The Association between Inflammatory Biomarkers and Cardiovascular Autonomic Dysfunction after Bacterial Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Subjects and Groups
2.3. Assessment of the Heart Rate Variability (HRV)
- HRV in response to the Valsalva maneuver (40 mmHg maintained for 10 s).
- HRV in response to metronome breathing (at 6 breaths per minute for 2 min).
- HRV in response to standing up.
- HRV in response to sustained handgrip (30% of maximum strength for 3 min).
2.4. Measurement of Serum Inflammatory Markers
2.5. Data Analysis
3. Results
3.1. Study Population
3.2. Cardiovascular Autonomic Function
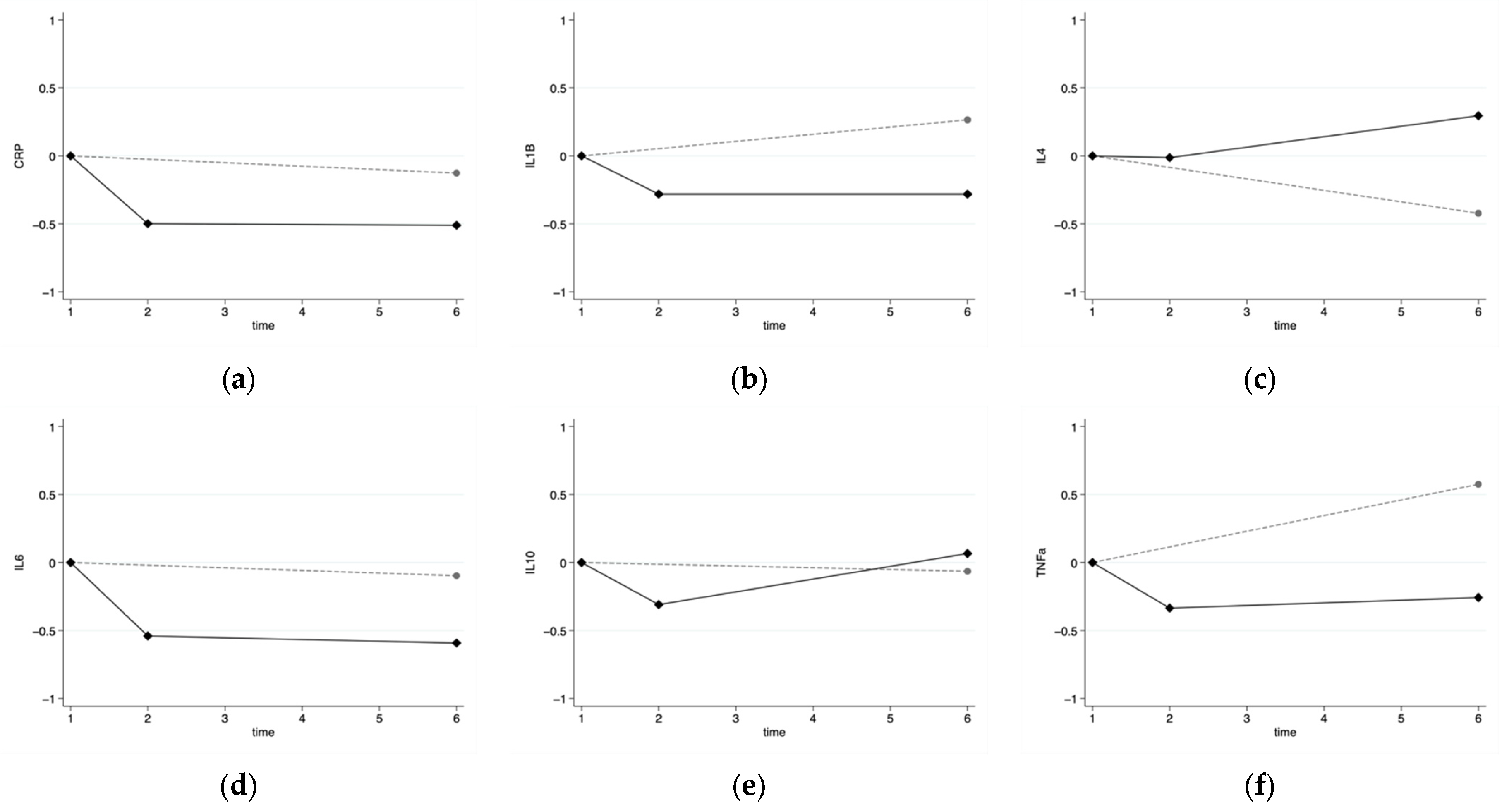
3.3. Inflammatory Markers
3.4. Cardiovascular Autonomic Dysfunction and Inflammatory Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haensel, A.; Mills, P.J.; Nelesen, R.A.; Ziegler, M.G.; Dimsdale, J.E. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 2008, 33, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Barone, L.; Scalone, G.; Pitocco, D.; Sgueglia, G.A.; Mollo, R.; Nerla, R.; Zaccardi, F.; Ghirlanda, G.; Crea, F. Inflammation-related effects of adjuvant influenza A vaccination on platelet activation and cardiac autonomic function. J. Intern. Med. 2010, 269, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. Neural circuitry and immunity. Immunol. Res. 2015, 63, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.; Tonkin, A. Clinical approach to autonomic dysfunction. Intern. Med. J. 2016, 46, 1134–1139. [Google Scholar] [CrossRef]
- Fairchild, K.D.; Srinivasan, V.; Moorman, J.R.; Gaykema, R.P.A.; Goehler, L.E. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am. J. Physiol. 2011, 300, R330–R339. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2014, 182, 15–41. [Google Scholar] [CrossRef]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. 2006, 6, 318–328. [Google Scholar] [CrossRef]
- Báez-Pagán, C.A.; Delgado-Velez, M.; Lasalde-Dominicci, J.A. Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. 2015, 10, 468–476. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat. Rev. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Huston, J.M.; Tracey, K.J. The pulse of inflammation: Heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J. Intern. Med. 2010, 269, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Van Westerloo, D.J.; Giebelen, I.A.J.; Florquin, S.; Daalhuisen, J.; Bruno, M.J.; De Vos, A.F.; Tracey, K.J.; Van Der Poll, T. The Cholinergic Anti-Inflammatory Pathway Regulates the Host Response during Septic Peritonitis. J. Infect. Dis. 2005, 191, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.; Lichtenstern, C.; Henrich, M.; Weigand, M.A.; Uhle, F. Loss of vagal tone aggravates systemic inflammation and cardiac impairment in endotoxemic rats. J. Surg. Res. 2014, 188, 480–488. [Google Scholar] [CrossRef]
- Ge, L.-S.; Chen, X.-X.; Wu, L.-P.; Zhou, D.-P.; Li, X.-W.; Lin, J.-F.; Li, Y.-C. Right Cervical Vagotomy Aggravates Viral Myocarditis in Mice Via the Cholinergic Anti-inflammatory Pathway. Front. Pharmacol. 2017, 8, 25. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Kessler, W.; Diedrich, S.; Menges, P.; Ebker, T.; Nielson, M.; Partecke, L.I.; Traeger, T.; Cziupka, K.; Van Der Linde, J.; Puls, R.; et al. The Role of the Vagus Nerve: Modulation of the Inflammatory Reaction in Murine Polymicrobial Sepsis. Mediat. Inflamm. 2012, 2012, 467620. [Google Scholar] [CrossRef]
- Marsland, A.L.; Gianaros, P.J.; Prather, A.A.; Jennings, J.R.; Neumann, S.A.; Manuck, S.B. Stimulated Production of Proinflammatory Cytokines Covaries Inversely with Heart Rate Variability. Psychosom. Med. 2007, 69, 709–716. [Google Scholar] [CrossRef]
- Williams, D.P.; Koenig, J.; Carnevali, L.; Sgoifo, A.; Jarczok, M.N.; Sternberg, E.M.; Thayer, J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain Behav. Immun. 2019, 80, 219–226. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; Dragoumanis, C.; Theodorou, V.; Gargaretas, C.; Pneumatikos, I. Relation of heart rate variability to serum levels of C-reactive protein, interleukin 6, and 10 in patients with sepsis and septic shock. J. Crit. Care 2009, 24, 625.e1–625.e7. [Google Scholar] [CrossRef]
- Taheishi, Y.; Hirasawa, H.; Oda, S.; Moriguchi, T.; Kuwaki, T. Blood interleukin—6 levels are inversely correlated with heart rate variability in septic patients. Crit. Care Med. 2005, 33, A163. [Google Scholar] [CrossRef]
- Tateishi, Y.; Oda, S.; Nakamura, M.; Watanabe, K.; Kuwaki, T.; Moriguchi, T.; Hirasawa, H. Depressed heart rate variability is associated with high IL-6 blood level and decline in the blood pressure in septic patients. Shock 2007, 28, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Ewing, D.J.; Clarke, B.F. Diagnosis and management of diabetic autonomic neuropathy. BMJ 1982, 285, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Newton, J.L. Autonomic dysfunction in chronic liver disease. Liver Int. 2009, 29, 483–489. [Google Scholar] [CrossRef]
- Mccraty, R.; Shaffer, F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health Risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, M.I.; Liu, N.; Prabhakar, S.M.; Chong, S.-L.; Lye, W.K.; Koh, Z.X.; Guo, D.; Rajesh, R.; Ho, A.F.W.; Ong, M.E.H. A novel heart rate variability based risk prediction model for septic patients presenting to the emergency department. Medicine 2018, 97, e10866. [Google Scholar] [CrossRef]
- Thanou, A.; Stavrakis, S.; Dyer, J.W.; Munroe, M.E.; James, J.A.; Merrill, J.T. Impact of heart rate variability, a marker for cardiac health, on lupus disease activity. Arthritis Res. Ther. 2016, 18, 197. [Google Scholar] [CrossRef]
- Chapleau, M.W.; Sabharwal, R. Methods of assessing vagus nerve activity and reflexes. Heart Fail. Rev. 2011, 16, 109–127. [Google Scholar] [CrossRef]
- Malik, M. Task Force of the European Society of Cardiology and The North American Society of Pacing and Electrophysiology. Heart Rate Variability Standards of measurement, physiological interpretation and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Junior, J.C.B.; Caruso, F.R.; Mendes, R.G.; Da Silva, T.R.; Biazon, T.M.P.D.C.; Rangel, F.; Phillips, S.A.; Arena, R.; Borghi-Silva, A. Noninvasive measurements of hemodynamic, autonomic and endothelial function as predictors of mortality in sepsis: A prospective cohort study. PLoS ONE 2019, 14, e0213239. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. The enteric nervous system: Another forgotten autonomic target in viral infections? Clin. Auton. Res. 2017, 27, 137–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Castilho, F.M.; Ribeiro, A.L.P.; Da Silva, J.L.P.; Nobre, V.; De Sousa, M.R. Heart rate variability as predictor of mortality in sepsis: A prospective cohort study. PLoS ONE 2017, 12, e0180060. [Google Scholar] [CrossRef]
- Pandey, N.R.; Bian, Y.-Y.; Shou, S.-T. Significance of blood pressure variability in patients with sepsis. World J. Emerg. Med. 2014, 5, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Sorenson, J.; Lanspa, M.; Grissom, C.K.; Mathews, V.; Brown, S.M. Systolic blood pressure variability in patients with early severe sepsis or septic shock: A prospective cohort study. BMC Anesthesiol. 2017, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.; Zanaboni, A.M.; Wiemken, T.; Nahas, A.; Uppatla, S.; Morlacchi, L.C.; Peyrani, P.; Blasi, F.; Ramirez, J. Criteria for clinical stability in hospitalised patients with community-acquired pneumonia. Eur. Respir. J. 2013, 42, 742–749. [Google Scholar] [CrossRef]
- Halm, E.A.; Fine, M.J.; Marrie, T.J.; Coley, C.M.; Kapoor, W.N.; Obrosky, D.S.; Singer, D.E. Time to Clinical Stability in Patients Hospitalized with Community-Acquired Pneumonia: Implications for practice guidelines. JAMA 1998, 279, 1452–1457. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44 (Suppl. 2), S27–S72. [Google Scholar] [CrossRef]
- Niederman, M.S.; Mandell, L.A.; Anzueto, A.; Bass, J.B.; Broughton, W.A.; Campbell, G.D.; Dean, N.; File, T.; Fine, M.J.; Gross, P.A.; et al. Guidelines for the Management of Adults with Community-acquired Pneumonia. Diagnosis, Assessment of Severity, Antimicrobial Therapy, and Prevention. Am. J. Respir. Crit. Care Med. 2001, 163, 1730–1754. [Google Scholar] [CrossRef]
- American Diabetes Association; American Academy of Neurology. Report and Recommendations of the San Antonio Conference on Diabetic Neuropathy. Consensus Statement. Diabetes 1988, 37, 1000–1004. [Google Scholar] [CrossRef]
- Dinarello, C.A. Targeting the Pathogenic Role of Interleukin 1β in the Progression of Smoldering/Indolent Myeloma to Active Disease. Mayo Clin. Proc. 2009, 84, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Sagawe, S.; Hecker, A.; Küllmar, M.; Askevold, I.; Damm, J.; Heldmann, S.; Pöhlmann, M.; Ruhrmann, S.; Sander, M.; et al. C-Reactive Protein Stimulates Nicotinic Acetylcholine Receptors to Control ATP-Mediated Monocytic Inflammasome Activation. Front. Immunol. 2018, 30, 1604. [Google Scholar] [CrossRef] [PubMed]
- Farrokhpour, M.; Kiani, A.; Mortaz, E.; Taghavi, K.; Farahbod, A.M.; Fakharian, A.; Kazempour-Dizaji, M.; Abedini, A. Procalcitonin and Proinflammatory Cytokines in Early Diagnosis of Bacterial Infections after Bronchoscopy. Open Access Maced. J. Med. Sci. 2019, 7, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Holub, M.; Lawrence, D.A.; Andersen, N.; Davidová, A.; Beran, O.; Marešová, V.; Chalupa, P. Cytokines and Chemokines as Biomarkers of Community-Acquired Bacterial Infection. Mediat. Inflamm. 2013, 2013, 190145. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Weidhase, L.; Wellhöfer, D.; Schulze, G.; Kaiser, T.; Drogies, T.; Wurst, U.; Petros, S. Is Interleukin-6 a better predictor of successful antibiotic therapy than procalcitonin and C-reactive protein? A single center study in critically ill adults. BMC Infect. Dis. 2019, 19, 150. [Google Scholar] [CrossRef]
- Jawa, R.S.; Anillo, S.; Huntoon, K.; Baumann, H.; Kulaylat, M. Interleukin-6 in Surgery, Trauma, and Critical Care Part II: Clinical Implications. J. Intensive Care Med. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Endeman, H.; Meijvis, S.C.A.; Rijkers, G.T.; van Velzen-Blad, H.; van Moorsel, C.H.M.; Grutters, J.C.; Biesma, D.H. Systemic cytokine response in patients with community-acquired pneumonia. Eur. Respir. J. 2011, 37, 1431–1438. [Google Scholar] [CrossRef]
- Lin, S.; Huang, Z.; Wang, M.; Weng, Z.; Zeng, D.; Zhang, Y.; Zhu, Y.; Jiang, J. Interleukin-6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. J. Crit. Care 2015, 30, 732–738. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Q.; Zhang, X.; Zhao, X.; Zhang, H.; Liu, A. Clinical Application of Serum Inflammatory Factors Combined with Dynamic Detection in the Diagnosis and Treatment of Neonatal Sepsis. Iran. J. Public Health 2021, 50, 325–332. [Google Scholar] [CrossRef]
- McNicholas, S.; Talento, A.F.; O’Gorman, J.; Hannan, M.M.; Lynch, M.; Greene, C.M.; Humphreys, H.; Fitzgerald-Hughes, D. Cytokine responses to Staphylococcus aureusbloodstream infection differ between patient cohorts that have different clinical courses of infection. Infect. Dis. 2014, 14, 580. [Google Scholar] [CrossRef] [PubMed]
- Jekarl, D.W.; Kim, J.Y.; Lee, S.; Kim, M.; Kim, Y.; Han, K.; Woo, S.H.; Lee, W.J. Diagnosis and evaluation of severity of sepsis via the use of biomarkers and profiles of 13 cytokines: A multiplex analysis. Clin. Chem. Lab. Med. 2015, 53, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Beneyto, L.A.P.; Luis, O.R.; Sánchez, C.S.; Simón, O.C.; Rentero, D.B.; Bayarri, V.M. Prognostic value of interleukin 6 for death of patients with sepsis. Med. Clin. 2016, 147, 281–286. [Google Scholar] [CrossRef]

| Characteristic 1 | Healthy Control Group | Infection Group |
|---|---|---|
| N | 37 | 13 |
| Age (years; mean (range)) | 52.4 (33–76) | 47.9 (24–69) |
| Sex (% females) | 67.6% | 46.2% |
| Smokers (%) | 16.2% | 38.5% |
| Alcohol intake >14 units/week (%) | 5.4% | 7.7% |
| Independent with ADLs (%) | 100% | 100% |
| White blood cells × 109/L | - 2 | 15.4 (5.9) |
| CRP (mg/L) | - 2 | 145 (107) |
| Oxygen sat (%) | - 2 | 96 (3) |
| Pyrexia (%) | - 2 | 61.5% |
| Group | Resting HRV | HRV in Breathing | HRV Valsalva | HRV Handgrip | HRV Standing | |
|---|---|---|---|---|---|---|
| Total sample | CRP | −0.153; p = 0.309 | −0.168; p = 0.269 | −0.303; p = 0.046 | −0.096; p = 0.539 | −0.252; p = 0.099 |
| IL1β | −0.050; p = 0.736 | 0.065; p = 0.667 | 0.124; p = 0.418 | −0.063; p = 0.684 | −0.032; p = 0.835 | |
| IL4 | 0.100; p = 0.504 | 0.170; p = 0.260 | 0.083; p = 0.586 | 0.087; p = 0.574 | 0.444; p = 0.002 | |
| IL6 | −0.226; p = 0.127 | −0.132; p = 0.383 | −0.003; p = 0.985 | −0.299; p = 0.049 | 0.129; p = 0.400 | |
| IL10 | −0.025; p = 0.869 | 0.195; p = 0.194 | 0.102; p = 0.505 | 0.182; p = 0.236 | 0.192; p = 0.207 | |
| TNFα | 0.028; p = 0.853 | −0.256; p = 0.086 | 0.308; p = 0.040 | −0.047; p = 0.763 | −0.225; p = 0.137 | |
| Infection group | CRP | −0.317; p = 0.406 | −0.224; p = 0.533 | −0.826; p = 0.011 | 0.000; p = 1.000 | −0.417; p = 0.265 |
| IL1β | −0.058; p = 0.873 | 0.378; p = 0.252 | −0.138; p = 0.724 | −0.407; p = 0.243 | 0.174; p = 0.631 | |
| IL4 | 0.394; p = 0.259 | −0.019; p = 0.956 | −0.257; p = 0.505 | 0.039; p = 0.915 | 0.575; p = 0.082 | |
| IL6 | −0.661; p = 0.038 | −0.464; p = 0.151 | −0.510; p = 0.160 | −0.322; p = 0.364 | −0.115; p = 0.751 | |
| IL10 | 0.355; p = 0.314 | 0.089; p = 0.794 | 0.312; p = 0.414 | 0.236; p = 0.511 | −0.082; p = 0.822 | |
| TNFα | −0.333; p = 0.347 | −0.309; p = 0.355 | 0.318; p = 0.404 | −0.103; p = 0.776 | −0.406; p = 0.244 |
| Group | Resting HRV | HRV in Breathing | HRV Valsalva | HRV Handgrip | HRV Standing | |
|---|---|---|---|---|---|---|
| Total sample | CRP | −0.160; p = 0.196 | −0.027; p = 0.853 | −0.258; p = 0.040 | −0.006; p = 0.967 | −0.325; p = 0.007 |
| IL1β | −0.110; p = 0.472 | 0.020; p = 0.877 | 0.258; p = 0.051 | −0.092; p = 0.533 | 0.079; p = 0.546 | |
| IL4 | 0.109; p = 0.490 | 0.258; p = 0.126 | 0.211; p = 0.112 | −0.099; p = 0.437 | 0.304; p = 0.032 | |
| IL6 | −0.261; p = 0.043 | −0.106; p = 0.426 | −0.060; p = 0.650 | −0.193; p = 0.134 | −0.148; p = 0.302 | |
| IL10 | −0.193; p = 0.227 | −0.025; p = 0.886 | −0.006; p = 0.963 | 0.003; p = 0.983 | 0.143; p = 0.320 | |
| TNFα | 0.039; p = 0.766 | −0.158; p = 0.296 | −0.110; p = 0.432 | 0.124; p = 0.423 | −0.245; p = 0.103 | |
| Only infection group | CRP | −0.427; p = 0.123 | −0.357; p = 0.235 | −0.517; p = 0.032 | −0.105; p = 0.704 | −0.490; p = 0.034 |
| IL1β | 0.218; p = 0.175 | 0.218; p = 0.159 | 0.306; p = 0.044 | 0.044; p = 0.808 | 0.306; p = 0.052 | |
| IL4 | 0.261; p = 0.431 | 0.220; p = 0.509 | 0.306; p = 0.368 | −0.410; p = 0.151 | 0.328; p = 0.259 | |
| IL6 | −0.315; p = 0.330 | −0.406; p = 0.226 | −0.273; p = 0.380 | −0.042; p = 0.909 | −0.448; p = 0.071 | |
| IL10 | −0.268; p = 0.415 | 0.094; p = 0.779 | 0.022; p = 0.949 | −0.076; p = 0.830 | 0.087; p = 0.786 | |
| TNFα | −0.336; p = 0.334 | −0.189; p = 0.558 | −0.308; p = 0.333 | 0.242; p = 0.446 | −0.287; p = 0.340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Colinas, M.; Gea, A.; Khattab, A.; Vassallo, M.; Allen, S.C.; Kwan, J. The Association between Inflammatory Biomarkers and Cardiovascular Autonomic Dysfunction after Bacterial Infection. Appl. Sci. 2022, 12, 3484. https://doi.org/10.3390/app12073484
Arias-Colinas M, Gea A, Khattab A, Vassallo M, Allen SC, Kwan J. The Association between Inflammatory Biomarkers and Cardiovascular Autonomic Dysfunction after Bacterial Infection. Applied Sciences. 2022; 12(7):3484. https://doi.org/10.3390/app12073484
Chicago/Turabian StyleArias-Colinas, Mónica, Alfredo Gea, Ahmed Khattab, Michael Vassallo, Stephen C. Allen, and Joseph Kwan. 2022. "The Association between Inflammatory Biomarkers and Cardiovascular Autonomic Dysfunction after Bacterial Infection" Applied Sciences 12, no. 7: 3484. https://doi.org/10.3390/app12073484
APA StyleArias-Colinas, M., Gea, A., Khattab, A., Vassallo, M., Allen, S. C., & Kwan, J. (2022). The Association between Inflammatory Biomarkers and Cardiovascular Autonomic Dysfunction after Bacterial Infection. Applied Sciences, 12(7), 3484. https://doi.org/10.3390/app12073484






