at 285 nm. The fragrance compound encapsulated in silica microcapsules, with silica coatings to reduce porosity, was added to a 15% SDS solution to mimic laundry detergent and fabric softener products. The leakage of the fragrance oil from the microcapsules was evaluated at various times and temperatures.
The fragrance silica microcapsules synthesized at room temperature were found to be the most suitable (Figure 1H). These microcapsules had dense less porous microcapsule shell structures because the reaction occurred slowly at the interface of the fragrance compound and the TEOS mixture [31,32]. However, even at room temperature, the fragrance oil leaked within 2 h at a higher silication rate due to the high temperature. Although the fragrance capsules synthesized at 50 °C and those at room temperature showed minor size differences, the rapid silica reaction presumably formed more porous structures at 50 °C (Figure 1A,B,D,E). Meanwhile, after ultrasonic homogenization into a fragrant nano-emulsion, the emulsion template reaction occurred, and the synthesized nanocapsules were narrowly dispersed to 400 nm in size (Figure 1C). However, these nanocapsules released the fragrance compound approximately 14 times faster than the microcapsules synthesized at room temperature, and the rate of leakage appeared to increase with increasing surface area (Figure 1E). The fragrance retention time of the microcapsules synthesized at room temperature (approximately 40 d) was too short for use in detergents; hence, the microcapsules required further treatment.
pH is also important for the formation of silica. The reaction rate is accelerated by increasing the pH; however, this also increases porosity to the extent that the structure of the microcapsules cannot be maintained. The reaction rate can be controlled by lowering the pH level; however, when the pH level decreases to <4, the hydrolysis rate exceeds the condensation rate [39]. Thus, the concentration of hydrolyzed TEOS is increased, and the rate of migration of hydrophilic TEOS to the exterior water phase is higher than the rate of incorporation at the interface. As a result, synthesizing the thick shell of silica microcapsules becomes difficult, and the hydrolyzed TEOS in the aqueous phase undergoes a sol–gel reaction, eventually resulting in the gelation of the entire phase. Furthermore, we confirmed that after preparing the fragrance emulsion, the pH level immediately decreased to <4, which resulted in the gelation of the entire phase after 3–4 d (data not provided).
In particular, monitoring the pH change during the reaction of silica microcapsules confirmed that the result ranged from pH 6.5 to pH 4.2 as the reaction proceeded. Microcapsules with the desired properties, such as a dense capsule wall and prolonged retention of the encapsulated compound, were synthesized at room temperature under mild conditions [36]. Therefore, unlike conventional microcapsules composed of melamine, polymethacrylate, and polyurethane/urea, the silica fragrance microcapsule we developed uses less energy (as it does not increase the temperature) and is eco-friendly (because it does not utilize or release dangerous compounds).
3.2. Enhanced Stability of Silica Microcapsules for Harsh Environments
To prevent or delay fragrance leakage from the microcapsules, we examined coating additional silica on the surfaces of previously synthesized silica microcapsules at room temperature. Hydrolyzed TEOS has stronger affinity with preformed silica surfaces compared with other hydrolyzed TEOS, resulting in seeded growth [40]. A thin silica layer grew on the surfaces of the silica microcapsules after post-silica coating (Figure 2A). The silica shells measured 102.3, 70.0, and 57.7 nm thick, on average, when additional TEOS concentrations of 5% (T5), 3% (T3), and 1% (T1), respectively, were added (Figure 2B). The untreated capsules (T0) had an average thickness of 42.3 nm. The thickness of the silica shell increased as the concentration of TEOS applied increased. However, although the thickness of T5 increased, SEM images revealed that this increase in thickness was less uniform than in the other samples. (Figure 2). Beyond a specific TEOS concentration, additional reactions occurred along with seeded growth on the surfaces of the microcapsules. The addition of more than 6% TEOS resulted in the formation of a gel throughout the phase (data not shown), which suggests that the maximum concentration of 5% TEOS can be added to the microcapsules.
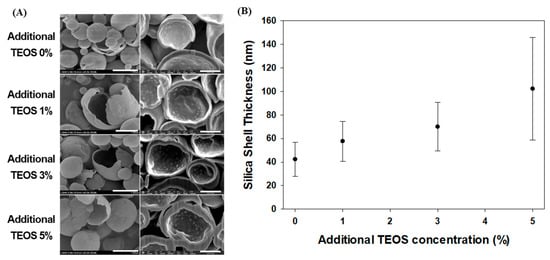
Figure 2.
(A) Scanning microscopy images of silica microcapsules synthesized following the addition of various concentrations of TEOS, (bar: left, 1 μm; right, 400 nm) and (B) silica shell thicknesses determined using the ImageJ software.
Stirring speed also had an effect on the reaction during the introduction of TEOS. Hydrophobic TEOS emulsifies into small particles that disperse throughout the phase. However, stirring the mixture at less than 200 rpm for less than 10 min led to phase separation with a large emulsified TEOS phase and non-uniform coating or gelation in the partial phase. In addition, when hydrolyzed sodium silicate was added directly to the phase in place of TEOS, the sol–gel reaction occurred throughout the phase rather than selectively on the silica microcapsule surface (data not shown).
TEOS presumably hydrolyzed and slowly dissolved in the aqueous phase at pH 4.2, then bound to the silanol groups of the silica microcapsules, which have a stronger affinity for TEOS than other hydrolyzed TEOS, resulting in a seeded growth reaction [41]. However, as the TEOS concentration increases, whether by the higher rate of dissolution of hydrolyzed TEOS in the aqueous phase or, if TEOS is oversupplied, by adding additional TEOS, increasing the reaction temperature, changing the pH to acidic or basic, or adding sodium silicate, as examples, the silica reaction occurs not only on the surface of the microcapsules but the sol–gel reaction also take place in the aqueous phase; thus, the amount of TEOS added, temperature, and pH needs to be adjusted.
3.3. Mesoporous Controlled Silica Microcapsules
BET analysis was also employed to determine the specific surface area and internal pore area of the synthesized silica microcapsules. The adsorption isotherms of the silica shells exhibit type II behavior (Figure 3A), indicating the presence of mesopores (2–50 nm) and macropores (>50 nm) [42]. The size of the macropore is similar to or larger than the measured thickness of the silica shell, therefore does not affect the internal structure of the shell that influences fragrance oil leakage, and can thus be ignored (Figure 3B). The adsorption hysteresis loops are non-overlapping and narrow, exhibiting the characteristics of a slit-like pore structure (H3 type). As the amount of added TEOS increases up to 3%, the overall absorbed volume decreases. Seeded growth occurs after hydrolysis of TEOS, which appears to fill both mesopores and external pores on the surface of the shell.
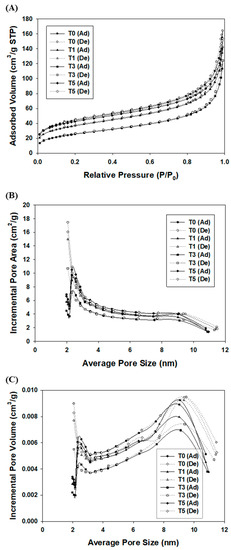
Figure 3.
(A) Nitrogen adsorption/desorption isotherms of silica microcapsules synthesized following the addition of various concentrations of TEOS. (B) Incremental pore areas and (C) incremental pore volumes as functions of average pore size.
The abovementioned data were calculated using the BET and t-plot methods. The total surface areas of the silica microcapsules and their respective surface areas are shown in Table 1. As the additional TEOS content increased from 0% to 3%, the total surface area of the silica shell decreased by 40.5%, and the micropore surface area decreased by a considerable 82.2%. The BJH distribution of average pore size versus incremental pore area and volume also revealed a reduction in both pore area and volume of both the macropores and mesopores (Figure 3B,C). The pores were clearly discernable by their distinct maxima centered at 2.36, 8.69, 44.2, and 101 nm in Figure S1. The pore sizes of 44.2 and 101 nm are not relevant because they are higher or similar to the thickness of the silica microcapsules, as described above. In particular, the reductions of 2.36 and 8.69 nm in the size of the mesopores were presumably directly related to the fragrance retention ability of the microcapsules.

Table 1.
Surface areas and porosities of fragrance-containing silica microcapsules synthesized following the addition of various amounts of TEOS.
The absorbed volume increased upon the introduction of additional 5% TEOS (T5) to the immature silica microcapsules (Figure 3A and Figure S2). The pores in the shells of the silica microcapsule were presumably filled by the addition of 3% TEOS as the capsule shell thickened. Further addition of TEOS resulted in a build-up of excess TEOS, forming a rough surface on the surface of the silica microcapsules (Figure 3A). Although the phase and number of additions were different, the results are surprisingly similar to those of Meaney et al. [33] when considering the concentration of TEOS added. In this system, multiple additions of TEOS are considered most effective. However, the addition of excess TEOS improves both the simplicity and the productivity of capsule synthesis.
3.4. Fragrance Retention Test
The primary goal of this work is to determine the correlation between capsule thickness and changes in porosity after the addition of TEOS, and their effect on fragrance retention. The fragrance retention of the silica microcapsule was assessed at a high temperature, representing harsher conditions than those in the previous experiment (Section 3.2). The concentration of the released fragrance compound was measured for up to four months at room temperature and 60 °C to determine the fragrance retention.
After 133 d, more than 60% of the fragrance compound remained within the T5 microcapsules, whereas 50% remained within the T3 microcapsules and only 25% remained within the T1 microcapsules at room temperature (Figure 4A). The fragrance encapsulated within the T0 microcapsules was completely released after 42 d. The fragrance leaked more rapidly at 60 °C (Figure 4B). Approximately 20% of the fragrance compound remained in the T5 microcapsules after 84 d. In contrast, all of the fragrance compound leaked from the T3, T1, and T0 microcapsules after 84, 40, and 7 d, respectively. Silica fragrance capsules with thicker shells exhibited more effective fragrance retention because of the additional silica at the surface of the microcapsule; however, this was only observed after the additional TEOS was adequately emulsified. A mechanical overhead stirrer speed of less than 200 rpm, or a short stirring time, resulted in lower fragrance retention (Table S1), which indicates that seeded growth occurs consistently when TEOS emulsions are uniformly distributed throughout the phase.
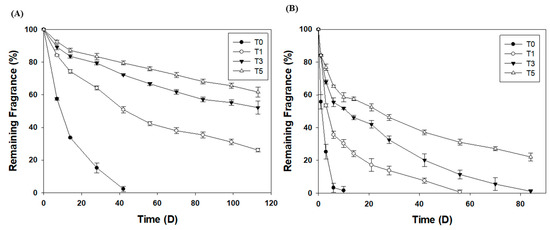
Figure 4.
Fragrance retention in 15% SDS solution (A) at room temperature and (B) at 60 °C.
Silica microcapsules for fragrance retention should ideally be devoid of pores; however, because silica has mesoporous properties, pores should be minimized to ensure fragrance retention for long periods in harsh environments. Despite the low number of pores in the synthesized microcapsules, hexyl cinnamaldehyde molecules can leak from the capsule through the few slit-like mesopores in the shell structure. As schematically represented in Figure 5, the addition of TEOS to the microcapsules at 25 °C under weakly acidic conditions significantly reduces the internal pore area because of the low rate of hydrolysis, while the silica formed by seeded growth is gradually deposited in the pores.
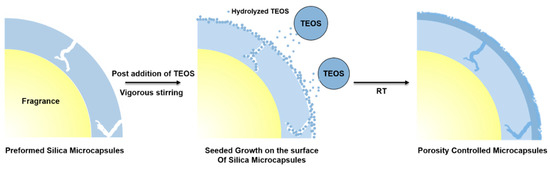
Figure 5.
Schematic representation of the mechanism of silica microcapsule synthesis in the presence of TEOS.
3.5. Qualitative Evaluation of Fragrance
We confirmed that silica fragrance capsules maintain fragrance for an extended period of time, even in harsh conditions. The silica capsules designed for the fabric softener also had to be evaluated to ensure that they worked as intended. The fragrance evaluation was carried out with 20 panelists to determine the residual fragrance. The ISO 5.12 and 5.3 methods were used to assess the degree of fragrance retention in the silica fragrance capsules at different time durations and temperatures in the cationic polymer solution that was used for the fabric softener [35]. In this evaluation, T1 was not included. The number of samples should be minimized because the olfactory sense, which is the easiest to exhaust among the five senses in humans, causes difficulty in accurately measuring the intensity of fragrance. Although T1 revealed superior fragrance preservation to control the fragrance retention test, T3 and T5 were assumed to be more significant.
Similar to the fragrance retention test, the fragrance intensity of the T5 microcapsule sample was more than moderate after 60 weeks and the fragrance intensity of the T3 microcapsule sample was weak, while the fragrance intensity of the T0 microcapsule sample was very weak within 8 weeks (Figure 6A). The fragrance of the sample after 8 weeks at 60 °C was similar to that in the previous in vitro fragrance retention test (Figure 6B). The fragrance retention in T5 was higher than that in T3, and the fragrance in T0 almost vanished after two weeks. The fragrance intensity could not be further evaluated, since DTHM hydrolyzes at 60 °C after 8 weeks; however, the silica fragrance capsules preserved the fragrance during the period of use as a fabric softener product. Silica fragrance capsules are used to confer fragrance in fabric softeners, and it has been concluded that they can be used for fabric softeners because of their superior long-term fragrance preservation and effective fragrance release based on user demand.
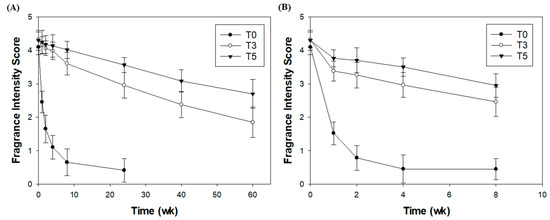
Figure 6.
Fragrance qualitative evaluation test in 10% solution of DTHM (A) at room temperature and (B) at 60 °C.
Silica microcapsules have been synthesized elsewhere [33]. In the inverse phase, it was possible to synthesize microcapsules with dense silica shells composed only of silica, which, in turn, prolonged content retention. Although their research revealed that sustained release might be feasible, most of the compounds present in microcapsules released within 100 h. However, for our purposes, the results were insufficient because we had to keep the ingredient in microcapsules for a long time. In contrast to the hydrophobic fragrance oil used in our study, the encapsulated hydrophilic solute is supposed to release readily in water, which is an external phase. Another distinction is that, as stated in Section 3.1, the formation rate of the shell of silica capsules is slow below pH 4, whereas their reaction conditions were 0.1 M HCl (pH 1). It is difficult to compare them because there are no data on their capsule thickness. However, due to differences in the synthesis environment, the thickness of the silica shell is presumably thinner than our result; therefore, the encapsulated compound is likely to be released quickly. Considering that the purpose of this study was to maintain fresh fragrance for extended periods even in harsh environments, microcapsules that achieve this objective were successfully synthesized in this study. Furthermore, the synthesis of these microcapsules is facile, requiring little energy and no pH adjustment, rendering the procedure an efficient and eco-friendly manufacturing approach.
4. Conclusions
Eco-friendly silica microcapsules exhibiting improved fragrance retention and significant perseverance in harsh environments, including high temperatures and surfactant concentrations, were developed. In contrast to the existing methods that add hydrophobic moieties or apply surface treatments with MTMS, DMDS, n-OTES, and APTES, we employed a seeded growth method by applying a silica coating to immature fragrance silica microcapsules synthesized from an O/W emulsion template consisting of a mixture of the fragrance compound and TEOS. The FIB-SEM images and BET analysis of the silica microcapsules revealed that the silica shells of the microcapsules thickened with increasing TEOS concentration, while the porosities of the microcapsules decreased. More than 60% of the fragrance was preserved by the thickest silica-coated microcapsules after approximately four months with high surfactant concentrations at room temperature. Furthermore, the fragrance test on participants revealed that the microcapsules could effectively preserve fragrance even under harsh conditions. We believe that our study will usher in a new era in which microplastics are phased out in favor of more environmentally friendly microcapsule alternatives. In particular, the findings of this study will facilitate improved fragrance retention in household items and cosmetics, contribute to the targeted delivery of pharmaceuticals that need to be isolated from external environments, and assist in the retention of self-healing or phase-conversion materials in construction applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12136759/s1, Figures S1 and S2: Eco-friendly silica microcapsules with improved fragrance retention; Table S1: Eco-friendly silica microcapsules with improved fragrance retention.
Author Contributions
Conceptualization, J.Y. and W.S.S.; data curation, J.Y.; formal analysis, J.Y.; investigation, J.Y. and W.S.S.; methodology, J.Y. and W.S.S.; project administration, N.G.K.; supervision, N.G.K.; validation, J.Y.; visualization, J.Y.; writing—original draft, J.Y.; writing—review and editing, J.Y., W.S.S. and N.G.K., All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Birnbaum, D.T.; Brannon-Peppas, L. Microparticle Drug Delivery Systems. In Drug Delivery Systems in Cancer Therapy; Humana Press: Totowa, NJ, USA, 2004; pp. 117–135. [Google Scholar]
- Patel, A.R.; Remijn, C.; Cabero, A.-I.M.; Heussen, P.C.; Hoorn, J.W.S.T.; Velikov, K.P. Novel All-Natural Microcapsules from Gelatin and Shellac for Biorelated Applications. Adv. Funct. Mater. 2013, 23, 4710–4718. [Google Scholar] [CrossRef]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Hitchcock, J.P.; Tasker, A.L.; Baxter, E.A.; Biggs, S.; Cayre, O.J. Long-Term Retention of Small, Volatile Molecular Species within Metallic Microcapsules. ACS Appl. Mater. Interfaces 2015, 7, 14808–14815. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M. Delivering value in the laundry room. C&EN Glob. Enterp. 2018, 96, 18–21. [Google Scholar] [CrossRef]
- Fei, X.; Zhao, H.; Zhang, B.; Cao, L.; Yu, M.; Zhou, J.; Yu, L. Microencapsulation mechanism and size control of fragrance microcapsules with melamine resin shell. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 300–306. [Google Scholar] [CrossRef]
- Tong, X.-M.; Zhang, T.; Yang, M.-Z.; Zhang, Q. Preparation and characterization of novel melamine modified poly(urea–formaldehyde) self-repairing microcapsules. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 91–97. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hayaty, M.; Navarchian, A.H. Morphological study of polymethyl methacrylate microcapsules filled with self-healing agents. Appl. Surf. Sci. 2017, 399, 721–731. [Google Scholar] [CrossRef]
- Alay, S.; Alkan, C.; Göde, F. Synthesis and characterization of poly(methyl methacrylate)/n-hexadecane microcapsules using different cross-linkers and their application to some fabrics. Thermochim. Acta 2011, 518, 1–8. [Google Scholar] [CrossRef]
- Koh, E.; Kim, N.-K.; Shin, J.; Kim, Y.-W. Polyurethane microcapsules for self-healing paint coatings. RSC Adv. 2014, 4, 16214–16223. [Google Scholar] [CrossRef]
- Tatiya, P.D.; Hedaoo, R.K.; Mahulikar, P.P.; Gite, V.V. Novel Polyurea Microcapsules Using Dendritic Functional Monomer: Synthesis, Characterization, and Its Use in Self-healing and Anticorrosive Polyurethane Coatings. Ind. Eng. Chem. Res. 2013, 52, 1562–1570. [Google Scholar] [CrossRef]
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly (methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stabil. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, N.; Jia, S.; Wang, C.; Wang, Y.; Qi, Y.; Wang, H.; Cui, X.; Hou, X.; Deng, T.S. Catalytic degradation of melamine–formaldehyde resins into valuable chemicals. Green Chem. 2021, 23, 7816–7824. [Google Scholar] [CrossRef]
- Katsumi, N.; Kusube, T.; Nagao, S.; Okochi, H. Accumulation of microcapsules derived from coated fertilizer in paddy fields. Chemosphere 2020, 267, 129185. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Annex, X.V. Restriction Report Proposal for a Restricton Substance; European Chemicals Agency (ECHA): Helsinki, Finland, 2019. [Google Scholar]
- Mitrano, D.M.; Wohlleben, W. Microplastic regulation should be more precise to incentivize both innovation and environmental safety. Nat. Commun. 2020, 11, 5324. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Jaafari, S.A.A.H.; Periasamy, V.S.; Almanaa, T.N.A.; Alshatwi, A.A. Fabrication of Biogenic Silica Nanostructures from Sorghum bicolor Leaves for Food Industry Applications. Silicon 2020, 12, 2829–2836. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, W.; Yun, J.; Lee, K.; Lee, J.; Yu, H.; Kim, J.H.; Kim, J.J.; Jang, J. Fabrication of Uniform Wrinkled Silica Nanopar-ticles and Their Application to Abrasives in Chemical Mechanical Planarization. ACS Appl. Mater. Interfaces 2018, 10, 11843–11851. [Google Scholar] [CrossRef]
- Dai, M.; Zhao, F.; Fan, J.; Li, Q.; Yang, Y.; Fan, Z.; Ling, S.; Yu, H.; Liu, S.; Li, J.; et al. A Nanostructured Moisture—Absorbing Gel for Fast and Large-Scale Passive Dehumidification. Adv. Mater. 2022, 34, 2200865. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Lee, K.-Y.; Ghorpade, R.V.; Park, H.-H. Superhydrophobic and Compressible Silica-polyHIPE Covalently Bonded Porous Networks via Emulsion Templating for Oil Spill Cleanup and Recovery. Sci. Rep. 2018, 8, 16783. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, Y.; Chen, X.; Li, Y.; Tian, Y.; Wang, H.; Li, X.; Yu, X.; Cao, Y. One-Pot Facile Synthesis of Double-Shelled Mesoporous Silica Microcapsules with an Improved Soft-Template Method for Sustainable Pest Management. ACS Appl. Mater. Interfaces 2021, 13, 39066–39075. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Ishii, T.; Koumoto, S. Synthesis of Monodisperse Mesoporous Silica Hollow Microcapsules and Their Release of Loaded Materials. Langmuir 2010, 26, 14334–14344. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Diab, R.; Joubert, O.; Canilho, N.; Pasc, A. Core–shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf. B Biointerfaces 2016, 140, 161–168. [Google Scholar] [CrossRef]
- Chen, K.; Xu, C.; Zhou, J.; Zhao, R.; Gao, Q.; Wang, C. Multifunctional fabric coatings with slow-releasing fragrance and UV resistant properties from ethyl cellulose/silica hybrid microcapsules. Carbohydr. Polym. 2019, 232, 115821. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; An, J.; Sun, D.; Tang, X.; Xiang, Y.; Yang, J. Robust microcapsules with polyurea/silica hybrid shell for one-part self-healing anticorrosion coatings. J. Mater. Chem. A 2014, 2, 11614–11620. [Google Scholar] [CrossRef]
- Mou, S.; Lu, Y.; Jiang, Y. A facile and cheap coating method to prepare SiO2/melamine-formaldehyde and SiO2/urea-formaldehyde composite microspheres. Appl. Surf. Sci. 2016, 384, 258–262. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Zhang, Z.; Vincent, B. Silica-Shell/Oil-Core Microcapsules with Controlled Shell Thickness and Their Breakage Stress. Langmuir 2009, 25, 7962–7966. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Liu, L.; Alva, G.; Jia, Y.; Fang, G. Synthesis and properties of microencapsulated octadecane with silica shell as shape–stabilized thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 160, 1–6. [Google Scholar] [CrossRef]
- Park, J.-H.; Oh, C.; Shin, S.-I.; Moon, S.-K.; Oh, S.-G. Preparation of hollow silica microspheres in W/O emulsions with polymers. J. Colloid Interface Sci. 2003, 266, 107–114. [Google Scholar] [CrossRef]
- Finnie, K.S.; Bartlett, J.R.; Barbé, C.J.A.; Kong, L. Formation of Silica Nanoparticles in Microemulsions. Langmuir 2007, 23, 3017–3024. [Google Scholar] [CrossRef]
- Meaney, S.; Tabor, R.; Follink, B. Synthesis and characterisation of robust emulsion-templated silica microcapsules. J. Colloid Interface Sci. 2017, 505, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Bruyninckx, K.; Dusselier, M. Sustainable Chemistry Considerations for the Encapsulation of Volatile Compounds in Laundry-Type Applications. ACS Sustain. Chem. Eng. 2019, 7, 8041–8054. [Google Scholar] [CrossRef]
- ISO 6330:2021; Textiles—Domestic Washing and Drying Procedures for Textile Testing. ISO: Geneva, Switzerland, 2021.
- Yokoi, T.; Wakabayashi, J.; Otsuka, Y.; Fan, W.; Iwama, M.; Watanabe, R.; Aramaki, K.; Shimojima, A.; Tatsumi, T.; Okubo, T. Mechanism of Formation of Uniform-Sized Silica Nanospheres Catalyzed by Basic Amino Acids. Chem. Mater. 2009, 21, 3719–3729. [Google Scholar] [CrossRef]
- Galgali, G.; Schlangen, E.; van der Zwaag, S. Synthesis and characterization of silica microcapsules using a sustainable solvent system template. Mater. Res. Bull. 2011, 46, 2445–2449. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Cihlář, J. Hydrolysis and polycondensation of ethyl silicates. 1. Effect of pH and catalyst on the hydrolysis and polycondensation of tetraethoxysilane (TEOS). Colloids Surf. A Physicochem. Eng. Asp. 1993, 70, 239–251. [Google Scholar] [CrossRef]
- Ghimire, P.P.; Jaroniec, M. Renaissance of Stöber method for synthesis of colloidal particles: New developments and opportunities. J. Colloid Interface Sci. 2020, 584, 838–865. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013; pp. 1–908. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).