Classification of Potato Varieties Drought Stress Tolerance Using Supervised Learning
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. The Pot Experiment
2.2. Plant Material
2.3. Modeling Methodology
3. Results
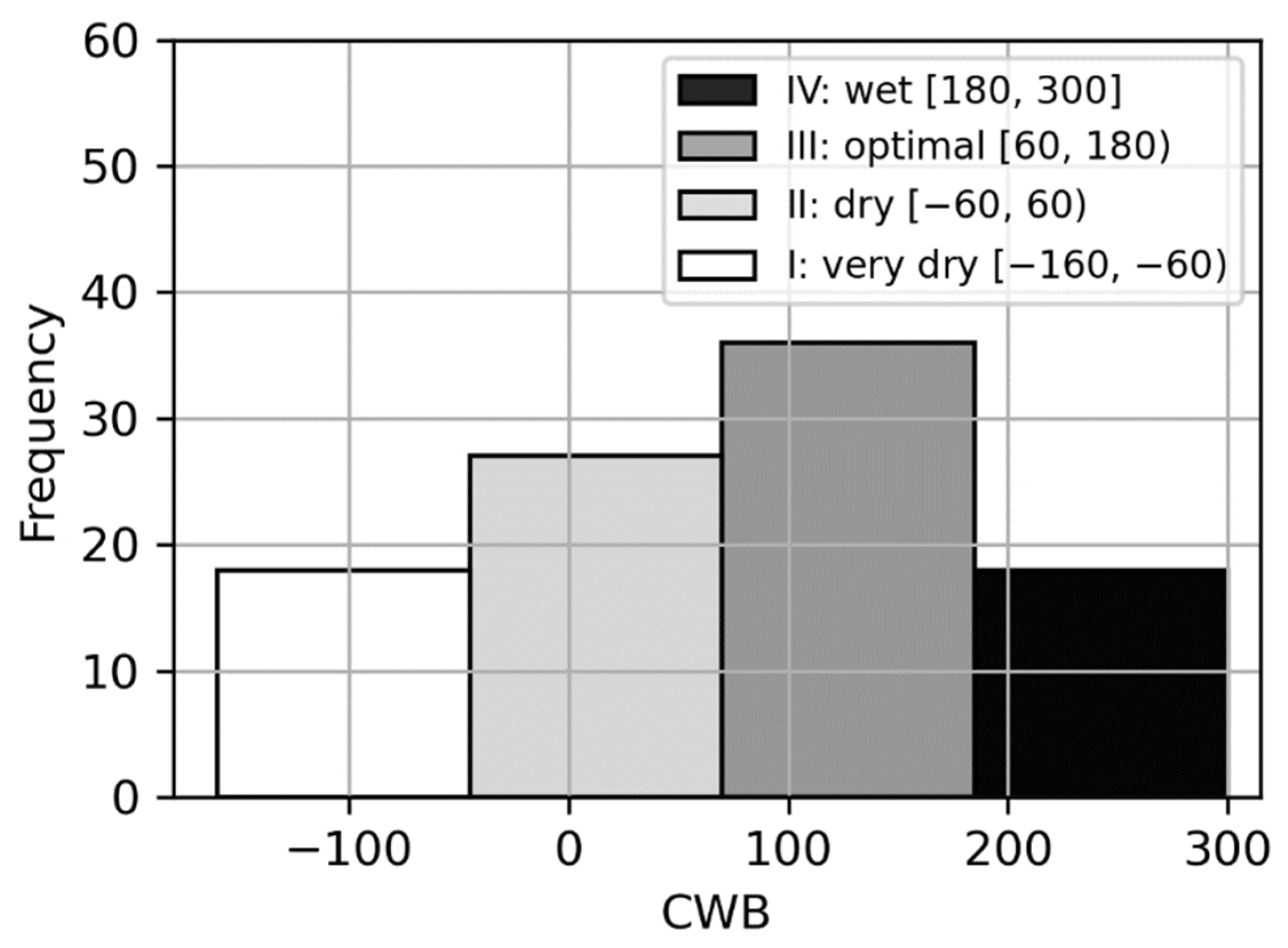
3.1. The Climatic Water Balance Determination
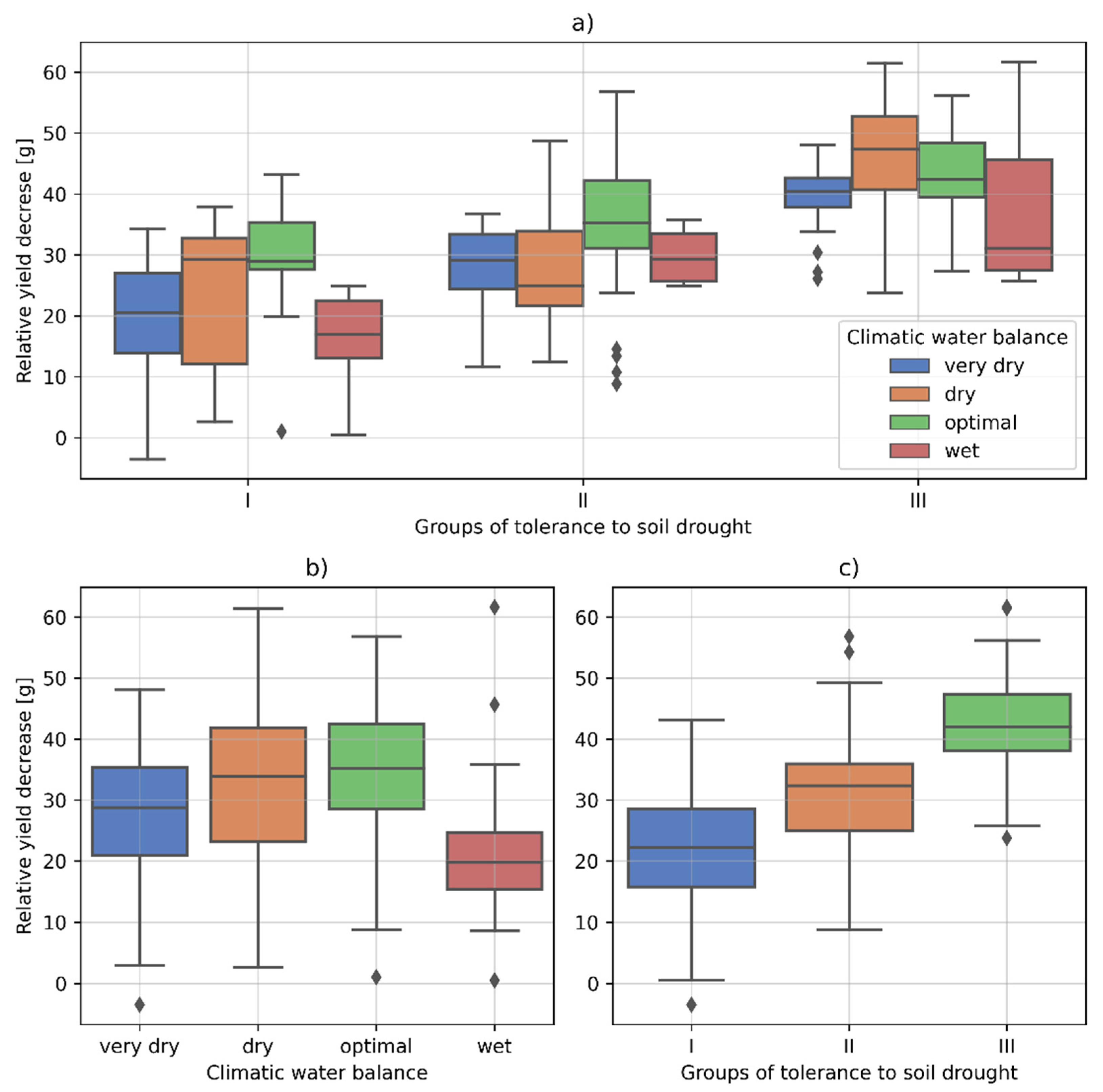
3.2. The Cultivars Differentiation into Groups with Different Tolerance to Soil Drought Based on the Yield Decrease
- Group I: resistant varieties with the lowest yield decrease, i.e., up to 30%;
- Group II: cultivars with medium tolerance to soil drought, where the yield decrease was in the range of 30–40%;
- Group III: susceptible cultivars where the yield drop exceeded 40%.
3.3. Yield Decrease Prediction Using the Varieties Tolerance to Soil Drought and the CWB
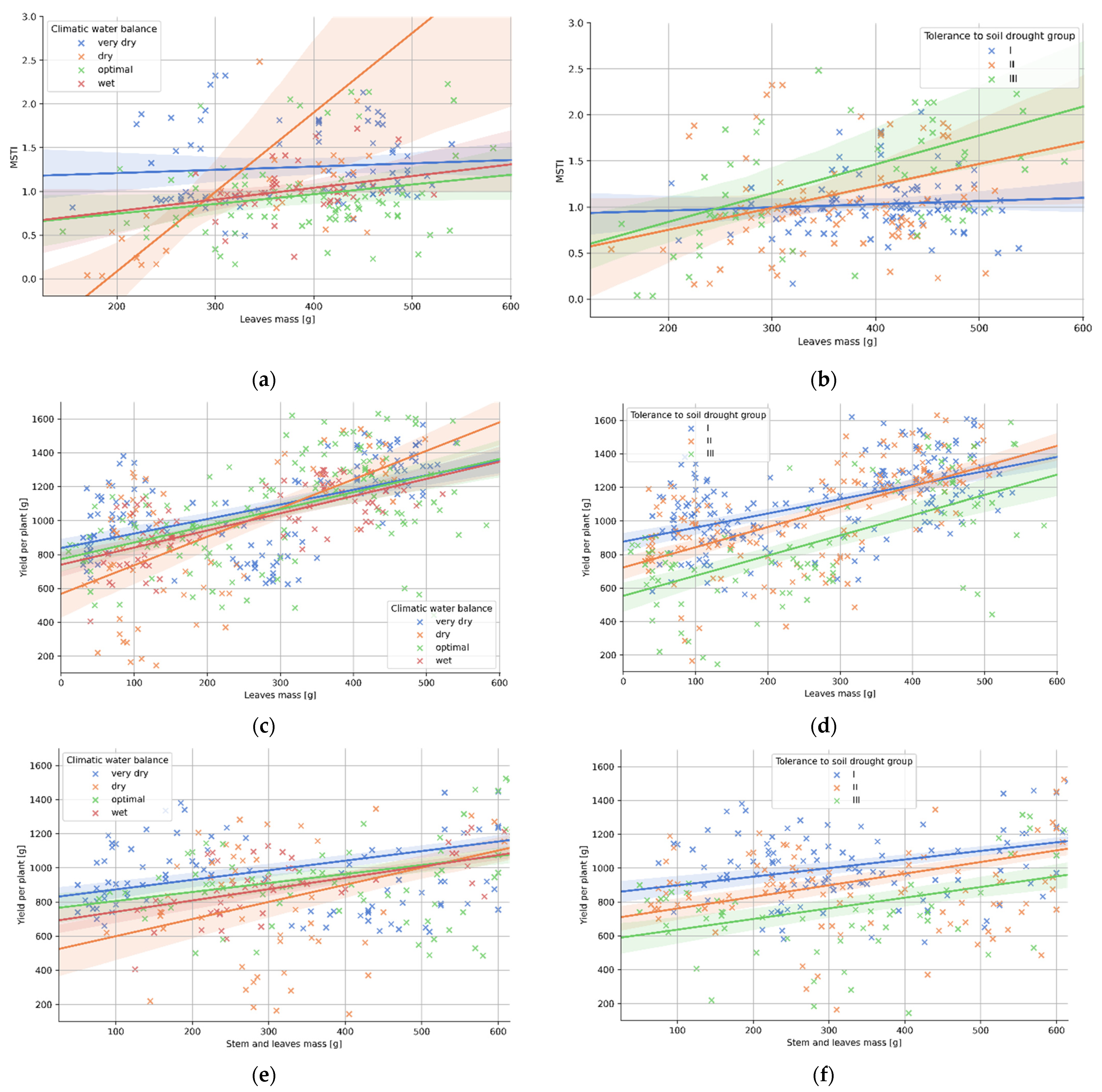
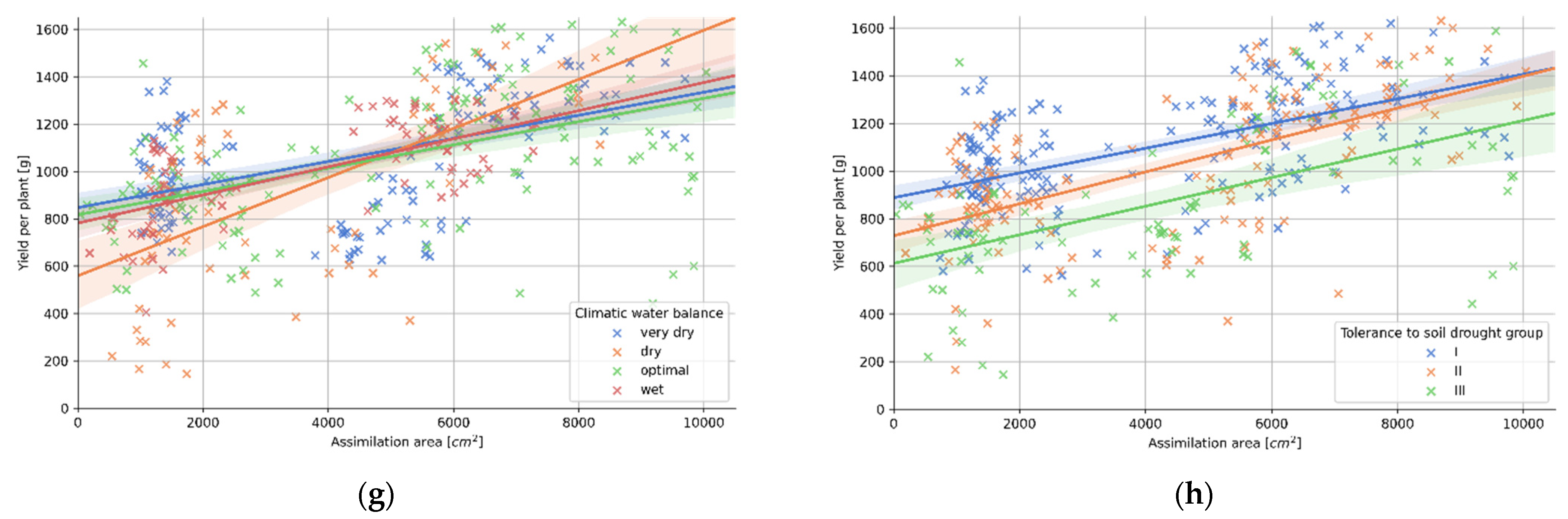
3.4. Relation between Plant Morphology and the Tolerance to Soil Drought
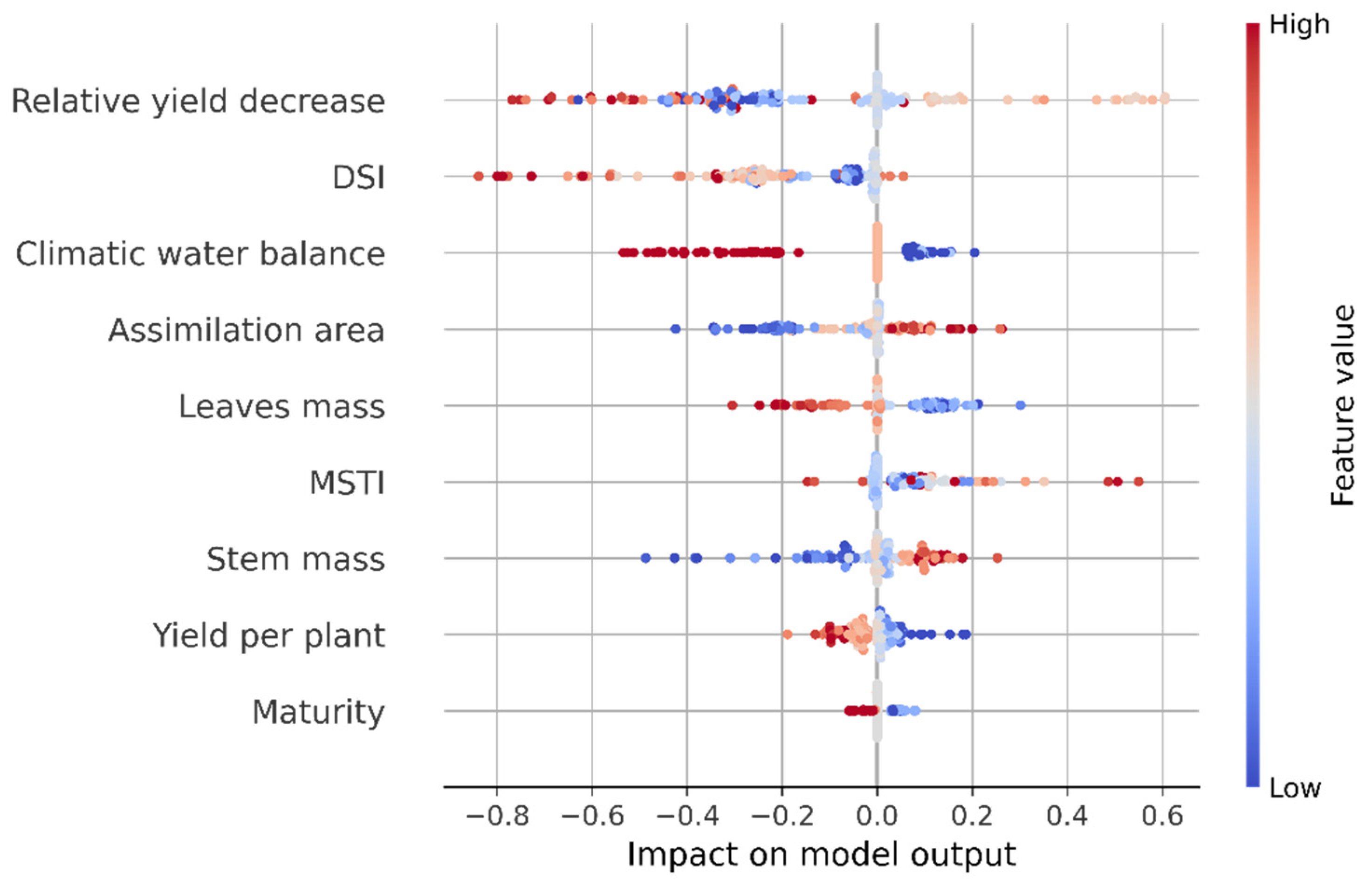
3.5. Machine Learning Models for Potato Variety Tolerance to Soil Drought Groups Classification
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, D.R.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. J. Agron. 2011, 103, 351–370. [Google Scholar] [CrossRef] [Green Version]
- Vandegeer, R.; Miller, R.E.; Bain, M.; Gleadow, R.M.; Cavagnaro, T.R. Drought adversely affects tuber development and nutritional quality of the staple crop cassava (Manihot esculenta Crantz). Funct. Plant Biol. 2012, 40, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, S.; Yada, R.Y.; Bizimungu, B.; Sullivan, J.A. Genotype by environment interaction effects on fibre components in potato (Solanum tuberosum L.). Euphytica 2012, 187, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Obidiegwu, J.; Bryan, G.; Jones, G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, N.; Brereton, A.; Fealy, R.; Sweeney, J. Possible CHANGE in Irish climate and its impact on barley and potato yields. Agric. For. Meteorol. 2003, 116, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–280. [Google Scholar] [CrossRef]
- Kabira, J.N.; Macharia, M.; Karanja, M.W.; Muriithi, L.M. Potato Seed: How to Grow and Market Healthy Planting Material; Technical Note; Agricultural Research Institute: Nairobi, Kenya, 2006; Volume 20. [Google Scholar]
- Van Dam, J.; Levin, I.; Struik, P.C.; Levy, D. Genetic characterisation of tetraploid potato (Solanum tuberosum L.) emphasizing genetic control of total glycoalkaloid content in the tubers. Euphytica 1999, 110, 67–76. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q.; Rajora, O.P.; Li, X.Q. Physiological and growth responses of potato cultivars to heat stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Ahn, Y.-J.; Claussen, K.; Zimmerman, J.L. Genotypic differences in the heat-shock response and thermotolerance in four potato cultivars. Plant Sci. 2004, 166, 901–911. [Google Scholar] [CrossRef]
- Struik, P.C. Responses of the potato plant to temperature. In Potato Biology and Biotechnology: Advances and Perspectives; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., MacKerron, D.K.L., Taylor, M.A., Ross, H.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 366–396. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Asanuma, K.; Kusutani, A.; Toyota, M. Effects of temperature at different growth stages on nonstructural carbohydrate, nitrate reductase activity and yield of potato. Environ. Control Biol. 2000, 38, 197–206. [Google Scholar] [CrossRef]
- Iwama, K.; Yamaguchi, J. Abiotic stress. In Handbook of Potato Production, Improvement and Post-Harvest Management; Gopal, J., Khurana, S.M.P., Eds.; Food Product Press: New York, NY, USA, 2006. [Google Scholar]
- Zarzyńska, K.; Boguszewska-Mańkowska, D.; Nosalewicz, A. Differences in size and architecture of the potato cultivars root system and their tolerance to drought stress. Plant Soil Environ. 2017, 63, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Vos, J.; Groenwold, J. Characteristics of photosynthesis and conductance of potato canopies and the effect of cultivar and transient drought. Field Crops Res. 1989, 20, 237–250. [Google Scholar] [CrossRef]
- Opena, G.B.; Porter, G.A. Soil management and supplemental irrigation effects on potato: II. root growth. Agron J. 1999, 91, 426–431. [Google Scholar] [CrossRef]
- Weisz, R.; Kaminski, J.; Smilowitz, Z. Water deficit effects on potato leaf growth and transpiration: Utilizing fraction extractable soil water for comparison with other crops. Am. Potato J. 1994, 71, 829–840. [Google Scholar] [CrossRef]
- Martinez, I.; Munoz, M.; Acuna, I.; Uribe, M. Evaluating the Drought Tolerance of Seven Potato Varieties on Volcanic Ash Soils in a Medium-Term Trial. Front. Plant Sci. 2021, 12, 1238. [Google Scholar] [CrossRef]
- Benos, L.; Tagarakis, A.C.; Dolias, G.; Berruto, R.; Kateris, D.; Bochtis, D. Machine Learning in Agriculture: A Comprehensive Updated Review. Sensors 2021, 21, 3758. [Google Scholar] [CrossRef]
- Ruszczak, B.; Smykała, K.; Dziubański, K. The Detection of Alternaria Solani Infection on Tomatoes Using Ensemble Learning. J. Ambient. Intell. Smart Environ. 2020, 12, 407–418. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Michalski, P.; Osuchowski, J. Evaluation of Power Insulator Detection Efficiency with the Use of Limited Training Dataset. Appl. Sci. 2020, 10, 2104. [Google Scholar] [CrossRef] [Green Version]
- Hassanpanah, D. Evaluation of potato advanced cultivars against water deficit stress under in vitro and in vivo condition. Biotechnology 2010, 9, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Koźmiński, C.; Michalska, B. Ćwiczenia z Agrometeorologii; PWN: Warszawa, Poland, 1999; p. 179. [Google Scholar]
- Radomski, C. Pomiary i Obliczanie Parowania. In Agrometeorologia; PWN: Warszawa, Poland, 1987; pp. 173–186. [Google Scholar]
- Sprenger, H.; Rudack, K.; Schudoma, C.; Neumann, A.; Seddig, S.; Peters, R.; Zuther, E.; Kopka, J.; Hincha, D.K.; Walther, D.; et al. Assessment of drought tolerance and its potential yield penalty in potato. Funct Plant Biol. 2015, 42, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Aliche, E.; Oortwijn, M.; Theeuwen, T.; Bachem, C.; Visser, R.; van der Linden, G. Drought response in field grown potatoes and the interactions between canopy growth and yield. Agric. Water Manag. 2018, 206, 20–30. [Google Scholar] [CrossRef]
- Mayvan, A.D.; Beheshti, S.A.; Masoom, M.H. Classification of vehicles based on audio signals using quadratic discriminant analysis and high energy feature vectors. Intern. J. Soft Comput. 2015, 6, 53–64. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, M.R.; Frigyik, B.A.; Rosset, S. Bayesian quadratic discriminant analysis. J. Mach. Learn. Res. 2007, 8, 1287–1314. [Google Scholar]
- Geurts, P.; Ernst, D.; Wehenkel, L. Extremely randomized trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Guestrin, C. XGBoost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef] [Green Version]
- Scikit Learn Documentation. Available online: https://scikit-learn.org (accessed on 25 June 2021).
- XGBoost Documentation. Available online: https://xgboost.readthedocs.io (accessed on 17 July 2021).
- SHAP (SHapley Additive exPlanations) Documentation. Available online: https://shap.readthedocs.io (accessed on 29 December 2021).
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.-Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving Potato Stress Tolerance and Tuber Yield Under a Climate Change Scenario—A Current Overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef]
- Głuska, A. Differentiation of root system size in potato cultivars. Biul. IHAR 2004, 232, 37–46. [Google Scholar]
- Boguszewska-Mańkowska, D.; Pieczyński, M.; Wyrzykowska, A.; Kalaji, H.M.; Sieczko, L.; Szweykowska-Kulińska, Z.; Zagdańska, B. Divergent strategies displayed by potato (Solanum tuberosum L.) cultivars to cope with soil drought. J. Agron. Crop Sci. 2018, 204, 13–30. [Google Scholar] [CrossRef] [Green Version]
- Zaki, H.E.M.; Radwan, K.S.A. Response of potato (Solanum tuberosum L.) cultivars to drought stress under in vitro and field conditions. Chem. Biol. Technol. Agric. 2022, 9, 1. [Google Scholar] [CrossRef]
- Schittenhelm, S.; Sourell, H.; Löpmeier, F.J. Drought resistance of potato cultivars with contrasting canopy architecture. Eur. J. Agron. 2006, 24, 193–202. [Google Scholar] [CrossRef]
- Jones, H.G.; Corlett, J.E. Current topics in drought physiology. J. Agric. Sci. 1992, 119, 291–296. [Google Scholar] [CrossRef]
- Vos, J.; Groenwold, J. Genetic Differences in water-use efficiency, stomatal conductance and carbon isotope fractionation in potato. Potato Res. 1989, 32, 113–121. [Google Scholar] [CrossRef]
- Jefferies, R.A.; MacKerron, D.K.L. Responses of potato genotypes to drought. II. leaf area index, growth and yield. Ann. Appl. Biol. 1993, 122, 105–112. [Google Scholar] [CrossRef]
- Wishart, J.; George, T.S.; Brown, L.K.; White, P.J.; Ramsay, G.; Jones, H.; Gregory, P.J. Filed Phenotyping of potato to assess root and shoot characteristics associated with drought tolerance. Plant Soil. 2014, 378, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Sprenger, H.; Erban, A.; Seddig, S.; Rudack, K.; Thalhammer, A.; Le, M.Q.; Walther, D.; Zuther, E.; Köhl, K.I.; Kopka, J.; et al. Metabolite and transcript markers for the prediction of potato drought tolerance. Plant Biotechnol. J. 2018, 16, 939–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, M.; Sprenger, H.; Zuther, E.; Peters, R.; Seddig, S.; Walther, D.; Kopka, J.; Hincha, D.K.; Köhl, K.I. Can Metabolite- and Transcript-Based Selection for Drought Tolerance in Solanum Tuberosum Replace Selection on Yield in Arid Environments? Front Plant Sci. 2020, 11, 1071. [Google Scholar] [CrossRef]
| Year | Tested Cultivar |
|---|---|
| 2005 | Andromeda, Asterix, Danusia, Korona, Rosalind, Satina, Saturna, Syrena, Wiking, Zebra, Zeus |
| 2006 | Andromeda, Asterix, Bartek, Cekin, Satina, Saturna, Syrena, Tajfun, Violet F, Zebra |
| 2007 | Medea, Miłek, Niagara, Satina, Tajfun, Violet F |
| 2008 | Aruba, Cyprian, Inwestor, Irga, Owacja, Tetyda |
| 2009 | Aruba, Cyprian, Flaming, Inwestor, Jutrzenka, Katahdine, Miłek, Niagara, Owacja, Tetyda |
| 2010 | Aruba, Cyprian, Flaming, Irga, Jutrzenka, Katahdine, Korona, Owacja, Tetyda |
| 2011 | Bursztyn, Cekin, Desiere, Gawin, Gustaw, Katahdine, Legenda, Lord, Michalina, Stasia, Tajfun |
| 2012 | Boryna, Gwiazda, Ignacy, Igor, Jurek, Kaszub, Lord, Michalina, Oberon, |
| 2013 | Bartek, Bogatka, Gwiazda, Igor, Jubilat, Jurek, Legenda, Malaga, Oberon, Owacja, Tajfun, Tetyda |
| 2014 | Tetyda, Cekin, Bogatka, Tajfun, Satina, Gawin, Owacja, Bartek, Gwiazda, Oberon |
| 2015 | Bogatka, Cekin, Etiuda, Finezja, Gawin, Gwiazda, Laskara, Magnolia, Malaga, Mazur, Oberon, Owacja, Satina, Tajfun |
| Climatic Water Balance | Year | V | VI | VII | VIII | V–VIII |
|---|---|---|---|---|---|---|
| very dry | 2015 | −13 | −60 | −8 | −85 | −166 |
| 2005 | 26 | −32 | −22 | −71 | −99 | |
| dry | 2006 | −22 | −24 | −109 | 123 | −32 |
| 2014 | −12 | 14 | −49 | 29 | −18 | |
| 2008 | 2 | −45 | 0 | 28 | −15 | |
| optimal | 2013 | 69 | 41 | −72 | 27 | 65 |
| 2012 | −11 | 38 | 18 | 30 | 75 | |
| 2009 | 13 | 20 | 27 | 24 | 84 | |
| 2007 | 27 | 50 | −5 | 24 | 96 | |
| wet | 2010 | 147 | −10 | 27 | 63 | 227 |
| 2011 | −33 | −36 | 255 | 21 | 207 |
| (a) Climatic Water Balance | Min | Max | Mean | Std | Q.25 | Q.5 | Q.75 | |
|---|---|---|---|---|---|---|---|---|
| very dry | −3.49 | 48.06 | 27.85 | 10.86 | 20.91 | 28.77 | 35.34 | |
| dry | 2.64 | 61.43 | 33.09 | 14.51 | 23.23 | 33.90 | 41.86 | |
| optimal | 1.02 | 56.83 | 35.12 | 10.71 | 28.54 | 35.24 | 42.45 | |
| wet | 0.50 | 61.64 | 20.78 | 10.37 | 15.45 | 19.84 | 24.64 | |
| (b) Tolerance to soil drought | Min | Max | Mean | Std | Q.25 | Q.5 | Q.75 | |
| Group I | −3.49 | 43.16 | 21.81 | 10.23 | 15.83 | 22.25 | 28.59 | |
| Group II | 43.16 | 56.83 | 31.02 | 9.68 | 24.96 | 32.36 | 35.92 | |
| Group III | 21.81 | 61.64 | 41.62 | 8.82 | 38.05 | 42.02 | 47.33 | |
| (c) Tolerance to soil drought | Climatic water balance | Min | Max | Mean | Std | Q.25 | Q.5 | Q.75 |
| Group I | very dry | −3.49 | 34.25 | 19.64 | 9.95 | 13.91 | 20.54 | 27.03 |
| dry | 2.64 | 37.92 | 22.79 | 14.18 | 12.14 | 29.22 | 32.75 | |
| optimal | 1.02 | 43.16 | 30.43 | 8.97 | 27.61 | 28.97 | 35.34 | |
| wet | 0.50 | 24.87 | 17.03 | 5.77 | 13.10 | 16.92 | 22.42 | |
| Group II | very dry | 11.70 | 36.79 | 28.36 | 6.77 | 24.40 | 29.11 | 33.42 |
| dry | 12.48 | 48.76 | 27.97 | 10.11 | 21.66 | 24.96 | 33.90 | |
| optimal | 8.85 | 56.83 | 34.78 | 11.17 | 31.15 | 35.24 | 42.17 | |
| wet | 24.90 | 35.82 | 29.85 | 5.28 | 25.68 | 29.33 | 33.50 | |
| Group III | very dry | 26.08 | 48.06 | 39.29 | 5.98 | 37.82 | 40.41 | 42.62 |
| dry | 23.76 | 61.43 | 45.70 | 10.27 | 40.70 | 47.33 | 52.71 | |
| optimal | 27.31 | 56.16 | 42.69 | 7.98 | 39.46 | 42.45 | 48.37 | |
| wet | 25.74 | 61.64 | 38.32 | 15.21 | 27.47 | 31.12 | 45.62 | |
| (a) Climatic Water Balance | p-Val. 1 | r | R2 | Std. Err. | a | b |
|---|---|---|---|---|---|---|
| very dry | 0.063 | 0.21 | 0.05 | 0.000 | 0.001 | 0.521 |
| dry | 0.469 | 0.08 | 0.00 | 0.001 | 0.000 | 1.134 |
| optimal | 0.004 | 0.50 | 0.25 | 0.002 | 0.009 | −1.736 |
| wet | 0.086 | 0.27 | 0.07 | 0.000 | 0.001 | 0.504 |
| (b) Tolerance to Soil Drought | p-Val. | r | R2 | Std. Err. | a | b |
| group I | 0.450 | 0.08 | 0.01 | 0.000 | 0.000 | 0.892 |
| group II | 0.029 | 0.24 | 0.06 | 0.001 | 0.002 | 0.272 |
| group III | 0.021 | 0.32 | 0.10 | 0.001 | 0.003 | 0.208 |
| (c) Climatic Water Balance | p-Val. | r | R2 | Std. Err. | a | b |
| very dry | 0.000 | 0.56 | 0.32 | 0.109 | 0.856 | 838.341 |
| dry | 0.000 | 0.62 | 0.39 | 0.262 | 1.686 | 568.129 |
| optimal | 0.000 | 0.51 | 0.26 | 0.145 | 0.984 | 772.845 |
| wet | 0.000 | 0.71 | 0.50 | 0.113 | 1.010 | 740.277 |
| (d) Tolerance to Soil Drought | p-Val. | r | R2 | Std. Err. | a | b |
| group I | 0.000 | 0.55 | 0.30 | 0.10 | 0.840 | 876.896 |
| group II | 0.000 | 0.62 | 0.39 | 0.13 | 1.207 | 722.849 |
| group III | 0.000 | 0.64 | 0.40 | 0.16 | 1.203 | 553.225 |
| (e) Climatic Water Balance | p-Val. | r | R2 | Std. Err. | a | b |
| very dry | 0.000 | 0.56 | 0.32 | 0.07 | 0.561 | 817.262 |
| dry | 0.000 | 0.60 | 0.36 | 0.17 | 1.004 | 498.879 |
| optimal | 0.000 | 0.44 | 0.20 | 0.09 | 0.527 | 752.745 |
| wet | 0.000 | 0.74 | 0.55 | 0.07 | 0.66 | 675.698 |
| (f) Tolerance to Soil Drought | p-Val. | r | R2 | Std. Err. | a | b |
| group I | 0.000 | 0.52 | 0.27 | 0.06 | 0.507 | 847.884 |
| group II | 0.000 | 0.58 | 0.34 | 0.08 | 0.686 | 692.679 |
| group III | 0.000 | 0.54 | 0.29 | 0.11 | 0.629 | 573.185 |
| (g) Climatic Water Balance | p-Val. | r | R2 | Std. Err. | a | b |
| very dry | 0.000 | 0.50 | 0.25 | 0.01 | 0.049 | 846.578 |
| dry | 0.000 | 0.63 | 0.39 | 0.02 | 0.104 | 558.749 |
| optimal | 0.000 | 0.50 | 0.25 | 0.01 | 0.049 | 816.544 |
| wet | 0.000 | 0.69 | 0.48 | 0.01 | 0.059 | 781.220 |
| (h) Tolerance to Soil Drought | p-Val. | r | R2 | Std. Err. | a | b |
| group I | 0.000 | 0.57 | 0.32 | 0.01 | 0.052 | 887.597 |
| group II | 0.000 | 0.64 | 0.41 | 0.01 | 0.067 | 727.828 |
| group III | 0.000 | 0.53 | 0.28 | 0.01 | 0.060 | 611.761 |
| Model | Config 1 | Accuracy | Precision | Recall | F1-Score |
|---|---|---|---|---|---|
| Extra Trees Classifier | 1 | 0.724 | 0.783 | 0.767 | 0.758 |
| Quadratic Discriminant An. | 1 | 0.635 | 0.687 | 0.674 | 0.651 |
| Random Forest | 1 | 0.692 | 0.734 | 0.721 | 0.721 |
| AdaBoost | 1 | 0.603 | 0.642 | 0.628 | 0.632 |
| Extreme boosting | 1 | 0.609 | 0.639 | 0.628 | 0.631 |
| Extra Trees Classifier | 2 | 0.871 | 0.904 | 0.893 | 0.890 |
| Quadratic Discriminant An. | 2 | 0.790 | 0.856 | 0.821 | 0.815 |
| Random Forest | 2 | 0.841 | 0.870 | 0.857 | 0.856 |
| AdaBoost | 2 | 0.645 | 0.819 | 0.786 | 0.783 |
| Extreme boosting | 2 | 0.746 | 0.820 | 0.786 | 0.770 |
| Extra Trees Classifier | 3 | 0.859 | 0.875 | 0.865 | 0.866 |
| Quadratic Discriminant An. | 3 | 0.791 | 0.845 | 0.802 | 0.803 |
| Random Forest | 3 | 0.820 | 0.832 | 0.823 | 0.824 |
| AdaBoost | 3 | 0.694 | 0.822 | 0.802 | 0.805 |
| Extreme boosting | 3 | 0.849 | 0.864 | 0.854 | 0.856 |
| Extra Trees Classifier | 4 | 0.845 | 0.847 | 0.830 | 0.820 |
| Quadratic Discriminant An. | 4 | 0.858 | 0.866 | 0.851 | 0.854 |
| Random Forest | 4 | 0.869 | 0.851 | 0.851 | 0.849 |
| AdaBoost | 4 | 0.761 | 0.837 | 0.830 | 0.832 |
| Extreme boosting | 4 | 0.828 | 0.812 | 0.809 | 0.799 |
| Extra Trees Classifier | 5 | 0.879 | 0.932 | 0.929 | 0.928 |
| Quadratic Discriminant An. | 5 | 0.900 | 0.916 | 0.893 | 0.890 |
| Random Forest | 5 | 0.905 | 0.940 | 0.929 | 0.926 |
| AdaBoost | 5 | 0.808 | 0.825 | 0.821 | 0.822 |
| Extreme boosting | 5 | 0.967 | 0.967 | 0.964 | 0.964 |
| Model | Feature | Weight |
|---|---|---|
| Extra Trees Classifier (initial features set of config 1) | Climatic water balance | 0.42 |
| Maturity | 0.21 | |
| Stem mass | 0.14 | |
| Assimilation area | 0.12 | |
| Leaves mass | 0.11 | |
| Extreme boosting (final selection of features config 5) | Climatic water balance | 0.20 |
| Relative yield decrease | 0.16 | |
| MSTI | 0.14 | |
| DSI | 0.13 | |
| Stem mass | 0.09 | |
| Maturity | 0.08 | |
| Assimilation area | 0.07 | |
| Leaves mass | 0.06 | |
| Yield per plant | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boguszewska-Mańkowska, D.; Ruszczak, B.; Zarzyńska, K. Classification of Potato Varieties Drought Stress Tolerance Using Supervised Learning. Appl. Sci. 2022, 12, 1939. https://doi.org/10.3390/app12041939
Boguszewska-Mańkowska D, Ruszczak B, Zarzyńska K. Classification of Potato Varieties Drought Stress Tolerance Using Supervised Learning. Applied Sciences. 2022; 12(4):1939. https://doi.org/10.3390/app12041939
Chicago/Turabian StyleBoguszewska-Mańkowska, Dominika, Bogdan Ruszczak, and Krystyna Zarzyńska. 2022. "Classification of Potato Varieties Drought Stress Tolerance Using Supervised Learning" Applied Sciences 12, no. 4: 1939. https://doi.org/10.3390/app12041939
APA StyleBoguszewska-Mańkowska, D., Ruszczak, B., & Zarzyńska, K. (2022). Classification of Potato Varieties Drought Stress Tolerance Using Supervised Learning. Applied Sciences, 12(4), 1939. https://doi.org/10.3390/app12041939






