Characteristics and Rates of Microbial Processes in Clays of Different Mineral and Elemental Composition in Relation to Safety Prediction for ESB Clay Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Experiments
2.2. Procedures
3. Results
3.1. Mineral and Elemental Composition of the Samples
3.2. Biological Availability of Biogenic Elements
3.3. Differences in the Composition of the Microbial Complex
3.4. Respiratory Activity of the Microflora of Clay Samples and Its Stimulation
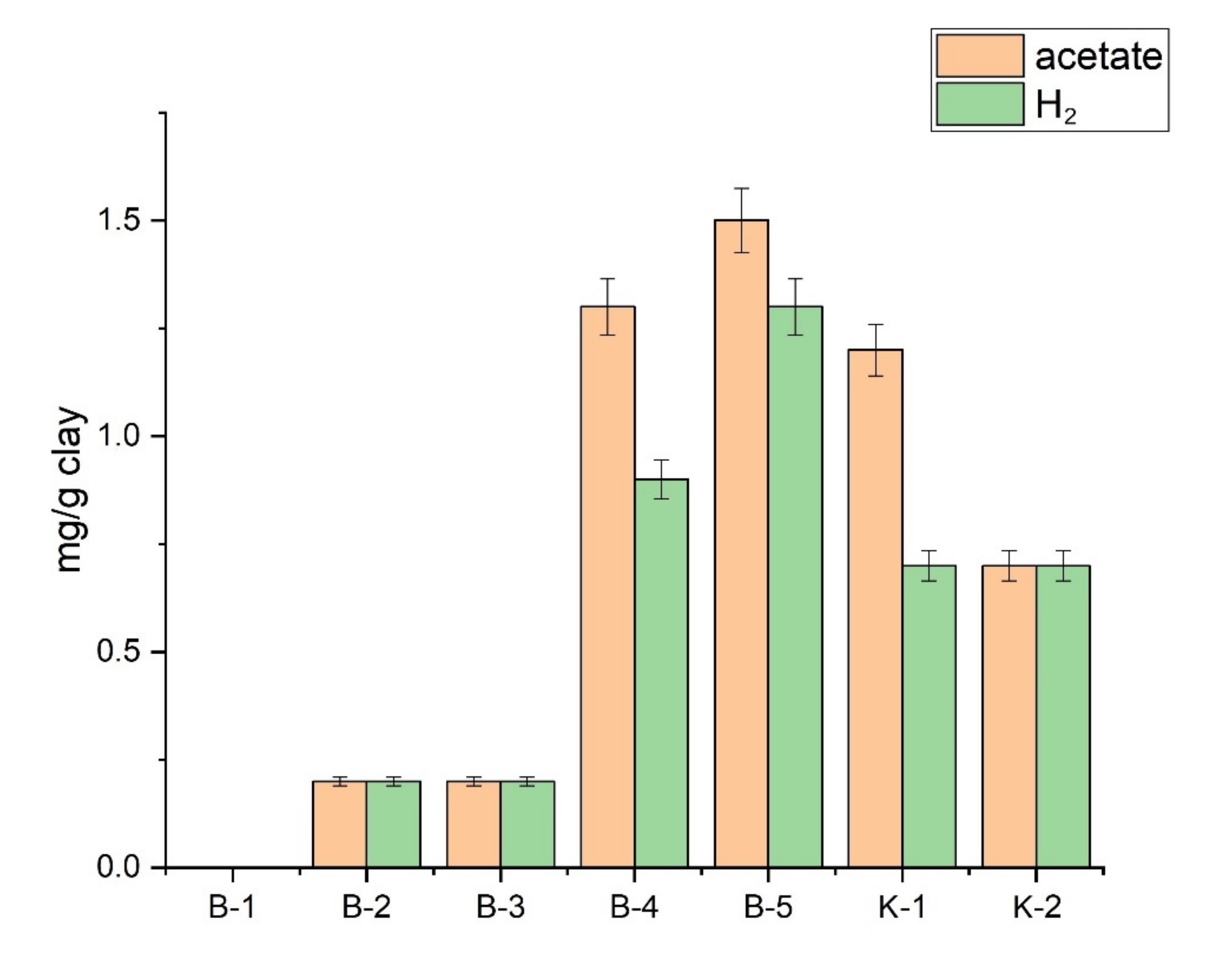
3.5. Clay Elements Leaching by Microbial Complex Stimulation
3.6. Biogenic Sulfide Formation
4. Discussion
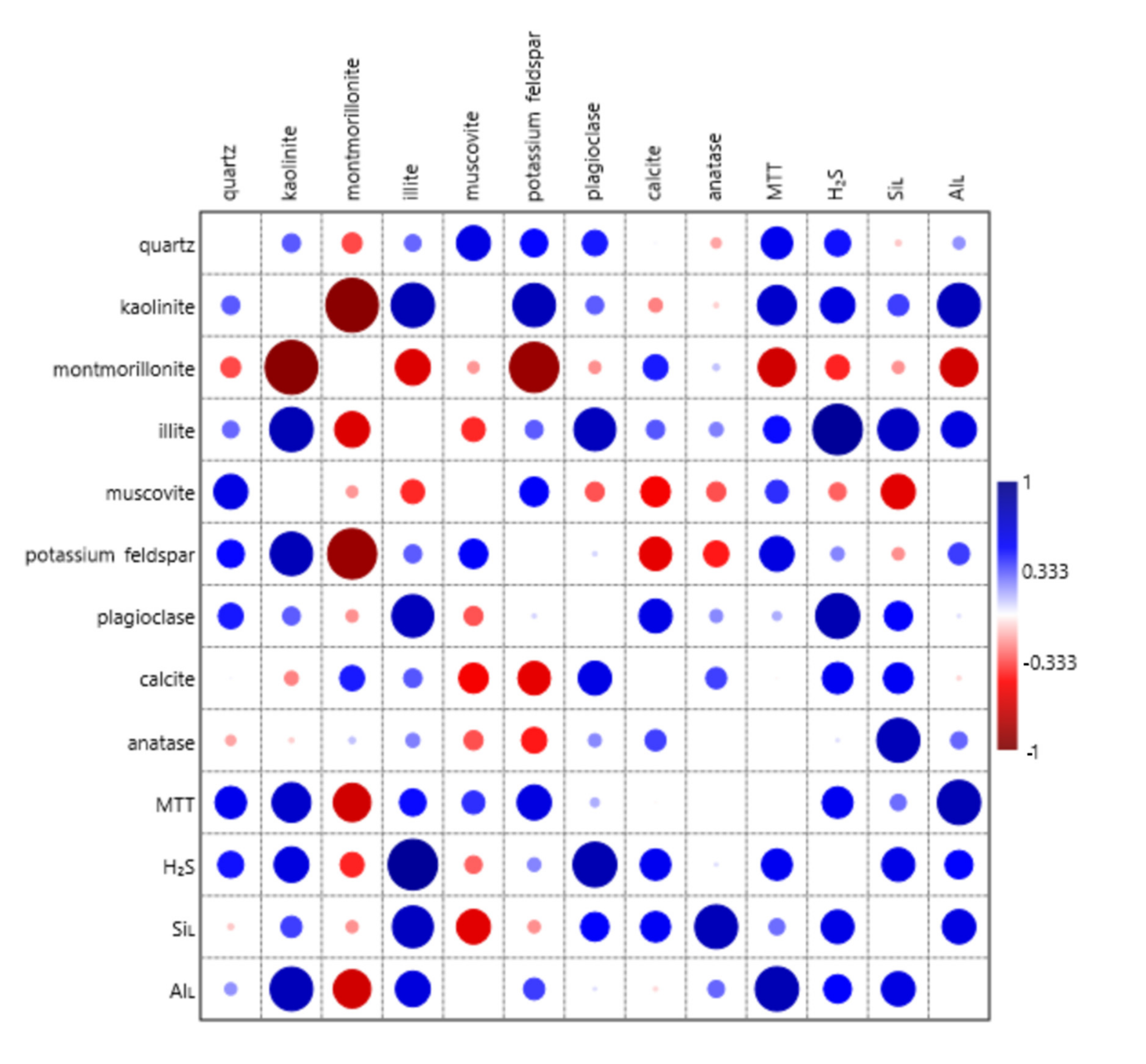
4.1. Correlations between the Composition of Clay Materials and Microbial Processes
4.2. Calculation of Overall Safety for Clay Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delage, P.; Cui, Y.J.; Tang, A.M. Clays in radioactive waste disposal. J. Rock Mech. Geotech. Eng. 2010, 2, 111–123. [Google Scholar] [CrossRef]
- Dohrmanna, R.; Kaufhold, S.; Lundqvist, B. The Role of Clays for Safe Storage of Nuclear Waste. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 677–710. [Google Scholar] [CrossRef]
- Pedersen, K. Microbial Processes in Radioactive Waste Disposal; SKB TR-00-04; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2000; 92p. [Google Scholar]
- Mijnendonckx, K.; Honty, M.; Wang, L.; Jacops, E.; Provoost, A.; Mysara, M.; Wouters, K.; De Craen, M.; Leys, N. An active microbial community in Boom Clay pore water collected from piezometers impedes validating predictive modelling of ongoing geochemical processes. J. Appl. Geochem. 2019, 106, 149–160. [Google Scholar] [CrossRef]
- Äspö Hard Rock Laboratory. Annual Report 2016; SKB TR-17-10; Swedish Nuclear Fuel and Waste Management Co.: Solna, Sweden, 2016; 130p. [Google Scholar]
- Leupin, O.X.; Bernier-Latmani, R.; Bagnoud, A.; Moors, H.; Leys, N.; Wouters, K.; Stroes-Gascoyne, S. Fifteen years of microbiological investigation in Opalinus Clay at the Mont Terri rock laboratory (Switzerland). Swiss J. Geosci. 2017, 110, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Meleshyn, A.Y.; Zakusin, S.V.; Krupskaya, V.V. Swelling Pressure and Permeability of Compacted Bentonite from 10th Khutor Deposit (Russia). Minerals 2021, 11, 742. [Google Scholar] [CrossRef]
- Belousov, P.; Chupalenkov, N.; Christidis, G.E.; Zakusina, O.; Zakusin, S.; Morozov, I.; Chernov, M.; Zaitseva, T.; Tyupina, E.; Krupskaya, V. Carboniferous bentonites from 10Th Khutor deposit (Russia): Composition, properties and features of genesis. Appl. Clay Sci. 2021, 215, 106308. [Google Scholar] [CrossRef]
- Characterization of Swelling Clays as Components of the Engineered Barriers System for Geological Repositories; IAEA-TECDOC-1718; IAEA: Vienna, Austria, 2013; 102p, Available online: https://www.iaea.org/publications/8525/characterization-of-swelling-clays-as-components-of-the-engineered-barrier-system-for-geological-repositories (accessed on 29 November 2021).
- Ilina, O.A.; Krupskaya, V.V.; Vinokurov, S.E.; Kalmykov, S.N. State-of-Art in the Development and Use of Clay Materials as Engineered Safety Barriers at Radioactive Waste Conservation and Disposal Facilities in Russia. Radioact. Waste 2019, 4, 71–84. [Google Scholar] [CrossRef]
- Martynov, K.V.; Zakharova, E.V.; Dorofeev, A.N.; Zubkov, A.A.; Prishchep, A.A. Use of Clay Materials in the Construction of Protective Barriers at Radiation Hazardous Facilities. Radioact. Waste 2020, 3, 39–53. (In Russian) [Google Scholar] [CrossRef]
- Stoulil, J.; Kaňok, J.; Kouřil, M.; Parschová, H.; Novák, P. Influence of temperature on corrosion rate and porosity of corrosion products of carbon steel in anoxic bentonite environment. J. Nucl. Mater. 2013, 443, 20–25. [Google Scholar] [CrossRef]
- Schwartz, M.O. Corrosion-Enhancing and Corrosion-Reducing Accessories in Bentonite Surrounding Copper-Shielded Containers for Nuclear Waste. J Hazard Toxic Radioact. Waste 2021, 25, 04021024. [Google Scholar] [CrossRef]
- Cuadros, J. Clay minerals interaction with microorganisms: A review. Clay Miner. 2017, 52, 235–261. [Google Scholar] [CrossRef] [Green Version]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between microorganisms and clay minerals: New insights and broader applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Naĭmark, E.B.; Eroshchev-Shak, V.A.; Chizhikova, N.P.; Kompantseva, E.I. Interaction of clay minerals with microorganisms: A review of experimental data. Zhurnal Obs. Biol. 2009, 70, 155–167. [Google Scholar]
- Dong, H.; Jaisi, D.P.; Kim, J.; Zhang, G. Microbe-clay mineral interactions. Am. Mineral. 2009, 94, 1505–1519. [Google Scholar] [CrossRef]
- Hong, H.; Fang, Q.; Cheng, L.; Wang, C.; Churchman, G.J. Microorganism-induced weathering of clay minerals in a hydromorphic soil. Geochim. Cosmochim. Acta 2016, 184, 272–288. [Google Scholar] [CrossRef]
- Fang, Q.; Churchman, G.J.; Hong, H.; Chen, Z.Q.; Liu, J.; Yu, J.; Han, W.; Wang, C.; Zhao, L.; Furnes, H. New insights into microbial smectite illitization in the Permo-Triassic boundary K-bentonites, South China. Appl. Clay Sci. 2017, 140, 96–111. [Google Scholar] [CrossRef]
- Huggett, J.M.; Cuadros, J. Low-temperature illitization of smectite in the late eocene and early oligocene of the Isle of Wight (Hampshire basin), U.K. Am. Mineral. 2005, 90, 1192–1202. [Google Scholar] [CrossRef]
- Wouters, K.; Moors, H.; Boven, P.; Leys, N. Evidence and characteristics of a diverse and metabolically active microbial community in deep subsurface clay borehole water. FEMS Microbiol. Ecol. 2013, 86, 458–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, S.P.; Barnett, M.J.; Field, L.P.; Milodowski, A.E. Subsurface Microbial Hydrogen Cycling: Natural Occurrence and Implications for Industry. Microorganisms 2019, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J. FEBEX-DP: Geochemical Modelling of Iron-Bentonite Interactions; Quintessa’s Contribution on Behalf of RWM QRS-1713A-R3, 1.3.; Quintessa Limited: Oxford, UK, 2017; p. 68. [Google Scholar]
- Bengtsson, A.; Blom, A.; Hallbeck, B.; Heed, C.; Johansson, L.; Stahlén, J.; Pedersen, K. Microbial Sulphide-Producing Activity in Water Saturated MX80, Asha and Calcigel Bentonite at Wet Densities from 1500 to 2000 kg m−3; SKB Report TR 16-09; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2016; pp. 1–50. [Google Scholar]
- Pedersen, K. Analysis of copper corrosion in compacted bentonite clay as a function of clay density and growth conditions for sulfate-reducing bacteria. J. Appl. Microbiol. 2010, 108, 1094–1104. [Google Scholar] [CrossRef]
- Chi, F.E.; Athar, R. In situ bacterial colonization of compacted bentonite under deep geological high-level radioactive waste repository conditions. Appl. Microbiol. Biotechnol. 2008, 79, 499–510. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Lukashenko, S.S.; Kudryavtsev, D.B.; Zakharova, L.Y.; Panteleeva, A.R.; Konovalov, A.I.; Jatskevich, E.I. Corrosion Inhibitor—Bactericide for Mineralized Hydrogen Sulfide and Carbon Dioxide Environments. RU 2503746 C1, 10 January 2014. [Google Scholar]
- Domb, A.J.; Brzezinska, M.S.; Walczak, M.; Jankiewicz, U.; Pejchalová, M. Antimicrobial Activity of Polyhexamethylene Guanidine Derivatives Introduced into Polycaprolactone. J. Polym. Environ. 2018, 26, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Kamorny, D.A.; Safonov, A.V.; Boldyrev, K.A.; Abramova, E.S.; Tyupina, E.A.; Gorbunova, O.A. Modification of the Cement Matrix with Organic Additives for Stabilizing Pertechnetate Ions. J. Nucl. Mater. 2021, 557, 153295. [Google Scholar] [CrossRef]
- Varlakov, A.P.; Gorbunova, O.A.; Barinov, A.S.; Iljin, V.A.; Efimov, K.M.; Gembitsky, P.A. Application of Polyhexamethyleneguanidine Type Biocides at Cementing the Radioactive Waste. In Proceedings of the ASME 2001 8th International Conference on Radioactive Waste Management and Environmental Remediation, Bruges, Belgium, 30 September–4 October 2001; pp. 701–703. [Google Scholar]
- Gribi, P.; Johnson, L.; Marschall, P.; Wersin, P. Safety Assessment for a KBS-3H Spent Nuclear Fuel Repository at OLKILUOTO; Process Report. SKB Rapport R-08-36; Swedish Nuclear Fuel and Waste Management Co.: Solna, Sweden, 2008; p. 320. [Google Scholar]
- Abramova, E.S.; Artemyev, G.D.; Popova, N.M.; Safonov, A.V. Microbial Processes in Engineering Clay Materials and Biocidal Additives to Prevent Them. Biotechnology 2021, 37, 65–74. [Google Scholar] [CrossRef]
- Olsson, S.; Karnland, O. Characterisation of Bentonites from Kutch, India and Milos, Greece—Some Candidate Tunnel Back-Fill Materials? Clay Technology AB, SKB Rapport R-09-53. Available online: https://skb.se/upload/publications/pdf/R-09-53.pdf (accessed on 29 November 2021).
- Krupskaya, V.V.; Biryukov, D.V.; Belousov, P.E.; Lekhov, V.A.; Romanchuk, A.Y.; Kalmykov, S.N. The use of natural clay materials to increase the nuclear and radiation safety level of nuclear legacy facilities. Radioact. Waste 2018, 2, 30–43. [Google Scholar]
- Belousov, P.E.; Krupskaya, V.V. Bentonite clays of Russia and neighboring countries. Georesursy Georesources 2019, 21, 79–90. [Google Scholar] [CrossRef]
- Patel, P.K. Lateritization and bentonitization of basalt in Kutch, Gujarat State, India. Sediment. Geol. 1987, 53, 327–346. [Google Scholar] [CrossRef]
- Laverov, N.P.; Yudintsev, S.V.; Kochkin, B.T.; Malkovsky, V.I. The Russian Strategy of using Crystalline Rock as a Repository for Nuclear Waste. Elements 2016, 12, 253–256. [Google Scholar] [CrossRef]
- Rozov, K.B.; Rumynin, V.G.; Nikulenkov, A.M.; Leskova, P.G. Sorption of 137Cs, 90Sr, Se, 99Tc, 152(154)Eu, 239(240)Pu on fractured rocks of the Yeniseysky site (Nizhne-Kansky massif, Krasnoyarsk region, Russia). J. Environ. Radioact. 2018, 192, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Trüper, H.G.; Schlegel, H.G. Sulphur metabolism in Thiorhodaceae I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Leeuwenhoek 1964, 30, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Dong, H. Clay–Microbe Interactions and Implications for Environmental Mitigation. Elements 2012, 8, 113–118. [Google Scholar] [CrossRef]
- Nishiyama, T.; Ueki, A.; Kaku, N.; Watanabe, K.; Ueki, K. Bacteroides graminisolvens sp. nov., a xylanolytic anaerobe isolated from a methanogenic reactor treating cattle waste. Int. J. Syst. Evol. 2009, 59, 1901–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H.; Hugenholtz, P. Fermentation of glycolate by a pure culture of a strictly anaerobic gram-positive bacterium belonging to the family Lachnospiraceae. Arch. Microbiol. 2003, 179, 321–328. [Google Scholar] [CrossRef]
- Abhishek, G.; Avishek, D.; Jayeeta, S.; Panigrahi, M.K.; Sar, P. Low-Abundance Members of the Firmicutes Facilitate Bioremediation of Soil Impacted by Highly Acidic Mine Drainage From the Malanjkhand Copper Project, India. Front. Microbiol. 2019, 9, 2882. [Google Scholar] [CrossRef]
- Cai, M.H.; Luo, G.; Li, J.; Li, W.T.; Li, Y.; Li, A.M. Substrate competition and microbial function in sulfate-reducing internal circulation anaerobic reactor in the presence of nitrate. Chemosphere 2021, 280, 130937. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Hu, C. Impacts of bacteria and corrosion on removal of natural organic matter and disinfection byproducts in different drinking water distribution systems. Int. Biodeterior. Biodegrad. 2017, 117, 52–59. [Google Scholar] [CrossRef]
- Singh, P.B.; Saini, H.S.; Kahlon, R.S. Pseudomonas: The versatile and adaptive metabolic network. In Pseudomonas: Molecular and Applied Biology, 1st ed.; Kahlon, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 81–126. [Google Scholar] [CrossRef]
- Wood, T.L.; Gong, T.; Zhu, L.; Miller, J.; Miller, D.S.; Yin, B.; Wood, T.K. Rhamnolipids from Pseudomonas aeruginosa disperse the biofilms of sulfate-reducing bacteria. npj Biofilms Microbiomes 2018, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelouas, A.; Grambow, B.; Fattahi, M.; Andres, Y.; Leclerc-Cessac, E. Microbial reduction of 99Tc in organic matter-rich soils. Sci. Total Environ. 2005, 336, 255–268. [Google Scholar] [CrossRef]

| Clay Type | Designation | Sample |
|---|---|---|
| Bentonite | B-1 | Kutch deposit (India) [33] |
| B-2 | Tagansk deposit, Dinozavrov (Kazakhstan) [34] | |
| B-3 | Ceraceous bentonite, Kamalinsk deposit (Krasnoyarsk region, Russia) [10] | |
| B-4 | 10th Khutor deposit (Khakassiya, Russia) | |
| B-5 | Zyryanskoye deposit of bentonite (Kurgan oblast, Russia) [10] | |
| Kaolin | K-1 | Kantatsk deposit (Krasnoyarsk region, Russia) [10] |
| K-2 | Kampanovsk deposit (Krasnoyarsk region, Russia) [11] |
| Sample | K | Na | Ca | Mg | Fe | S | C * | N | P | C/N |
|---|---|---|---|---|---|---|---|---|---|---|
| B-1 | 0.06 | 1.40 | 1.50 | 2.87 | 14.0 | 0.05 | 0.19 | 0 | 0 | 0 |
| B-2 | 0.05 | 1.07 | 1.45 | 3.33 | 3.2 | 0.05 | 0.14 | 0 | 0 | 0 |
| B-3 | 1.08 | 0.1 | 1.68 | 3.42 | 3.4 | <0.02 | 0.01 | 0 | 0.05 | 0 |
| B-4 | 0.71 | 0.91 | 2.05 | 2.73 | 2.4 | 0.04 | 0.34 | 0.01 | 0.1 | 34.2 |
| B-5 | 0.78 | 0.57 | 3.93 | 1.73 | 4.2 | 0.08 | 0.86 | 0.03 | 0.2 | 25.2 |
| K-1 | 1.01 | 0.68 | 1.54 | 2.87 | 3.4 | <0.02 | 0.41 | 0.03 | 0.1 | 13.7 |
| K-2 | 1.3 | 0.03 | 0.30 | 0.53 | 1.6 | <0.02 | 0.11 | 0 | 0 | 0 |
| Sample | Quartz | Clay Minerals | Feldspars | Other Minerals | Residual * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kaolinite | Montmorillonite | Illite | Muscovite | PFS | Plagioclases | Calcite | Anatase | |||
| B-1 | 4 | - | 78 | - | - | - | - | 1.5 | 1.5 | 15 |
| B-2 | 9 | - | 89.5 | - | - | - | - | 1.5 | - | - |
| B-3 | 27 | 5 | 60 | - | 1 | 6 | - | - | - | 1 |
| B-4 | 11.5 | 4 | 71 | 1 | - | 4 | 5 | 3 | - | 0.5 |
| B-5 | 16 | 6 | 67 | 3 | - | 0.5 | 1.5 | 5 | 1 | - |
| K-1 | 25 | 24 | 15 | 5 | - | 5 | 6 | 1.5 | 1 | 17.5 |
| K-2 | 8.5 | 81 | 2 | 2 | - | 6 | - | - | - | 0.5 |
| Sample | Na | K | Ca | Mg | Fe | S | P | ∑ Leaching | pH |
|---|---|---|---|---|---|---|---|---|---|
| B-1 ws | 8.4 ± 0.2 | 1.3 ± 0.03 | 5.2 ± 0.13 | 11.5 ± 0.29 | 2.8 ± 0.07 | l.d.l | l.d.l | 29.2 | 7.8 |
| as | 77 ± 0.18 | 3.6 ± 0.07 | 105 ± 2.6 | 186.55 ± 4.7 | 1260 ± 31.5 | 0.75 ± 0.01 | l.d.l | 2007 | - |
| B-2 ws | 26.5 ± 0.66 | 1 ± 0.03 | 19.5 ± 0.49 | 3.1 ± 0.08 | 0.1 ± 0.003 | l.d.l | l.d.l | 50.3 | 8.2 |
| as | 58.85 ± 1.42 | 3 ± 0.09 | 101.5 ± 2.5 | 216.45 ± 5.6 | 288 ± 8.64 | 1.1 ± 0.03 | l.d.l | 910 | - |
| B-3 ws | 5.2 ± 0.13 | 2.3 ± 0.06 | 6.1 ± 0.15 | 2.9 ± 0.07 | 0.6 ± 0.02 | l.d.l | l.d.l | 17.2 | 6.3 |
| as | 5.5 ± 0.13 | 64.8 ± 1.7 | 117.6 ± 2.9 | 222.3 ± 5.3 | 306 ± 10.16 | l.d.l | 2.25 ± 0.05 | 968 | - |
| B-4 ws | 10.7 ± 0.27 | 7.3 ± 0.18 | 2.6 ± 0.07 | 3.4 ± 0.09 | 2.5 ± 0.06 | l.d.l | l.d.l | 26.7 | 8.5 |
| as | 50.05 ± 1.29 | 42.6 ± 1.09 | 143.5 ± 3.8 | 177.45 ± 4.55 | 213.6 ± 4.83 | 0.68 ± 0.01 | 4.5 ± 0.09 | 880 | - |
| B-5 ws | 11.7 ± 0.29 | 2.2 ± 0.06 | 13.5 ± 0.34 | 5.9 ± 0.15 | 0.3 ± 0.008 | l.d.l | l.d.l | 33.6 | 7.4 |
| as | 31.35 ± 0.8 | 46.8 ± 1.29 | 275.1 ± 6.89 | 112.45 ± 2.8 | 369.6 ± 8.79 | 1.44 ± 0.04 | 9 ± 0.11 | 1127 | - |
| K-1 ws | 13.2 ± 0.33 | 4.3 ± 0.11 | 4.8 ± 0.12 | 10.9 ± 0.27 | 0.2 ± 0.005 | l.d.l | l.d.l | 33.7 | 7.4 |
| as | 37.4 ± 0.9 | 60.6 ± 1.6 | 107.8 ± 2.7 | 186.55 ± 4.6 | 265.2 ± 6.55 | l.d.l | 4.7 ± 0.09 | 950 | - |
| K-2 ws | 14 ± 0.35 | 1.9 ± 0.05 | 3.3 ± 0.08 | 2.1 ± 0.05 | 2.6 ± 0.07 | l.d.l | l.d.l | 24 | 7.0 |
| as | 16.5 ± 0.41 | 61.8 ± 1.57 | 21 ± 0.52 | 34.45 ± 0.8 | 120 ± 3.15 | l.d.l | l.d.l | 376 |
| B-1 | B-2 | B-3 | B-4 | B-5 | K-1 | K-2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysobacter | 76 | Bacillus | 68 | Massilia | 40 | Bacteroides | 24 | Noviherbaspirillum | 21 | Pseudomonas | 68 | Bacillus | 5 |
| Escherichia-Shigella | 3 | Massilia | 18 | Noviherbaspirillum | 11 | Pseudomonas | 4 | Hydrogenophaga | 25 | Bacillus | 16 | Bosea | 6 |
| Ralstonia | 1 | Noviherbaspirillum | 3 | Aquaspirillum | 3 | Acinetobacter | 3 | Brevundimonas | 13 | Unknown | 8 | Cupriavidus | 9 |
| Herbaspirillum | 1 | Aquaspirillum | 1 | Cupriavidus | 1 | Lachnospiraceae | 22 | Caldimonas | 2 | Ralstonia | 2 | Ralstonia | 9 |
| Cupriavidus | 1 | Cupriavidus | 1 | Pseudomonadales | 1 | Ruminococcaceae | 24 | Acidovorax | 2 | Cupriavidus | 1 | Ramlibacter | 5 |
| Aquabacterium | 1 | Pseudomonadales | 1 | Xanthomonadales | 1 | Actinobacteria | 3 | Pelomonas | 3 | Streptococcus | 2 | Gemmatimonas | 1 |
| Sphigomonas | 3 | Acinetobacter | 1 | Methylobacterium | 1 | Hungatella | 3 | Methyloversatilis | 5 | Sphingomonas | 1 | Nocardioides | 12 |
| Devosia | 1 | Psychrobacter | 1 | Afipia | 1 | Brevundimonas | 1 | Thiobacillus | 2 | Rhizobium | 1 | Massilia | 8 |
| Gemmobacter | 1 | Escherichia | 3 | Sphingomonas | 2 | Xanthomonadales | 1 | Xanthomonadales | 4 | Alloprevotella | 1 | Domibacillus | 1 |
| Ensifer | 1 | Klebsiella | 1 | Brevundimonas | 2 | Unknown | 15 | Sphingobium | 3 | Pajaroellobacter | 2 | ||
| Hydrobacter | 1 | Unknown | 2 | Hydrobacter | 8 | Rhizobiales | 11 | Rhizobium gr. | 20 | ||||
| Ensifer | 1 | Corynebacterium | 1 | Oleomonas | 2 | Rubellimicrobium | 5 | ||||||
| Corynebacterium | 2 | Actinomyces | 1 | Actinobacteria | 1 | Unknown | 17 | ||||||
| Brachybacterium | 1 | Lawsonella | 1 | Lachnospiraceae | 1 | ||||||||
| Bacillus | 2 | Bacillus | 14 | Pseudomonas | 1 | ||||||||
| Clostridiales | 1 | Streptococcus | 3 | Unknown | 4 | ||||||||
| Actinobacteria | 1 | Lactobacillales | 2 | ||||||||||
| Unknown | 2 | Unknown | 6 | ||||||||||
| Sample | MTTinit | KMTTox | KMTTanox |
|---|---|---|---|
| B-1 | 1.2 ± 0.03 | 3.4 | 13.18 |
| B-2 | 0.7 ± 0.02 | 3.0 | 6.6 |
| B-3 | 1.0 ± 0.03 | 10.8 | 11.5 |
| B-4 | 0.7 ± 0.02 | 12.0 | 1.9 |
| B-5 | 0.7 ± 0.02 | 29.0 | 21.71 |
| K-1 | 1.8 ± 0.05 | 3.5 | 0.6 |
| K-2 | 1.8 ± 0.05 | 9.0 | 6.1 |
| Sample | Acetate | Н2 | PO4−3 | SO4−2 | CO2 |
|---|---|---|---|---|---|
| B-1 | 3.1 | 2.9 | 1.8 | 1.5 | 1.3 |
| B-2 | 4.3 | 3.1 | 2.3 | 2.5 | 1.4 |
| B-3 | 2.9 | 2.2 | 1.6 | 1.3 | 1.2 |
| B-4 | 3.6 | 2.9 | 2.6 | 2.2 | 1.1 |
| B-5 | 5.5 | 3.9 | 2.9 | 3.1 | 1.6 |
| K-1 | 4.2 | 2.9 | 2.5 | 2.5 | 1.1 |
| K-2 | 3.3 | 2.5 | 1.9 | 1.5 | 1.2 |
| Sample | K | Na | Si | Ca | Al | Fe | ∑ |
|---|---|---|---|---|---|---|---|
| B-1 | 2.91 | 1.19 | 2.43 | 1.28 | 3.89 | 16.22 | 27.89 |
| B-2 | 1.29 | 1.01 | 0.72 | 0.46 | 2.5 | 0.19 | 6.17 |
| B-3 | 1.38 | 1.02 | 0.71 | 0.65 | 2.63 | 0.37 | 6.65 |
| B-4 | 1.02 | 1 | 1.87 | 0.33 | 2.27 | 2.5 | 8.9 |
| B-5 | 1.92 | 1.04 | 9.21 | 1.89 | 16.73 | 0.87 | 31.66 |
| K-1 | 2.14 | 1.1 | 4.62 | 1.44 | 6.22 | 0.44 | 15.96 |
| K-2 | 3.09 | 1.2 | 2.02 | 0.52 | 6.25 | 0.3 | 12.94 |
| Parameters | B-1 | B-2 | B-3 | B-4 | B-5 | K-1 | K-2 | |
|---|---|---|---|---|---|---|---|---|
| Biogenic elements in clays | Fe | 4 | 4 | 4 | 2 | 5 | 1 | 1 |
| S | 3 | 3 | 1 | 2 | 5 | 1 | 1 | |
| C | 2 | 3 | 1 | 2 | 5 | 4 | 1 | |
| N | 1 | 1 | 1 | 1 | 3 | 2 | 1 | |
| P | 1 | 1 | 1 | 2 | 4 | 2 | 1 | |
| Acid leachate ∑ | 5 | 3 | 3 | 3 | 4 | 3 | 1 | |
| Bioleachates | Kʟ | 5 | 1 | 2 | 1 | 3 | 3 | 5 |
| Siʟ | 3 | 1 | 1 | 2 | 5 | 3 | 2 | |
| Alʟ | 2 | 1 | 1 | 1 | 5 | 3 | 3 | |
| Caʟ | 3 | 1 | 2 | 1 | 5 | 4 | 1 | |
| Feʟ | 5 | 1 | 1 | 2 | 1 | 1 | 1 | |
| Naʟ | 2 | 1 | 4 | 1 | 2 | 5 | 4 | |
| MTTinit | 3 | 1 | 2 | 1 | 1 | 5 | 5 | |
| MTTox | 1 | 1 | 3 | 1 | 5 | 2 | 3 | |
| MTTanox | 2 | 2 | 5 | 1 | 5 | 1 | 5 | |
| H₂S | 1 | 1 | 1 | 3 | 5 | 3 | 2 | |
| Total | 43 | 26 | 33 | 26 | 63 | 43 | 37 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramova, E.; Popova, N.; Artemiev, G.; Zharkova, V.; Zakharova, E.; Safonov, A. Characteristics and Rates of Microbial Processes in Clays of Different Mineral and Elemental Composition in Relation to Safety Prediction for ESB Clay Materials. Appl. Sci. 2022, 12, 1843. https://doi.org/10.3390/app12041843
Abramova E, Popova N, Artemiev G, Zharkova V, Zakharova E, Safonov A. Characteristics and Rates of Microbial Processes in Clays of Different Mineral and Elemental Composition in Relation to Safety Prediction for ESB Clay Materials. Applied Sciences. 2022; 12(4):1843. https://doi.org/10.3390/app12041843
Chicago/Turabian StyleAbramova, Elena, Nadezhda Popova, Grigoriy Artemiev, Viktoria Zharkova, Elena Zakharova, and Alexey Safonov. 2022. "Characteristics and Rates of Microbial Processes in Clays of Different Mineral and Elemental Composition in Relation to Safety Prediction for ESB Clay Materials" Applied Sciences 12, no. 4: 1843. https://doi.org/10.3390/app12041843
APA StyleAbramova, E., Popova, N., Artemiev, G., Zharkova, V., Zakharova, E., & Safonov, A. (2022). Characteristics and Rates of Microbial Processes in Clays of Different Mineral and Elemental Composition in Relation to Safety Prediction for ESB Clay Materials. Applied Sciences, 12(4), 1843. https://doi.org/10.3390/app12041843








