Widespread Ability of Ligninolytic Fungi to Degrade Hazardous Organic Pollutants as the Basis for the Self-Purification Ability of Natural Ecosystems and for Mycoremediation Technologies
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi and Cultivation Conditions
2.2. Chromatography
2.3. Spectrophotometric Analysis
2.4. Infrared Spectrometry
2.5. Enzymatic Analysis
2.6. Emulsifying Activity Measurements
2.7. Polysaccharide Concentration Measurements
2.8. Phytotoxicity Estimation
2.9. Chemicals
3. Results and Discussion
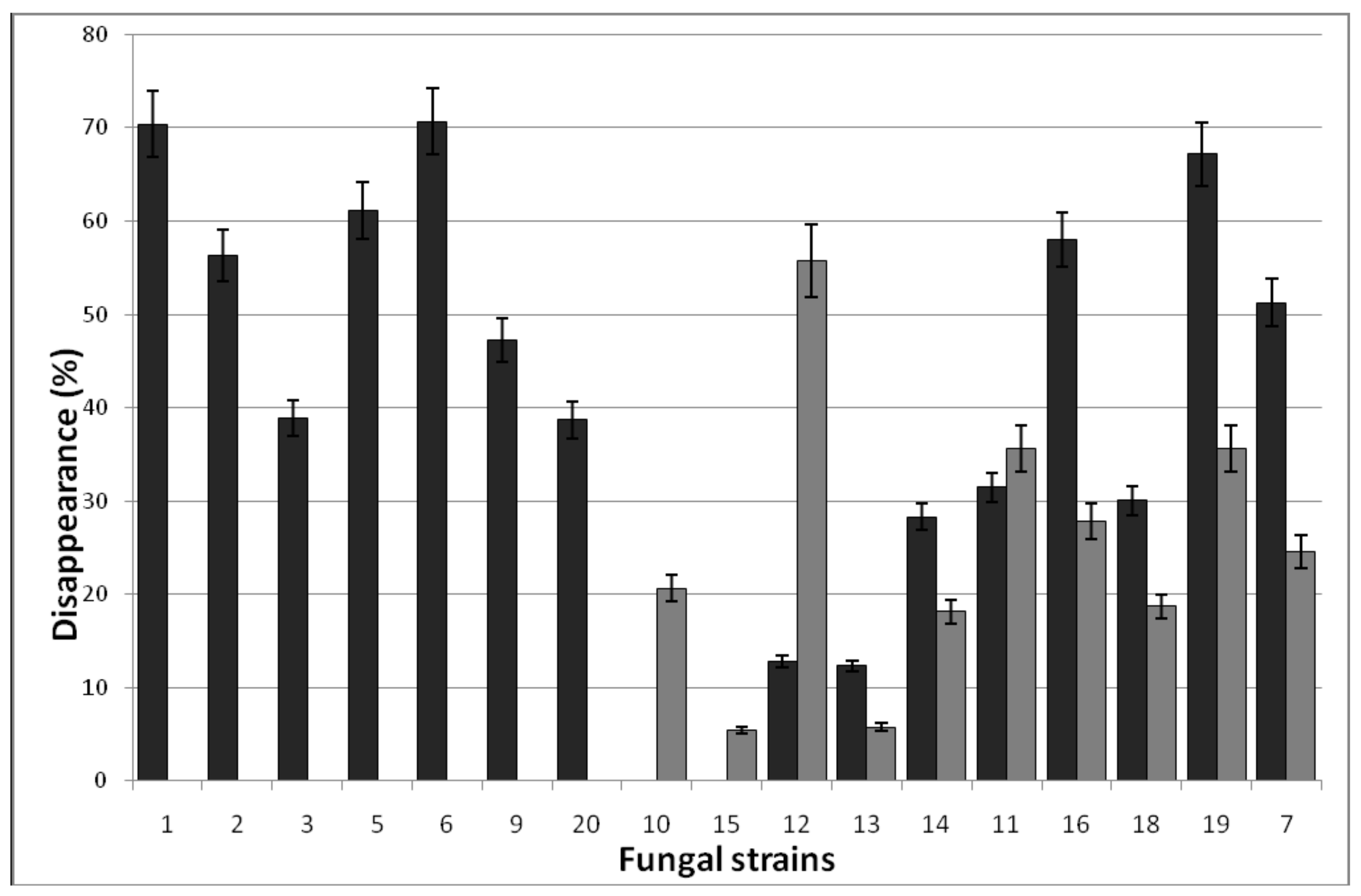
3.1. Pollutant Disappearance Experiments
3.1.1. Polycyclic Aromatic Hydrocarbons (PAHs)
3.1.2. Decolorization of Anthraquinone-Type Dyes
3.1.3. Oil Disappearance
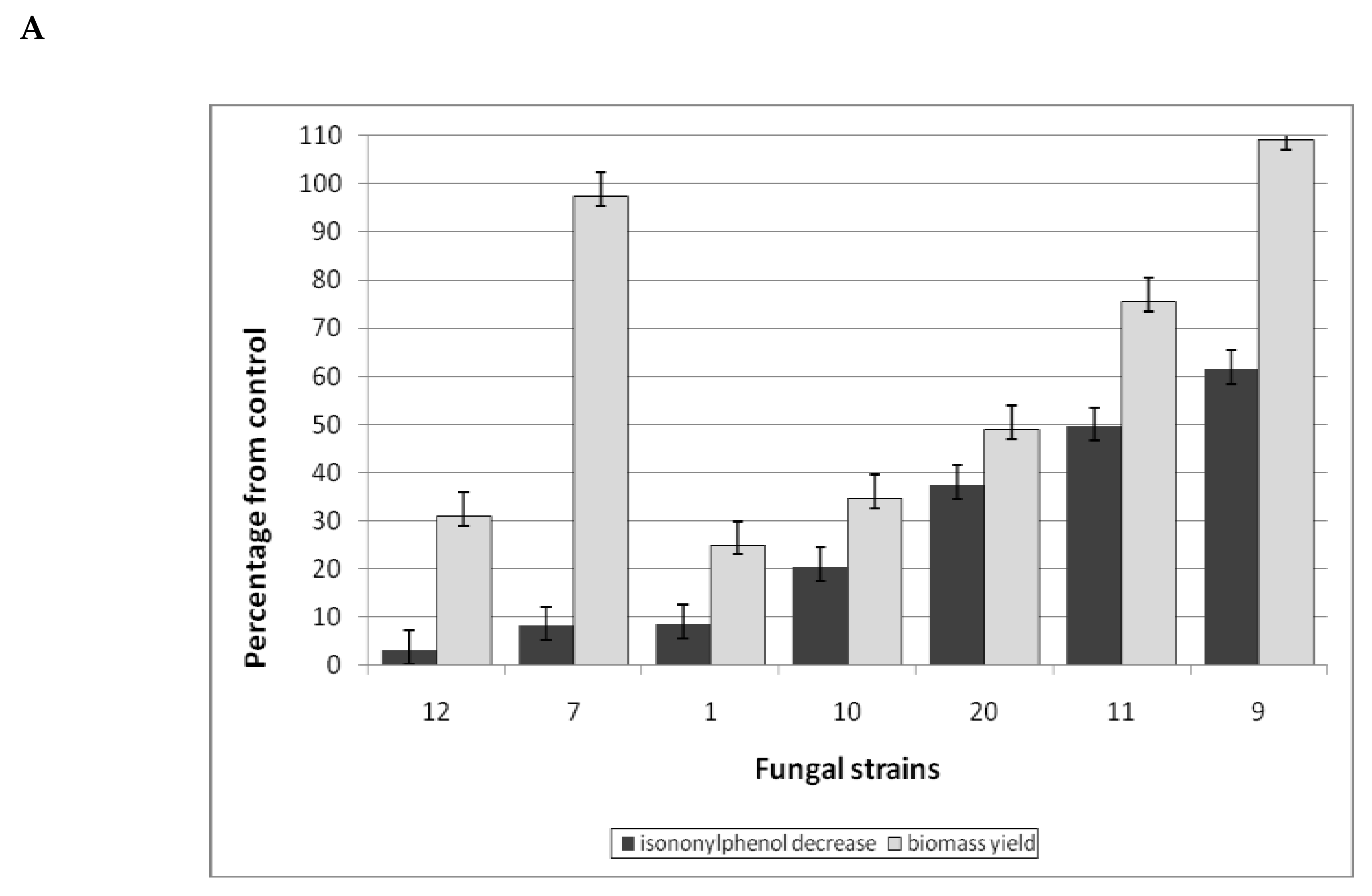
3.1.4. Surfactant Degradation
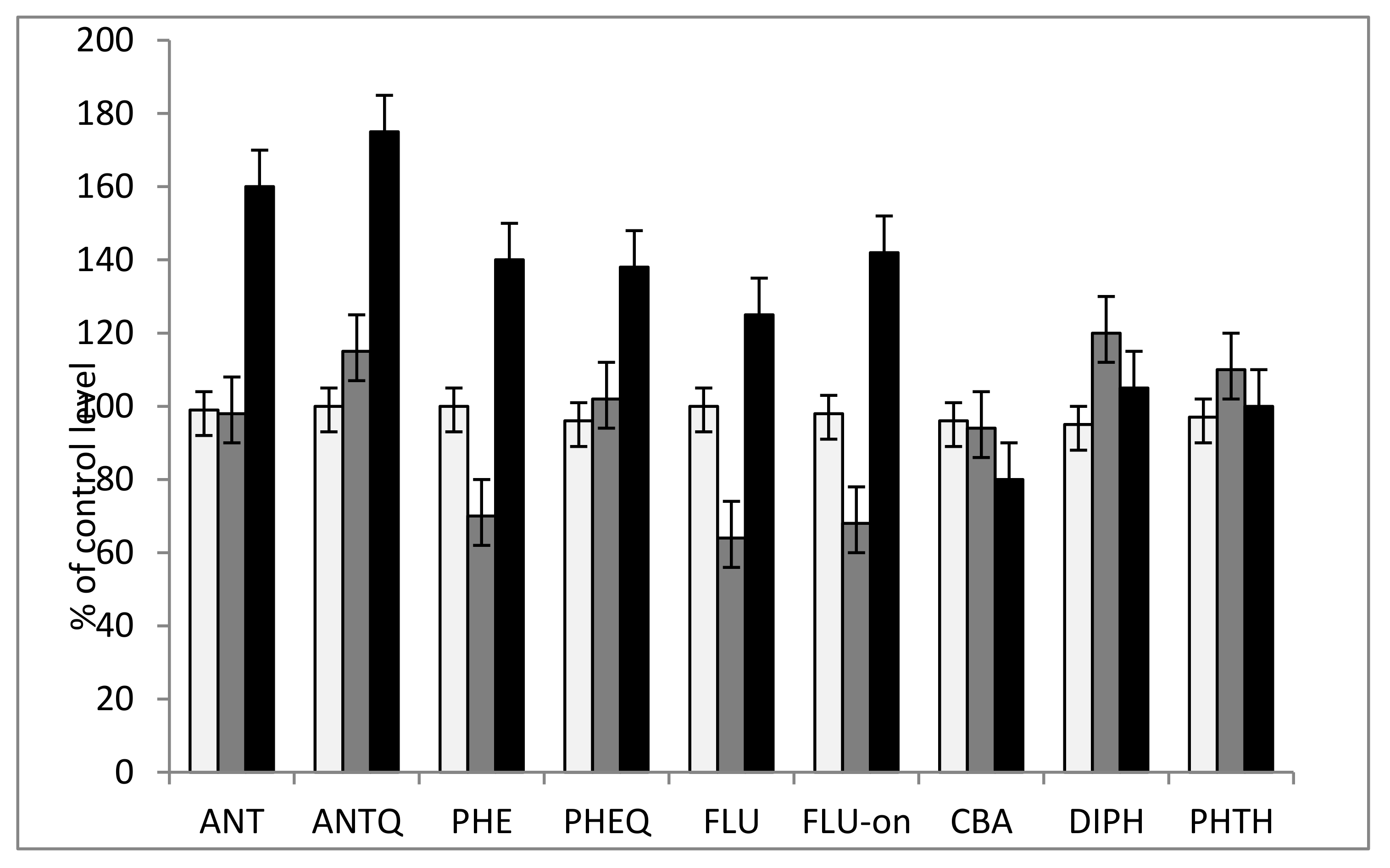
3.2. The Study of the Phytotoxicity of Parent Compounds and Metabolites Revealed
3.3. Study of Adaptive Properties of Fungi
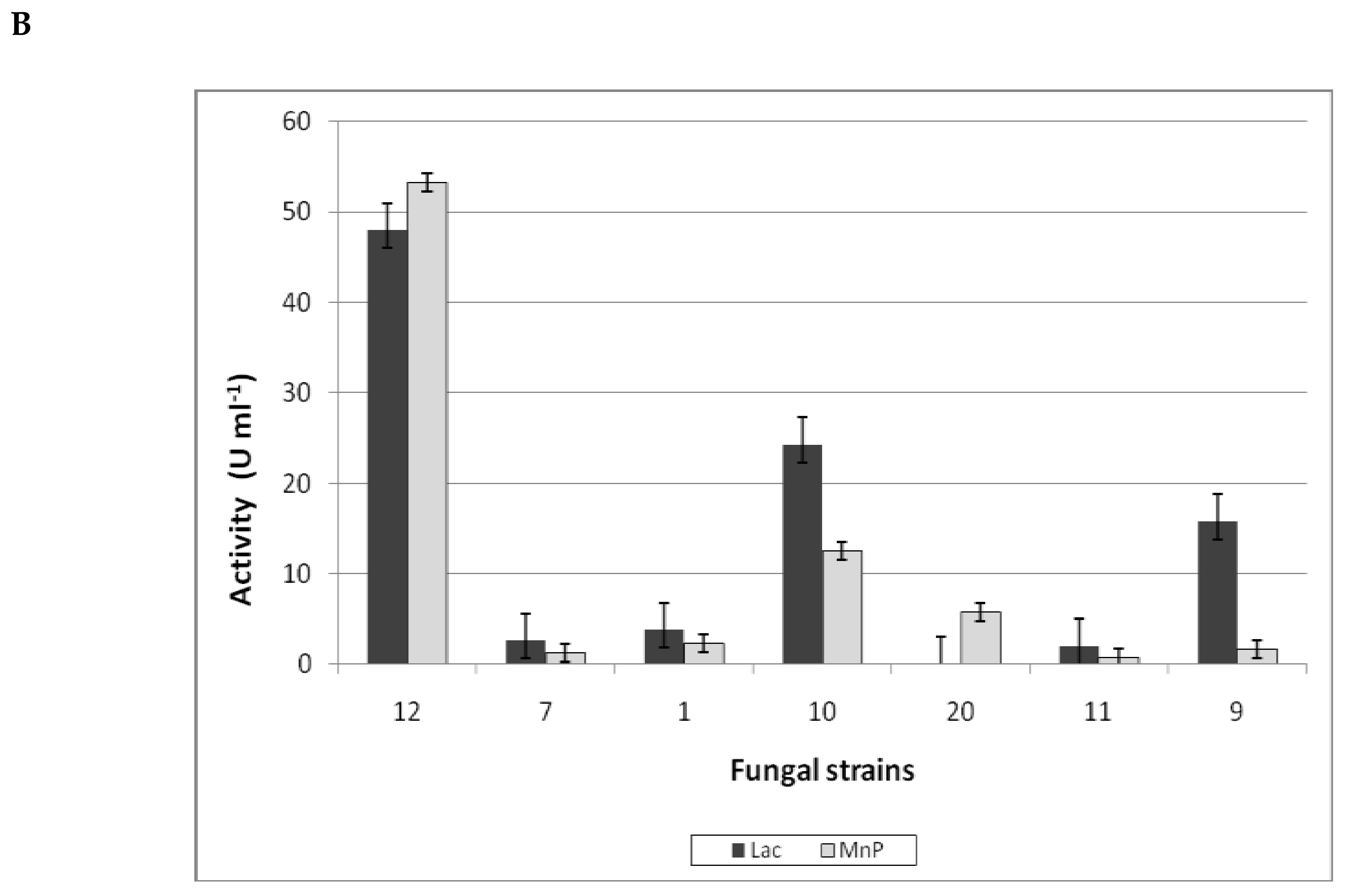
3.3.1. Ligninolytic Enzymes Production
3.3.2. Emulsifying Compounds Production
3.3.3. Extracellular Polysaccharides Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
Abbreviations
| PAHs | polycyclic aromatic hydrocarbons |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt |
| DMP | 2,6-dimethoxyphenol |
References
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Treu, R.; Falandysz, J. Mycoremediation of hydrocarbons with basidiomycetes—A review. J. Environ. Sci. Health Part B 2017, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, G.; Vagge, G.; Cutroneo, L.; Greco, G.; Di Piazza, S.; Faga, M.; Zotti, M.; Capello, M. Fungi as potential tool for polluted port sediment remediation. Environ. Sci. Pollut. Res. 2019, 26, 35602–35609. [Google Scholar] [CrossRef] [PubMed]
- Noman, E.; Al-Gheethi, A.; Mohamed, R.M.S.R.; Atalip, B. Myco-Remediation of Xenobiotic Organic Compounds for a Sustainable Environment: A Critical Review. Top. Curr. Chem. 2019, 377, 17. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Mannan, M.A.-U. Mycoremediation: Expunging environmental pollutants. Biotechnol. Rep. 2020, 26, e00452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-remediation: A mechanistic understanding of contaminants alleviation from natural environment and future prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef]
- Wong, D.W.S. Structure and Action Mechanism of Ligninolytic Enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef]
- Pozdnyakova, N.N. Involvement of the Ligninolytic System of White-Rot and Litter-Decomposing Fungi in the Degradation of Polycyclic Aromatic Hydrocarbons. Biotechnol. Res. Int. 2012, 2012, 1–20. [Google Scholar] [CrossRef]
- Wang, X.; Yao, B.; Su, X. Linking Enzymatic Oxidative Degradation of Lignin to Organics Detoxification. Int. J. Mol. Sci. 2018, 19, 3373. [Google Scholar] [CrossRef]
- Yateem, A.; Balba, M.; Al-Awadhi, N.; El-Nawawy, A. White rot fungi and their role in remediating oil-contaminated soil. Environ. Int. 1998, 24, 181–187. [Google Scholar] [CrossRef]
- Ishikhuemhen, O.; Anoliefo, G.; Oghale, O. Bioremediation of crude oil polluted soil by the white rot fungus Pleurotus tuber-reqium (Fr.) Sing. Environ. Sci. Pollut. Res. 2003, 10, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ogbo, E.; Okhuoya, J. Biodegradation of aliphatic, aromatic, resinic and asphaltic fractions of crude oil contaminated soils by Pleurotus tuber-regium Fr. Singer—A white rot fungus. Afr. J. Biotechnol. 2008, 4, 4291–4297. [Google Scholar]
- Okerentugba, P.O.; Orji, F.; Ibiene, A.A.; Elemo, G.N. Spend mushroom compost for bioremediation of petroleum hydrocarbon polluted soil: A review. Glob. Adv. Res. J. Environ. Sci. Toxicol. 2015, 4, 001–007. [Google Scholar]
- Mohammadi-Sichani, M.M.; Assadi, M.M.; Farazmand, A.; Kianirad, M.; Ahadi, A.M.; Ghahderijani, H.H. Bioremediation of soil contaminated crude oil by Agaricomycetes. J. Environ. Health Sci. Eng. 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Grasso, D.; Subramaniam, K.; Pignatello, J.; Yang, Y.; Ratté, D. Micellar desorption of polynuclear aromatic hydrocarbons from contaminated soil. Colloids Surf. A Physicochem. Eng. Asp. 2001, 194, 65–74. [Google Scholar] [CrossRef]
- Sartoros, C.; Yerushalmi, L.; Béron, P.; Guiot, S.R. Effects of surfactant and temperature on biotransformation kinetics of anthracene and pyrene. Chemosphere 2005, 61, 1042–1050. [Google Scholar] [CrossRef]
- Ying, G.-G.; Williams, B.; Kookana, R. Environmental fate of alkylphenols and alkylphenol ethoxylates—A review. Environ. Int. 2002, 28, 215–226. [Google Scholar] [CrossRef]
- Subramanian, V.; Yadav, J.S. Role of P450 Monooxygenases in the Degradation of the Endocrine-Disrupting Chemical Nonylphenol by the White Rot Fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 2009, 75, 5570–5580. [Google Scholar] [CrossRef]
- Baboshin, M.A.; Golovleva, L.A. Aerobic bacterial degradation of polycyclic aromatic hydrocarbons (PAHs) and its kinetic aspects. Microbiology 2012, 81, 639–650. [Google Scholar] [CrossRef]
- Song, H.-G. Comparison of pyrene biodegradation by white rot fungi. World J. Microbiol. Biotechnol. 1999, 15, 669–672. [Google Scholar] [CrossRef]
- Arun, A.; Raja, P.P.; Arthi, R.; Ananthi, M.; Kumar, K.S.; Eyini, M. Polycyclic aromatic hydrocarbons (PAHs) biodegradation by basidiomycetes fungi, pseudomonas isolate, and their cocultures: Comparative in vivo and in silico approach. Appl. Biochem. Biotechnol. 2008, 151, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Arun, A.; Eyini, M. Comparative studies on lignin and polycyclic aromatic hydrocarbons degradation by basidiomycetes fungi. Bioresour. Technol. 2011, 102, 8063–8070. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Delgado, C.; Alfaro-Barta, I.; Eymar, У. Combination of biochar amendment and mycoremediation for polycyclic aromatic hydrocarbons immobilization and biodegradation in creosote-contaminated soil. J. Hazard. Mater. 2015, 285, 259–266. [Google Scholar] [CrossRef]
- Bezalel, L.; Hadar, Y.; Cerniglia, C.E. Enzymatic Mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 1997, 63, 2495–2501. [Google Scholar] [CrossRef]
- Kirk, T.; Croan, S.; Tien, M.; Murtagh, K.E.; Farrell, R.L. Production of multiple ligninases by Phanerochaete chrysosporium: Effect of selected growth conditions and use of a mutant strain. Enzym. Microb. Technol. 1986, 8, 27–32. [Google Scholar] [CrossRef]
- Polunina, A.G.; Kushik, G.I. Metody analiza organicheskogo veshchestva porod, nefti i gaza (Methods of Analyslis of Organic Matter in Rocks, Oil, and Gas). Tyumen’ Tr. Zap.-Sib. NIGNI 1977, 122, 6–7. (In Russian) [Google Scholar]
- Klimenko, N.A.; Panchenko, N.P. Opredelenie nPAV v stochnih vodah (Determination of non-ionic surfactants in waster waters). Text. Ind. 1971, 2, 85–86. (In Russian) [Google Scholar]
- Wang, P.; Nong, X.-H.; Ge, J.-H. Aerobic biodegradation of nonylphenol ethoxylates in shaking-flask test. Electron. J. Biotechnol. 2011, 14, 1. [Google Scholar] [CrossRef]
- Pozdnyakova, N.N.; Jarosz-Wilkolazka, A.; Polak, J.; Graz, M.; Turkovskaya, O.V. Decolourisation of anthraquinone-and anthracene-type dyes by versatile peroxidases from Bjerkandera fumosa and Pleurotus ostreatus D1. Biocatal. Biotransform. 2015, 33, 69–80. [Google Scholar] [CrossRef]
- Niku-Paavola, M.; Karhunen, E.; Salola, P.; Raunio, V. Ligninolytic enzymes of the white rot fungus Phlebia radiate. Biochem. J. 1988, 254, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Heinfling, A.; Martinez, M.; Martinez, A.; Bergbauer, M.; Szewzyk, U. Purification and characterization of peroxidases from dye-decolorizing fungus Bjerkandera adusta. FEMS Microbiol. Lett. 1998, 16, 543–550. [Google Scholar]
- Tien, M.; Kirk, K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Nat. Acad. Sci. USA 1984, 81, 2280–2284. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacillus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.B. A note on the use of dinitrosalicylic acid for determining the products of enzymatic reactions. Appl. Microbiol. Biotechnol. 1988, 29, 494–496. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zeng, J.; Lin, X.; Zhang, J.; Zhu, H.; Chen, H.; Wong, M.H. Successive transformation of benzo[a]pyrene by laccase of Trametes versicolor and pyrene-degrading Mycobacterium strains. Appl. Microbiol. Biotechnol. 2012, 97, 3183–3194. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Steffen, K.T.; Schubert, S.; Tuomela, M.; Hatakka, A.; Hofrichter, M. Enhancement of bioconversion of high-molecular mass polycyclic aromatic hydrocarbons in contaminated non-sterile soil by litter-decomposing fungi. Biodegradation 2006, 18, 359–369. [Google Scholar] [CrossRef]
- Kabiersch, G.; Rajasärkkä, J.; Ullrich, R.; Tuomela, M.; Hofrichter, M.; Virta, M.; Hatakka, A.; Steffen, K. Fate of bisphenol A during treatment with the litter-decomposing fungi Stropharia rugosoannulata and Stropharia coronilla. Chemosphere 2011, 83, 226–232. [Google Scholar] [CrossRef]
- Fariba, M.; Simin, N.; Alireza, M.; Ramin, N.; Doustmorad, Z.; Gholam, K.; Abdolkarim, C. Phytoremediation of petroleum-polluted soils: Application of Polygonum aviculare and its root-associated (penetrated) fungal strains for bioremediation of petroleum-polluted soils. Ecotoxicol. Environ. Saf. 2010, 73, 613–619. [Google Scholar]
- Jacques, R.J.; Okeke, B.C.; Bento, F.M.; Teixeira, A.S.; Peralba, M.C.; Camargo, F.A. Microbial consortium bioaugmentation of a polycyclic aromatic hydrocarbons contaminated soil. Bioresour. Technol. 2008, 99, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ishikawa, K.; Hirai, M.; Shoda, M. Characteristics of a newly isolated fungus, Geotrichum candidum Dec 1, which decolorizes various dyes. J. Ferment. Bioeng. 1995, 79, 601–607. [Google Scholar] [CrossRef]
- Potin, O.; Veignie, E.; Rafin, C. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by Cladosporium sphaerospermum isolated from an aged PAH contaminated soil. FEMS Microbiol. Ecol. 2004, 51, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Serrano, C.; Pires, T.; Mestrinho, E.; Dias, L.; Teixeira, D.M.; Caldeira, A.T. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci. Total Environ. 2012, 435–436, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Vroumsia, T.; Steiman, R.; Seigle-Murandi, F.; Benoit-Guyod, J.-L. Effects of culture parameters on the degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4-dichlorophenol (2,4-DCP) by selected fungi. Chemosphere 1999, 39, 1397–1405. [Google Scholar] [CrossRef]
- Krivobok, S.; Miriouchkine, E.; Seigle-Murandi, F.; Benoit-Guyod, J.-L. Biodegradation of Anthracene by soil fungi. Chemosphere 1998, 37, 523–530. [Google Scholar] [CrossRef]
- Pozdnyakova, N.N.; Varese, G.C.; Prigione, V.; Dubrovskaya, E.V.; Balandina, S.A.; Turkovskaya, O.V. Degradative properties of two newly isolated strains of the ascomycetes Fusarium oxysporum and Lecanicillium aphanocladii. Int. Microbiol. 2019, 22, 103–110. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Gramss, G.; Mascher, К. Mutual influence of soil basidiomycetes and white mustard plants on their enzymatic and catabolic activities. J. Basic Microbiol. 2011, 51, 40–51. [Google Scholar] [CrossRef]
- Hammel, K.; Green, B.; Gai, W.Z. Ring fission of anthracene by a eukaryote. Proc. Natl. Acad. Sci. USA 1991, 88, 10605–10608. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Guo, J.; Zeng, G.; Zhong, X.; Sun, G. Decolourization of anthraquinone dye by Shewanella decolorationis S12. Appl. Microbiol. Biotechnol. 2006, 71, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Eichlerová, I.; Homolka, L.; Benada, O.; Kofroňová, O.; Hubálek, T.; Nerud, F. Decolorization of Orange G and Remazol Brilliant Blue R by the white rot fungus Dichomitus squalens: Toxicological evaluation and morphological study. Chemosphere 2007, 69, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y.; Matsushima, Y.; Shoda, M. Complete decolourization of the anthraquinone dye Reactive blue 5 by the concerted action of two peroxidases from Thanatephorus cucumeris Dec1. Appl. Microbiol. Biotechnol. 2006, 73, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Peroxidase mediated decolourization and remediation of wastewater containing industrial dyes: A review. Rev. Environ. Sci. Biotechnol. 2010, 9, 117–140. [Google Scholar] [CrossRef]
- Liu, W.; Chao, Y.; Yang, X.; Bao, H.; Qian, S. Biodecolourization of azo, anthraquinonic and triphenylmethane dyes by white rot fungi and laccase-secreting engineered strain. J. Ind. Microbiol. Biotechnol. 2004, 31, 127–132. [Google Scholar] [CrossRef]
- Ogbo, E.; Tabuanu, A.; Ubebe, R. Phytotoxicity assay of diesel fuel-spiked substrates remediated with Pleurotus tuber-regium using Zea mays. Int. J. Appl. Res. Nat. Prod. 2010, 3, 12–16. [Google Scholar]
- Bovio, E.; Gnavi, G.; Prigione, V.; Spina, F.; Denaro, R.; Yakimov, M.; Calogero, R.; Crisafi, F.; Varese, G.C. The culturable mycobiota of a Mediterranean marine site after an oil spill: Isolation, identification and potential application in bioremediation. Sci. Total Environ. 2017, 576, 310–318. [Google Scholar] [CrossRef]
- Márquez-Rocha, F.J.; Hernández-Rodríguez, V.Z.; Vazquez-Duhalt, R. Biodegradation of soil-adsorbed polycyclic aromatic hydrocarbons by the white rot fungus Pleurotus ostreatus. Biotechnol. Lett. 2000, 22, 469–472. [Google Scholar] [CrossRef]
- Bhattacharaya, S.; Angayarkanni, J.; Das, A.; Palaniswamy, M. Mycoremediation of benzo[a]pyrene by Pleurotus ostreatus isolated from Wayanad district in Kerala, India. Int. J. Pharm. Bio. Sci. 2012, 2, 84–93. [Google Scholar]
- Zheng, Z.; Obbard, J.P. Polycyclic Aromatic Hydrocarbon Removal from Soil by Surfactant Solubilization and Phanerochaete chrysosporium Oxidation. J. Environ. Qual. 2002, 31, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-S.; Song, H.-G. Degradation of Alkylphenols by White Rot Fungus Irpex lacteus and Its Manganese Peroxidase. Appl. Biochem. Biotechnol. 2012, 168, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Zakupra, V.A. Methods of analysis and control in the production of surface-active substances. M. Chem. 1977, 368. (In Russian) [Google Scholar]
- Perov, P.A.; Glukhova, L.Y.; Markova, E.I.; Kudasova, E.I.; Chernih, V.A. IR, NMR and Raman Spectra of Surfactants, Raw Materials and Preparations Based on Them: Catalog.; TsNIITEneftekhim: Moscow, Russia, 1989; p. 233. (In Russian) [Google Scholar]
- Nakanishi, K. Infrared spectra and structure of organic compounds/K. Nakanishi. Per. English. M. Mir 1965, 216. (In Russian) [Google Scholar]
- Shipman, L. Ab Initio Quantum Mechanical Characterization of the Ground Electronic State of Benzo[a]pyrene. Implications for the Mechanism of Polynuclear Aromatic Hydrocarbon Oxidation to Epoxides by Cytochrome P450, Polynuclear Aromatic Hydrocarbons; Jones, P., Leber, P., Eds.; Michigan: Ann Arbor Science: Ann Arbor, MI, USA, 1979; Volume 3, pp. 139–143. [Google Scholar]
- Cerniglia, C. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv. Appl. Microbiol. 1984, 30, 31–71. [Google Scholar]
- McConkey, B.J.; Duxbury, C.L.; Dixon, D.G.; Greenberg, B.M. Toxicity of a pah photooxidation product to the bacteria photobacterium phosphoreum and the duckweed lemna gibba: Effects of phenanthrene and its primary photoproduct, phenanthrenequinone. Environ. Toxicol. Chem. 1997, 16, 892–899. [Google Scholar] [CrossRef]
- Baboshin, M.A.; Baskunov, B.P.; Finkel’shtein, Z.I.; Golovlev, E.L.; Golovleva, L.A. Microbial transformation of antracene and phenanthrene. Microbiol. Mosc. 2005, 74, 303–309. [Google Scholar] [CrossRef]
- Muratova, A.; Pozdnyakova, N.; Makarov, O.; Baboshin, M.; Baskunov, B.; Myasoedova, N.; Golovleva, L.; Turkovskaya, O. Degradation of phenanthrene by the rhizobacterium Ensifer meliloti. Biodegradation 2014, 25, 787–795. [Google Scholar] [CrossRef]
- Kireeva, N.A.; Kuzyakhmetov, G.G.; Miftakhova, A.M.; Vodop’yanov, V.V. Fitotoksichnost’ antropogenno zagryaznennykh pochv (Phytotoxicity of Anthropogenically Contaminated Soils). Ufa Gilem 2003, 113. (In Russian) [Google Scholar]
- Šašek, V.; Cajthaml, T.; Bhatt, M. Use of fungal technology in soil remediation: A Case Study. Water Air Soil Pollut. Focus 2003, 3, 5–14. [Google Scholar] [CrossRef]
- Muratova, A.; Pozdnyakova, N.; Golubev, S.; Wittenmayer, L.; Makarov, O.; Merbach, W.; Turkovskaya, O. Oxidoreductase activity of sorghum root exudates in a phenanthrene-contaminated environment. Chemosphere 2009, 74, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shoda, M. Purification and Characterization of a Novel Peroxidase from Geotrichum candidum Dec 1 Involved in Decolorization of Dyes. Appl. Environ. Microbiol. 1999, 65, 1029–1035. [Google Scholar] [CrossRef]
- Cajthaml, T. Biodegradation of endocrine-disrupting compounds by ligninolytic fungi: Mechanisms involved in the degradation. Environ. Microbiol. 2015, 17, 4822–4834. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.N.; Nikiforova, S.V.; Makarov, O.E.; Chernyshova, M.P.; Pankin, K.E.; Turkovskaya, O.V. Influence of cultivation conditions on pyrene degradation by the fungus Pleurotus Ostreatus D1. World J. Microbiol. Biotechnol. 2009, 26, 205–211. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Dubrovskaya, E.; Chernyshova, M.; Makarov, O.; Golubev, S.; Balandina, S.; Turkovskaya, O. The degradation of three-ringed polycyclic aromatic hydrocarbons by wood-inhabiting fungus Pleurotus ostreatus and soil-inhabiting fungus Agaricus bisporus. Fungal Biol. 2018, 122, 363–372. [Google Scholar] [CrossRef]
- Pozdnyakova, N.N.; Chernyshova, M.; Grinev, V.S.; Landesman, E.O.; Koroleva, O.V.; Turkovskaya, O.V. Degradation of fluorene and fluoranthene by the basidiomycete Pleurotus ostreatus. Appl. Biochem. Microbiol. 2016, 52, 621–628. [Google Scholar] [CrossRef]
- Nikiforova, S.V.; Pozdnyakova, N.N.; Turkovskaya, O.V. Emulsifying Agent Production During PAHs Degradation by the White Rot Fungus Pleurotus Ostreatus D1. Curr. Microbiol. 2009, 58, 554–558. [Google Scholar] [CrossRef]
- Bhardwaj, S.S.C.A.H.K.C.G. Biosurfactants from Fungi: A Review. J. Pet. Environ. Biotechnol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Veignie, E.; Vinogradov, E.; Sadovskaya, I.; Coulon, C.; Rafin, C. Preliminary Characterizations of a Carbohydrate from the Concentrated Culture Filtrate from Fusarium solani and Its Role in Benzo[a]Pyrene Solubilization. Adv. Microbiol. 2012, 2, 375–381. [Google Scholar] [CrossRef][Green Version]
- Seviour, R.J.; McNeil, B.; Fazenda, M.; Harvey, L.M. Operating bioreactors for microbial exopolysaccharide production. Crit. Rev. Biotechnol. 2010, 31, 170–185. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Martínez, M.; Almendros, G.; Gonzalez-Vila, F.J.; Martínez, A.T. Hyphal-sheath polysaccharides in fungal deterioration. Sci. Total Environ. 1995, 167, 315–328. [Google Scholar] [CrossRef][Green Version]
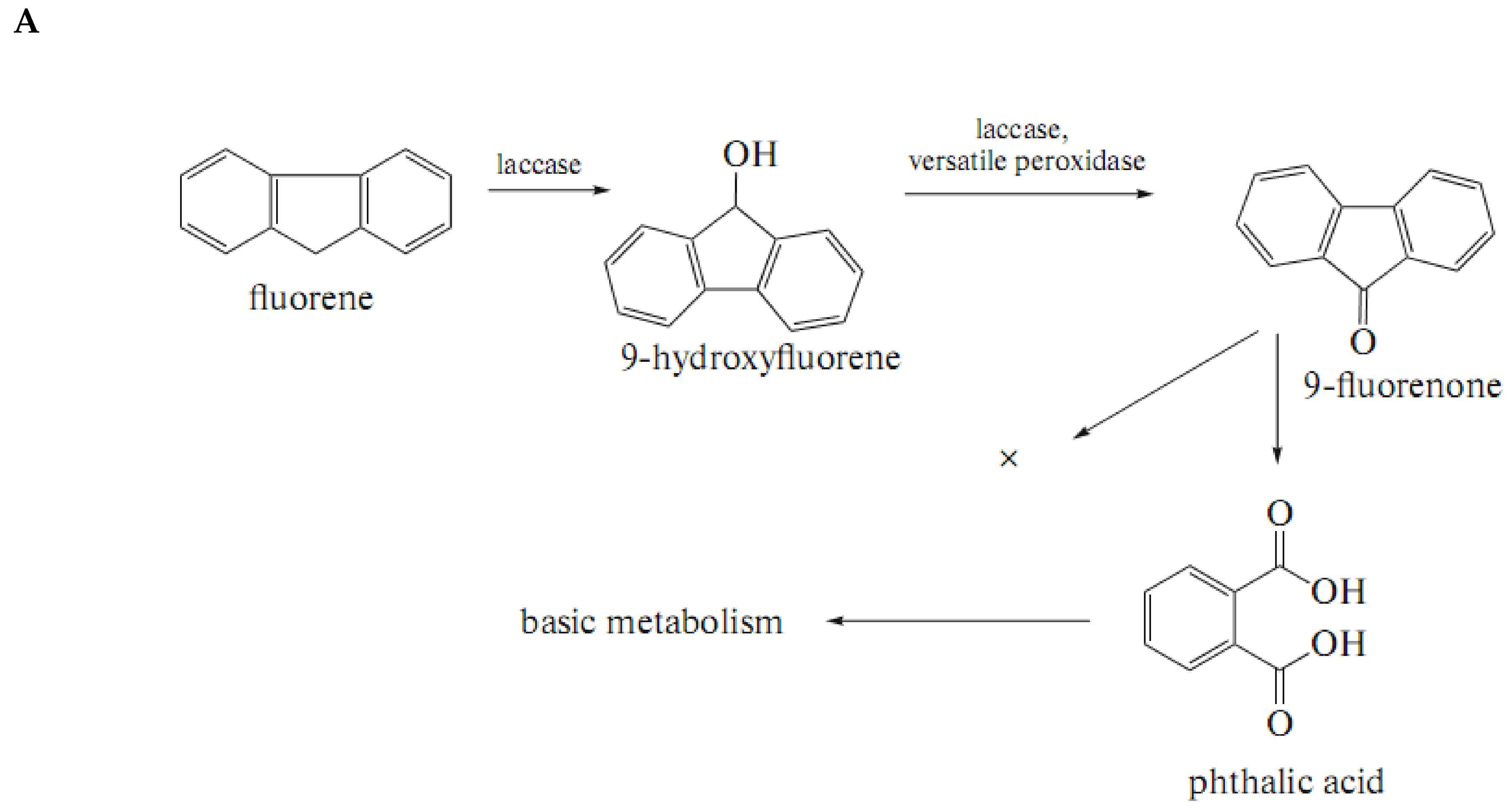
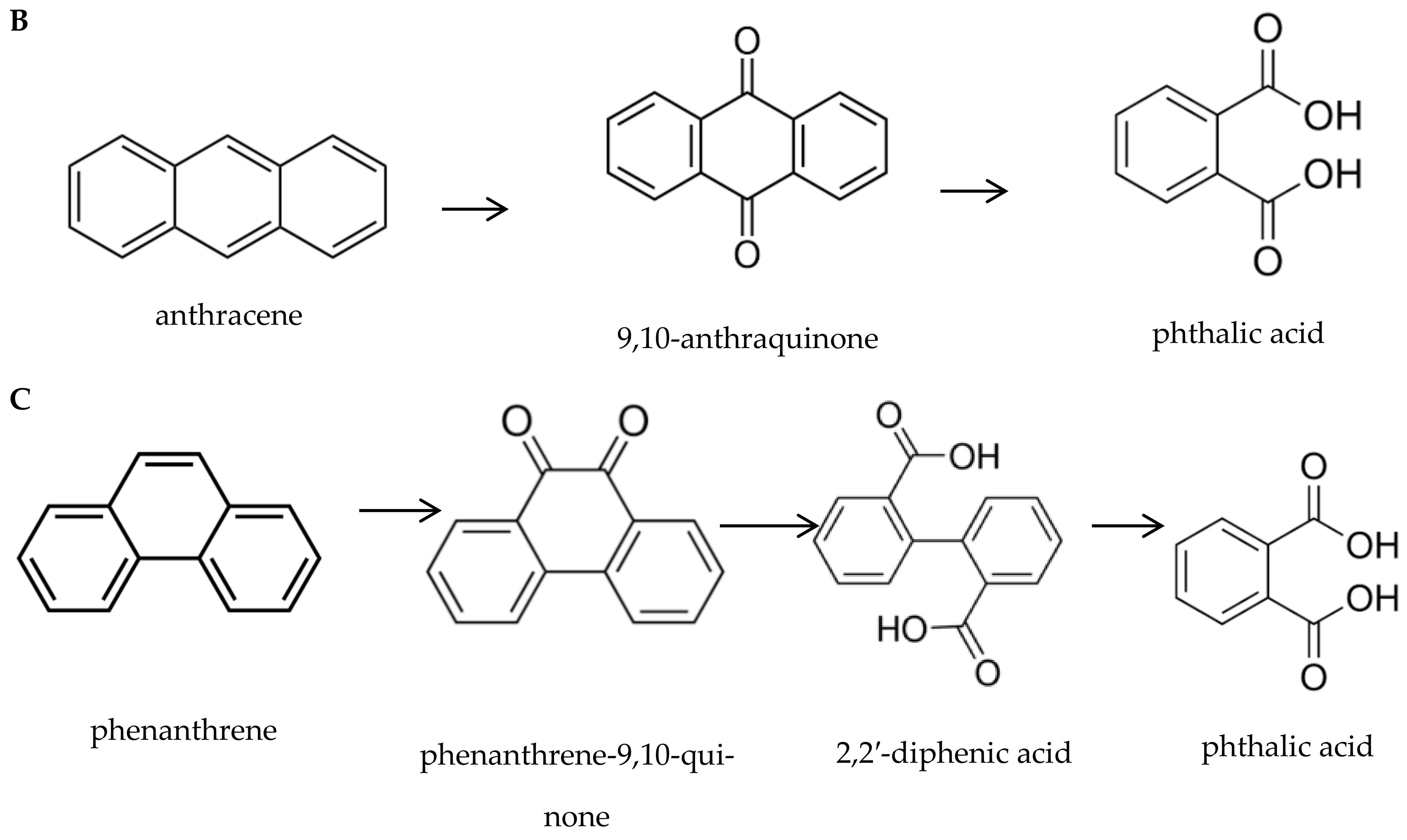
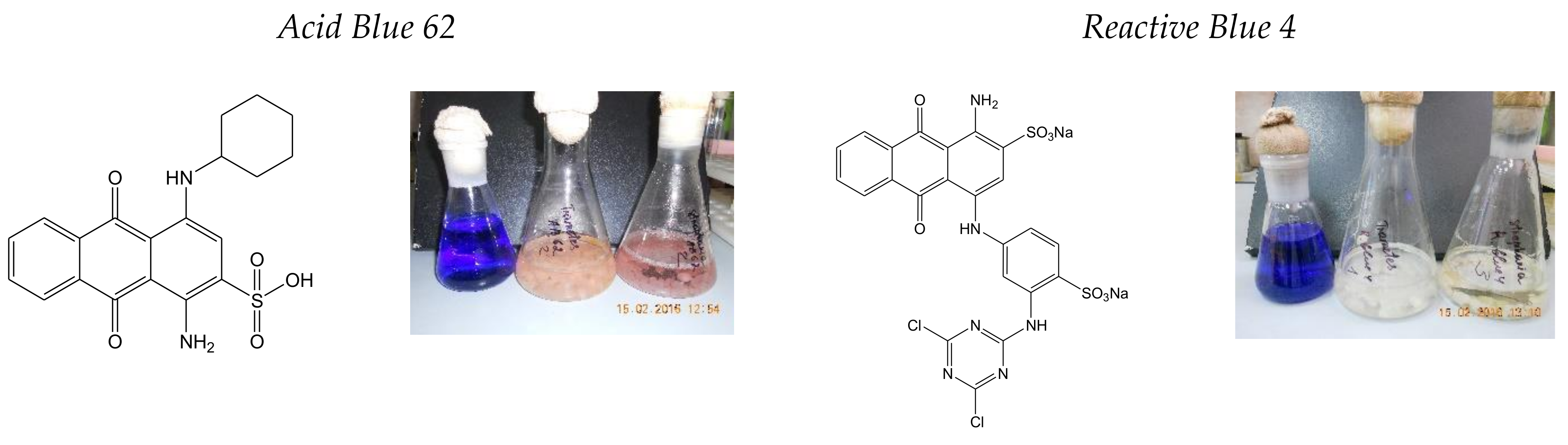
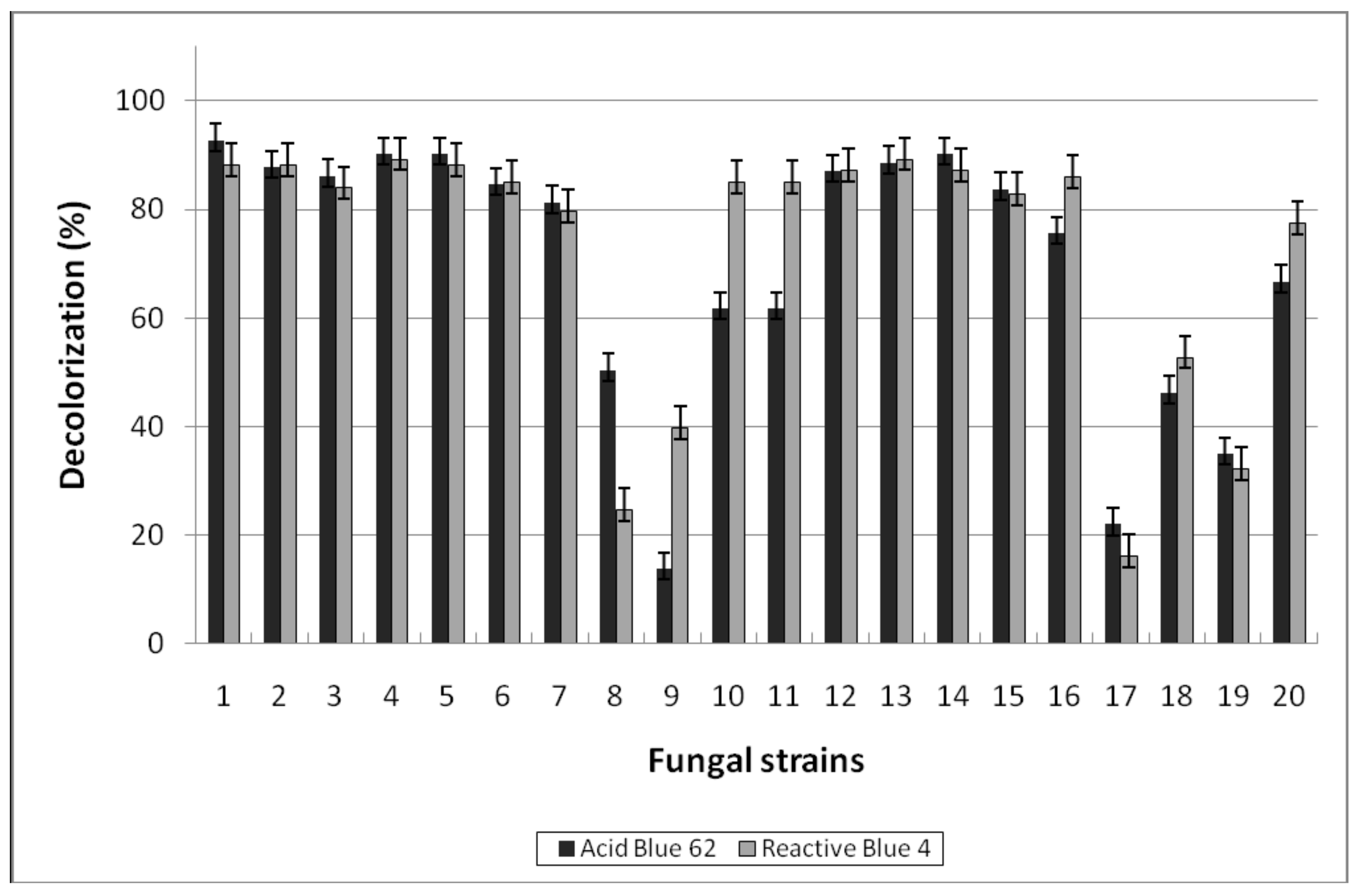

| № | Fungi | PAH Disappearance (%) | ||
|---|---|---|---|---|
| ANT | PHEN | FLU | ||
| 1 | Bjerkandera adusta MUT 3398 | 61.6 ± 3.0 | 82.8 ± 4.8 | 96.2 ± 7.2 |
| 2 | Cladosporium herbarum MUT 3238 | 94.1 ± 1.0 | 95.4 ± 2.5 | 98.0 ± 2.4 |
| 3 | Fusarium oxysporum IBPPM 543 | 0 ± 2.3 | 20.8 ± 1.2 | 41.9 ± 3.5 |
| 4 | Geotrichum candidum MUT4803 | 0 ± 3.0 | 17.7 ± 1.3 | 16.8 ± 7.1 |
| 5 | Lecanicillium aphanocladii IBPPM 542 | 39.8 ± 12.1 | 62.8 ± 2.8 | 81.3 ± 3.0 |
| 6 | Lenzites betulina LE-BIN 2047 | 89.3 ± 8.6 | 74.4 ± 18.8 | 99.2 ± 5.7 |
| 7 | Pleurotus ostreatus f. Florida IBPPM 540 | 50.9 ± 5.7 | 70.8 ± 2.1 | 93.9 ± 5.4 |
| 8 | Pleurotus ostreatus MUT2977 | 92.0 ± 3.2 | 71.2 ± 13.7 | 93.0 ± 1.3 |
| 9 | Pleurotus ostreatus 336 | 56.7 ± 1.8 | 71.3 ± 1.6 | 86.6 ± 2.9 |
| 10 | Pleurotus ostreatus D1 | 85.3 ± 4.2 | 100 ± 6.8 | 100 ± 5.3 |
| 11 | Pleurotus ostreatus LE-BIN0432 | 36.7 ± 11.8 | 79.3 ± 15.5 | 94.8 ± 18.9 |
| 12 | Schizophyllum commune IBPPM 541 | 49.5 ± 2.6 | 39.8 ± 8.5 | 74.2 ± 9.4 |
| 13 | Steccherinum murashkinskyi LE-BIN 1963 | 71.8 ± 2.6 | 76.1 ± 7.2 | 91.5 ± 6.5 |
| 14 | Stropharia rugosoannulata DSM11372 | 49.5 ± 3.1 | 55.2 ± 4.9 | 72.2 ± 7.0 |
| 15 | Trametes gibbosa LE-BIN 1911 | 17.0 ± 6.3 | 45.3 ± 7.2 | 51.8 ± 18.8 |
| 16 | Trametes hirsuta LE-BIN 072 | 37.8 ± 2.6 | 59.6 ± 4.4 | 90.4 ± 2.5 |
| 17 | Trametes maxima LE-BIN 0275 | 84.6 ± 2.1 | 78.8 ± 15.6 | 95.4 ± 22.9 |
| 18 | Trametes ochracea LE-BIN 093 | 16.2 ± 5.6 | 48.5 ± 19.8 | 85.2 ± 22.1 |
| 19 | Trametes versicolor DSM11372 | 57.1 ± 7.8 | 79.8 ± 2.7 | 96.8 ± 7.1 |
| 20 | Trametes versicolor MUT3403 | 86.4 ± 13.2 | 60.9 ± 8.6 | 87.8 ± 2.8 |
| Fungal Strains | Activity, U/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (without Pollutant) | Pollutants | ||||||||
| ANT | PHEN | FLU | Neonol | INP | AB62 | RB4 | Oil | ||
| B. adusta: LiP | 0 | 42.3 | 9.5 | 37.2 | 49.9 | 94.4 | 0 | 0 | 46.8 |
| MnP | 0 | 3.2 | 3.4 | 21.5 | 318.2 | 135.0 | 8.0 | 15.0 | 58.3 |
| C. herbarum: PerOx | 0 | 12.3 | 10.4 | 12.2 | 13.1 | 5.8 | 4.7 | 2.0 | 11.2 |
| F. oxysporum: PerOx | 0 | 0.9 | 3.8 | 0.5 | 7.4 | 0.2 | 82.4 | 18.1 | 4.8 |
| G. candidum: PerOx | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L. aphanocladii: PerOx | 0 | 12.4 | 7.6 | 12.2 | 7.8 | 8.0 | 8.0 | 3.8 | 6.2 |
| L. betulina: Lac | 4.8 | 5.3 | 1.0 | 2.4 | 3.1 | 2.9 | 2.2 | 1.0 | 15.4 |
| MnP | 3.7 | 21.2 | 22.5 | 21.4 | 7.5 | 1.3 | 12.6 | 4.8 | 14.4 |
| P. ostreatus Florida: Lac | 49.9 | 7.3 | 46.2 | 16.9 | 336.4 | 3.8 | 18.8 | 8.4 | 86.4 |
| MnP | 242.5 | 38.4 | 78.8 | 14.2 | 741.0 | 2.3 | 5.1 | 5.0 | 136.8 |
| P. ostreatus MUT2977: | |||||||||
| Lac | 40.1 | 24.9 | 288.5 | 158.2 | 33.3 | 1.4 | 23.4 | 3.6 | 24.0 |
| MnP | 392.6 | 118.5 | 494.9 | 291.7 | 167.0 | 4.6 | 5.4 | 1.8 | 44.0 |
| P. ostreatus 336: Lac | 98.9 | 33.2 | 3.0 | 0.6 | 3.5 | 1.1 | 73.6 | 7.1 | 56.7 |
| MnP | 145.6 | 25.9 | 7.1 | 12.2 | 11.5 | 1.2 | 5.1 | 3.6 | 217.9 |
| P. ostreatus D1: Lac | 49.1 | 30.1 | 40.1 | 18.0 | 52.3 | 6.8 | 55.2 | 7.2 | 29.5 |
| MnP | 403.0 | 112.1 | 456.9 | 355.8 | 283.0 | 17.5 | 68.8 | 10.0 | 139.4 |
| P. ostreatus LE-BIN0432: | |||||||||
| Lac | 64.0 | 16.9 | 2.7 | 1.5 | 34.0 | 3.0 | 88.0 | 5.8 | 18.4 |
| MnP | 450.1 | 57.0 | 26.2 | 24.0 | 8.2 | 3.0 | 5.1 | 2.7 | 2.5 |
| S. commune: Lac | 26.8 | 11.1 | 13.2 | 15.9 | 15.4 | 5.0 | 8.8 | 7.9 | 14.2 |
| MnP | 86.3 | 3.0 | 27.5 | 3.4 | 1.6 | 0.4 | 20.8 | 12.3 | 1.0 |
| LiP | 12.5 | ||||||||
| S. murashkinskyi: Lac | 10.1 | 13.6 | 44.0 | 18.0 | 215.1 | 31.8 | 12.2 | 15.6 | 24.0 |
| MnP | 12.8 | 14.2 | 16.6 | 22.3 | 249.7 | 35.7 | 18.3 | 18.8 | 29.1 |
| S. rugosoannulata: Lac | 2.1 | 5.1 | 4.8 | 2.7 | 34.7 | 11.7 | 15.5 | 13.2 | 8.7 |
| MnP | 7.2 | 1.3 | 2.5 | 1.0 | 104.0 | 43.7 | 17.3 | 14.7 | 23.6 |
| LiP | 58.0 | ||||||||
| T. gibbosa: Lac | 23.3 | 26.4 | 26.5 | 24.8 | 39.0 | 3.1 | 20.0 | 22.3 | 99.5 |
| MnP | 48.4 | 61.5 | 70.7 | 41.2 | 7.5 | 0.6 | 14.9 | 9.6 | 17.5 |
| T. hirsuta: Lac | 32.2 | 18.5 | 13.7 | 26.3 | 174.8 | 9.8 | 39.0 | 4.0 | 98.5 |
| MnP | 56.6 | 49.0 | 110.8 | 199.3 | 202.0 | 14.6 | 7.5 | 0.6 | 17.7 |
| T. maxima: Lac | 15.5 | 13.7 | 3.1 | 6.6 | 308.9 | 26.6 | 13.9 | 4.2 | 12.2 |
| MnP | 16.1 | 14.4 | 6.5 | 31.5 | 72.3 | 6.9 | 42.5 | 14.2 | 18.6 |
| T. ochracea: Lac | 9.3 | 7.2 | 7.1 | 6.0 | 394.1 | 31.3 | 9.4 | 10.5 | 4.8 |
| MnP | 50.1 | 166.3 | 48.3 | 32.2 | 50.4 | 6.3 | 5.5 | 2.8 | 3.2 |
| T. versicolor DSM11372: | |||||||||
| Lac | 3.4 | 126.0 | 141.6 | 73.7 | 1.9 | 2.0 | 35.5 | 4.0 | 8.2 |
| MnP | 24.1 | 9.6 | 15.3 | 10.4 | 17.5 | 0.8 | 17.5 | 1.2 | 18.3 |
| LiP | 16.6 | 23.7 | |||||||
| T. versicolor MUT3403: | |||||||||
| Lac | 76.7 | 103.4 | 17.6 | 164.0 | 32.0 | 48.0 | 32.1 | 48.1 | 49.5 |
| MnP | 47.9 | 128.5 | 64.1 | 86.4 | 149.5 | 53.2 | 14.9 | 5.3 | 13.2 |
| Fungi | Emulsifying Activity (E48, %)  | ||||
|---|---|---|---|---|---|
| ANT | PHEN | FLU | INP | oil | |
| B. adusta MUT 3398 | 21.8 ± 3.4 | 28.2 ± 3.2 | 34.0 ± 3.1 | 30.4 ± 13.0 | 7,6 ± 1,3 |
| C. herbarum MUT 3238 | 34.0 ± 5.5 | 54.6 ± 13. | 24.2 ± 1.3 | 13.2 ± 3.3 | 32.3 ± 3.6 |
| F. oxysporum IBPPM 543 | 47.1 ± 2.3 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 27.0 ± 3,4 |
| G. candidum MUT4803 | 0 ± 0 | 34,3 ± 5,7 | 0 ± 0 | 54,2 ± 7,7 | 6,5 ± 3,8 |
| Lec. aphanocladii IBPPM 542 | 19.3 ± 5.0 | 12.5 ± 3.9 | 6.2 ± 2.8 | 0 ± 0 | 18.2 ± 3.7 |
| Len. betulina LE-BIN 2047 | 69.8 ± 12.3 | 21.9 ± 1.3 | 38.2 ± 4.9 | 47.5 ± 8.4 | 60 ± 5.5 |
| P. ostreatus f. Florida IBPPM 540 | 65.7 ± 6.9 | 38.1 ± 3.4 | 0.7 ± 0.2 | 26.7 ± 3.6 | 30.1 ± 6.8 |
| P. ostreatus MUT 2977 | 3.0 ± 5.2 | 0 ± 0 | 5.0 ± 8.7 | 12.3 ± 4.9 | 23.2 ± 3.0 |
| P. ostreatus 336 | 21.6 ± 3.7 | 21.1 ± 2.5 | 22.4 ± 4.3 | 18.6 ± 2.9 | 27.1 ± 3.3 |
| P. ostreatus D1 | 3.5 ± 1.0 | 2.0 ± 0.4 | 2.5 ± 0.2 | 26.1 ± 1.8 | 38.2 ± 5.6 |
| P. ostreatus LE-BIN 0432 | 21.3 ± 3.0 | 27.7 ± 2.5 | 32.4 ± 1.3 | 28.1 ± 1.9 | 37.1 ± 3.8 |
| Sch. commune IBPPM 541 | 12.4 ± 3.4 | 3.3 ± 0.3 | 23.3 ± 1.4 | 28.4 ± 1.9 | 29.1 ± 2.3 |
| St. murashkinskyi LE-BIN 1963 | 42.0 ± 1.0 | 27.0 ± 3.4 | 38.7 ± 4.2 | 19.6 ± 5.1 | 21.4 ± 4.1 |
| Str. rugosoannulata DSM11372 | 41.8 ± 1.9 | 38.7 ± 11.9 | 26.9 ± 3.8 | 9.8 ± 3.8 | 44.3 ± 12.7 |
| T. gibbosa LE-BIN 1911 | 17.8 ± 6.7 | 6.3 ± 3.8 | 2.0 ± 3.4 | 0 ± 0 | 52.3 ± 12.1 |
| T. hirsuta LE-BIN 072 | 34.2 ± 1.6 | 59.4 ± 4.4 | 21.2 ± 1.1 | 60.7 ± 0.6 | 35.2 ± 3.6 |
| T. maxima LE-BIN 0275 | 38.1 ± 2.3 | 49.3 ± 5.0 | 35.8 ± 1.9 | 40.1 ± 0.9 | 42.2 ± 1.8 |
| T. ochracea LE-BIN 093 | 22.4 ± 1.5 | 6.3 ± 2.8 | 0.4 ± 0.4 | 0 ± 0 | 52.6 ± 9.3 |
| T. versicolor DSM11269 | 12.0 ± 1.9 | 9.2 ± 2.2 | 15.4 ± 7.5 | 39.4 ± 2.3 | 21.7 ± 2.8 |
| T. versicolor MUT 3403 | 1.4 ± 2.5 | 11.6 ± 2.3 | 2.6 ± 2.3 | 19.5 ± 3.8 | 29.7 ± 3.6 |
| Fungi | Properties | ||||||
|---|---|---|---|---|---|---|---|
| Degradation | Lac/MnP/LiP | Emulsifying Compounds | |||||
| PAHs | Dyes | Neonol | INP | Oil | |||
| B. adusta MUT 3398 | + | + | + | + | |||
| C. herbarum MUT 3238 | + | + | + | + | + | ||
| F. oxysporum IBPPM 543 | + | + | |||||
| G. candidum MUT4803 | + | ||||||
| Lec. aphanocladii IBPPM 542 | + | + | + | ||||
| Len. betulina LE-BIN 2047 | + | + | + | + | |||
| P. ostreatus f. Florida IBPPM 540 | + | + | + | + | + | + | |
| P. ostreatus MUT2977 | + | + | + | + | |||
| P. ostreatus 336 | + | + | + | + | |||
| P. ostreatus D1 | + | + | + | + | |||
| P. ostreatus LE-BIN0432 | + | + | + | + | |||
| Sch. commune IBPPM 541 | + | + | + | + | + | ||
| St. murashkinskyi LE-BIN 1963 | + | + | + | ||||
| Str. rugosoannulata DSM11372 | + | + | + | + | + | ||
| T. gibbosa LE-BIN 1911 | + | + | |||||
| T. hirsuta LE-BIN 072 | + | + | + | ||||
| T. maxima LE-BIN 0275 | + | + | + | + | |||
| T. ochracea LE-BIN 093 | + | ||||||
| T. versicolor DSM11372 | + | + | + | + | + | + | |
| T. versicolor MUT3403 | + | + | + | + | + | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozdnyakova, N.; Dubrovskaya, E.; Schlosser, D.; Kuznetsova, S.; Sigida, E.; Grinev, V.; Golubev, S.; Kryuchkova, E.; Varese, G.C.; Turkovskaya, O. Widespread Ability of Ligninolytic Fungi to Degrade Hazardous Organic Pollutants as the Basis for the Self-Purification Ability of Natural Ecosystems and for Mycoremediation Technologies. Appl. Sci. 2022, 12, 2164. https://doi.org/10.3390/app12042164
Pozdnyakova N, Dubrovskaya E, Schlosser D, Kuznetsova S, Sigida E, Grinev V, Golubev S, Kryuchkova E, Varese GC, Turkovskaya O. Widespread Ability of Ligninolytic Fungi to Degrade Hazardous Organic Pollutants as the Basis for the Self-Purification Ability of Natural Ecosystems and for Mycoremediation Technologies. Applied Sciences. 2022; 12(4):2164. https://doi.org/10.3390/app12042164
Chicago/Turabian StylePozdnyakova, Natalia, Ekaterina Dubrovskaya, Dietmar Schlosser, Svetlana Kuznetsova, Elena Sigida, Vyacheslav Grinev, Sergei Golubev, Elena Kryuchkova, Giovanna Cristina Varese, and Olga Turkovskaya. 2022. "Widespread Ability of Ligninolytic Fungi to Degrade Hazardous Organic Pollutants as the Basis for the Self-Purification Ability of Natural Ecosystems and for Mycoremediation Technologies" Applied Sciences 12, no. 4: 2164. https://doi.org/10.3390/app12042164
APA StylePozdnyakova, N., Dubrovskaya, E., Schlosser, D., Kuznetsova, S., Sigida, E., Grinev, V., Golubev, S., Kryuchkova, E., Varese, G. C., & Turkovskaya, O. (2022). Widespread Ability of Ligninolytic Fungi to Degrade Hazardous Organic Pollutants as the Basis for the Self-Purification Ability of Natural Ecosystems and for Mycoremediation Technologies. Applied Sciences, 12(4), 2164. https://doi.org/10.3390/app12042164








