Pre-Disinfection of Poly-Methyl-Methacrylate (PMMA) Reduces Volatile Sulfides Compounds (VSC) Production in Experimental Biofilm In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Material
2.2. Predisinfection Protocol
2.3. Experimental Protocol
2.4. Malodor Scores
2.5. VSC Levels
2.6. Salivary Proteins Degradation
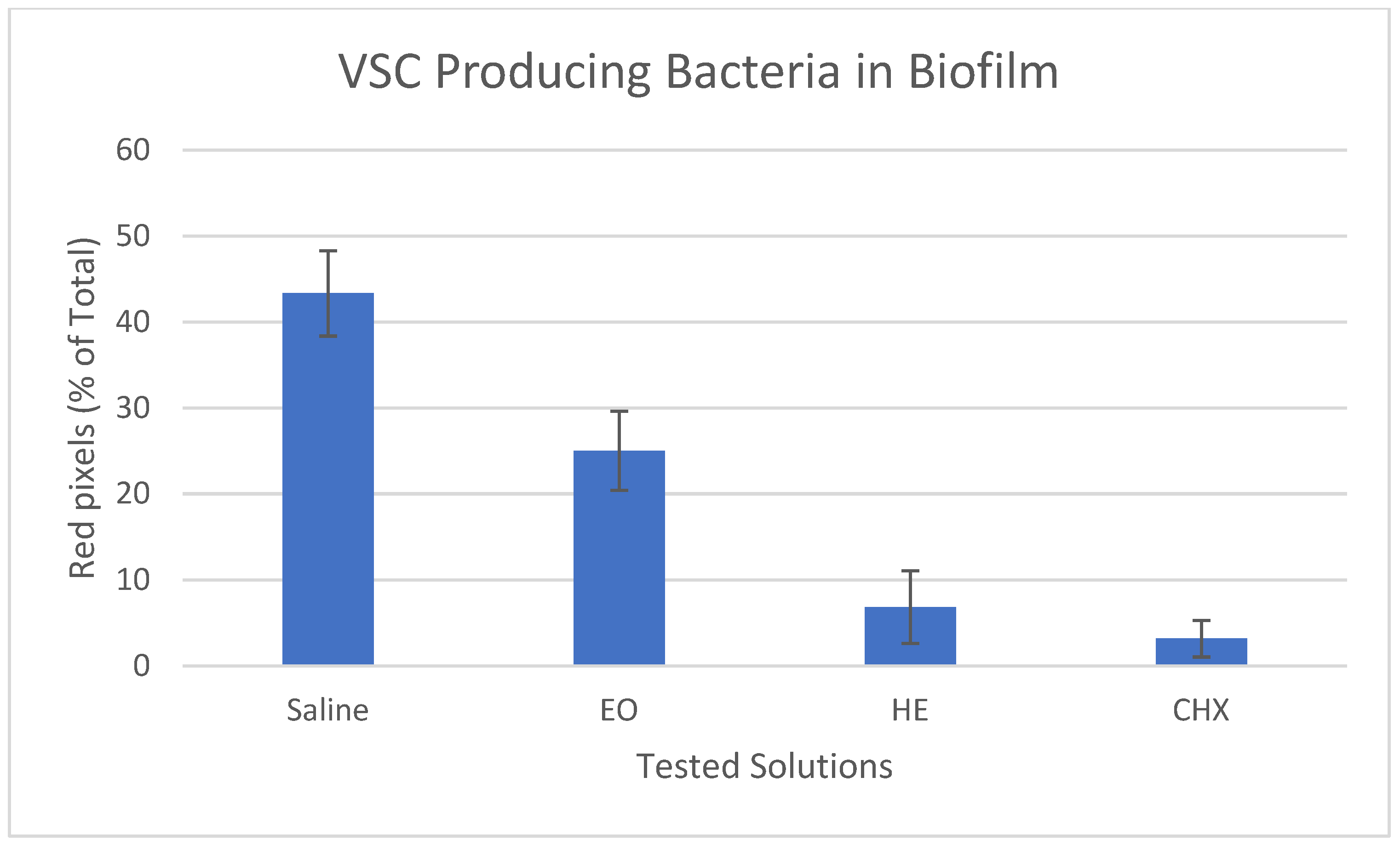
2.7. VSC-Producing Bacteria in Biofilm
2.8. Statistical Analysis
3. Results
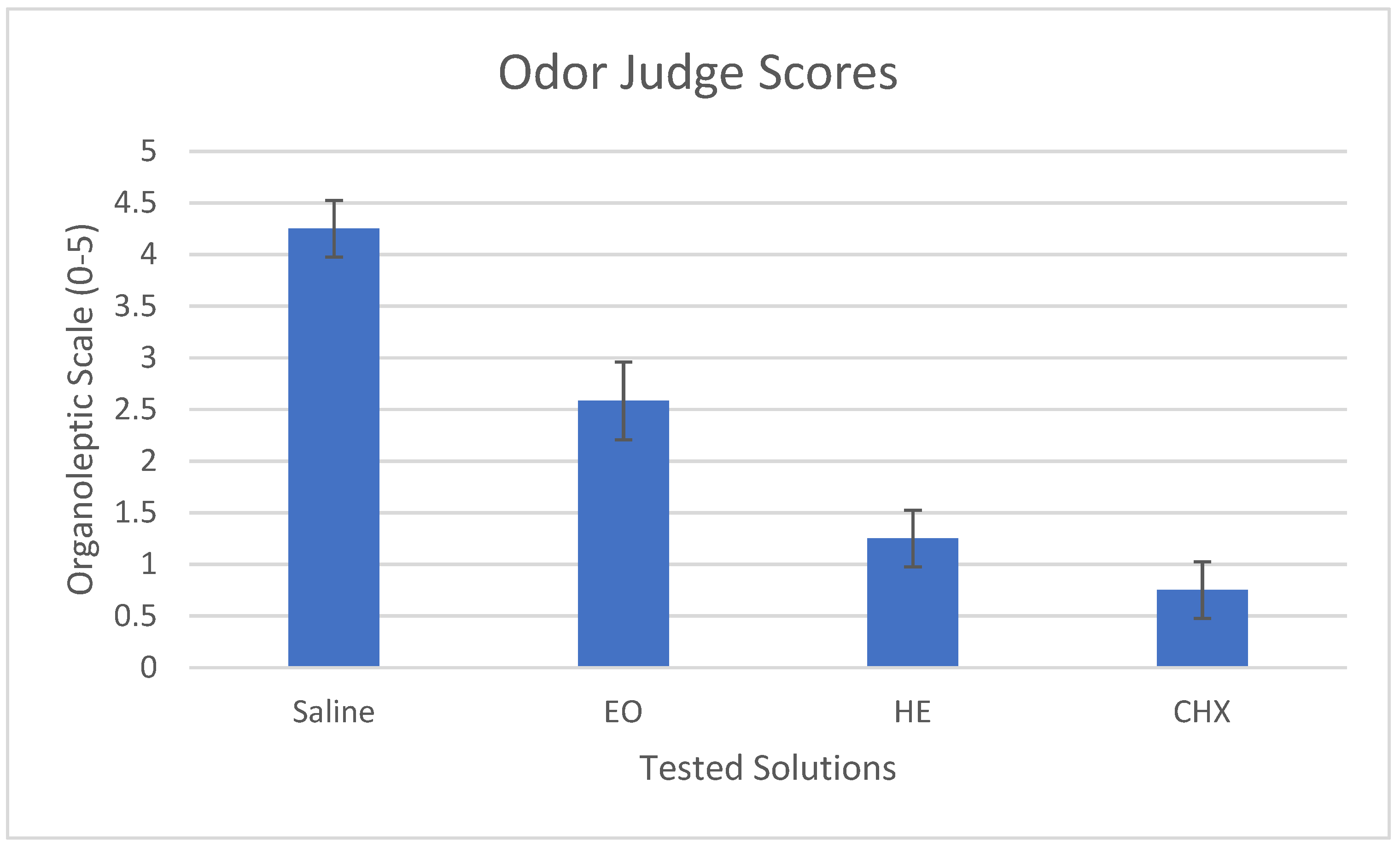
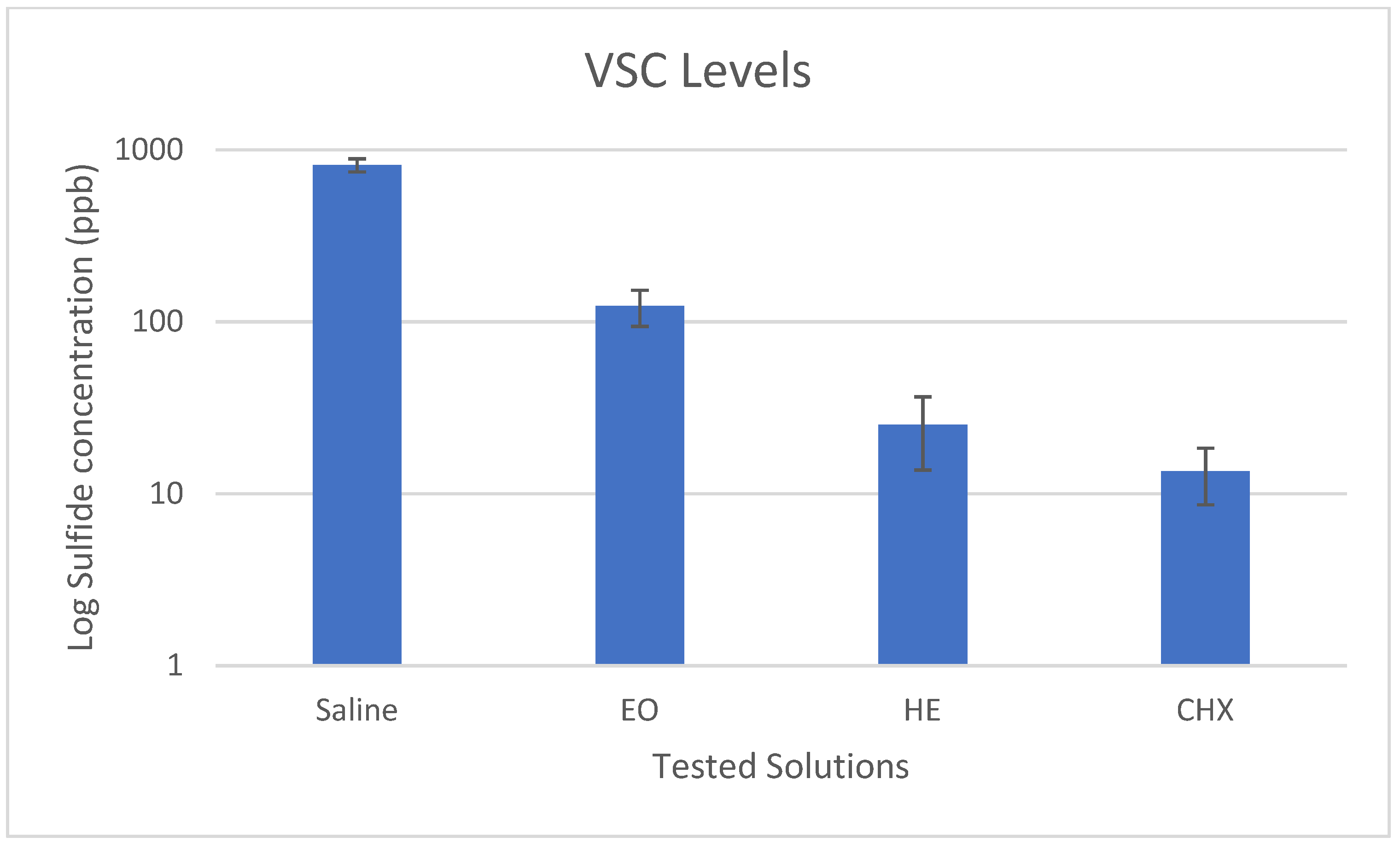
3.1. Malodor and VSC levels
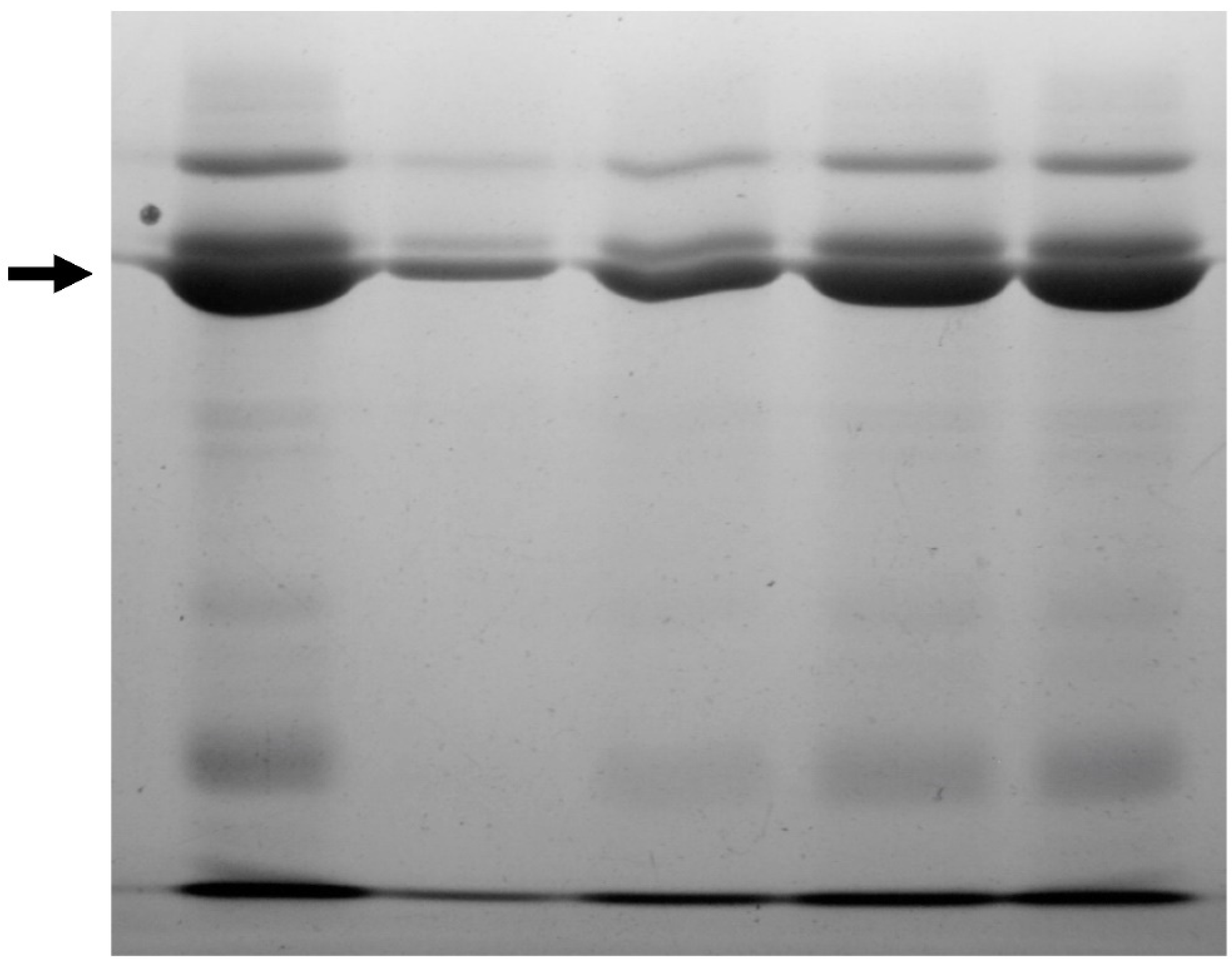
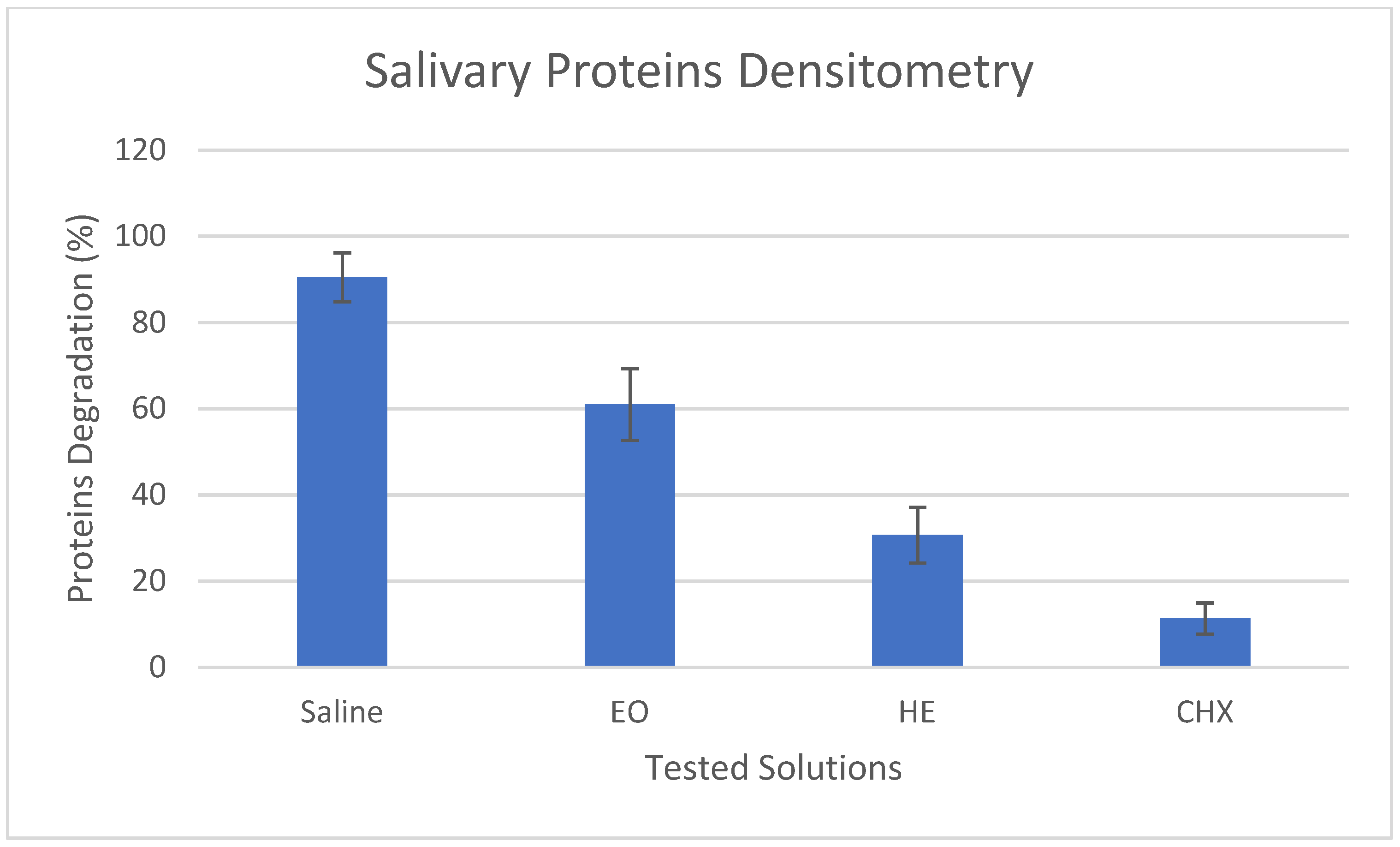
3.2. Salivary Proteins Degradation
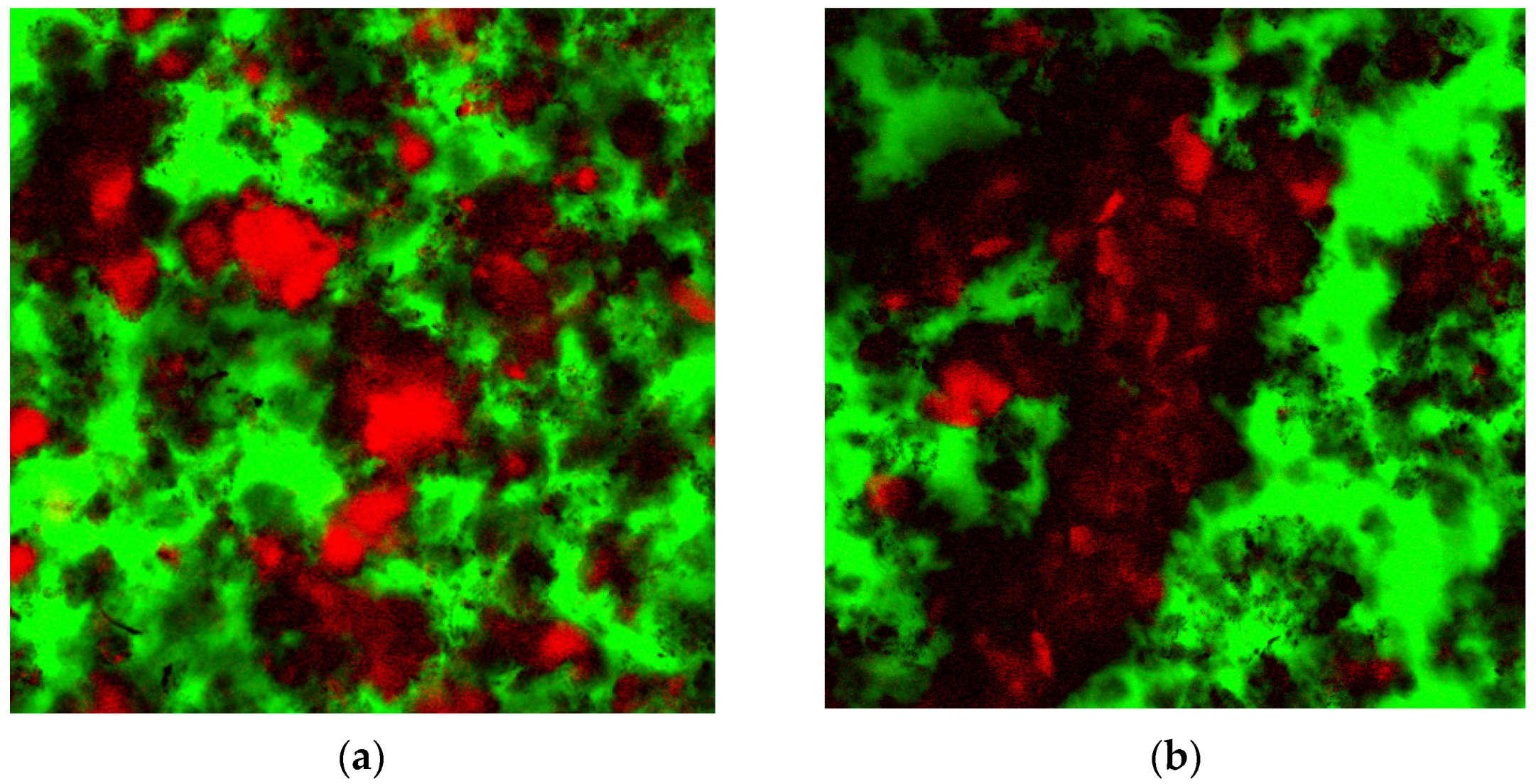
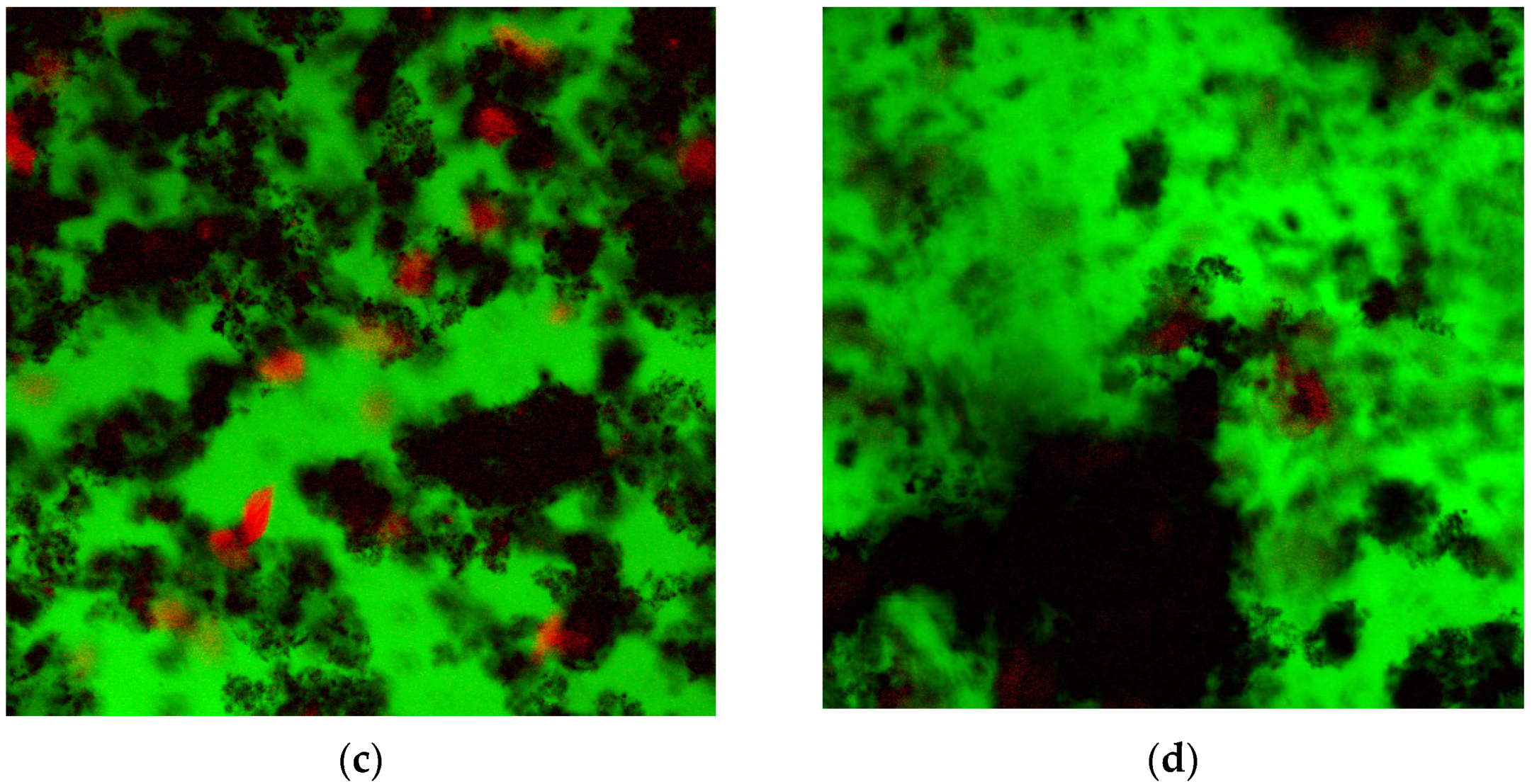
3.3. Confocal Laser Scanning Microscopy (CLSM)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seemann, R.; Conceicao, M.D.; Filippi, A.; Greenman, J.; Lenton, P.; Nachnani, S.; Quirynen, M.; Roldan, S.; Schulze, H.; Sterer, N.; et al. Halitosis management by the general dental practitioner--results of an international consensus workshop. J. Breath Res. 2014, 8, 017101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterer, N.; Shaharabany, M.; Rosenberg, M. β-Galactosidase activity and H(2)S production in an experimental oral biofilm. J. Breath Res. 2009, 3, 016006. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M.; Shahin, S.Y.; Alsaqer, B.T.; Ali, A.A. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: A new approach for denture stomatitis prevention. Int. J. Nanomed. 2017, 12, 5409–5419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N. Oral Microbiome Metabolism: From “Who Are They?” to “What Are They Doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Alzoman, H.; Rashid Habib, S.; Alghamdi, S.; Al-Juhani, H.; Daabash, R.; Al-Khalid, W.; Al-Askar, M.; Al-Johany, S. Relationship between Fixed Dental Crowns and Volatile Sulphur Compounds. Int. J. Environ. Res. Public Health 2021, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sterer, N.; Rubinstein, Y. Effect of various natural medicinals on salivary protein putrefaction and malodor production. Quintessence Int. 2006, 37, 653–658. [Google Scholar]
- Carvalho, M.D.; Tabchoury, C.M.; Cury, J.A.; Toledo, S.; Nogueira-Filho, G.R. Impact of mouthrinses on morning bad breath in healthy subjects. J. Clin. Periodontol. 2004, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Acharya, S.; Verma, E.; Singhal, D.; Singla, N. Efficacy of chlorhexidine, hydrogen peroxide and tulsi extract mouthwash in reducing halitosis using spectrophotometric analysis: A randomized controlled trial. J. Clin. Exp. Dent. 2019, 11, e457–e463. [Google Scholar] [CrossRef] [PubMed]
- Addy, M.; Mahdavi, S.A.; Loyn, T. Dietary staining in vitro by mouthrinses as a comparative measure of antiseptic activity and predictor of staining in vivo. J. Dent. 1995, 23, 95–99. [Google Scholar] [CrossRef]
- Van Strydonck, D.A.C.; Slot, D.E.; Van der Velden, U.; Van der Weijden, F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: A systematic review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosner, O.; Melamed, G.; Livne, S.; Jeffet, U.; Dolev, E.; Izhack, G.B.; Heller, H.; Sterer, N. Pre-Disinfection of Poly-Methyl-Methacrylate (PMMA) Reduces Volatile Sulfides Compounds (VSC) Production in Experimental Biofilm In Vitro. Appl. Sci. 2022, 12, 1947. https://doi.org/10.3390/app12041947
Rosner O, Melamed G, Livne S, Jeffet U, Dolev E, Izhack GB, Heller H, Sterer N. Pre-Disinfection of Poly-Methyl-Methacrylate (PMMA) Reduces Volatile Sulfides Compounds (VSC) Production in Experimental Biofilm In Vitro. Applied Sciences. 2022; 12(4):1947. https://doi.org/10.3390/app12041947
Chicago/Turabian StyleRosner, Ofir, Guy Melamed, Shiri Livne, Uziel Jeffet, Eran Dolev, Gil Ben Izhack, Hadas Heller, and Nir Sterer. 2022. "Pre-Disinfection of Poly-Methyl-Methacrylate (PMMA) Reduces Volatile Sulfides Compounds (VSC) Production in Experimental Biofilm In Vitro" Applied Sciences 12, no. 4: 1947. https://doi.org/10.3390/app12041947
APA StyleRosner, O., Melamed, G., Livne, S., Jeffet, U., Dolev, E., Izhack, G. B., Heller, H., & Sterer, N. (2022). Pre-Disinfection of Poly-Methyl-Methacrylate (PMMA) Reduces Volatile Sulfides Compounds (VSC) Production in Experimental Biofilm In Vitro. Applied Sciences, 12(4), 1947. https://doi.org/10.3390/app12041947





