Abstract
Soil salinization has become a major problem for agriculture worldwide, especially because this phenomenon is continuously expanding in different regions of the world. Salinity is a complex mechanism, and in the soil ecosystem, it affects both microorganisms and plants, some of which have developed efficient strategies to alleviate salt stress conditions. Currently, various methods can be used to reduce the negative effects of this problem. However, the use of biological methods, such as plant-growth-promoting bacteria (PGPB), phytoremediation, and amendment, seems to be very advantageous and promising as a remedy for sustainable and ecological agriculture. Other approaches aim to combine different techniques, as well as the utilization of genetic engineering methods. These techniques alone or combined can effectively contribute to the development of sustainable and eco-friendly agriculture.
1. Introduction
Among the most important concerns regarding agriculture in the 21st century is soil salinization [1]. According to some recent forecasts, environmental salinization mechanisms will continue to be enhanced as a result of global climate change [2]. The latter is having a significant impact on certain agricultural areas. Its consequences on soil salinity are most severe in arid, semi-arid, and coastal agricultural regions [3]. Salt accumulation in soil is a worldwide issue. Salt-affected soils occur as a result of a variety of processes that can be very localized and complicated in nature. Indicators have been designed to give insight into regions at risk of salinization on a global scale [4]. Increased salinity in agricultural regions is a major environmental hazard that threatens food security [5]. Saline soils are a serious problem for agriculture because they convert agronomically valuable land into unproductive land. According to the United Nations Environment Program, about 20% of agricultural land and 50% of farmland throughout the globe is salt stressed. Soil salinization is limiting the amount of land that can be utilized for agriculture by 1–2% every year, with arid and semi-arid zones being the worst impacted [6]. Soil salinization is expected to damage 1–10 billion hectares globally, posing a danger to the agricultural productivity needed to feed the world’s growing population [7], and that salinity stress harms agricultural yield on more than 20% of irrigated land throughout the world [8]. According to assessments, salinity and related issues, such as sodicity, waterlogging, and droughts, cause around 2000 hectares of land to yield reduced agricultural output every day [9].
Salinity is a major threat that reduces the capacity of all types of terrestrial ecosystems to provide services by threatening biodiversity, lowering agriculture production, degrading the environment, contaminating groundwater below standard levels, increasing flood risks, creating food security concerns, and limiting a community’s economic growth [10]. Furthermore, salinity is one of nature’s most powerful abiotic factors, and it harms both plants and microbes [11]. It can be difficult to distinguish the effects of salinity from those of other factors that may co-vary with salinity when studying the impacts of salinity on soil microbial communities [12]. However, plant life is hampered by high saline levels from germination to the final stage of the plant [13]. High salt intake impairs a variety of physiological and metabolic processes in plants, potentially threatening their viability [14]. Thus, salinity is one of the abiotic factors that has an impact on plant yield; hence, efforts are being undertaken to develop more resistant crops. It has become critical to devise techniques to convert coastal zones and degraded land resources into cultivable land [15]. Various remediation approaches have been successfully implemented and are in use, but there is still no one-size-fits-all technological solution for all circumstances [16]. Scraping, flushing, leaching, and applying an amendment (e.g., gypsum and CaCl2) are common procedures for reclaiming saline soil, although they have limited effectiveness and have negative consequences for agro-ecosystems [14]. An innovative concept known as “biosaline agriculture” has been gaining traction over the last few decades. Different halophytes (salt-tolerant plants) are produced as an alternative for conventional crop plants utilizing saline/brackish water irrigation [17]. The sustainability of agriculture without a compromise of environmental quality, agro-ecosystem functioning, and biodiversity protection are all key challenges in today’s agriculture [18]. Thus, the capacity of halotolerant or halophilic PGPB to grow in resistance to high salt levels through the use of osmoregulatory mechanisms to retain regular cell functioning has become crucial [13]. Moreover, for the restoration of saline soils, phytoremediation may be a cost-effective solution [19]. Salt-affected soils can be restored [20] by increasing the nutritional content of the soil and the quantity of soil organisms; an organic amendment to saline–alkali soil can boost the development of salt-tolerant plants directly or indirectly [2]. The ability of biochar–manure compost to relieve salt stress in soils has also been established [21].
Various breeding strategies and genetic engineering technologies are being used in studies to enhance saline-resistant crops [22]. Genetic research might lead to the development of salt-tolerant crops, which could boost agricultural productivity in saline areas and allow agriculture to expand to previously unsuitable areas [23]. In addition, many innovative strategies, such as blending treatment types, mixed plant cultures, and biostimulation, are being employed to increase the efficiency and quality of the restoration of salt-affected soils [16]. However, due to the long-term nature and high cost of such approaches, there is a need to establish simple and low-cost biological solutions that may be applied on a short-term basis [24]. This article deals with the different current aspects of agricultural soil salinization, mainly exploring the different impacts and mechanisms related to this phenomenon, as well as the biological methods used to remediate agricultural saline soils.
2. Soil Salinization
Salinization occurs when water soluble salts, such as sodium (Na+), chloride (Cl−), potassium (K+), sulfate (SO42−), magnesium (Mg2+), carbonate (CO32−), bicarbonate (HCO3−), and calcium (Ca2+), accumulate in the soil [6]. The most common salt species in saline soils is sodium chloride, which has a negative impact on plant production or causes death. When the electrical conductivity (EC) of the saturation soil paste is 4 dS m−1 (about 40 mM NaCl), the soil is categorized as saline [25,26].
The entire amount of salt-affected land on the planet is estimated to be 900 million hectares, or 6% of the total land mass [27]. Soil salinization is limiting the amount of land that can be utilized for agriculture by 1–2% every year, with arid and semi-arid areas being the worst impacted [6]. According to the Food and Agricultural Organization (FAO), the salinization of arable land will lead to 30% land loss over the next 25 years and up to 50% by 2050 if preventive measures are not adopted [28].
In particular, the majority of salt-affected areas are found in arid and semi-arid regions of the globe. The majority of nations impacted by salinity are found in sub-Saharan Africa, and Central and Middle East Asia. Soil salinization is a major problem in developing nations with dry climates (such as India, Bolivia, Pakistan, Egypt, Peru, Tunisia, and Morocco); industrialized nations, however, are less affected by salt but are not insusceptible. Salinization has already harmed crop output in 20–30% of irrigated farmland, with an additional 1.5 million hectares being damaged each year [29].
Soil salinity is becoming more of a concern throughout the world, resulting in soil deterioration and a global yearly agricultural production loss of more than USD 12 billion owing to this phenomenon [30,31].
2.1. Effects of Salinity on Plants
Plant life is hampered by high saline levels from germination to the final stage of the plant. Nutrient absorption, phytohormone production, root and shoot regulation, and the replication of DNA are all hampered as a result [13]. The common consequences of salinity include oxidative stress, which is characterized by the formation of reactive oxygen species that can affect enzymes, bio-membranes, nucleic acids, and proteins [32].
In addition, premature senescence is caused by the toxic effects of Na+ concentration, which results in a reduction in photosynthetic efficiency and reduced metabolic activities. In membrane transport and enzymatic processes, Na+ competes with K+, limiting plant development. The majority of plant cells have strategies to prevent the negative impacts of Na+ concentration by maintaining K+ and actively rejecting Na+ in roots and/or redistributing Na+ in shoot vacuoles [25,26,27,28,29,30,31,32,33].
2.2. Impacts of Salinity on Soil Microorganisms
Microbial composition, diversity, numbers, and functions are all adversely affected by salinity [33]. It is common to notice a decline in enzymatic activity or microbial biomass [34]. The susceptibility of soil enzyme functions to salt differs; salinity severely decreases the functions of β-glucosidase, alkaline phosphatase, and urease [4]. The dose–response connections between salinity and bacterial growth, as well as quantitative distributions of the trait salt tolerance among populations, have been established. The relationship between community salt tolerance and soil salinity was found to be substantial, suggesting that salinity has a considerable filtering influence on microbial populations [19].
Actinobacteria prevailed in saline soil, whereas Proteobacteria predominated in non-saline soil, according to a biodiversity study of soil microbiome utilizing pyrosequencing of the 16S rRNA gene. Bacteriodetes, Thaumarchaeota, Firmicutes, and Acidobacteria were found to be the most common phyla in both saline and non-saline soils, whereas Cyanobacteria, Verrucomicrobia, and Gemmatimonadetes were the least common [12]. In fungi, a low osmolality reduces spore germination and hyphae development, and it affects the morphology [35] and expression of genes [36].
Another negative impact of salt is the disruption of the symbiotic relationship among plants and useful microbes. A disruption in plant–bacteria connections, for example, is induced by changes in proteins implicated in the first adhesion phases (adsorption and anchoring) of bacteria to plant roots in symbiotic relationships, as well as the suppression of nitrogen fixation and nodulation functions [37].
Consequently, the impact is usually more prominent in the rhizosphere due to increased water absorption by plants as a result of transpiration. The basic reason is that living at high salt amounts has a substantial bio-energetic taxation because microbes must preserve osmotic balance between the cytoplasm and the surrounding environment, excluding sodium ions from within the cell. As a consequence, adequate energy for osmoadaptation is necessary [38,39].
3. Impact of Global Climate Change on Soil Salinization
The combined impacts of severe weather events and salinization provide a serious challenge [40]. However, it is difficult to determine the influence of climate change on soil salinization growth [7]. Sea-level rise (SLR) and precipitation changes across wide swaths of coastal areas are both possible consequences of global climate change (GCC). Soil salinization and agricultural productivity will be affected by SLR and precipitation fluctuations. Low-lying coastal locations in South and Southeast Asia, as well as regions in sub-Saharan Africa, are particularly more vulnerable [41]. The salinization of hydromorphic soils will be enhanced by climatic change conditions caused by global warming and an expansion in aridity [42]. Through the physical, chemical, and biological aspects of soil, salinization has the capacity to affect soil health [43].
Because of increased temperature and variations in precipitation (amount and frequency), climate change is projected to have a major effect on soils and ecosystems, changing biogeochemical and hydrological processes [44]. Apparently, increasing groundwater pumping is required to fulfill the growing crop water needs as temperatures rise. Annual precipitation levels in the eastern Mediterranean are expected to decrease by 20% by 2050, increasing the danger of summer droughts [45]. Climate change is anticipated to accelerate the pace of soil salinization over the world. This will result in the use of lower quality water, elevated irrigation-induced salinization, the intensification of dryland salinization (due to the expansion of arid and semi-arid regions and desertification), and sea-level rise, directly contaminating nearby soils or indirectly affecting soils via saline intrusion in aquifers [40].
For example, in Australia, one of the countries most affected by both salinity and climate change, there are an estimated 1.7 million hectares of salinization or at hazard of salinization above the verified 1.047 million ha of salt-affected soils [46]. Thus, inventorying and monitoring climate change impacts on salinity are critical to determine the scope of the problem, identify trends, and develop irrigation and crop management measures that will keep these areas’ agricultural production maintained [47].
4. Bio-Phytoremediation by Plants
4.1. Halophyte Plants
Phytodesalination is a plant-based method for removing excess salt from contaminated areas and reclaiming land for farming [3]. It is a low-cost, environmentally friendly way to clean salt-affected places utilizing a variety of plants, depending on the degree of the pollution [16]. Halophytes are salt-loving plants with special traits that enable them to grow in severe environments [15]. However, halophytes are a diverse group of plants that make up just 1% of the world’s flora. They demonstrate habitat diversity, abiotic stress responses, and dispersion across flowering plant groups [17]. According to Ismail [48], plants can be classified as halophytes if they can complete their life cycle at 200 mM NaCl, and based on this, 350 species are classified as halophytes, divided into 20 orders and 256 families [49].
There have been various studies on salt-tolerant crops [1,2,3,4,5,6,7,8,9,10]. Halophytes have been widely studied in order to obtain better knowledge of their salt adaptability from physiological, morphological, and biochemical perspectives [40,41,42,43,44,45,46,47,48,49,50]. However, the management and compartmentalization of ions, the manufacture of suitable solutes, the activation of antioxidative enzymes, the stimulation of plant hormones, and alterations in photosynthetic systems are all processes that most plants possess to mitigate the deleterious impacts of salinity [51,52].
Numerous stress-related genes were discovered to be constitutively transcribed at greater levels in T. halophila than in A. thaliana in a comparative transcriptome investigation conducted by Taji et al. [51], in which they suggested an effective transcriptional regulatory pathway for salt stress response in halophytes. In addition, halophytes have a multitude of cis-regulatory components and stress-inducible motifs that were discovered in many stress-sensitive genes [53].
4.2. Plants’ Mechanisms for Desalination
Salt resistance is a multifaceted characteristic with numerous interacting characteristics, and little is understood about the regulatory networks that are implicated [54]. In particular, plant response to salt stress comprises a number of physiological, metabolic, and molecular pathways. Halophytes use a variety of physiological and molecular strategies, comprising variations in photosynthetic and transpiration rates, the sequestration of Na+ to extracellular or vacuole, the control of stomata aperture and stomatal density, and the concentration and production of phytohormones, as well as the relevant gene expression underpinning these physiological features, such as the transduction of stress signals, transcription factor control, transporter gene stimulation and expression, and synthetase inhibition or activation [55].
Certain plants, including halophytes and glycophytes, employ the process of salt exclusion [56], which involves preventing sodium from entering the cytoplasm outside the cell by restricting saline element admission and rejecting it in the apoplasmic component [57,58]. Furthermore, other processes, such as ion compartmentalization, osmoregulation by osmolytes, succulence formation, selective ion absorption and transport, antioxidant reaction, redox control, and energy status, all participate in salt adaptation strategies in halophytes [59]. It has been demonstrated that mucilage synthesis in the halophyte Kosteletzkya virginica is positively connected with salt amounts, and polysaccharide content varies among plant parts and treatments (Figure 1) [60].
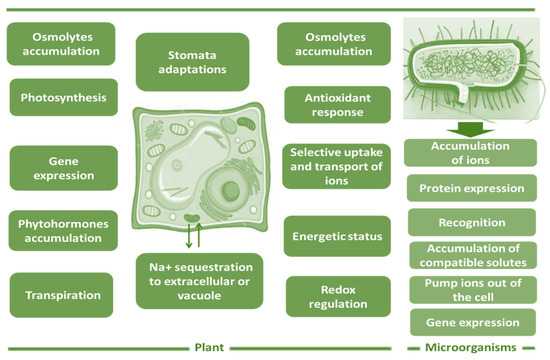
Figure 1.
Strategies implicated in soil biodesalination by plants and microorganisms.
Some of these regulatory systems are also functional in non-halophytes, while others are halophyte-specific attributes that have developed [61,62]. It is evident that various species of halophytes use more than one of these regulatory systems. One set may be active in one category while another set is prevalent in another, resulting in the categorization of halophytes as salt excluders (e.g., Rhizophora mangle), salt includers (frequently possessing mechanisms of salt recretion, e.g., species of genus Tamarix), and salt accumulators (some species of genus Atriplex) [63].
4.3. Genetic Engineering for Biodesalination
Some well-proven, extensively utilized, and cost-effective conventional ameliorative measures (e.g., conservation agriculture and use of ecological conditioners) aid in the combat against salinity and other constraints, particularly in developing nations [64]. In recent decades, biologically engineered plants that are completely resistant to saline–alkali conditions have emerged as a new research focus both at home and abroad, with the objective of increasing plant productivity in saline–alkali soil [65]. Breeding and genetic concepts, such as the collection and incorporation of salt-tolerant genotype(s) through traditional breeding, marker-assisted screening, molecular and transgenic strategies, and genome editing, have been in focus for a long time, and some remedies for the reduction of salt stress in crops have been successfully implemented [64].
The usual engineering approach to the problem is no longer sufficient. Genetic technology allows for the development of salt-tolerant crops, and, when combined with environmental modification, it might boost agricultural productivity in saline areas and expand agriculture to previously unsuitable areas [66]. For instance, water channel proteins (aquaporins) are important for water uptake/transport, particularly under stress circumstances; the overexpression of the wheat TaNIP aquaporin gene in recombinant Arabidopsis was found to improve salt resistance relative to wild-type seedlings [11]; and the wheat TaAQP8 aquaporin gene was found to increase recombinant tobacco salt resistance [23].
The realistic or perceived danger of horizontal gene transfer to associated wild or cultivated plants is a significant obstacle to the field implementation of transgenic plants for bioremediation. As a result, the next generation of transgenic plants will very certainly include methods that prohibit such a transmission, such as the incorporation of transgenes into chloroplast DNA or the usage of conditioned lethality genes [67].
There is significant genetic variation between halophytic genotypes [68], and the genotypes of some populations can function better in suboptimal environments [69]. Modern genetic manipulation technologies are now being used as an attempt to increase the biomass of salt-resistant plants [70]. The elaboration of expressed sequence tags (ESTs) and cDNA libraries utilizing multiple genomic strategies, such as serial analysis of gene expression (SAGE), suppressive subtractive hybridization (SSH), representational difference analysis (RDA), and differential display reverse transcription-polymerase chain reaction (DDRTPCR) [71], has provided a massive database for the study of the genetic network involved in halophytes’ abiotic stress resistance mechanisms [72,73].
Various genes involved in stress tolerance have been identified from halophytes and cloned or overexpressed in bacterial systems, as well as sensitive glycophyte cultivars, using this methodology to boost stress tolerance and agricultural productivity [74]. The identification of genes operating in the network has relied heavily on the screening for mutations that impact the plant’s response to stress. Designed screens include those aiming to identify mutations that increase or reduce salt sensitivity. The adoption of DNA microarray technology has also been useful, since it allows researchers to track changes in gene expression in response to stress and to identify genes that are either activated or repressed by the treatments [75].
Thus, the following techniques have been utilized to generate salt-tolerant crops through genetic engineering: boosting the plant’s capacity to restrict salt ion intake from the soil; raising the salt ion active extrusion rate; and improving salt ion compartmentalization in the cell vacuole, where they have no effect on cellular processes. Osmoprotectant genes have also been the subject of genetic modification investigations, although while over-expression enhances salt tolerance in some circumstances, it also affects plant development in the absence of stress, resulting in lower output, which is a highly undesirable feature for farmers [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76].
5. Bio-Phytoremediation by Microorganisms
5.1. Halophilic Microorganisms
Extremely saline settings are one-of-a-kind habitats for discovering new microbial species. Salt-resistant microbiomes have been found in a variety of saline environments. Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria, Euryarchaeota, and Spirochaetes were among the microorganisms found in a variety of salt-resistant microorganisms (Table 1) [77]. In addition to their genetic and physiological adaptations to salt, halophytes’ salinity tolerance is influenced by complicated ecological processes. As a result, prokaryotes and fungi that live in the roots and leaves of plants could have a substantial impact on plant development. Archaea and bacteria, as well as members of the fungal kingdom, are designed to be able to acclimatize to a wide range of variations in external osmolarity [78].

Table 1.
Halotolerant microorganisms and their beneficial effects on plant growth under saline conditions.
These germs also serve as ideal models for the determination of stress tolerance, adaptability, and response mechanisms, which may then be incorporated into agricultural plants to cope with climate change-related stresses [24]. Worldwide, salt-resistant plant microbiomes (rhizospheric, endophytic, and epiphytic) have been identified and described for their resistance to abiotic salt stress and other favorable characteristics [79].
The processes that allow microbes to grow and survive in salt environments are essentially the same across groups. The main strategies comprise avoiding high salt concentrations through specialized membrane or cell wall structures, pumping ions out of the cell via “salting-out” mechanisms or adapting their intracellular environment by accumulating non-toxic organic osmolytes, and adapting proteins and enzymes to high levels of solute ions [78]. Exopolysaccharides are excreted in large amounts by various rhizobacterial species, which contribute to reduce salt stress through unknown mechanisms [80].
A microbe must be able to detect and respond to change in order to survive in a changing environment. In saline conditions, Chryseobacterium balustinum, a PGP bacterium, has been shown to improve root surface, total nitrogen composition, and biological nitrogen fixation (BNF) in Lupinus albus seedlings [81]. The plant-associated microbiome has also been shown to be an important component in understanding plant adaptability to a salty environment [82]. Microbes associated with halophytes in the rhizosphere can withstand salt levels of up to 30%, and this relationship among plants and microbes may help improve soil fertility and quality [18].
5.2. Microbia’s Mechanisms for Desalination
Microbes, such as fungi and prokaryotes, which belong to the domains bacteria and Archaea, can adapt to a wide variety of changes in external osmolarity. Until recently, it was believed that Archaea (Halobacteriacea’s family) were the only active aerobic heterotrophs at or near NaCl saturation [40]. In addition, the majority of halophyte studies have mainly focused on the physiological and genetic control of salt tolerance [78]. In particular, halophilic bacteria are acclimated to grow appropriately in hypersaline conditions. Certain strategies are used by these bacteria in order to survive and grow. Osmotic imbalance is recognized by an osmosensor, which could be macromolecules performing structural alterations to adapt to the shifting extracellular functions of water or modifications in cell structure [90].
The first strategy is to accumulate potassium/chloride ions in order to maintain cell intracellular osmotic equilibrium under high-salt environments [91]. This is a frequent adaption in very anaerobic halophilic bacteria [92]. However, the majority of halotolerant and moderately halophilic bacteria employ a second strategy: compatible solute condensation. These complicated solutes (which include sugars, polyols, amino acids, carbohydrates, and their derivatives) may be acquired from the medium or produced by organisms [93].
Pumping ions out of the cell is another mechanism used to avoid excessive salt amounts in the cytoplasm. Na+/H+ antiporters, such as NhaA, an antiporter found in E. coli and other bacteria [94], help to pump out excess sodium ions [78]. Current analysis of salt-induced alterations in proteome maps showed a greater complexity of microorganism osmolyte-accumulating pathways. For example, under salt stress, Halomonas sp. AAD12 revealed substantial differences in the expression of proteins implicated in osmoregulation, stress reaction, energy production, and transport [95].
To deal with internal high salt amounts, fungi also accumulate compatible solutes. This has been studied in the salt-sensitive yeast S. cerevisiae and in several halotolerant filamentous fungi; it was shown that the majority of glycerol is generated as salt amounts increase [96]. Numerous H. werneckii genes that react to moderately and excessively high salt quantities were found using subtractive hybridization [97]; 13 of them had no homology to sequences in databases, demonstrating that the black yeast has extremely particular adaptations to hypersalinity.
5.3. Halotolerant Microorganisms for Saline Soil-Based Agriculture
Microbes might play a key role in establishing environmentally friendly crop management systems. However, in order to develop strategies for their effective use in agricultural production, we must better leverage their special features of resistance to extremes, genetic variety, and association with crop plants [24]. In particular, the use of plant-growth-promoting rhizobacteria (PGPR) coupled with salt-tolerant plants (halophytes) as probiotics for salty soil agriculture is a potential alternative to traditional methods [22].
Several investigations have shown that salinity-tolerant PGPRs derived from the rhizosphere soils of different halophytic species have the capability to be used as bioinoculants in the growth of glycophytic salt-resistant crops in salt-dominated agricultural regions [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98]. The utilization of microbial bioinoculants to promote plant health in salinized soils has the potential to reduce salt stress and enhance plant development while also boosting disease resistance (Figure 2) [99].
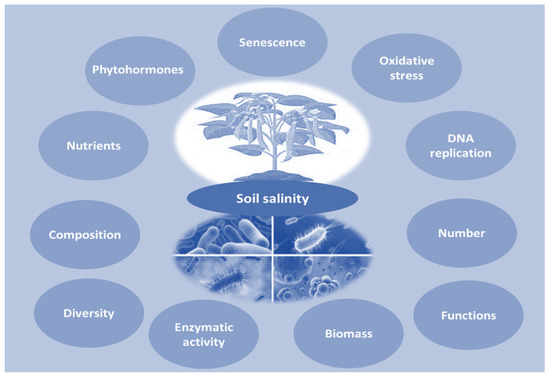
Figure 2.
Adverse impacts of soil salinity on plants and microorganisms.
Salicornia brachiata has yielded a number of salt-resistant bacteria in addition to the wild rice type. Haererehalobacter, Zhihengliuella, Rhizobium radiobacter, and Brachybacterium saurashtrense are among these bacteria. Plant-growth-promoting impacts on S. brachiata have been well documented, including increased P. solubilization, IAA production, and ACC deaminase biosynthesis under saline conditions. Moreover, under saline environments, inoculating Solanum lycopersicum with P. putida UW4 boosted shoot biomass [100]. Inoculating durum wheat (Triticum durum var. waha) with the rhizospheric bacteria Azospirillum brasilense NH, which was initially isolated from salt-affected soil in northern Algeria, greatly improved growth under saline soil conditions [101]. Under saline environments, multi-strain inoculation (PGPR) resulted in significant maize growth and yield improvement. At 6 dSm−1, rhizobacterial strains (alone or in combination) increased 1000-grain weight and fresh and dry plant biomass by 35%, 36%, and 48%, respectively. The dry matter of the crop (40%) was unregistered with Rhizobium strain Mn2 [102].
5.4. Microbial Bioremediation under Abiotic Stress Conditions
It is important to understand the relationships in plant development that promote rhizobacteria and abiotic and biotic factors in the mechanisms of bioremediation and energy production [103]. Various pressures arise from diverse surrounding conditions, such as heavy metals, drought, UV light, freezing, salinity, hypoxia, too-low and high temperatures, and bright light [104,105]. There are few or no studies dedicated to the use of microorganisms under various abiotic stresses, particularly in the case of uncontrolled bioremediation techniques, such as in situ methods. Undoubtedly, bioremediation involving the utilization of microorganisms and different abiotic stresses, as well as their effects on the latter, does not act separately in nature.
Microorganisms may be able to play a key role in this regard if we can take the use of their unique features of extreme tolerance, ubiquity, genetic variety, and interaction with crop plants, as well as develop ways for their successful deployment in agriculture production [24]. Bacteria and Archaea, as well as members of the fungal kingdom, are known to be able to adapt to a wide range of variations in external osmolarity. Increases in soil salinity have been linked to changes in microbiota composition, and research into the functional interactions between plants and microorganisms that contribute to salt stress resistance is gaining traction [78]. A range of physiological, metabolic, and molecular pathways are also involved in plant adaptation and resistance to salt stress [57].
The efficiency of bioremediation is determined by a number of factors, including amendments, such as activated carbon and biochar [106], the type of organisms used, the prevailing environmental parameters at the polluted site, and the level of contaminants present. Temperature, low nutrient concentrations, and other conditions that are difficult to manage can obstruct their use and the efficacy of the bioremediation process [107]. In the ecosystem, representatives of the genera Pseudomonas, Flavobacterium, Bacillus, Azotobacter, Microbacterium, Hydrogenomonas, Achromobacter, and Xanthomonas are the most common co-metabolizers [108]. The incomparable metabolic power of such organisms highlights the strong implementable ability of PGP members of such genera to alleviate crop stress caused by contaminated soils, paving the way for bioremediation [109].
To deal with toxic solutes and survive under stressful conditions, microbes have evolved a multitude of adaptive networks [110]. It has been shown that halophile-produced enzymes can be important biological substances, such as phytohormones and exopolysaccharides, which are important in plant–microbiome interactions [111]. These are also beneficial for the bioremediation of pollutants in salty environments [112,113]. In a study, the screening of microbial multi-abiotic stress tolerance led to the selection of the strain Pseudomonas azotoformans BioRPaz-3: pH (6, 7, 8, and 10), temperature (20 °C, 30 °C, 40 °C, and 45 °C), salinity up to 600 mM, drought up to 2.4 Mpa, Pb (100 µg/mL, 200 µg/mL, and 500 µg/mL), and Cu (200 µg/mL, 500 µg/mL, and 1000 µg/mL). This strain can be used efficiently for Pb and Cu bioremediation [114]. Different species of PGPB have been identified, facilitating heavy metal remediation and crop performance under drought and salinity stress [115].
6. Soil Desalination Combined Methods
For more than three decades, scientists and policymakers throughout the world explored long-term strategies, such as physicochemical, conventional breeding, and genetic engineering, using state-of-the-art molecular techniques to manage soil salinity. Nonetheless, they failed due to non-technical feasibility assessments, durability and affordability concerns, and field-level sustainability limits [102]. Thus, the combination of different desalination methods seems to be a very promising approach in order to remediate the global problem of agricultural soil salinization. In the study conducted by Lastiri-Hernández et al. [116], S. verrucosum was shown to enhance the physicochemical qualities of a moderately salty and clay-textured soil, mostly at depths ranging from 0 to 30 cm. When S. verrucosum is combined with two inorganic amendments (CaSO4•2 H2O and Polisul-C), its phytodesalination ability increases. Moreover, the introduction of organic amendments (vermicompost and cattle dung) may alter soil organism richness in saline–alkali soil [10].
Biochar, in association with manure compost and pyroligneous solution, was found to increase maize production and contribute to the management of salt stress in saline areas [117]. It is a recalcitrant supply of soil organic carbon that is combusted at relatively high temperatures under low oxygen conditions. Carbon (C) sequestration, bioenergy generation, soil conditioner, and biomass waste management are all benefits of pyrolyzing biomass [2]. In maize, biochar and Pseudomonas sp. were found to reduce sodium by 24% and 50%, respectively, while increasing proline, soil moisture level, and peroxidase (POD) activity. The use of fertilizer enhanced electrolyte leakage by 73%. In a combination treatment with biochar and Pseudomonas, the impact of fertilizer was 3–4 fold greater for proline and POD activity. To reduce salt stress, Pseudomonas sp. and biochar can be used in addition to fertilizer [21].
7. Soil Desalination Perspectives
Agro-ecosystems are extremely vulnerable to salinization. Furthermore, salinity is frequently associated with a variety of other environmental constraints, such as drought, waterlogging, acidity, pollution, and nutrient insufficiency [66]. Thus, the combination of bioremediation and plant growth stimulation might be a helpful strategy to solve this global agricultural challenge. After bio-formulation, the most attractive and required PGPB in the sector will be the most productive for commercialization. In the case of consortia, the strains must be regulated such that their ratios in the final product remain stable. Mixing strains at or at the ending of their development cycles may produce the most consistent results. However, consortia, may give advantages over single-strain inoculum due to strain relationships [92].
The complexities and other processes implicated in desalination remain unknown. For example, it has been illustrated that some halophytes use excretion mechanisms to eliminate excess salt ions from their sensitive tissues; that, in some cases, these glandular configurations are not always particular to Na+ and Cl−; and that other toxic components, such as cadmium, zinc, lead, and copper, are concentrated and excreted by salt glands or trichomes on the surfaces of leaves—a new phytoremediation process that is termed “phytoexcretion” [118].
Exogenous utilization of phytohormones has also been demonstrated to be a highly attractive and useful technique to combat environmental challenges, such as salt stress [119]. Similarly, exogenous jasmonic acid treatment seems to have the capacity to ameliorate salt-induced negative effects in plants through the enhancement of physiological characteristics [120], as phytohormonal imbalance is a common salt-induced plant reaction. Phytohormones are a diverse group of natural organics (for example, gibberellins, cytokinins ethylene, auxin, brassinosteroids, and strigolactones, and jasmonic, abscisic, and salicylic acids) that control growth and development under normal conditions, and they are essential in signaling transduction pathways during different environmental stresses [121,122].
Biomimicry for desalination entails the development of novel technologies based on biological systems or their components as prototypes [123]. Moreover, continued improvement in biotechnology and ecoengineering provides some of the most effective and innovative solutions against salinity (e.g., genome editing, nanomaterials, marker-assisted breeding, and plant–microbial associations), though many knowledge gaps and ethical frontiers must be overcome before these suggested solutions can be successfully transferred to industrial-scale food production [66].
8. Conclusions
It seems that soil salinization will be a real challenge in the coming years, especially because of its interdependence with other growing natural phenomena, such as water stress and climate change. Microorganisms and plants provide a very promising cost and eco-friendly alternative to solve these problems. The development of this approach through other disciplines, such as genomics and genetic engineering (genetically improved plants and microorganisms used for desalination), can effectively contribute to an improvement in the yield of expected desalination.
Other techniques of soil biodesalination are very promising for alleviating the effects of affected lands. Nanotechnology is one method of choice, as it currently finds different applications in the agronomic field. The combination of various methods seems to be more effective. The great variability, sustainability, and interdependence of the parameters affecting soil biodesalination-related processes can be described as major disadvantages that require good control in in situ and ex situ assays.
Author Contributions
The first author S.M. wrote the draft; the second author E.-h.N. completed the draft until the final manuscript. The third C.C. revised it and added some necessary details. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Portuguese funds through Fundação para a Ciência e a Tecnologia (project UIDB/00329/2020).
Conflicts of Interest
No conflict of interest to declare.
References
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2009, 44, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Saddhe, A.A.; Manuka, R.; Nikalje, G.C.; Penna, S. Halophytes as a potential resource for phytodesalination. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–21. [Google Scholar]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.J.J.; Malfanova, N.; Kamilova, F.; Berg, G. Plant growth promotion by microbes. In Molecular Microbial Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; Wiley Blackwell: Singapore, 2013; pp. 561–574. [Google Scholar]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Teh, S.Y.; Koh, H.L. Climate change and soil salinization: Impact on agriculture, water and food security. Intern. J. Agric. For. Plant 2016, 2, 1–9. [Google Scholar]
- Mahmood, A.; Kataoka, R. Potential of biopriming in enhancing crop productivity and stress tolerance. In Advances in Seed Priming; Rakshit, A., Singh, H., Eds.; Springer: Singapore, 2018; pp. 127–145. [Google Scholar]
- Cheeseman, J.M. Mechanisms of salinity tolerance in plants. Plant Physiol. 1988, 87, 547–550. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zheng, C.; Zhang, Y.; Sun, Z. Organic amendment application influence soil organism abundance in saline alkali soil. Eur. J. Soil Biol. 2013, 54, 32–40. [Google Scholar] [CrossRef]
- Gao, Z.; He, X.; Zhao, B.; Zhou, C.; Liang, Y.; Ge, R.; Shen, Y.; Huang, Z. Overexpressing a putative aquaporin gene from wheat, TaNIP, enhances salt tolerance in transgenic arabidopsis. Plant Cell Physiol. 2010, 51, 767–775. [Google Scholar] [CrossRef]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef]
- Sun, Y.; Kong, X.; Li, C.; Liu, Y.; Ding, Z. Potassium Retention under Salt Stress Is Associated with Natural Variation in Salinity Tolerance among Arabidopsis Accessions. PLoS ONE 2015, 10, e0124032. [Google Scholar] [CrossRef]
- Jha, B.; Gontia, I.; Hartmann, A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth–promoting potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Bhaskar, S.D.; Yadav, K.; Penna, S. Halophytes: Prospective plants for future. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 221–234. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wu, Z.; Zheng, Y.; Kaleem, I.; Li, C. Growth promotion and protection against salt stress by Pseudomonas putida Rs-198 on cotton. Eur. J. Soil Biol. 2010, 46, 49–54. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mirza, B.S.; Mehnaz, S.; Mirza, M.S.; Mclean, J.; Malik, K.A. Impact of soil salinity on the microbial structure of halophyte rhizosphere microbiome. World J. Microbiol. Biotechnol. 2018, 34, 1–17. [Google Scholar] [CrossRef]
- Babar, M.; Rasul, S.; Aslam, K.; Abbas, R.; Manzoor, I.; Hanif, M.K.; Naqqash, T. Mining of halo-tolerant plant growth promoting rhizobacteria and their impact on wheat (Triticum aestivum L.) under saline conditions. J. King Saud Univ. Sci. 2021, 33, 101372. [Google Scholar] [CrossRef]
- Fazal, A.; Bano, A. Role of plant growth-promoting rhizobacteria (PGPR), biochar, and chemical fertilizer under salinity stress. Commun. Soil Sci. Plant Anal. 2016, 47, 1985–1993. [Google Scholar] [CrossRef]
- Panda, A.; Parida, A.K. Development of Salt Tolerance in Crops Employing Halotolerant Plant Growth–Promoting Rhizobacteria Associated with Halophytic Rhizosphere Soils. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Kumar, M., Etesami, H., Kumar, V., Eds.; Springer: Singapore, 2019; pp. 75–101. [Google Scholar]
- Hu, W.; Yuan, Q.; Wang, Y.; Cai, R.; Deng, X.; Wang, J.; Zhou, S.; Chen, M.; Chen, L.; Huang, C.; et al. Overexpression of a wheat aquaporin gene, TaAQP8, enhances salt stress tolerance in transgenic tobacco. Plant Cell Physiol. 2012, 53, 2127–2141. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, J.; Singh, D. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Flowers, T. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- World Commission on Dams (WCD). Dams and Development: A New Framework for Decision Making. Report of the World Commission on Dams Earthscan; Earthscan Publications Ltd.: London, UK, 2000. [Google Scholar]
- Riadh, K.; Wided, M.; Hans-Werner, K.; Chedly, A. Responses of halophytes to environmental stresses with special emphasis to salinity. Adv. Bot. Res. 2010, 53, 117–145. [Google Scholar]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Bensidhoum, L.; Nabti, E.H. Plant growth-promoting Bacteria for improving crops under saline conditions. In Microorganisms in Saline Environments: Strategies and Functions; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 329–352. [Google Scholar]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi: A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Borneman, J.; Skroch, P.W.; O’Sullivan, K.M.; Palus, J.A.; Rumjanek, N.G.; Jansen, J.L.; Nienhuis, J.; Triplett, E.W. Molecular microbial diversity of an agricultural soil in Wisconsin. Appl. Environ. Microbiol. 1996, 62, 1935–1943. [Google Scholar] [CrossRef]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Pan, C.; Liu, C.; Zhao, H.; Wang, Y. Changes of soil physico-chemical properties and enzyme activities in relation to grassland salinization. Eur. J. Soil Biol. 2013, 55, 13–19. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, H.; Tang, M.J.; Shen, S.H. Proteome analysis of an ectomycorrhizal fungus Boletus edulis under salt shock. Mycol. Res. 2007, 111, 939–946. [Google Scholar] [CrossRef]
- Nabti, E.; Schmid, M.; Hartmann, A. Application of halotolerant bacteria to restore plant growth under salt stress. In Halophile: Biodiversity and Sustainable Exploitation; Maheshwari, D.K., Saraf, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 235–359. [Google Scholar]
- Oren, A. Molecular ecology of extremely halophilic archaea and bacteria. FEMS Microbiol. Ecol. 2002, 39, 1–7. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, H.; Yu, B.; Liu, X.; Li, Y.; Ji, S.; Zhang, C.L. Microbial response to salinity change in Lake Chaka, a hypersaline lake on Tibetan plateau. Environ. Microbiol. 2007, 9, 2603–2621. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.T. Phytoremediation of salt-affected soils: A review of processes, applicability, and the impact of climate change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.V.; Kirkby, M.J. Application of salinization indicators and initial development of potential global soil salinization scenario under climatic change. Glob. Biogeochem. Cycles 2003, 17, 1–13. [Google Scholar] [CrossRef]
- Okur, B.; Örçen, N. Soil salinization and climate change. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: New York, NY, USA, 2020; pp. 331–350. [Google Scholar]
- Patil, A.; Lamnganbi, M. Impact of climate change on soil health: A review. Int. J. Chem. Stud. 2018, 6, 2399–2404. [Google Scholar]
- Gelybó, G.; Tóth, E.; Farkas, C.; Horel, Á.; Kása, I.; Bakacsi, Z. Potential impacts of climate change on soil properties. Agrokem. Talajt. 2018, 67, 121–141. [Google Scholar] [CrossRef]
- Ashour, E.K.; Al-Najar, H. The impact of climate change and soil salinity in irrigation water demand in the Gaza strip. J. Earth Sci. Clim. Chang. 2012, 3, 2. [Google Scholar] [CrossRef]
- Jardine, A.; Speldewinde, P.; Carver, S.; Weinstein, P. Dryland salinity and ecosystem distress syndrome: Human health implications. EcoHealth 2007, 4, 10–17. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Srivastava, N. Reclamation of saline and sodic soil through phytoremediation. In Environmental Concerns and Sustainable Development; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020; pp. 279–306. [Google Scholar]
- Ismail, S.; Rao, N.K.; Dagar, J.C. Identification, evaluation, and domestication of alternative crops for saline environments. In Research Developments in Saline Agriculture; Dagar, J., Yadav, R., Sharma, P., Eds.; Springer: Singapore, 2019; pp. 505–536. [Google Scholar]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Taji, T.; Komatsu, K.; Katori, T.; Kawasaki, Y.; Sakata, Y.; Tanaka, S.; Kobayashi, M.; Toyoda, A.; Seki, M.; Shinozaki, K. Comparative genomic analysis of 1047 completely sequenced cDNAs from an Arabidopsis-related model halophyte, Thellungiella halophila. BMC Plant Biol. 2010, 10, 261. [Google Scholar] [CrossRef]
- Tiwari, V.; Patel, M.K.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Functional characterization of the tau class Glutathione-S-Transferases gene (SbGSTU) promoter of Salicornia brachiata under salinity and osmotic Stress. PLoS ONE 2016, 11, e0148494. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, S.; Franken, P.; Witzel, K. Properties of the halophyte microbiome and their implications for plant salt tolerance. Funct. Plant Biol. 2013, 40, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Manousaki, E.; Kalogerakis, N. Halophytes present new opportunities in phytoremediation of heavy metals and saline soils. Ind. Eng. Chem. Res. 2011, 50, 656–660. [Google Scholar] [CrossRef]
- Xu, C.; Tang, X.; Shao, H.; Wang, H. Salinity tolerance mechanism of economic halophytes from physiological to molecular hierarchy for improving food quality. Curr. Genom. 2016, 17, 207–214. [Google Scholar] [CrossRef]
- Alem, C.; Amri, A. Importance de la stabilité des membranes cellulaires dans la tolérance à la salinité chez l’orge. Rev. Biol. Biotechnol. 2005, 4, 20–31. [Google Scholar]
- Munns, R. Strategies for Crop Improvement in Saline Soils. In Salinity and Water Stress. Tasks for Vegetation Sciences; Ashraf, M., Ozturk, M., Athar, H., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 44, pp. 1–16. [Google Scholar]
- Castiglione, S.; Oliva, G.; Vigliotta, G.; Novello, G.; Gamalero, E.; Lingua, G.; Cicatelli, A.; Guarino, F. Effects of compost amendment on glycophyte and halophyte crops grown on saline soils: Isolation and characterization of rhizobacteria with plant growth promoting features and high salt resistance. Appl. Sci. 2021, 11, 2125. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Srivastava, A.K.; Pandey, G.K.; Suprasanna, P. Halophytes in biosaline agriculture: Mechanism, utilization, and value addition. Land Degrad. Dev. 2018, 29, 1081–1095. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Han, R.M.; Classen, B.; Quetin-Leclerq, J.; Mahy, G.; Ruan, C.J.; Qin, P.; Perez-Alfocea, F.; Lutts, S. Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: Localization and composition in relation to salt stress. J. Plant Physiol. 2010, 167, 382–392. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Nelson, D.E.; Jensenay, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099–1111. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Nilsen, E.T. The Physiology of Plants under Stress, Soil and Biotic Factors; Wiley: New York, NY, USA, 2000; p. 227. [Google Scholar]
- Breckle, S.W. Salinity, halophytes and salt affected natural ecosystems. In Salinity: Environmen—Plant—Molecules; Lauchli, A., Luttge, U., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 53–77. [Google Scholar]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar]
- Li, P.S.; Kong, W.L.; Wu, X.Q. Salt Tolerance Mechanism of the Rhizosphere Bacterium JZ-GX1 and Its Effects on Tomato Seed Germination and Seedling Growth. Front. Microbiol. 2021, 12, 1342. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Norlyn, J.D.; Rush, D.W.; Kingsbury, R.W.; Kelley, D.B.; Cunningham, G.A.; Wrona, A.F. Saline culture of crops: A genetic approach. Science 1980, 210, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Davison, J. Risk mitigation of genetically modified bacteria and plants designed for bioremediation. J. Ind. Microbiol. Biotechnol. 2005, 32, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Llanes, A.; Bonercarrere, V.; Capdevielle, F.; Vidal, S.; Luna, V. Genetic diversity in a natural population of the halophytic legume Prosopis strombulifera revealed by AFLP fingerprinting. B Soc. Argent. Bot. 2011, 46, 305–312. [Google Scholar]
- Maron, J.L.; Vilà, M.; Bommarco, R.; Elmendorf, S.; Beardsley, P. Rapid evolution of an invasive plant. Ecol. Monogr. 2004, 74, 261–280. [Google Scholar] [CrossRef]
- Munir, N.; Abideen, Z.; Sharif, N. Development of halophytes as energy feedstock by applying genetic manipulations. All Life 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Breyne, P.; Zabeau, M. Genome-wide expression analysis of plant cell cycle modulated genes. Curr. Opin. Plant Biol. 2001, 4, 136–142. [Google Scholar] [CrossRef]
- Wang, L.W.; Showalter, A.M. Cloning and saltinduced, ABA-independent expression of choline monooxygenase in Atriplex prostrate. Physiol. Plant 2004, 120, 405–412. [Google Scholar] [CrossRef]
- Popova, O.V.; Yang, O.; Dietz, K.J.; Golldack, D. Differential transcript regulation in Arabidopsis thaliana and the halotolerant Lobularia maritima indicates genes with potential function in plant salt adaptation. Gene 2008, 423, 142–148. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Suprasanna, P. Prospects of halophytes in understanding and managing abiotic stress tolerance. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 29–56. [Google Scholar]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Kour, D.; Rana, K.L.; Kumar, V.; Singh, B.; Chauahan, V.S.; Sugitha, T.C.K.; Saxena, A.K.; Dhaliwal, H.S. Plant microbiome and its beneficial multifunctional plant growth promoting attributes. Int. J. Environ. Sci. Nat. Resour. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Gutierrez-Manero, F.J.; Probanza, A.; Ramos, B.; Colon-Flores, J.J.; Lucas-Garcia, J.A. Effects of culture filtrates of rhizobacteria isolated from wild lupine on germination, growth, and biological nitrogen fixation of lupine seedlings. J. Plant Nutr. 2003, 26, 1101–1115. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. Multidiscip. J. Microb. Ecol. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Yuan, Z.; Druzhinina, I.S.; Labbe, J.; Redman, R.; Qin, Y.; Rodriguez, R.; Zhang, C.; Tuskan, G.A.; Lin, F. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Pankaj, U.; Singh, D.N.; Mishra, P.; Babu, C.V.; Shanker, K.; Verma, R.K. Autochthonous halotolerant plant growth-promoting rhizobacteria promote bacoside A yield of Bacopa monnieri (L.) Nash and phytoextraction of salt-affected soil. Pedosphere 2020, 30, 671–683. [Google Scholar] [CrossRef]
- Alexander, A.; Singh, V.K.; Mishra, A. Halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 2530. [Google Scholar] [CrossRef]
- Abdelkader, A.F.; Esawy, M.A. Case study of a biological control: Geobacillus caldoxylosilyticus (IRD) contributes to alleviate salt stress in maize (Zea mays L.) plants. Acta Physiol. Plant 2011, 33, 2289–2299. [Google Scholar] [CrossRef]
- Abdelwahab, R.A.I.; Cherif, A.; Cristina, C.; Nabti, E. Extracts from seaweeds and Opuntia ficusindica cladodes enhance diazotrophic-PGPR halotolerance, their enzymatic potential, and their impact on wheat germination under salt stress. Pedosphere 2017, 160, 60333-3. [Google Scholar]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from Chenopodium quinoa willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Khan, M.A.; Lee, K.E.; Kang, S.M.; Dhungana, S.K.; Bhusal, N.; Lee, I.J. The halotolerant rhizobacterium-Pseudomonas koreensis MU2 enhances inorganic silicon and phosphorus use efficiency and augments salt stress tolerance in soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Bilal, M.; Hassani, D.; Iqbal, H.M.; Wang, H.; Huang, D. Mitigation of salt stress in white clover (Trifolium repens) by Azospirillum brasilense and its inoculation effect. Bot. Stud. 2017, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Mishra, A.; Jha, B. Halotolerant Rhizobacteria: A Promising Probiotic for Saline Soil-Based Agriculture. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Kumar, M., Etesami, H., Kumar, V., Eds.; Springer: Singapore, 2019; pp. 53–73. [Google Scholar]
- Cherif-Silini, H.; Silini, A.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Bouremani, N.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Tailoring Next Generation Plant Growth Promoting Microorganisms as Versatile Tools beyond Soil Desalinization: A Road Map towards Field Application. Sustainability 2021, 13, 4422. [Google Scholar] [CrossRef]
- Hnelt, I.; Volker, M. Molecular mechanisms of adaptation of the moderately Halophilic bacterium Halobacillis halophilus to its environment. Life 2013, 3, 234–243. [Google Scholar] [CrossRef]
- Oren, A. Adaptation of Halophilic archaea to life at high salt concentrations. Salin. Environ. Plants Mol. 2004, 306, 81–96. [Google Scholar]
- Shivanand, P.; Mugeraya, G. Halophilic bacteria and their compatible solutes Osmoregulation and potential applications. Curr. Sci. 2011, 100, 1516–1521. [Google Scholar]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 2005, 435, 1197–1202. [Google Scholar] [CrossRef]
- Ceylan, S.; Yilan, G.; Akbulut, B.S.; Poli, A.; Kazan, D. Interplay of adaptive capabilities of Halomonas sp AAD12 under salt stress. J. Biosci. Bioeng. 2012, 114, 45–52. [Google Scholar] [CrossRef]
- Blomberg, A.; Adler, L. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 1992, 33, 145–212. [Google Scholar]
- Vaupotic, T.; Plemenitas, A. Differential gene expression and HogI interaction with osmoresponsive genes in the extremely halotolerant black yeast Hortaea werneckii. BMC Genom. 2007, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Smith, M.D.; Glick, B.R.; Liang, Y. Effects of ACC deaminase containing rhizobacteria on plant growth and expression of Toc GTPases in tomato (Solanum lycopersicum) under salt stress. Botany 2014, 92, 775–781. [Google Scholar] [CrossRef]
- Nabti, E.; Sahnoune, M.; Ghoul, M.; Fischer, D.; Hofmann, A.; Rothballer, M.; Schmid, M.; Hartmann, A. Restoration of growth of durum wheat (Triticum durum var waha) under saline conditions due to inoculation with the rhizosphere bacterium Azospirillum brasilense NH and extracts of the marine alga Ulva lactuca. J. Plant Growth Regul. 2010, 29, 6–22. [Google Scholar]
- Iqbal, H.I.; Zahir, Z.A.; Rehman, O.; Khalid, R.; Waheed, A.; Raza, R.A.; Saleem, S.; Rashid, M.; Alvi, S.T.; Munir, A. Improving the growth and yield of maize through multi-strain inoculation (PGPR) under saline conditions. Pak. J. Agric. Res. 2021, 34, 400–406. [Google Scholar] [CrossRef]
- Hayat, K.; Bundschuh, J.; Jan, F.; Menhas, S.; Hayat, S.; Haq, F.; Shah, M.A.; Chaudhary, H.J.; Ullah, A.; Zhang, D.; et al. Combating soil salinity with combining saline agriculture and phytomanagement with salt-accumulating plants. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1085–1115. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 3, 737–752. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Bussan, D.D.; Sessums, R.F.; Cizdziel, J.V. Activated carbon and biochar reduce mercury methylation potentials in aquatic sediments. Bull. Environ. Contam Toxicol. 2016, 96, 536–539. [Google Scholar] [CrossRef]
- Kumar, S.; Belbase, S.; Sinha, A.; Singh, M.K.; Mishra, B.K.; Kumar, R. Bioremediation potential of Rhizobacteria associated with plants under abiotic metal stress. In Soil Bioremediation: An Approach Towards Sustainable Technology; Parray, J.A., Mahmoud, A.H.A.E., Sayyed, R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2021; pp. 213–255. [Google Scholar]
- Beam, H.W.; Perry, J.J. Co-Metabolism as a factor in microbial degradation of cycloparaffinic hydrocarbons. Arch. Microbiol. 1973, 91, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Fiedurek, J.; Trytek, M.; Szczodrak, J. Strain improvement of industrially important microorganisms based on resistance to toxic metabolites and abiotic stress. J. Basic Microbiol. 2017, 57, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Abdulla Malik, K.; Mehnaz, S. Microbiome of Halophytes: Diversity and Importance for Plant Health and Productivity. Microbiol. Biotechnol. Lett. 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Dastgheib, S.M.M.; Amoozegar, M.A.; Khajeh, K.; Ventosa, A. A halotolerant Alcanivorax sp. strain with potential application in saline soil remediation. Appl. Microbiol. Biotechnol. 2011, 90, 305–312. [Google Scholar] [CrossRef]
- Bergi, J.; Trivedi, R. Bioremediation of Saline Soil by Cyanobacteria. In Microbial Bioremediation & Biodegradation; Shah, M.P., Ed.; Springer: Singapore, 2020; pp. 447–465. [Google Scholar]
- Mokrani, S.; Nabti, E.H. Heavy metal resistance and bioremediation capacity of rhizospheric strain BioRPaz-3 Pseudomonas azotoformans endowed with antifungal activities and multi-abiotic stress tolerance in in vitro trials. SN Appl. Sci. 2020, 2, 1985. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ahmad, H. Plant growth promoting bacteria: Role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 2018, 37, 1599–1609. [Google Scholar] [CrossRef]
- Lastiri-Hernández, M.A.; Alvarez-Bernal, D.; Bermúdez-Torres, K.; Cárdenas, G.C.; Ceja-Torres, L.F. Phytodesalination of a moderately saline soil combined with two inorganic amendments. Bragantia 2019, 78, 579–586. [Google Scholar] [CrossRef]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Pan, G. Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2014, 35, 45–52. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Halophytes—An emerging trend in phytoremediation. Int. J. Phytoremedia. 2011, 13, 959–969. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Jasmonates: Mechanisms and functions in abiotic stress tolerance of plants. Biocatal. Agric. Biotechnol. 2019, 20, 101210. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop. J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Maru, M.; Sahle-Demissie, E.; Zewge, F. A review on biodesalination using halophytic microalgae: Opportunities and challenges. J. Water Supply Res. Technol. Aqua. 2021, 70, 1–22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).