New Isolated Autochthonous Strains of S. cerevisiae for Fermentation of Two Grape Varieties Grown in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Genetic Identification of Yeast Strains Isolated from the Natural Environment
2.2. Wine Fermentation
2.2.1. White Wine Fermentation
2.2.2. Red Wine Fermentation
2.3. Physicochemical Analyzes of the Obtained Wines
2.3.1. Determination of Ethyl Alcohol Content
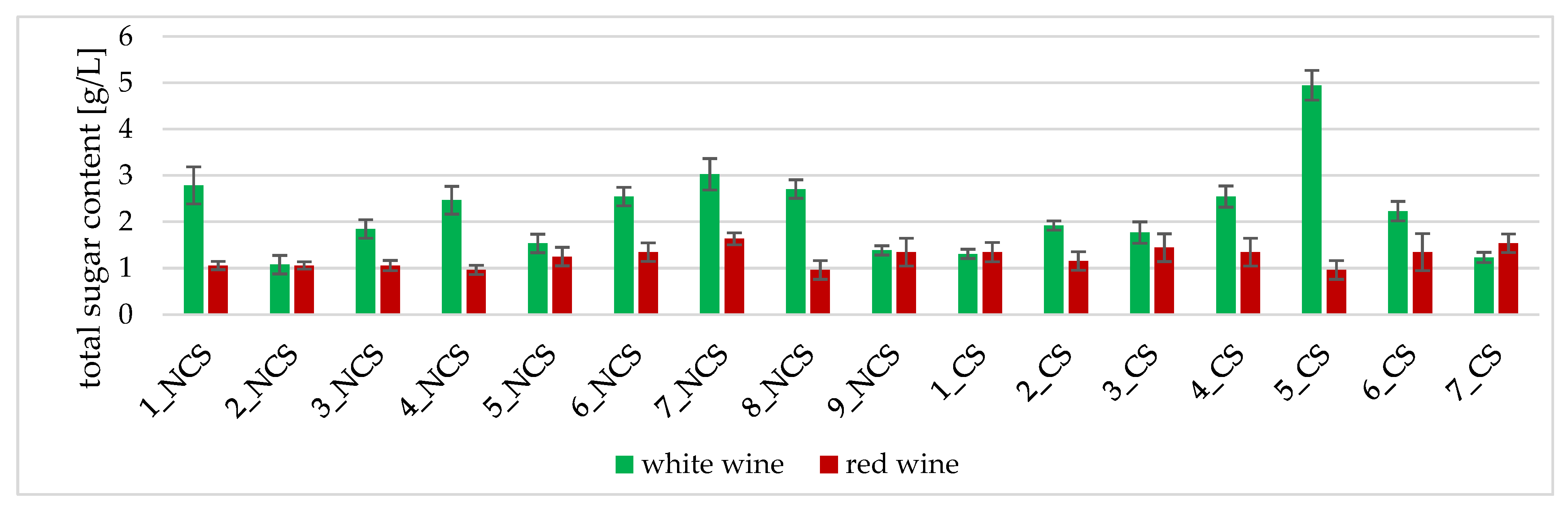
2.3.2. Total Sugar Content Determination
2.3.3. Determination of the pH of Wine
2.3.4. Determination of Total Acidity
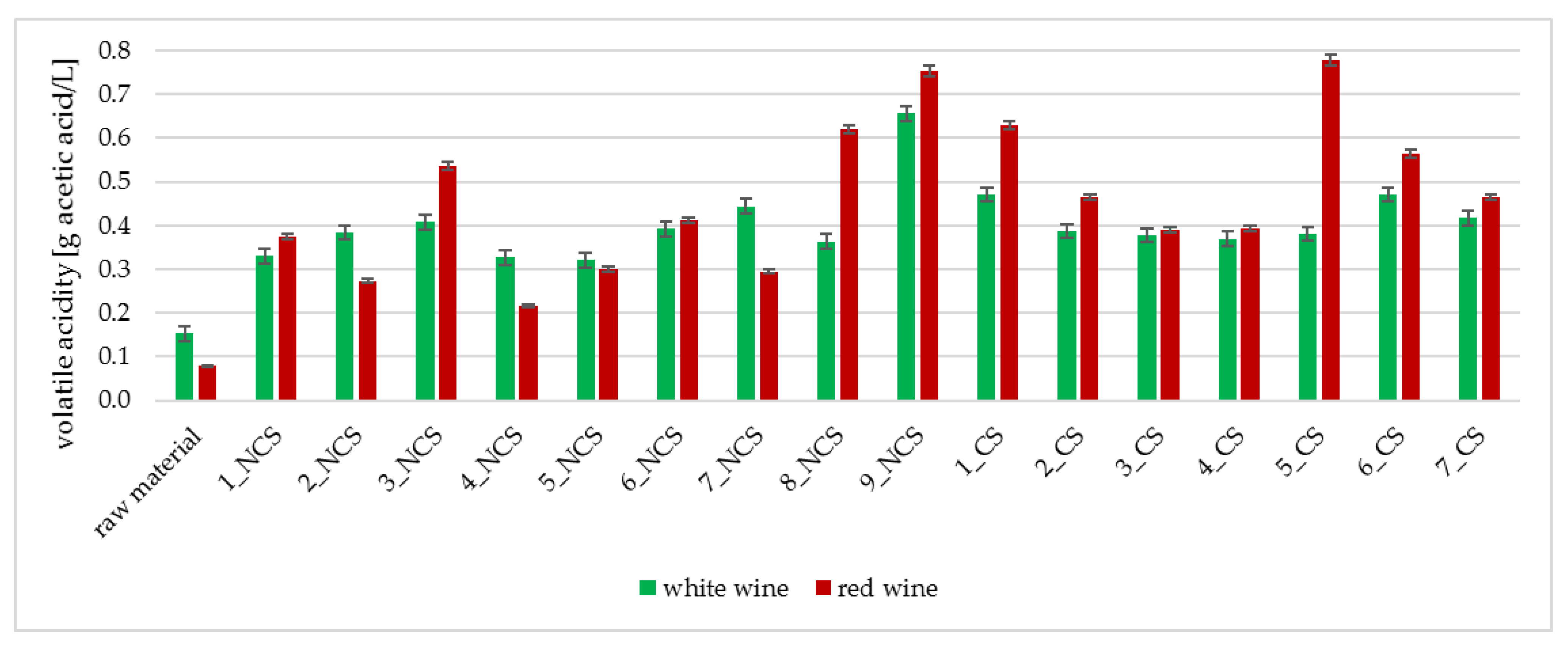
2.3.5. Determination of Volatile Acidity
2.4. Determination of Total Polyphenol Content
2.5. Chromatographic Analyses
2.5.1. Method of Analytical Determination of Selected Polyphenolic Compounds
2.5.2. Method of Analytical Determination of Organic Acids
2.5.3. Method of Analytical Determination of Wine Fermentation By-Products
2.6. Statistical Analysis
3. Results and Discussion
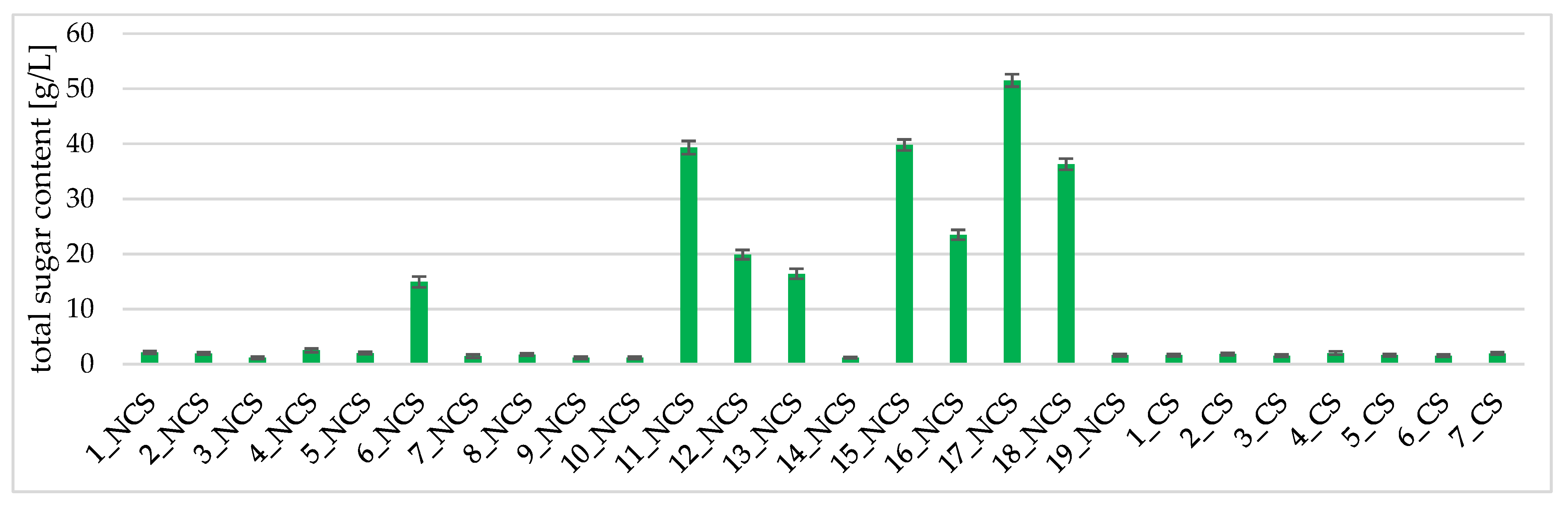
3.1. Isolation and Genetic Identification of Native Yeast and Determination of their Fermentative Potential
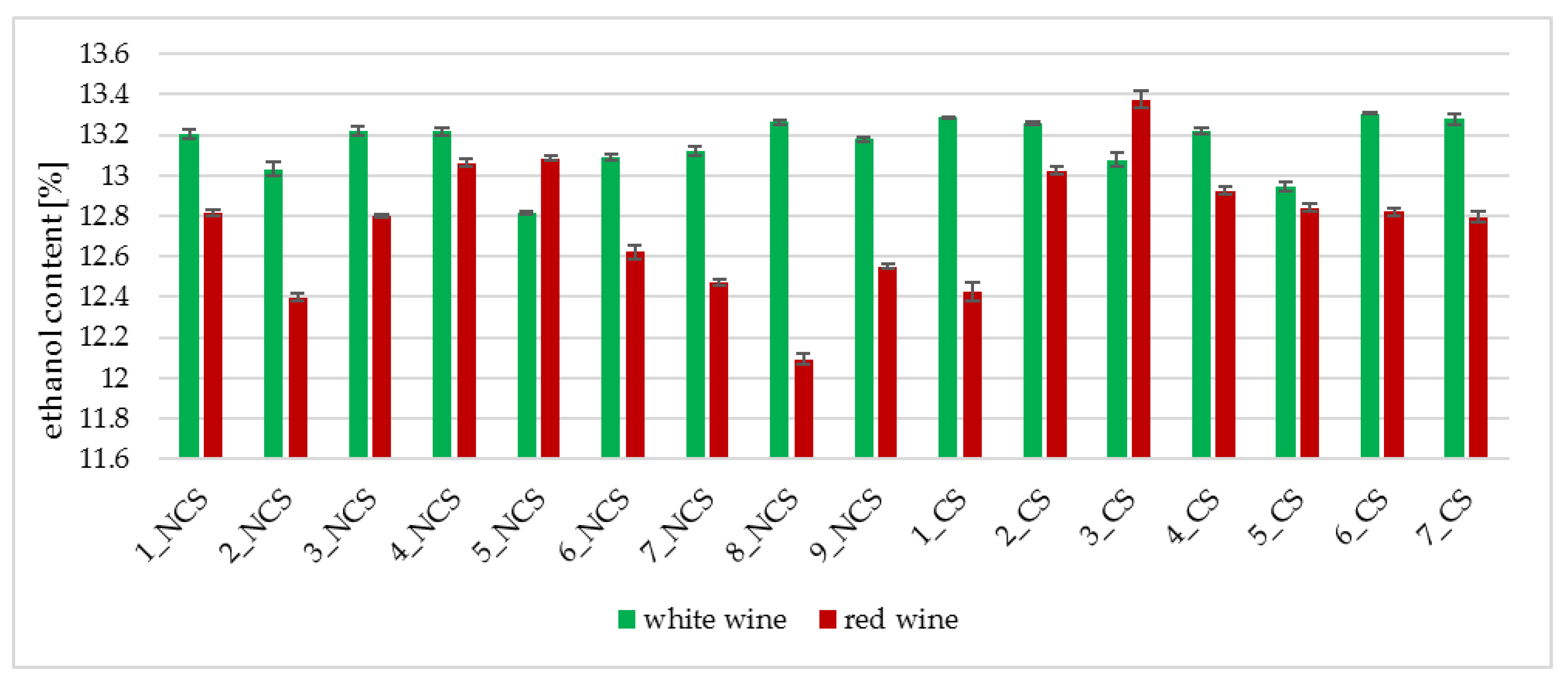
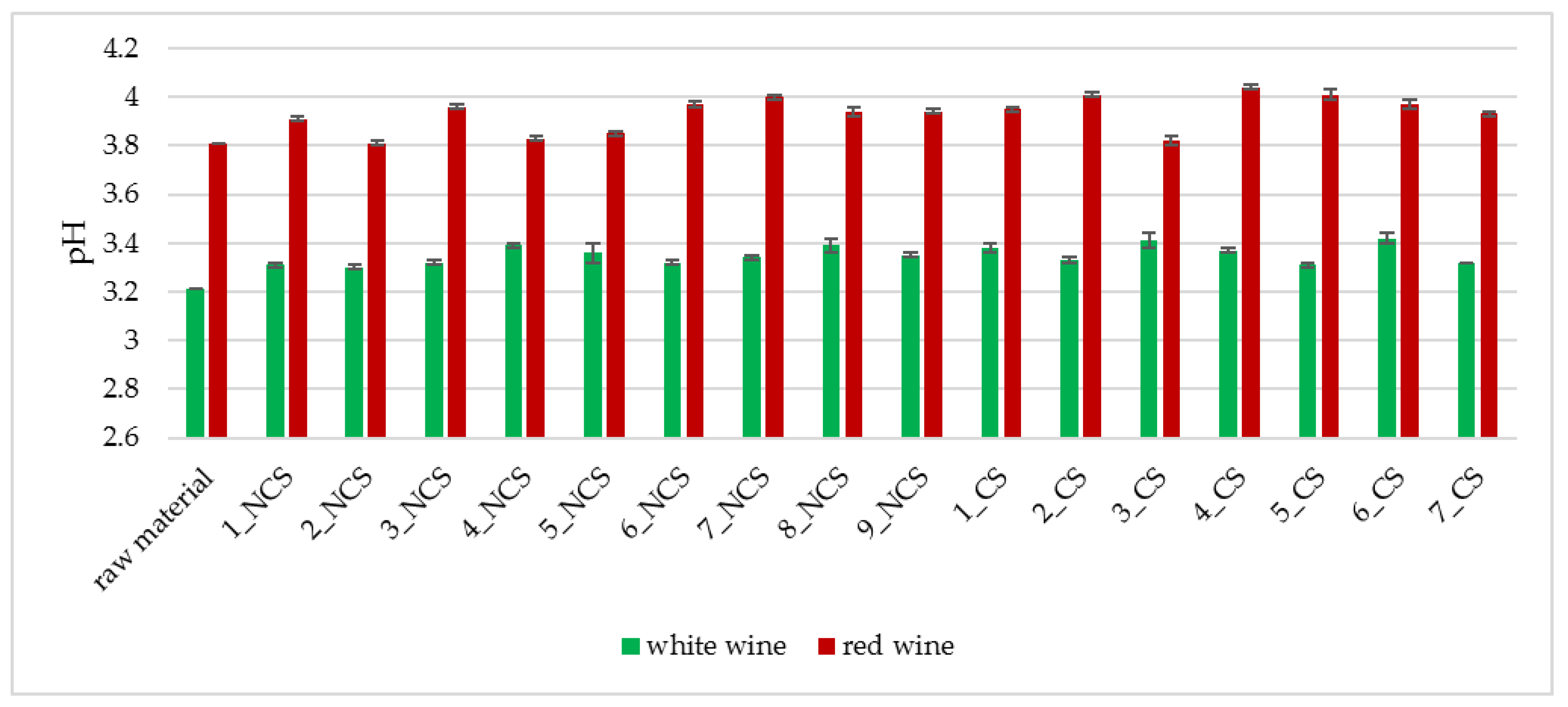
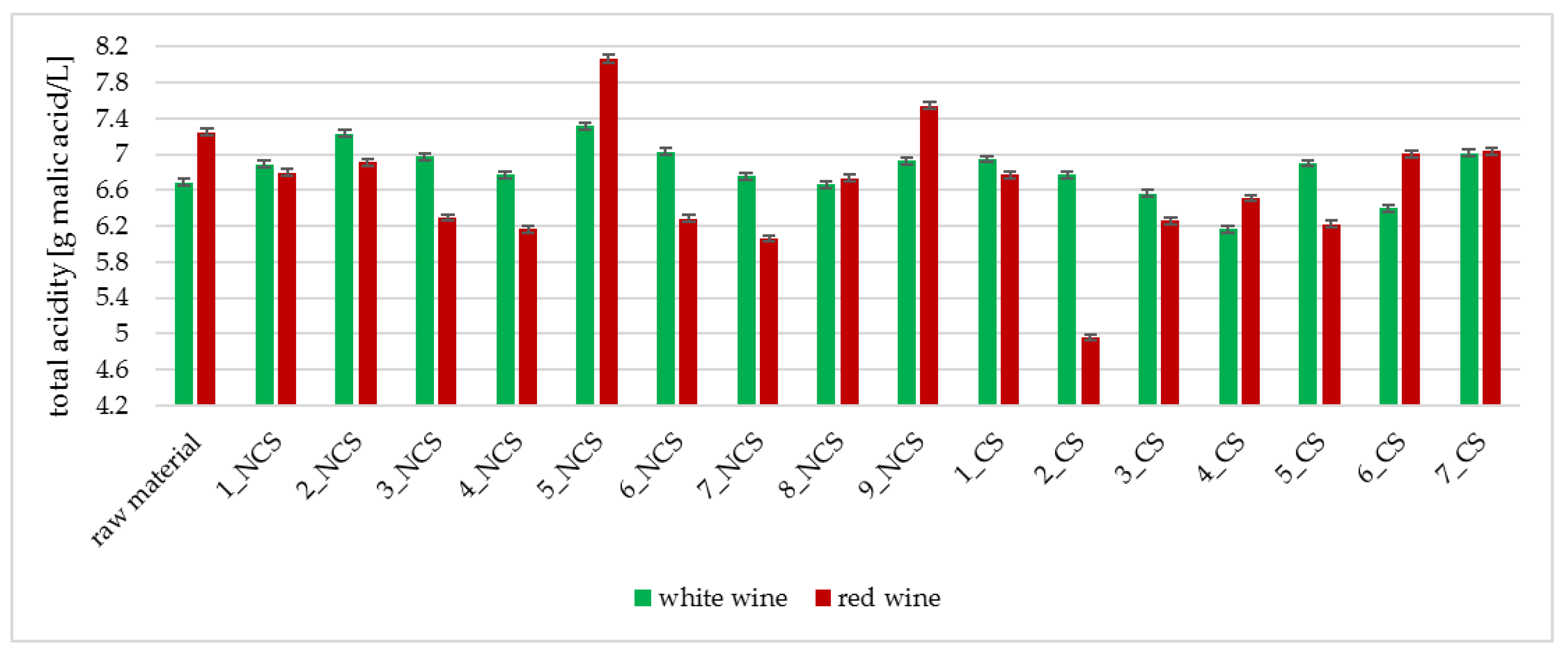
3.2. Physicochemical Analyses of the Obtained Wines
3.3. Determination of Selected Polyphenolic Compounds and Total Polyphenol Content
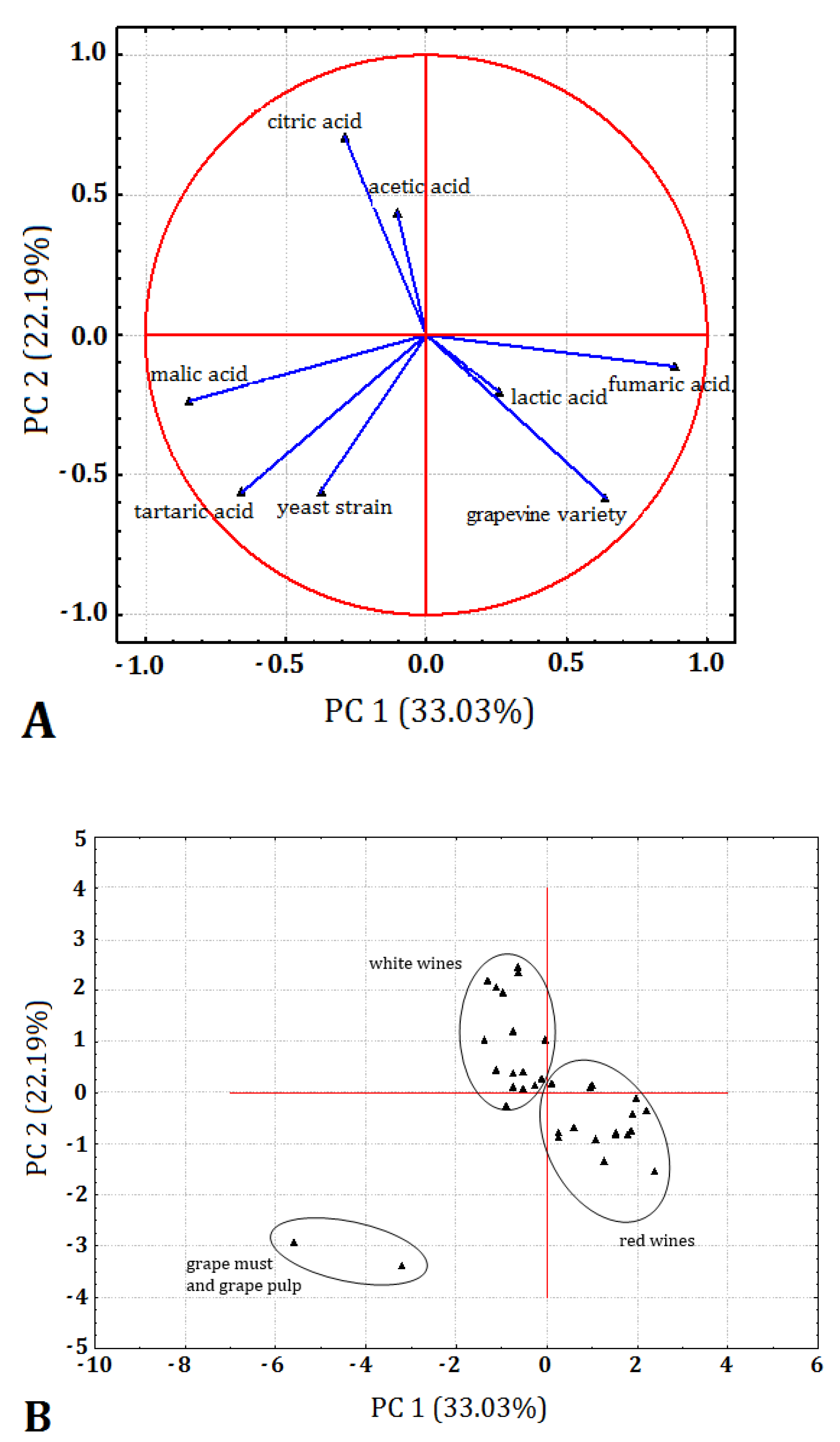
3.4. Determination of Selected Organic Acids
3.5. Determination of Wine Fermentation By-Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cioch, M.; Skotniczy, M.; Kuchta, T.; Satora, P. Charakterystyka wybranych parametrów win gronowych pozyskiwanych z winnic południowej Polski [Characteristic of selected parameters of grape wines obtained from the vineyards of suthern Poland]. Postępy Tech. Przetwórstwa Spożywczego 2018, 1, 14–18. [Google Scholar]
- Schaefer, R.; Olsen, J.; Thach, L. Exploratory wine consumer behavior in a transitional market: The case of Poland. Wine Econ. Policy 2018, 7, 54–64. [Google Scholar] [CrossRef]
- Greinert, A.; Kostecki, J.; Vystavna, Y. The history of viticultural land use as a determinant of contemporary regional development in Western Poland. Land Use Policy 2019, 85, 249–258. [Google Scholar] [CrossRef]
- National Support Center for Agriculture (KOWR). Available online: https://www.kowr.gov.pl/interwencja/wino (accessed on 28 February 2022).
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavor. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Morata, A.; Benito, S.; Loira, I.; Palomero, F.; González, M.C.; Suárez-Lepe, J.A. Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. Int. J. Food Microbiol. 2012, 159, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Minebois, R.; Pérez-Torrado, R.; Querol, A. A time course metabolism comparison among Saccharomyces cerevisiae, S. uvarum and S. kudriavzevii species in wine fermentation. Food Microbiol. 2020, 90, 103484. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tan, F.; Chu, R.; Li, G.; Li, L.; Yang, T.; Zhang, M. The effect of non–Saccharomyces yeasts on biogenic amines in wine. Trends Food Sci. Tech. 2021, 116, 1029–1040. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.C.; Coulon, J.; Thibon, C.; Masneuf-Pomarède, I. Non–Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT Food Sci. Technol. 2021, 149, 111781. [Google Scholar] [CrossRef]
- Balmaseda, A.; Rozès, N.; Leal, M.A.; Bordons, A.; Reguant, C. Impact of changes in wine composition produced by non-Saccharomyces on malolactic fermentation. Int. J. Food Microbiol. 2021, 337, 108954. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla, B.; Zulian, L.; Ferreres, À.; Pastor, R.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Sequential inoculation of native non–Saccharomyces and Saccharomyces cerevisiae strains for wine making. Front. Microbiol. 2017, 8, 1293. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Schirone, M.; Fasoli, G.; Aguzzi, I.; Corsetti, A.; Suzzi, G. Biogeographical characterization of Saccharomyces cerevisiae wine yeast by molecular methods. Front. Microb. 2013, 4, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capece, A.; Granchi, L.; Guerrini, S.; Mangani, S.; Romaniello, R.; Vincenzini, M.; Romano, P. Diversity of Saccharomyces cerevisiae strains isolated from two Italian wine–producing regions. Front. Microb. 2016, 7, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Celis, M.; Ruiz, J.; Martín-Santamaría, M.; Alonso, A.; Marquina, D.; Navascués, E.; Gómez-Flechoso, M.Á.; Belda, I.; Santos, A. Diversity of Saccharomyces cerevisiae yeasts associated to spontaneous and inoculated fermenting grapes from Spanish vineyards. Lett. Appl. Microbiol. 2019, 68, 580–588. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Influence of native Saccharomyces cerevisiae strains from DO “Vinos de Madrid” in the volatile profile of white wines. Fermentation 2019, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- Çelik, Z.D.; Erten, H.; Cabaroglu, T. The influence of selected autochthonous Saccharomyces cerevisiae strains on the physicochemical and sensory properties of narince wines. Fermentation 2019, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Blanco, P.; Mirás-Avalos, J.M.; Pereira, E.; Orriols, I. Fermentative aroma compounds and sensory profiles of Godello and Albariño wines as influenced by Saccharomyces cerevisiae yeast strains. J. Sci. Food Agric. 2013, 93, 2849–2857. [Google Scholar] [CrossRef]
- Elbakush, A.E.; Güven, D. Evaluation of ethanol tolerance in relation to intracellular storage compounds of Saccharomyces cerevisiae using FT–IR spectroscopy. Process Biochem. 2021, 101, 266–273. [Google Scholar] [CrossRef]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive laboratory evolution principles and applications in industrial biotechnology. Biotechnol. Adv. 2021, 54, 107795. [Google Scholar] [CrossRef]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. Trac Trend. Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The role of yeasts and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Kunicka-Styczyńska, A. In the search for novel wine yeast with deacidification activity. Ferment. Technol. 2012, 1, e106. [Google Scholar]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef] [Green Version]
- Giovinazzo, G.; Grieco, F. Functional properties of grape and wine polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Norton, E.L. Chemistry and Reactivity of Tannins in Vitis spp.: A Review. Molecules 2020, 25, 2110. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Choi, Y.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [Green Version]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Wojdyło, A.; Samoticha, J.; Chmielewska, J. The influence of different strains of Oenococcus oeni malolactic bacteria on profile of organic acids and phenolic compounds of red wine cultivars Rondo and Regent growing in a cold region. J. Food Sci. 2020, 85, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Bene, Z.; Kállay, M.; Horváth, B.O.; Nyitrai-Sárdy, D. Comparison of selected phenolic components of white qvevri wines. Mitt. Klosterneubg. Rebe Wein Obstbau Früchteverwertung 2019, 69, 76–82. [Google Scholar]
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Nofer, J. Effect of different yeast strains and temperature of fermentation on basic enological parameters, polyphenols and volatile compounds of aurore white wine. Foods 2019, 8, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markoski, M.M.; Garavaglia, J.; Oliveira, A.; Olivaes, J.; Marcadenti, A. Molecular properties of red wine compounds and cardiometabolic benefits. Nutr. Metab. Insights 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.A.; Campos, F.M.; Figueiredo, A.R.; Hogg, T.A. Ability of lactic acid bacteria to produce volatile phenols. Am. J. Enol. Viticul. 2006, 57, 166–171. [Google Scholar]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunicka-Styczyńska, A.; Rajkowska, K. Fermentative stability of wine yeast Saccharomyces sensu stricto complex and their hybrids. Food Technol. Biotech. 2012, 50, 222–229. [Google Scholar]
- Tarko, T.; Duda-Chodak, A.; Satora, P.; Sroka, P.; Gojniczek, I. Chemical composition of cool–climate grapes and enological parameters of cool–climate wines. Fruits 2014, 69, 75–86. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics, 1st ed.; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Katarína, F.; Katarína, M.; Katarína, Ď.; Ivan, Š.; Fedor, M. Influence of yeast strain on aromatic profile of Gewürztraminer wine. LWT Food Sci. Technol. 2014, 59, 256–262. [Google Scholar] [CrossRef]
- Mateos, J.R.; Pérez-Nevado, F.; Fernández, M.R. Influence of Saccharomyces cerevisiae yeast strain on the major volatile compounds of wine. Enzyme Microb. Tech. 2006, 40, 151–157. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, L.; Pan, X.; Lao, F.; Liao, X.; Wu, J. Alterations of phenolic compounds in red raspberry juice induced by high–hydrostatic–pressure and high–temperature short–time processing. Innov. Food Sci. Emerg. 2021, 67, 102569. [Google Scholar] [CrossRef]
- Ordinance of the Minister of Agriculture and Rural Development of 21 May 2013 on the Detailed Method of Producing Fermented Wine Beverages and the Methods of Analyzing these Beverages for Official Control of Commercial Quality [w Sprawie Szczegółowego Sposobu Wyrobu Fermentowanych Napojów Winiarskich Oraz Metod Analiz Tych Napojów do Celów Urzędowej Kontroli w Zakresie Jakości Handlowej], Dz.U. 2013 poz. 624. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130000624 (accessed on 8 February 2022).
- Valášek, P.; Mlček, J.; Adámková, A.; Křivánková, M.; Adámek, M.; Sedláčková, E. Comparison of contents of selected esters, higher alcohols and total content of polyphenolic substances in wines of the varieties ‘Chardonnay’ and ‘Riesling’ by vintage. Mitt. Klosterneubg. Rebe Wein Obstbau Früchteverwertung 2019, 69, 115–123. [Google Scholar]
- Burin, V.M.; Arcari, S.G.; Bordignon-Luiz, A.M.T.; Costa, L.L.F. Determination of some phenolic compounds in red wine by RP-HPLC: Method development and validation. J. Chromatogr. Sci. 2011, 49, 647–651. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO/IEC 17025:2018-02. Ogólne Wymagania Dotyczące Kompetencji Laboratoriów Badawczych i Wzorcujących [General Requirements for the Competence of Testing and Calibration Laboratories]; Polish Committee for Standardization: Warsaw, Poland, 2018. (In Polish) [Google Scholar]
- Capece, A.; Romaniello, R.; Siesto, G.; Pietrafesa, R.; Massari, C.; Poeta, C.; Romano, P. Selection of indigenous Saccharomyces cerevisiae strains for Nero d’Avola wine and evaluation of selected starter implantation in pilot fermentation. Int. J. Food Microbiol. 2010, 144, 187–192. [Google Scholar] [CrossRef]
- Vigentini, I.; Barrera Cardenas, S.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of native yeast strains for in–bottle fermentation to face the uniformity in sparkling wine production. Front. Microbiol. 2017, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Fariña, L.; Dellacassa, E.; Carrau, F. Non–Saccharomyces and Saccharomyces strains co–fermentation increases acetaldehyde accumulation: Effect on anthocyanin–derived pigments in Tannat red wines. Yeast 2016, 33, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co–culture of non–Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co–fermented with commercial non–Saccharomyces inocula. Aust. J. Grape Wine R. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, D.; Lee, P.R.; Liu, S.Q. Assessment of volatile and non–volatile compounds in durian wines fermented with four commercial non–Saccharomyces yeasts. J. Sci. Food Agric. 2016, 96, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Infante, J.J.; Molina, M.; Domínguez, M.; Rebordinos, L.; Cantoral, J.M. Genomic characterization and selection of wine yeast to conduct industrial fermentations of a white wine produced in a SW Spain winery. J. Appl. Microbiol. 2010, 108, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Miśniakiewicz, M.; Ptasińska, J. Analiza jakości wina dostępnego na polskim rynku [Analysis of the quality of wine available on the Polish market]. Zesz. Nauk. Uniw. Ekon. W Krakowie 2008, 781, 131–154. [Google Scholar]
- King, E.S.; Dunn, R.L.; Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Prefer. 2013, 28, 235–243. [Google Scholar] [CrossRef]
- Sokolowsky, M.; Fischer, U. Evaluation of bitterness in white wine applying descriptive analysis, time–intensity analysis, and temporal dominance of sensations analysis. Anal. Chim. Acta 2012, 732, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Demiglio, P.; Pickering, G.J. The influence of ethanol and pH on the taste and mouthfeel sensations elicited by red wine. Food Agric. Environ. 2008, 6, 143–150. [Google Scholar]
- Fontoin, H.; Saucier, C.; Teissedre, P.L.; Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Prefer. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Pena-Neira, A.; Lopez-Solis, R. Enhancement of both salivary protein–enological tannin interactions and astringency perception by ethanol. J. Agr. Food Chem. 2010, 58, 3729–3735. [Google Scholar] [CrossRef] [PubMed]
- Goldner, M.C.; Zamora, M.C.; Lira, P.D.L.; Gianninoto, H.; Bandoni, A. Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 2009, 24, 243–257. [Google Scholar] [CrossRef]
- Dharmadhikari, M. Composition of grapes. Vineyard Vintage View Mo State Univ. 1994, 9, 3–8. [Google Scholar]
- Gajek, M.; Pawlaczyk, A.; Szynkowska-Jozwik, M.I. Multi–elemental analysis of wine samples in relation to their type, origin, and grape variety. Molecules 2021, 26, 214. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem. J. 2009, 91, 187–192. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Volschenk, H.; Van Vuuren, H.J.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef]
- Margalit, Y. Concepts in Wine Chemistry, 3rd ed.; Board and Bench Publishing: San Francisco, CA, USA, 2016; pp. 11–20. [Google Scholar]
- Izajasz-Parchanska, M.; Cioch, M.; Tuszynski, T. Monitoring parametrów dojrzałości technologicznej winogron na terenie małopolskiej Winnicy Srebrna Góra, w sezonie wegetacyjnym 2012 [Monitoring parameters of technological maturity of grapes in Małopolska Silver Mountain Vineyard in the growing season 2012]. Acta Agrophysica 2014, 21, 263–278. [Google Scholar]
- Lisek, J. Climatic factors affecting development and yielding of grapevine in central Poland. J. Fruit Ornam. Plant Res. 2008, 16, 285–293. [Google Scholar]
- Cruz-Pio, L.E.; Poveda, M.; Alberto, M.R.; Ferrer, S.; Pardo, I. Exploring the biodiversity of two groups of Oenococcus oeni isolated from grape musts and wines: Are they equally diverse? Syst. Appl. Microbiol. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Jordão, A.M.; Vilela, A.; Cosme, F. From sugar of grape to alcohol of wine: Sensorial impact of alcohol in wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef] [Green Version]
- Austrian Wine. Available online: https://www.austrianwine.com/our-wine/wine-law/residual-sugar-content (accessed on 3 November 2021).
- German Wines. Available online: https://www.germanwines.de/knowledge/taste-quality/levels-of-sweetness-in-wine/ (accessed on 3 November 2021).
- Wang, X.J.; Li, Y.K.; Song, H.C.; Tao, Y.S.; Russo, N. Phenolic matrix effect on aroma formation of terpenes during simulated wine fermentation–Part I: Phenolic acids. Food Chem. 2021, 341, 128288. [Google Scholar] [CrossRef] [PubMed]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Minnaar, P.P.; Du Plessis, H.W.; Jolly, N.P.; Van Der Rijst, M.; Du Toit, M. Non-Saccharomyces yeast and lactic acid bacteria in Co-inoculated fermentations with two Saccharomyces cerevisiae yeast strains: A strategy to improve the phenolic content of Syrah wine. Food Chem. X 2019, 4, 100070. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Robak, J.; Fortuna, T.; Buksa, K. Characterization of Polish wines produced from the multispecies hybrid and Vitis vinifera L. grapes. Int. J. Food Prop. 2015, 18, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Kapusta, I.; Cebulak, T.; Oszmiański, J. Characterization of polish wines produced from the interspecific hybrid grapes grown in south-east Poland. Eur. Food Res. Technol. 2018, 244, 441–455. [Google Scholar] [CrossRef]
- Wojdyło, A.; Samoticha, J.; Chmielewska, J. Effect of different pre–treatment maceration techniques on the content of phenolic compounds and color of Dornfelder wines elaborated in cold climate. Food Chem. 2021, 339, 127888. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska-Iwanek, J.; Gąstoł, M.; Wanat, A.; Krośniak, M.; Jancik, M.; Zagrodzki, P. Wine of cool–climate areas in south Poland. S. Afr. J. Enol. Vitic. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Stratil, P.; Kubáň, V.; Fojtová, J. Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech J. Food Sci. 2018, 26, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Jurasz, E. Polish wines: Characteristics of cool–climate wines. J. Food Compos. Anal. 2010, 23, 463–468. [Google Scholar] [CrossRef]
- Moreira, J.L.; Santos, L. Analysis of organic acids in wines by Fourier–transform infrared spectroscopy. Anal. Bioanal. Chem. 2005, 382, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Zotou, A.; Loukou, Z.; Karava, O. Method development for the determination of seven organic acids in wines by reversed-phase high performance liquid chromatography. Chromatographia 2004, 60, 39–44. [Google Scholar] [CrossRef]
- Mato, I.; Suárez-Luque, S.; Huidobro, J.F. Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chem. 2007, 102, 104–112. [Google Scholar] [CrossRef]
- Peres, R.G.; Moraes, E.P.; Micke, G.A.; Tonin, F.G.; Tavares, M.F.M.; Rodriguez-Amaya, D.B. Rapid method for the determination of organic acids in wine by capillary electrophoresis with indirect UV detection. Food Control 2009, 20, 548–552. [Google Scholar] [CrossRef]
- Ohira, S.I.; Kuhara, K.; Shigetomi, A.; Yamasaki, T.; Kodama, Y.; Dasgupta, P.K.; Toda, K. On–line electrodialytic matrix isolation for chromatographic determination of organic acids in wine. J. Chromatogr. A 2014, 1372, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeravik, J.; Fohlerova, Z.; Milovanovic, M.; Kubesa, O.; Zeisbergerova, M.; Lacina, K.; Petrovic, A.; Glatz, Z.; Skladal, P. Various instrumental approaches for determination of organic acids in wines. Food Chem. 2016, 194, 432–440. [Google Scholar] [CrossRef]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non–volatiles composition of the aromatic grape variety Albariño. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Tubío, M.; Alvarez, K.; Lissarrague, J.R.; Oliveira, J.M. Rootstock effect on volatile composition of Albariño wines. Appl. Sci. 2021, 11, 2135. [Google Scholar] [CrossRef]
- Zamúz, S.; Vilanova, M. Volatile compounds after spontaneous fermentation of musts from Vitis vinifera cv. Albariño grapes cultivated in different origins from Rías Baixas AOC, Spain. Flavour Fragr. J. 2006, 21, 743–748. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Bouzas-Cid, Y.; Trigo-Córdoba, E.; Orriols, I.; Falqué, E. Irrigation effects on the volatile composition and sensory profile of Albariño wines from two different terroirs. Eur. Food Res. Technol. 2019, 245, 2157–2171. [Google Scholar] [CrossRef]
- Del Torno-de Román, L.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Gluconic acid determination in wine by electrochemical biosensing. Sens. Actuators B Chem. 2013, 176, 858–862. [Google Scholar] [CrossRef]
- Robles, A.D.; Fabjanowicz, M.; Płotka-Wasylka, J.; Konieczka, P. Organic acids and polyphenols determination in Polish wines by ultrasound-assisted solvent extraction of porous membrane-packed liquid samples. Molecules 2019, 24, 4376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Jurasz, E. Physicochemical and antioxidant properties of selected polish grape and fruit wines. Acta Sci. Pol. Technol. Aliment. 2008, 7, 35–45. [Google Scholar]
- Bartowsky, E.J.; Pretorius, I.S. Microbial Formation and Modification of Flavor and Off-Flavor Compounds in Wine. In Biology of Microorganisms on Grapes, in Must and in Wine, 1st ed.; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 209–232. [Google Scholar]
- Vilanova, M.; Pretorius, I.S.; Henschke, P.A. Influence of diammonium phosphate addition to fermentation on wine biologicals. In Processing and Impact on Active Components in Food, 1st ed.; Preedy, V., Ed.; Academic Press: London, UK, 2015; pp. 483–491. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 2, pp. 51–64. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 1, pp. 53–78. [Google Scholar]
- International Organization of Vine and Wine. Available online: https://www.oiv.int/public/medias/3741/e-code-annex-maximum-acceptable-limits.pdf (accessed on 20 February 2022).
- Jackson, R.S. Wine Science: Principles, Practice, Perception, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; pp. 232–275. [Google Scholar]
| Strain Symbol | Isolation Source | Yeast Identification Acc. to 26S rRNA Sequencing | Yeast Strain Number | GenBank No. | |
|---|---|---|---|---|---|
| Voivodship | Grape Varieties | ||||
| 1_NCS | vineyard (Lubuskie voivodship) | Solaris | Saccharomyces cerevisiae | KKP 858 | OK632015 |
| 2_NCS | vineyard (Lubuskie voivodship) | Solaris | Saccharomyces cerevisiae | KKP 859 | OK632063 |
| 3_NCS | vineyard (Lubuskie voivodship) | Solaris | Saccharomyces cerevisiae | KKP 860 | OK982922 |
| 4_NCS | vineyard (Lubuskie voivodship) | Regent | Saccharomyces cerevisiae | KKP 855 | OK631982 |
| 5_NCS | vineyard (Lubuskie voivodship) | Regent | Saccharomyces cerevisiae | KKP 854 | OK576273 |
| 6_NCS | vineyard (Lubuskie voivodship) | Regent | Saccharomyces cerevisiae | KKP 862 | OK639175 |
| 7_NCS | vineyard (Lubuskie voivodship) | Regent | Saccharomyces cerevisiae | KKP 861 | OK669126 |
| 8_NCS | vineyard (Lubuskie voivodship) | Seyval blanc | Saccharomyces cerevisiae | KKP 857 | OK632020 |
| 9_NCS | vineyard (Świętokrzyskie voivodship) | Solaris | Saccharomyces cerevisiae | KKP 3077 | OK524219 |
| 10_NCS | vineyard (Lubuskie voivodship) | Regent | Saccharomyces cerevisiae | KKP 856 | OK632033 |
| 11_NCS | vineyard (Lubuskie voivodship) | Regent | Saccharomyces cerevisiae | KKP 562 | OK639178 |
| 12_NCS | vineyard (Świętokrzyskie voivodship) | Solaris | Metschnikowia sp. | KKP 853 | OK524308 |
| 13_NCS | vineyard (Świętokrzyskie voivodship) | Solaris | Hanseniaspora uvarum | KKP 863 | OK524309 |
| 14_NCS | vineyard (Świętokrzyskie voivodship) | Seyval blanc | Hanseniaspora uvarum | KKP 869 | OK639177 |
| 15_NCS | vineyard (Świętokrzyskie voivodship) | Regent | Hanseniaspora uvarum | KKP 865 | OK526377 |
| 16_NCS | vineyard (Świętokrzyskie voivodship) | Regent | Hanseniaspora uvarum | KKP 866 | OK526381 |
| 17_NCS | vineyard (Świętokrzyskie voivodship) | Regent | Hanseniaspora uvarum | KKP 867 | OK526424 |
| 18_NCS | vineyard (Świętokrzyskie voivodship) | Seyval blanc | Hanseniaspora uvarum | KKP 864 | OK526376 |
| 19_NCS | vineyard (Świętokrzyskie voivodship) | Regent | Hanseniaspora uvarum | KKP 868 | OK631966 |
| 1_CS | commercial strain | Saccharomyces cerevisiae * | |||
| 2_CS | commercial strain | Saccharomyces cerevisiae * | |||
| 3_CS | commercial strain | Saccharomyces cerevisiae * | |||
| 4_CS | commercial strain | Saccharomyces cerevisiae * | |||
| 5_CS | commercial strain | Saccharomyces cerevisiae * | |||
| 6_CS | commercial strain | Saccharomyces cerevisiae * | |||
| 7_CS | commercial strain | Saccharomyces cerevisiae * | |||
| Strain Symbol | Ethanol [%] * | pH | Total Acidity [g malic acid/L] | Strain Symbol | Ethanol [%] * | pH | Total Acidity [g malic acid/L] |
|---|---|---|---|---|---|---|---|
| 1_NCS | 5.894 ± 0.010 a | 3.66 ± 0,02 c | 4.449 ± 0.240 | 14_NCS | 0.178 ± 0.005 r | 3.47 ± 0.01 ab | 1.536 ± 0.083 |
| 2_NCS | 5.826 ± 0.012 b | 3.68 ± 0.02 c | 4.529 ± 0.245 | 15_NCS | 2.325 ± 0.005 p | 3.61 ± 0.02 bc | 3.055 ± 0.165 |
| 3_NCS | 5.681 ± 0.038 ef | 3.61 ± 0.01 bc | 4.516 ± 0.244 | 16_NCS | 2.428 ± 0.004 o | 3.53 ± 0.00 ab | 5.420 ± 0.293 |
| 4_NCS | 5.411 ± 0.013 i | 3.60 ± 0.01 bc | 4.583 ± 0.247 | 17_NCS | 2.934 ± 0.011 m | 3.48 ± 0.03 ab | 5.568 ± 0.301 |
| 5_NCS | 5.640 ± 0.008 fg | 3.59 ± 0.04 bc | 4.462 ± 0.241 | 18_NCS | 2.626 ± 0.006 n | 3.57 ± 0.02 bc | 3.665 ± 0.198 |
| 6_NCS | 4.422 ± 0.018 j | 3.59 ± 0.01 bc | 4.703 ± 0.254 | 19_NCS | 0.212 ± 0.001 r | 3.45 ± 0.03 a | 4.697 ± 0.254 |
| 7_NCS | 5.835 ± 0.006 b | 3.66 ± 0.01 c | 4.429 ± 0.239 | 1_CS | 5.548 ± 0.012 h | 3.71 ± 0.01 c | 4.181 ± 0.226 |
| 8_NCS | 5.612 ± 0.011 g | 3.57 ± 0.03 abc | 4.677 ± 0.253 | 2_CS | 5.681 ± 0.013 ef | 3.60 ± 0.00 bc | 4.817 ± 0.260 |
| 9_NCS | 5.655 ± 0.016 fg | 3.64 ± 0.01 bc | 4.549 ± 0.246 | 3_CS | 5.719 ± 0.010 de | 3.50 ± 0.00 ab | 4.435 ± 0.239 |
| 10_NCS | 0.092 ± 0.009 s | 3.51 ± 0.00 ab | 4.791 ± 0.159 | 4_CS | 5.810 ± 0.024 bc | 3.58 ± 0.03 bc | 4.643 ± 0.251 |
| 11_NCS | 1.655 ± 0.005 q | 3.49 ± 0.01 ab | 2.995 ± 0.162 | 5_CS | 5.673 ± 0.015 ef | 3.55 ± 0.01 abc | 4.851 ± 0.262 |
| 12_NCS | 3.250 ± 0.004 k | 3.56 ± 0.02 bc | 5.273 ± 0.285 | 6_CS | 5.741 ± 0.018 d | 3.55 ± 0.01 abc | 4.837 ± 0.261 |
| 13_NCS | 3.124 ± 0.004 l | 3.52 ± 0.02 ab | 3.759 ± 0.203 | 7_CS | 5.766 ± 0.023 cd | 3.51 ± 0.02 ab | 5.333 ± 0.288 |
| Strain Symbol | Gallic Acid [mg/L] | Chlorogenic Acid [mg/L] | Caffeic Acid [mg/L] | Vanillic Acid [mg/L] | Total Polyphenols at 726 nm [mg/L] |
|---|---|---|---|---|---|
| Grape must | - | - | - | - | 331.04 ± 0.21 j |
| 1_NCS | 21.19 ± 2.10 b | 7.01 ± 0.28 a | 0.68 ± 0.18 a | 3.63 ± 1.18 a | 264.94 ± 0.16 bcd |
| 2_NCS | 24.35 ± 0.77 b | 6.84 ± 0.01 a | 2.48 ± 0.02 d | 2.87 ± 0.05 a | 278.45 ± 0.07 g |
| 3_NCS | 26.25 ± 8.69 b | 6.45 ± 0.13 a | 0.51 ± 0.08 a | 2.97 ± 0.74 a | 299.92 ± 1.65 i |
| 4_NCS | 20.82 ± 0.43 b | 6.62 ± 0.01 a | 0.94 ± 0.44 abc | 2.60 ± 0.33 a | 261.04 ± 0.53 abc |
| 5_NCS | 22.53 ± 1.59 b | 39.85 ± 8.55 b | 0.67 ± 0.03 a | 2.90 ± 0.83 a | 271.65 ± 0.69 ef |
| 6_NCS | 23.15 ± 1.02 b | 20.28 ± 18.80 ab | 1.87 ± 0.29 bcd | 1.07 ± 0.00 a | 267.41 ± 1.69 cde |
| 7_NCS | 20.97 ± 0.10 b | 6.64 ± 0.01 a | 0.36 ± 0.41 a | 2.27 ± 0.94 a | 277.73 ± 0.70 g |
| 8_NCS | 22.84 ± 0.19 b | 6.87 ± 0.01 a | 1.93 ± 0.79 cd | 2.73 ± 0.18 a | 269.10 ± 2.29 de |
| 9_NCS | 19.65 ± 0.18 ab | 33.18 ± 1.10 b | 0.42 ± 0.02 a | 2.55 ± 0.83 a | 275.08 ± 0.39 fg |
| 1_CS | 24.06 ± 0.38 b | 34.35 ± 0.44 b | 0.60 ± 0.15 a | 3.97 ± 1.04 a | 279.37 ± 0.84 g |
| 2_CS | 21.11 ± 1.86 b | 7.04 ± 0.16 a | 0.66 ± 0.26 ab | 2.79 ± 0.33 a | 261.10 ± 6.75 ab |
| 3_CS | 22.54 ± 0.52 b | 7.27 ± 0.24 a | 1.04 ± 0.21 abc | 4.21 ± 1.28 a | 280.16 ± 1.05 g |
| 4_CS | 10.85 ± 0.37 a | 7.81 ± 0.52 a | 0.85 ± 0.24 abc | 3.24 ± 0.87 a | 291.88 ± 0.37 h |
| 5_CS | 22.98 ± 0.99 b | 6.65 ± 0.10 a | 1.23 ± 0.33 abc | 2.82 ± 0.62 a | 257.53 ± 0.56 a |
| 6_CS | 20.83 ± 0.87 b | 6.55 ± 0.00 a | 0.42 ± 0.09 a | 3.14 ± 0.51 a | 271.51 ± 1.05 ef |
| 7_CS | 23.39 ± 1.96 b | 6.89 ± 0.01 a | 1.06 ± 0.09 abc | 3.93 ± 0.38 a | 271.10 ± 0.43 ef |
| Strain Symbol | Gallic Acid [mg/L] | Chlorogenic Acid [mg/L] | Caffeic Acid [mg/L] | Vanillic Acid [mg/L] | Total Polyphenols at 726 nm [mg/L] |
|---|---|---|---|---|---|
| Grape pulp | - | - | - | - | 1304.37 ± 5.80 a |
| 1_NCS | 86.21 ± 5.36 defg | 52.43 ± 0.29 ef | <0.10 a | <0.10 a | 1979.33 ± 7.42 c |
| 2_NCS | 60.18 ± 1.56 a | 45.33 ± 0.26 de | 1.39 ± 0.00 b | 1.70 ± 0.19 c | 1858.59 ± 5.64 b |
| 3_NCS | 65.77 ± 1.88 abc | 32.91 ± 0.06 c | <0.10 a | 1.14 ± 0.05 b | 2168.44 ± 7.95 d |
| 4_NCS | 76.86 ± 0.80 bcde | 61.00 ± 7.68 gh | <0.10 a | <0.10 a | 2264.30 ± 2.56 e |
| 5_NCS | 80.48 ± 0.98 cdef | 65.26 ± 0.21 hi | <0.10 a | <0.10 a | 2193.63 ± 4.57 d |
| 6_NCS | 63.49 ± 2.01 ab | 62.91 ± 0.19 gh | 2.15 ± 0.02 c | 2.14 ± 0.01 d | 2504.96 ± 7.81 f |
| 7_NCS | 71.62 ± 0.01 abcd | 45.05 ± 0.12 de | <0.10 a | 1.58 ± 0.08 c | 2666.30 ± 4.72 j |
| 8_NCS | 71.32 ± 0.56 abcd | 71.50 ± 0.45 i | <0.10 a | 1.69 ± 0.08 c | 2539.93 ± 53.66 fg |
| 9_NCS | 103.70 ± 4.44 h | 56.70 ± 0.31 fgh | <0.10 a | <0.10 a | 2811.33 ± 1.02 k |
| 1_CS | 85.62 ± 0.96 defg | 23.37 ± 0.04 b | <0.10 a | <0.10 a | 2979.70 ± 5.41 l |
| 2_CS | 101.29 ± 1.19 gh | 65.40 ± 0.64 hi | <0.10 a | <0.10 a | 2606.15 ± 4.05 i |
| 3_CS | 71.89 ± 0.40 abcd | 12.27 ± 0.01 a | <0.10 a | <0.10 a | 1851.85 ± 4.03 b |
| 4_CS | 95.59 ± 4.78 fgh | 17.17 ± 0.05 ab | <0.10 a | <0.10 a | 3028.96 ± 4.85 m |
| 5_CS | 194.76 ± 3.85 i | 38.36 ± 0.01 cd | <0.10 a | <0.10 a | 2167.33 ± 2.19 d |
| 6_CS | 92.68 ± 8.79 efgh | 46.21 ± 0.51 e | <0.10 a | <0.10 a | 2554.30 ± 4.35 gh |
| 7_CS | 73.00 ± 9.30 abcd | 44.98 ± 0.09 de | <0.10 a | <0.10 a | 2588.30 ± 1.48 h |
| Strain Symbol | Citric Acid [g/L] | Tartaric Acid [g/L] | Malic Acid [g/L] | Lactic Acid [g/L] | Acetic Acid [g/L] | Fumaric Acid [mg/L] |
|---|---|---|---|---|---|---|
| Grape must | <0.10 a | 1.73 ± 0.01 b | 4.52 ± 0.30 b | <0.10 a | <0.10 a | <0.65 a |
| 1_NCS | <0.10 a | 0.96 ± 0.18 ab | 2.04 ± 0.30 a | <0.10 a | <0.10 a | 3.09 ± 0.23 de |
| 2_NCS | <0.10 a | 0.94 ± 0.34 ab | 2.24 ± 0.40 a | <0.10 a | 0.10 ± 0.00 a | 2.93 ± 0.10 de |
| 3_NCS | <0.10 a | 0.95 ± 0.21 ab | 2.13 ± 0.33 a | <0.10 a | 0.10 ± 0.00 a | 3.09 ± 0.23 de |
| 4_NCS | <0.10 a | 1.06 ± 0.44 ab | 2.12 ± 0.39 a | <0.10 a | <0.10 a | 2.92 ± 0.08 de |
| 5_NCS | <0.10 a | 1.01 ± 0.31 ab | 2.38 ± 0.37 a | <0.10 a | <0.10 a | 2.40 ± 0.19 cd |
| 6_NCS | <0.10 a | 0.68 ± 0.00 a | 2.06 ± 0.24 a | <0.10 a | 0.11 ± 0.01 a | 3.41 ± 0.16 e |
| 7_NCS | <0.10 a | 0.83 ± 0.16 a | 2.28 ± 0.37 a | <0.10 a | 0.11 ± 0.01 a | 1.81 ± 0.16 bc |
| 8_NCS | <0.10 a | 0.87 ± 0.18 a | 2.09 ± 0.21 a | <0.10 a | 0.11 ± 0.01 a | 3.05 ± 0.18 de |
| 9_NCS | <0.10 a | 0.89 ± 0.22 ab | 1.83 ± 0.26 a | <0.10 a | 0.22 ± 0.13 a | <0.65 a |
| 1_CS | <0.10 a | 0.69 ± 0.13 a | 2.17 ± 0.33 a | <0.10 a | 0.13 ± 0.04 a | 3.36 ± 0.32 e |
| 2_CS | <0.10 a | 0.65 ± 0.12 a | 2.12 ± 0.23 a | <0.10 a | 0.11 ± 0.01 a | 3.33 ± 0.39 e |
| 3_CS | <0.10 a | 0.84 ± 0.13 a | 2.04 ± 0.25 a | <0.10 a | 0.10 ± 0.00 a | 2.84 ± 0.28 de |
| 4_CS | <0.10 a | 0.81 ± 0.18 a | 1.92 ± 0.29 a | <0.10 a | 0.11 ± 0.01 a | 1.66 ± 0.06 bc |
| 5_CS | <0.10 a | 0.80 ± 0.23 a | 2.26 ± 0.45 a | <0.10 a | 0.10 ± 0.00 a | 1.17 ± 0.01 ab |
| 6_CS | <0.10 a | 0.67 ± 0.14 a | 1.51 ± 0.08 a | <0.10 a | 0.12 ± 0.02 a | 1.53 ± 0.33 b |
| 7_CS | <0.10 a | 0.75 ± 0.04 a | 2.11 ± 0.23 a | <0.10 a | 0.12 ± 0.03 a | 2.80 ± 0.06 de |
| Strain Symbol | Citric Acid [g/L] | Tartaric Acid [g/L] | Malic Acid [g/L] | Lactic Acid [g/L] | Acetic Acid [g/L] | Fumaric Acid [mg/L] |
|---|---|---|---|---|---|---|
| Grape pulp | <0.10 a | 1.95 ± 0.08 c | 2.55 ± 0.14 e | <0.10 a | <0.10 a | <0.65 a |
| 1_NCS | <0.10 a | 0.79 ± 0.14 ab | 1.60 ± 0.01 bcd | <0.10 a | <0.10 a | 5.55 ± 0.07 efg |
| 2_NCS | <0.10 a | 0.69 ± 0.14 ab | 1.68 ± 0.01 bcd | <0.10 a | <0.10 a | 5.22 ± 0.02 def |
| 3_NCS | <0.10 a | 0.74 ± 0.09 ab | 1.52 ± 0.22 abcd | <0.10 a | <0.10 a | 4.95 ± 0.23 de |
| 4_NCS | <0.10 a | 0.70 ± 0.00 ab | 1.86 ± 0.06 cd | <0.10 a | <0.10 a | 5.49 ± 0.37 efg |
| 5_NCS | <0.10 a | 0.45 ± 0.06 a | 1.64 ± 0.02 bcd | <0.10 a | <0.10 a | 5.83 ± 0.10 fgh |
| 6_NCS | <0.10 a | 0.87 ± 0.22 ab | 1.09 ± 0.08 a | 0.23 ± 0.13 b | 0.11 ± 0.01 ab | 4.61 ± 0.35 d |
| 7_NCS | <0.10 a | 1.01 ± 0.27 ab | 1.58 ± 0.02 bcd | 2.95 ± 0.08 c | <0.10 a | 5.91 ± 0.17 gh |
| 8_NCS | <0.10 a | 0.64 ± 0.05 ab | 1.60 ± 0.00 bcd | <0.10 a | <0.10 a | 6.38 ± 0.02 h |
| 9_NCS | <0.10 a | 1.22 ± 0.26 b | 1.65 ± 0.06 bcd | <0.10 a | 0.16 ± 0.08 bc | 2.83 ± 0.11 b |
| 1_CS | <0.10 a | 0.93 ± 0.03 ab | 1.44 ± 0.15 abc | <0.10 a | 0.18 ± 0.04 bc | 3.62 ± 0.08 c |
| 2_CS | <0.10 a | 0.72 ± 0.05 ab | 1.54 ± 0.23 bcd | <0.10 a | <0.10 a | 5.01 ± 0.10 de |
| 3_CS | <0.10 a | 0.54 ± 0.19 a | 1.90 ± 0.14 d | <0.10 a | <0.10 a | 2.81 ± 0.01 b |
| 4_CS | <0.10 a | 0.94 ± 0.11 ab | 1.28 ± 0.01 ab | <0.10 a | <0.10 a | 3.80 ± 0.13 c |
| 5_CS | <0.10 a | 0.74 ± 0.08 ab | 1.29 ± 0.02 ab | <0.10 a | 0.21 ± 0.01 c | 3.79 ± 0.02 c |
| 6_CS | <0.10 a | 0.71 ± 0.16 ab | 1.69 ± 0.13 bcd | <0.10 a | 0.15 ± 0.04 bc | 3.28 ± 0.04 bc |
| 7_CS | <0.10 a | 0.88 ± 0.15 ab | 1.80 ± 0.00 cd | <0.10 a | 0.10 ± 0.00 ab | 3.21 ± 0.08 bc |
| Organic Acids [g/L] | Spain [84] | Brazil [85] | German [86] | Czech Republic [87] | ||||
|---|---|---|---|---|---|---|---|---|
| White | Red | White | Red | White | Red | White | Red | |
| tartaric | 3.09 | 2.70 | 1.34 | 2.21 | 1.05 | 1.89 | 2.61 | 1.86 |
| malic | 1.97 | 0.39 | 2.63 | 2.24 | 0.78 | 0.58 | 6.22 | 0.12 |
| acetic | 0.21 | 0.48 | 0.92 | 1.00 | 0.37 | 0.42 | 1.67 | 0.70 |
| lactic | 0.62 | 0.84 | 3.93 | 7.31 | 0.47 | 0.17 | 0.22 | 3.78 |
| citric | 0.46 | 0.60 | - * | - * | 1.58 | - * | 0.56 | 0.27 |
| Strain Symbol | Acetaldehyde [mg/100 mL] | Ethyl Acetate [mg/100 mL] | Methanol [mg/100 mL] | 1–Propanol [mg/100 mL] | Iso–Butanol [mg/100 mL] | Amyl Alcohols [mg/100 mL] |
|---|---|---|---|---|---|---|
| 1_NCS | 3.536 ± 0.413 abc | 1.913 ± 0.230 defg | 4.526 ± 0.533 ab | 0.622 ± 0.072 bcde | 5.878 ± 0.704 de | 32.935 ± 3.894 f |
| 2_NCS | 3.025 ± 0.135 ab | 1.154 ± 0.050 ab | 3.255 ± 0.151 a | 0.422 ± 0.016 a | 3.843 ± 0.167 bc | 21.770 ± 0.961 cd |
| 3_NCS | 3.885 ± 0.155 abc | 1.789 ± 0.074 defg | 4.646 ± 0.223 b | 0.643 ± 0.017 de | 5.381 ± 0.212 de | 29.925 ± 1.178 ef |
| 4_NCS | 3.957 ± 0.077 abc | 2.297 ± 0.045 gh | 4.986 ± 0.088 b | 0.682 ± 0.009 e | 6.037 ± 0.117 e | 33.204 ± 0.651 f |
| 5_NCS | 4.408 ± 0.779 cd | 1.570 ± 0.271 bcde | 3.930 ± 0.670 ab | 0.453 ± 0.076 ab | 3.408 ± 0.599 b | 21.367 ± 3.763 bcd |
| 6_NCS | 3.005 ± 0.182 a | 1.235 ± 0.086 abc | 4.092 ± 0.280 ab | 0.587 ± 0.044 abcde | 5.180 ± 0.357 de | 28.032 ± 1.953 def |
| 7_NCS | 5.853 ± 0.709 e | 1.710 ± 0.223 cdef | 4.362 ± 0.553 ab | 0.513 ± 0.065 abcde | 2.139 ± 0.260 a | 15.244 ± 1.893 abc |
| 8_NCS | 3.347 ± 0.278 abc | 1.763 ± 0.144 def | 4.255 ± 0.285 ab | 0.563 ± 0.045 abcde | 4.788 ± 0.398 cd | 26.869 ± 2.221 def |
| 9_NCS | 3.529 ± 0.056 abc | 2.600 ± 0.029 h | 3.953 ± 0.030 ab | 0.572 ± 0.006 abcde | 2.731 ± 0.051 ab | 15.067 ± 0.204 abc |
| 1_CS | 4.221 ± 0.543 abcd | 1.201 ± 0.151 abc | 4.578 ± 0.548 b | 0.554 ± 0.080 abcde | 3.202 ± 0.411 ab | 23.864 ± 3.041 de |
| 2_CS | 4.018 ± 0.244 abc | 2.140 ± 0.128 fgh | 4.569 ± 0.265 b | 0.641 ± 0.024 cde | 5.199 ± 0.323 de | 29.820 ± 1.880 ef |
| 3_CS | 3.408 ± 0.692 abc | 2.004 ± 0.389 efg | 4.231 ± 0.875 ab | 0.618 ± 0.127 bcde | 4.744 ± 0.898 cd | 25.646 ± 4.923 de |
| 4_CS | 5.531 ± 0.147 de | 1.484 ± 0.036 bcde | 4.565 ± 0.155 b | 0.472 ± 0.011 abc | 5.374 ± 0.140 de | 28.135 ± 0.802 def |
| 5_CS | 4.689 ± 0.716 cde | 1.403 ± 0.214 abcd | 4.122 ± 0.591 ab | 0.467 ± 0.069 ab | 2.683 ± 0.405 ab | 14.391 ± 2.182 ab |
| 6_CS | 7.267 ± 0.704 f | 1.721 ± 0.166 cdef | 4.371 ± 0.341 ab | 0.476 ± 0.045 abcd | 2.097 ± 0.203 a | 11.632 ± 1.124 a |
| 7_CS | 4.405 ± 0.150 bcd | 0.908 ± 0.028 a | 4.874 ± 0.139 b | 0.648 ± 0.021 e | 3.891 ± 0.122 bc | 26.356 ± 0.869 def |
| Strain Symbol | Acetaldehyde [mg/100 mL] | Ethyl Acetate [mg/100 mL] | Methanol [mg/100 mL] | 1–Propanol [mg/100 mL] | Iso–Butanol [mg/100 mL] | Amyl Alcohols [mg/100 mL] |
|---|---|---|---|---|---|---|
| 1_NCS | 3.288 ± 0.330 ab | 1.149 ± 0.117 abc | 9.090 ± 0.635 ab | 2.092 ± 0.196 abc | 5.924 ± 0.586 abcd | 33.724 ± 3.323 de |
| 2_NCS | 4.201 ± 0.096 bc | 1.064 ± 0.018 ab | 7.330 ± 0.108 a | 2.072 ± 0.034 abc | 5.944 ± 0.116 abcde | 43.978 ± 0.854 fg |
| 3_NCS | 15.558 ± 0.312 f | 1.497 ± 0.027 bcd | 9.621 ± 0.572 ab | 2.449 ± 0.049 bcd | 6.753 ± 0.138 bcdefg | 36.292 ± 0.714 def |
| 4_NCS | 1.965 ± 0.152 ab | 1.897 ± 0.132 d | 9.445 ± 0.672 ab | 1.916 ± 0.136 ab | 8.127 ± 0.571 efgh | 42.783 ± 2.985 fg |
| 5_NCS | 1.557 ± 0.133 a | 1.565 ± 0.127 bcd | 9.312 ± 0.762 ab | 2.506 ± 0.221 bcd | 4.756 ± 0.411 ab | 39.372 ± 3.368 efg |
| 6_NCS | 16.867 ± 2.297 f | 5.719 ± 0.792 f | 17.957 ± 2.480 de | 6.028 ± 0.829 f | 7.360 ± 1.053 cdefg | 27.460 ± 3.767 abcd |
| 7_NCS | 10.549 ± 1.228 e | 1.386 ± 0.168 bcd | 14.372 ± 1.677 cd | 2.816 ± 0.319 cd | 9.716 ± 1.151 hi | 42.716 ± 5.003 fg |
| 8_NCS | 6.934 ± 0.137 d | 1.229 ± 0.101 abcd | 8.807 ± 0.153 ab | 2.099 ± 0.040 abc | 8.720 ± 0.168 gh | 47.138 ± 0.904 g |
| 9_NCS | 3.127 ± 0.193 ab | 4.045 ± 0.245 e | 23.032 ± 1.455 f | 2.363 ± 0.151 bcd | 5.391 ± 0.338 abc | 19.635 ± 1.221 ab |
| 1_CS | 11.578 ± 1.509 e | 1.553 ± 0.197 bcd | 16.512 ± 2.037 de | 2.417 ± 0.320 bcd | 8.046 ± 1.027 defgh | 31.862 ± 4.078 cde |
| 2_CS | 6.744 ± 1.076 d | 1.132 ± 0.187 abc | 8.515 ± 1.391 ab | 1.418 ± 0.236 a | 6.362 ± 1.032 bcdef | 27.881 ± 4.511 bcd |
| 3_CS | 3.077 ± 0.172 ab | 1.135 ± 0.073 abc | 12.005 ± 0.793 bc | 1.712 ± 0.123 ab | 3.965 ± 0.266 a | 18.684 ± 1.240 a |
| 4_CS | 1.066 ± 0.160 a | 0.575 ± 0.076 a | 18.490 ± 2.403 de | 1.409 ± 0.192 a | 11.675 ± 1.516 i | 32.995 ± 4.301 cde |
| 5_CS | 17.050 ± 0.296 f | 1.791 ± 0.032 cd | 10.234 ± 0.163 abc | 2.974 ± 0.051 d | 8.186 ± 0.130 fgh | 32.920 ± 0.539 cde |
| 6_CS | 6.099 ± 0.183 cd | 6.858 ± 0.198 g | 20.451 ± 0.620 ef | 2.899 ± 0.086 cd | 8.437 ± 0.256 fgh | 27.691 ± 0.840 bcd |
| 7_CS | 6.186 ± 0.668 cd | 1.044 ± 0.116 ab | 18.672 ± 2.011 e | 3.933 ± 0.430 e | 5.172 ± 0.554 ab | 24.491 ± 2.542 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcicki, M.; Świder, O.; Choińska, R.; Bujak, M.; Sokołowska, B.; Szczepańska, M.; Bartosiak, E.; Roszko, M.Ł.; Juszczuk-Kubiak, E. New Isolated Autochthonous Strains of S. cerevisiae for Fermentation of Two Grape Varieties Grown in Poland. Appl. Sci. 2022, 12, 3483. https://doi.org/10.3390/app12073483
Wójcicki M, Świder O, Choińska R, Bujak M, Sokołowska B, Szczepańska M, Bartosiak E, Roszko MŁ, Juszczuk-Kubiak E. New Isolated Autochthonous Strains of S. cerevisiae for Fermentation of Two Grape Varieties Grown in Poland. Applied Sciences. 2022; 12(7):3483. https://doi.org/10.3390/app12073483
Chicago/Turabian StyleWójcicki, Michał, Olga Świder, Renata Choińska, Marzena Bujak, Barbara Sokołowska, Magdalena Szczepańska, Elżbieta Bartosiak, Marek Łukasz Roszko, and Edyta Juszczuk-Kubiak. 2022. "New Isolated Autochthonous Strains of S. cerevisiae for Fermentation of Two Grape Varieties Grown in Poland" Applied Sciences 12, no. 7: 3483. https://doi.org/10.3390/app12073483
APA StyleWójcicki, M., Świder, O., Choińska, R., Bujak, M., Sokołowska, B., Szczepańska, M., Bartosiak, E., Roszko, M. Ł., & Juszczuk-Kubiak, E. (2022). New Isolated Autochthonous Strains of S. cerevisiae for Fermentation of Two Grape Varieties Grown in Poland. Applied Sciences, 12(7), 3483. https://doi.org/10.3390/app12073483









