Antibacterial, Antiradical and Antiproliferative Potential of Green, Roasted, and Spent Coffee Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield, Total Phenolic Content, and Antiradical Scavenging Activity
2.2. Antibacterial Activity
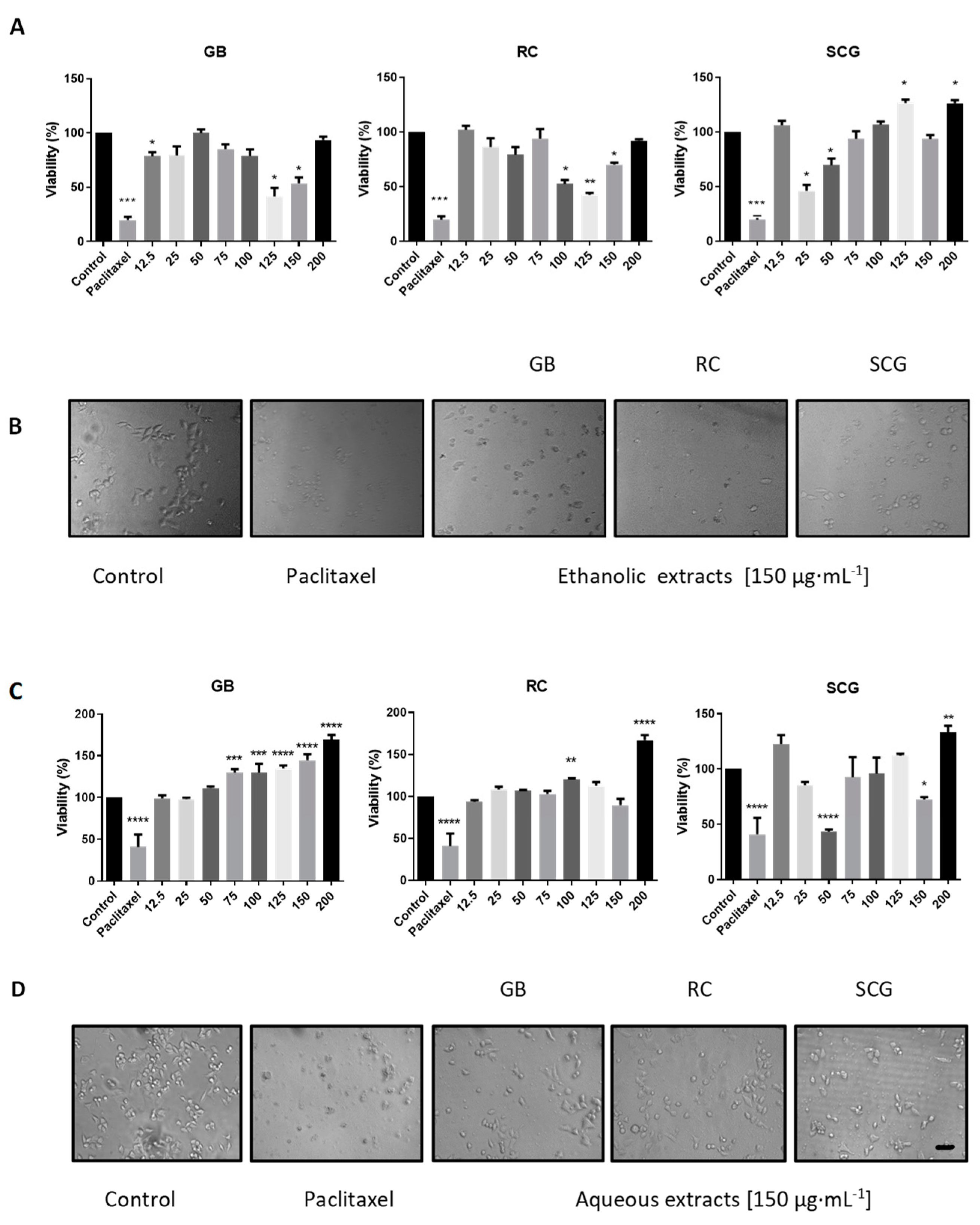
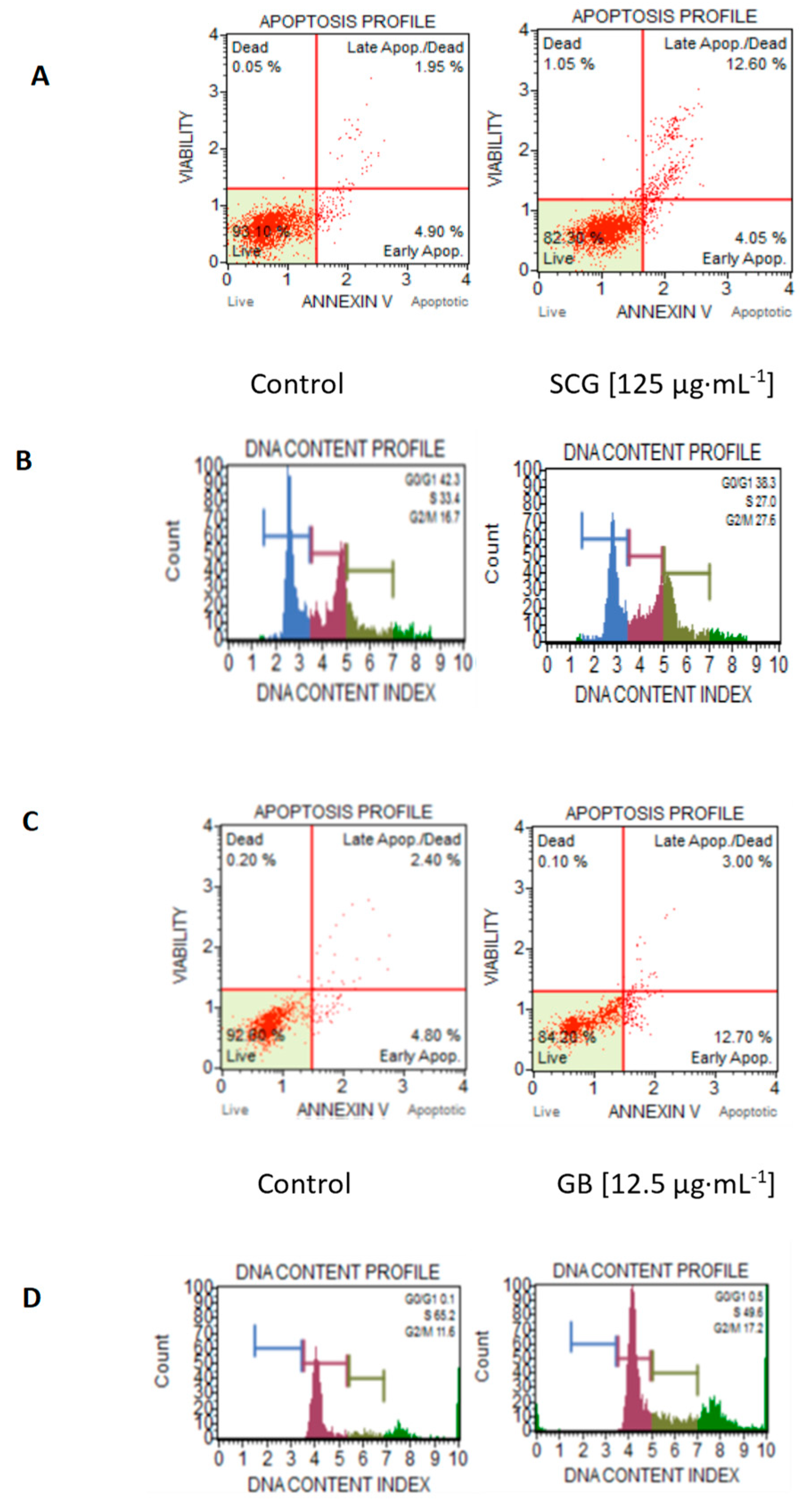
2.3. Analysis of In Vitro Antiproliferative Activity, Morphological Changes, Apoptosis, and Cell-Cycle Arrest
3. Materials and Methods
3.1. Materials
3.2. Preparation of Coffee Sample Extracts
3.3. Determination of Total Phenolic Content and Antiradical Scavenging Activity
3.4. Antibacterial Activity
3.5. Cell Proliferation Assay and Morphological Changes
3.6. Apoptosis and the Analysis of Cell-Cycle Arrest
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Tilahun, A.; Soo, B. Influence of pretreatment and modifiers on subcritical water liquefaction of spent coffee grounds: A green waste valorization approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Daglia, M.; Cuzzoni, M.T.; Dacarro, C. Antibacterial activity of coffee. J. Agric. Food Chem. 1994, 42, 2270–2272. [Google Scholar] [CrossRef]
- Monente, C.; Bravo, J.; Vitas, A.I.; Arbillaga, L.; De Peña, M.P.; Cid, C. Coffee and spent coffee extracts protect against cell mutagens and inhibit growth of food-borne pathogen microorganisms. J. Funct. Foods 2015, 12, 365–374. [Google Scholar] [CrossRef]
- Amigo-Benavent, M.; Wang, S.; Mateos, R.; Sarriá, B.; Bravo, L. Antiproliferative and cytotoxic effects of green coffee and yerba mate extracts, their main hydroxycinnamic acids, methylxanthine and metabolites in different human cell lines. Food Chem. Toxicol. 2017, 106, 125–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Zamora, A.; Pastoriza, S.; Rufián-Henares, J.A. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT-Food Sci. Technol. 2015, 61, 12–18. [Google Scholar] [CrossRef]
- Hernández-Arriaga, A.M.; Dave Oomah, B.; Campos-Vega, R. Microbiota source impact in vitro metabolite colonic production and anti-proliferative effect of spent coffee grounds on human colon cancer cells (HT-29). Food Res. Int. 2017, 97, 191–198. [Google Scholar] [CrossRef]
- Balzano, M.; Loizzo, M.R.; Tundis, R.; Lucci, P.; Nunez, O.; Fiorini, D.; Giardinieri, A.; Frega, N.G.; Pacetti, D. Spent espresso coffee grounds as a source of anti-proliferative and antioxidant compounds. Innov. Food Sci. Emerg. Technol. 2020, 59, 102254. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Chaves-Ulate, E.C.; Esquivel-Rodríguez, P. Chlorogenic acids present in coffee: Antioxidant and antimicrobial capacity. Agron. Mesoam. 2019, 30, 299–311. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R.; Farmaco, D.; Superiore, I.; Elena, V.R.; Rome, I.-; Chimica, I.; et al. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Coelho, C.; Ribeiro, M.; Cruz, A.C.S.; Domingues, M.R.M.; Coimbra, M.A.; Bunzel, M.; Nunes, F.M. Nature of phenolic compounds in coffee melanoidins. J. Agric. Food Chem. 2014, 62, 7843–7853. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef] [Green Version]
- Bartel, C.; Mesias, M.; Morales, F.J. Investigation on the extractability of melanoidins in portioned espresso coffee. Food Res. Int. 2015, 67, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Andrade, K.S.; Gonalvez, R.T.; Maraschin, M.; Ribeiro-Do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef]
- Getachew, A.T.; Cho, Y.J.; Chun, B.S. Effect of pretreatments on isolation of bioactive polysaccharides from spent coffee grounds using subcritical water. Int. J. Biol. Macromol. 2018, 109, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Páscoa, R.N.M.J.; Magalhães, L.M.; Lopes, J.A. FT-NIR spectroscopy as a tool for valorization of spent coffee grounds: Application to assessment of antioxidant properties. Food Res. Int. 2013, 51, 579–586. [Google Scholar] [CrossRef]
- Ozuna, C.; Mulík, S.; Valdez-rodríguez, B.; Rosario, M.; Fernández-lópez, C.L. The effect of organic farming on total phenols, total flavonoids, brown compounds and antioxidant activity of spent coffee grounds from Mexico. Biol. Agric. Hortic. 2020, 36, 107–118. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Głowacka, R.; Górska, A.; Irkowska-Wojdyła, M.; Wołosiak, R.; Majewska, E.; Derewiaka, D. The influence of brewing method on bioactive compounds residues in spent coffee grounds of different roasting degree and geographical origin. J. Food Sci. Technol. 2019, 54, 3008–3014. [Google Scholar] [CrossRef]
- Sedem, C.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Stefani, T.; Garza-González, E.; Rivas-Galindo, V.M.; Rios, M.Y.; Alvarez, L.; Camacho-Corona, M.D.R. Hechtia glomerata Zucc: Phytochemistry and activity of its extracts and major constituents against resistant bacteria. Molecules 2019, 24, 3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.M.; Nunan, E.A.; Glória, M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Sanin, A.; Rubio, S. Valorization of spent coffee grounds by supramolecular solvent extraction. Sep. Purif. Technol. 2019, 228, 115759. [Google Scholar] [CrossRef]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of in vitro digestion on bioactive compounds, antioxidant and antimicrobial activities of coffee (Coffea arabica L.) pulp aqueous extract. Food Chem. 2021, 348, 129094. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tomé, M.; Jiménez-Monreal, A.M.; García-Jiménez, L.; Almela, L.; García-Diz, L.; Mariscal-Arcas, M.; Murcia, M.A. Assessment of antimicrobial activity of coffee brewed in three different ways from different origins. Eur. Food Res. Technol. 2011, 233, 497–505. [Google Scholar] [CrossRef]
- Pedras, B.M.; Nascimento, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Semi-continuous extraction/hydrolysis of spent coffee grounds with subcritical water. J. Ind. Eng. Chem. 2019, 72, 453–456. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Morales, F.J. Antimicrobial activity of melanoidins against Escherichia coli is mediated by a membrane-damage. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinsinwar, S.; Vadivel, V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 8279–8297. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Chung, L.Y.; Navaratnam, P. Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus. Fitoterapia 2014, 94, 48–54. [Google Scholar] [CrossRef]
- Diederich, M.; Cerella, C. Non-canonical programmed cell death mechanisms triggered by natural compounds. Semin. Cancer Biol. 2016, 40–41, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Hutachok, N.; Koonyosying, P.; Pankasemsuk, T.; Angkasith, P.; Chumpun, C.; Fucharoen, S.; Srichairatanakool, S. Chemical Analysis, Toxicity Study, and Free-Radical Scavenging and Iron-Binding Assays Involving Coffee (Coffea arabica) Extracts. Molecules 2021, 26, 26. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? npj Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- De Souza, L.D.S.; Carrero Horta, I.P.; De Souza Rosa, L.; Barbosa Lima, L.G.; Santos Da Rosa, J.; Montenegro, J.; Da Silva Santos, L.; Nana De Castro, R.B.; Freitas-Silva, O.; Teodoro, A.J. Effect of the roasting levels of: Coffea arabica L. extracts on their potential antioxidant capacity and antiproliferative activity in human prostate cancer cells. RSC Adv. 2020, 10, 30115–30126. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular antioxidant and anti-inflammatory effects of coffee extracts with different roasting levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Ding, Y.M.; Hueng, D.Y.; Chen, J.Y.; Chen, Y. Caffeine suppresses the progression of human glioblastoma via cathepsin B and MAPK signaling pathway. J. Nutr. Biochem. 2016, 33, 63–72. [Google Scholar] [CrossRef]
- Ku, B.M.; Lee, Y.K.; Jeong, J.Y.; Ryu, J.; Choi, J.; Kim, J.S.; Cho, Y.W.; Roh, G.S.; Kim, H.J.; Cho, G.J.; et al. Caffeine inhibits cell proliferation and regulates PKA/GSK3β pathways in U87MG human glioma cells. Mol. Cells 2011, 31, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J.; et al. The cytotoxicity of kahweol in HT-29 human colorectal cancer cells is mediated by apoptosis and suppression of heat shock protein 70 expression. Biomol. Ther. (Seoul) 2015, 23, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukla, M.; Polaniak, R.; Birkner, E. The impact of coffee and its selected bioactive compounds on the development and progression of colorectal cancer in vivo and in vitro. Molecules 2018, 23, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef]
- Montenegro, J.; dos Santos, L.S.; de Souza, R.G.G.; Lima, L.G.B.; Mattos, D.S.; Viana, B.P.P.B.; da Fonseca Bastos, A.C.S.; Muzzi, L.; Conte-Júnior, C.A.; Gimba, E.R.P.; et al. Bioactive compounds, antioxidant activity and antiproliferative effects in prostate cancer cells of green and roasted coffee extracts obtained by microwave-assisted extraction (MAE). Food Res. Int. 2021, 140, 110014. [Google Scholar] [CrossRef] [PubMed]
- Mojica, B.E.; Fong, L.E.; Biju, D.; Muharram, A.; Davis, I.M.; Vela, K.O.; Rios, D.; Osorio-Camacena, E.; Kaur, B.; Rojas, S.M.; et al. The impact of the roast levels of coffee extracts on their potential anticancer activities. J. Food Sci. 2018, 83, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Dave Oomah, B. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Ramalakshmi, K.; Rao, L.J.M.; Takano-Ishikawa, Y.; Goto, M. Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem. 2009, 115, 79–85. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, M.K.; Kim, Y.S.; Lee, S.K. In vitro protein expression changes in RAW 264.7 cells and HUVECs treated with dialyzed coffee extract by immunoprecipitation high performance liquid chromatography. Sci. Rep. 2018, 8, 13841. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Sudeep, H.V.; Venkatesh, B.J.; Shyam Prasad, K. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HCT-116 cells. Food Sci. Hum. Wellness 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Bravo, J.; Arbillaga, L.; De Peña, M.P.; Cid, C. Antioxidant and genoprotective effects of spent coffee extracts in human cells. Food Chem. Toxicol. 2013, 60, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.-J.; Heo, Y.-S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis 2011, 32, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.; Habermeyer, M.; Kemény, M.; Weyand, U.; Niederberger, E.; Frank, O.; Hofmann, T. Maillard reaction products modulating the growth of human tumor cells in vitro. Chem. Res. Toxicol. 2003, 16, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Salazar-Pineda, D.T.; Castro-Alarcón, N.; Moreno-Godínez, M.E.; del Pilar Nicasio-Torres, M.; Pérez-Hernández, J.; Alvarez-Fitz, P. Antibacterial and anti-inflammatory activity of extracts and fractions from agave cupreata. Int. J. Pharmacol. 2017, 13, 1063–1070. [Google Scholar] [CrossRef]
- Jiménez-Hernández, J.; Estrada-Bahena, E.B.; Maldonado-Astudillo, Y.I.; Talavera-Mendoza, Ó.; Arámbula-Villa, G.; Azuara, E.; Álvarez-Fitz, P.; Ramírez, M.; Salazar, R. Osmotic dehydration of mango with impregnation of inulin and piquin-pepper oleoresin. LWT-Food Sci. Technol. 2017, 79, 609–615. [Google Scholar] [CrossRef]

| Sample | Yield (%) | TPC (μg CAE·mg−1 Dry Extract) | ABTS (IC50 μg·mL−1) | DPPH (IC50 μg·mL−1) | |
|---|---|---|---|---|---|
| GB | 19.28 ± 0.21 b | 196.05 ± 1.21 c | 10.76 ± 0.14 bc | 87.60 ± 1.68 ab | |
| Ethanolic | RC | 17.57 ± 0.10 c | 244.97 ± 8.66 b | 13.21 ± 3.60 b | 59.11 ± 7.11 c |
| SCG | 11.82 ± 0.34 e | 298.33 ± 1.41 a | 7.50 ± 0.58 c | 44.88 ± 5.81 c | |
| GB | 22.17 ± 0.39 a | 160.57 ± 1.82 e | 19.54 ± 0.33 a | 96.79 ± 5.12 a | |
| Aqueous | RC | 23.28 ± 0.79 a | 183.00 ± 3.10 d | 6.32 ± 1.45 c | 79.55 ± 8.70 b |
| SCG | 12.80 ± 0.42 d | 187.13 ± 1.01 cd | 9.99 ± 2.87 bc | 51.20 ± 6.13 c |
| MIC (mg∙mL−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC | Methicillin-Resistant | ||||||||||
| Extract | Sample | EC1 | EClo | Sd | Sa1 | Ef | Kp | EC2 | Sh | Sho | Sa2 |
| GB | 8 | >8 | >8 | >8 | >8 | >8 | 8 | >8 | >8 | 8 | |
| Ethanolic | RC | 8 | >8 | 8 | 2 | 8 | 4 | 4 | 4 | 2 | 4 |
| SCG | 8 | 8 | 8 | 8 | 8 | 2 | 2 | 2 | 2 | 2 | |
| GB | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | >8 | |
| Aqueous | RC | >8 | >8 | >8 | >8 | >8 | 4 | 4 | 4 | 4 | 4 |
| SCG | >8 | 8 | 8 | 4 | 8 | 2 | 4 | 4 | 8 | 4 | |
| C− | + | + | + | + | + | + | + | + | + | + | |
| C+ | − | − | − | − | − | − | − | − | − | − | |
| Concentration | Apoptosis (%) | Cell Cycle Phases (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | Extract | Sample | (µg·mL−1) | LC | Ap | Sub G0/G1 | G1-S | G2-M |
| Control | -- | -- | 93.1 | 4.9 | 42.3 | 33.4 | 16.7 | |
| GB | 25 | 90.5 | 9 | 32.9 | 35.6 | 23.1 | ||
| GB | 125 | 85.8 | 13.5 | 47.5 | 21.2 | 26.6 | ||
| C33A | Ethanolic | RC | 25 | 92.8 | 6.8 | 37.6 | 24.4 | 32 |
| RC | 125 | 85.5 | 13.9 | 42.7 | 20.7 | 32.2 | ||
| SCG | 25 | 92.4 | 7.4 | 36.6 | 22.8 | 35.1 | ||
| SCG | 125 | 82.0 | 12.6 | 38.3 | 27 | 27.6 | ||
| Control | -- | -- | 92.6 | 4.8 | 0.1 | 65.2 | 11.6 | |
| Ethanolic | GB | 12.5 | 84.2 | 12.7 | 0.5 | 49.6 | 17.2 | |
| RC | 12.5 | 91.3 | 8.7 | 0.2 | 53.8 | 16 | ||
| A549 | SCG | 12.5 | 95.1 | 4.7 | 0.9 | 50.1 | 15.5 | |
| Aqueous | GB | 200 | 94.4 | 4.5 | 0.3 | 64.2 | 12 | |
| RC | 200 | 91.2 | 7.4 | 0.9 | 62.6 | 17.5 | ||
| SCG | 200 | 87.1 | 10.3 | 0.5 | 58.7 | 14 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Hernández, G.C.; Alvarez-Fitz, P.; Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Parra-Rojas, I.; Flores-Alfaro, E.; Salazar, R.; Ramírez, M. Antibacterial, Antiradical and Antiproliferative Potential of Green, Roasted, and Spent Coffee Extracts. Appl. Sci. 2022, 12, 1938. https://doi.org/10.3390/app12041938
Díaz-Hernández GC, Alvarez-Fitz P, Maldonado-Astudillo YI, Jiménez-Hernández J, Parra-Rojas I, Flores-Alfaro E, Salazar R, Ramírez M. Antibacterial, Antiradical and Antiproliferative Potential of Green, Roasted, and Spent Coffee Extracts. Applied Sciences. 2022; 12(4):1938. https://doi.org/10.3390/app12041938
Chicago/Turabian StyleDíaz-Hernández, Gema C., Patricia Alvarez-Fitz, Yanik I. Maldonado-Astudillo, Javier Jiménez-Hernández, Isela Parra-Rojas, Eugenia Flores-Alfaro, Ricardo Salazar, and Mónica Ramírez. 2022. "Antibacterial, Antiradical and Antiproliferative Potential of Green, Roasted, and Spent Coffee Extracts" Applied Sciences 12, no. 4: 1938. https://doi.org/10.3390/app12041938
APA StyleDíaz-Hernández, G. C., Alvarez-Fitz, P., Maldonado-Astudillo, Y. I., Jiménez-Hernández, J., Parra-Rojas, I., Flores-Alfaro, E., Salazar, R., & Ramírez, M. (2022). Antibacterial, Antiradical and Antiproliferative Potential of Green, Roasted, and Spent Coffee Extracts. Applied Sciences, 12(4), 1938. https://doi.org/10.3390/app12041938






