Bentonite–Concrete Interactions in Engineered Barrier Systems during the Isolation of Radioactive Waste Based on the Results of Short-Term Laboratory Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Bentonites of the 10th Khutor Deposit
2.2. Concrete
2.3. Composition of the Synthetic Water
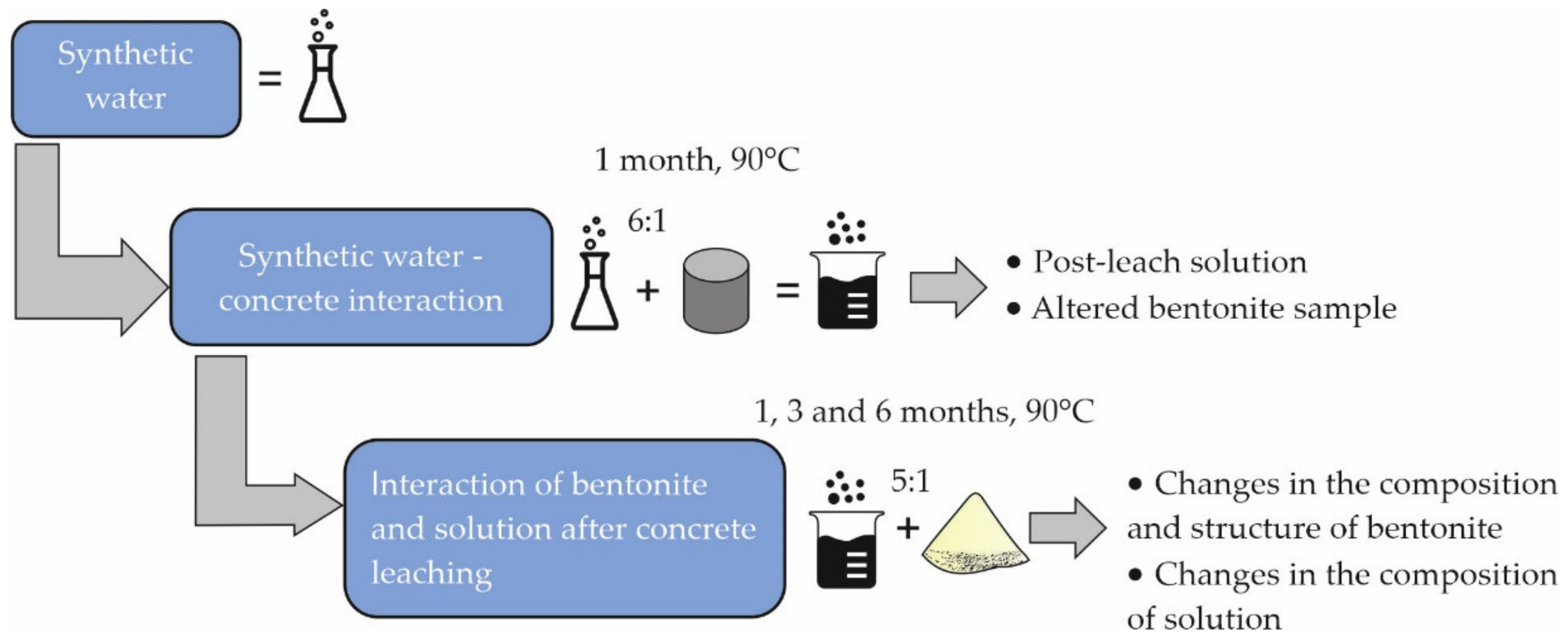
2.4. Experiment Progress
2.5. Methods
2.6. Geochemical Modeling
3. Results and Discussion
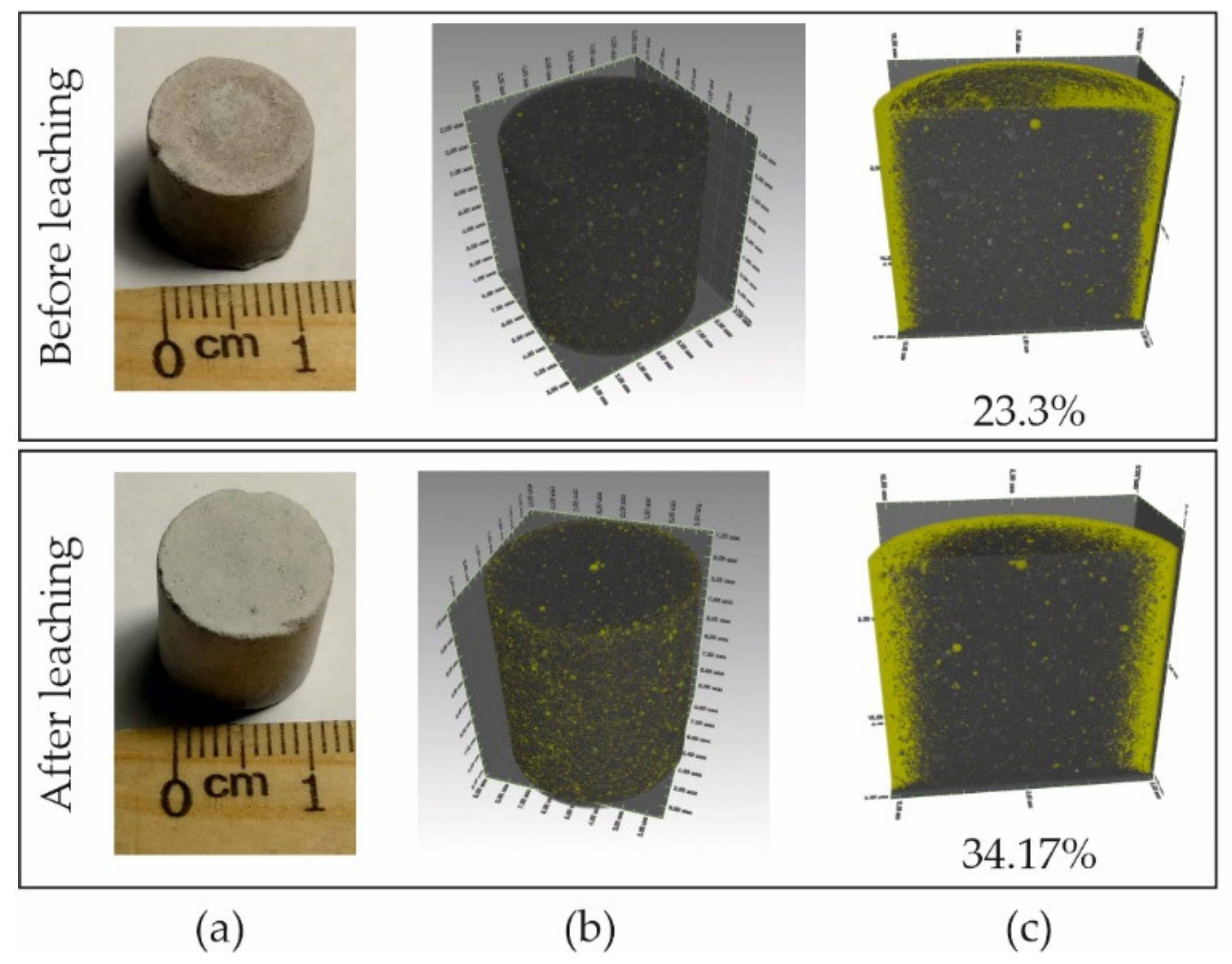
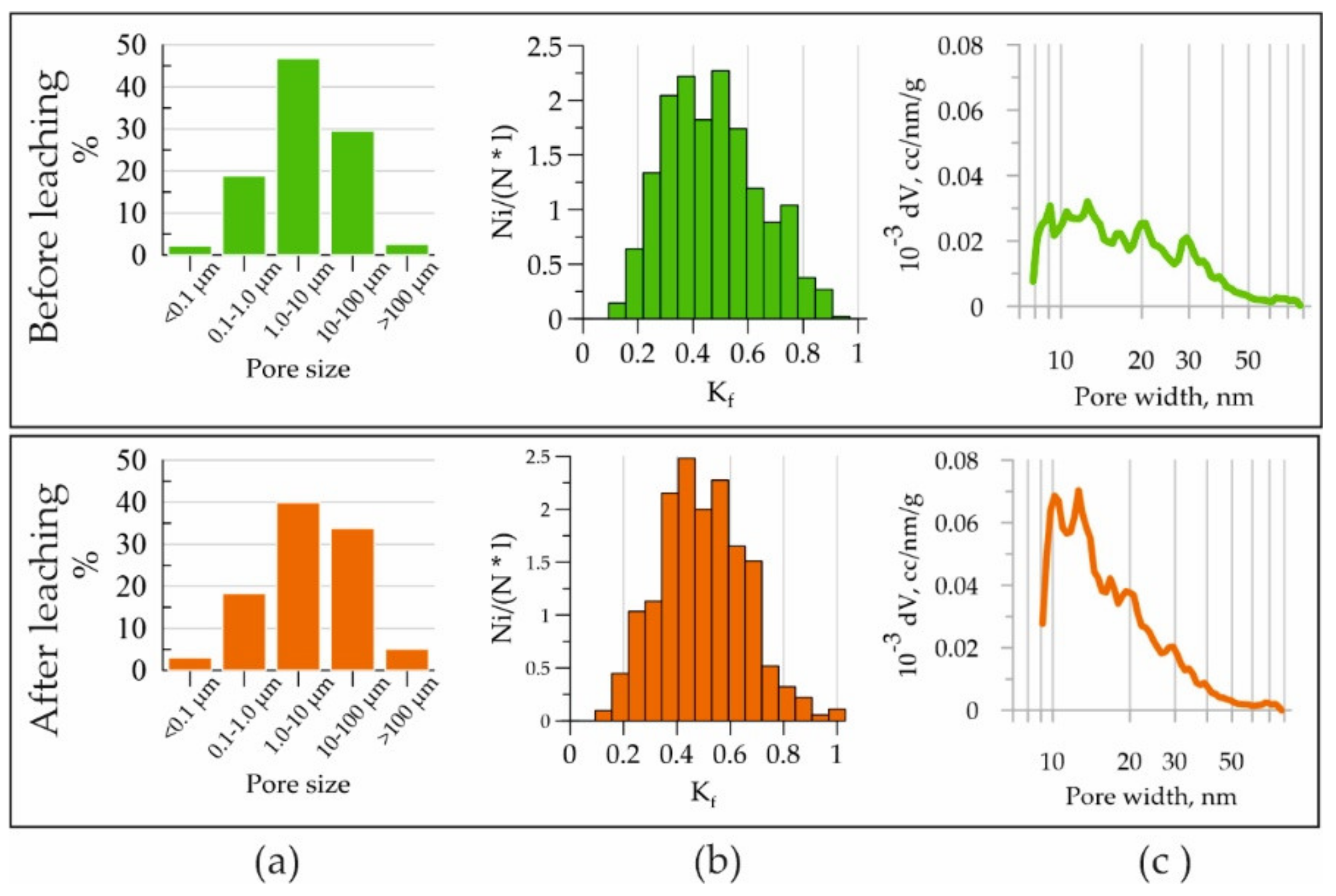
3.1. Changes in the Structure of the Pore Space of Concrete during Leaching Experiments
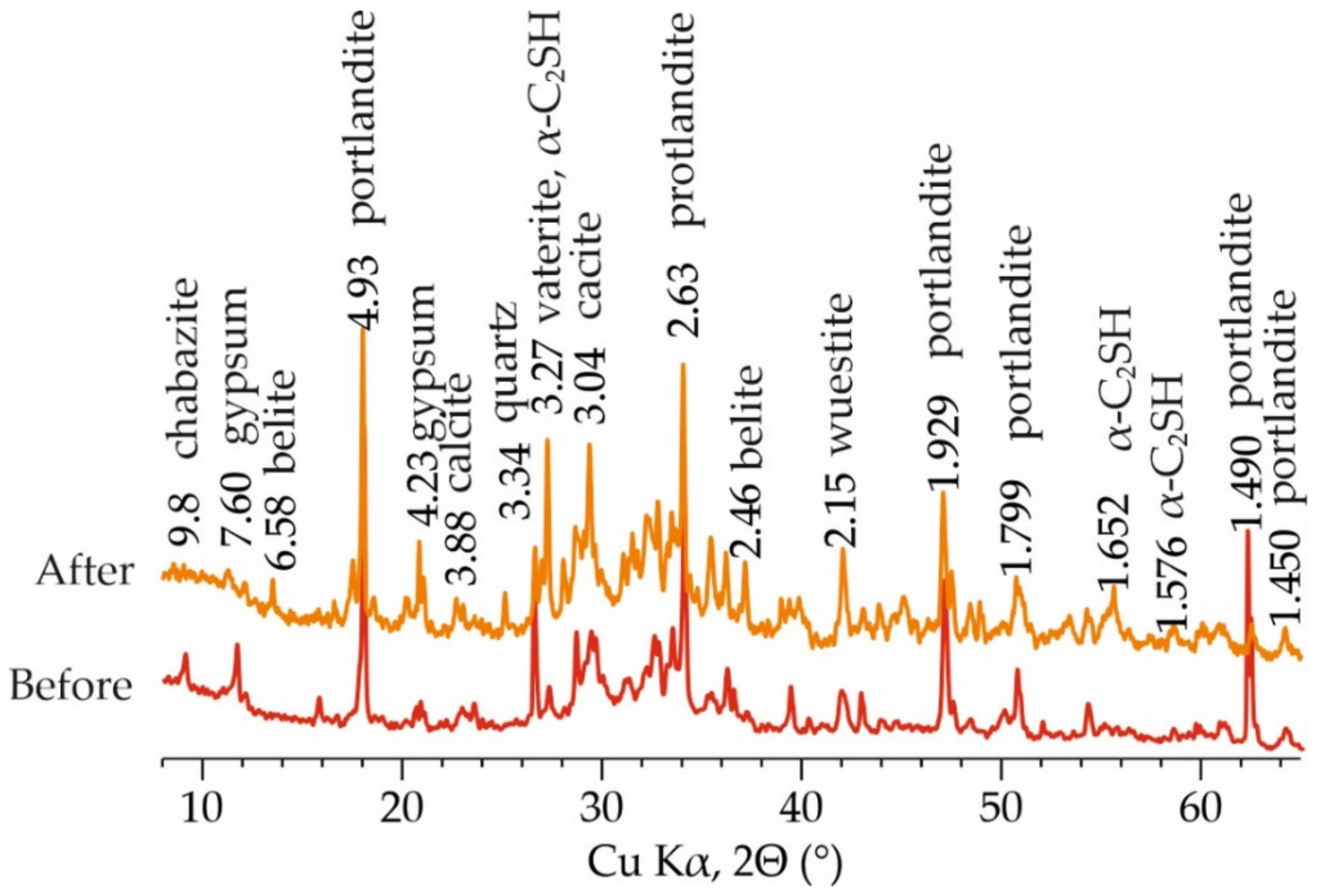
3.2. Change in Concrete Composition during Leaching Experiments
3.3. Changes in the Composition and Structure of Bentonite during Experiments
3.4. Modeling of Bentonite Evolution Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scientific and Technical Basis for the Geological Disposal of Radioactive Wastes; Technical Reports Series; International Atomic Energy Agency: Vienna, Austria, 2003.
- Sellin, P.; Leupin, O.X. The Use of Clay as an Engineered Barrier in Radioactive-Waste Management a Review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Tsebakovskaya, N.S.; Utkin, S.S.; Kapyrin, I.V.; Medyantsev, N.V.; Shamina, A.V. Review of Foreign Practices for Disposal of Spent Nuclear Fuel and Radioactive Waste; Komtekhprint: Moscow, Russia, 2015. [Google Scholar]
- Dixon, D.; Sandén, T.; Jonsson, E.; Hansen, J. Backfilling of Deposition Tunnels: Use of Bentonite Pellets; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2011. [Google Scholar]
- Boerjesson, L.; Gunnarsson, D.; Johannesson, L.-E.; Jonsson, E. Design, Production and Initial State of the Buffer; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2010. [Google Scholar]
- Keto, P.; Dixon, D.; Jonsson, E.; Gunnarsson, D. R-09-52 Assessment of Backfill design for KBS-3V Repository; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2009. [Google Scholar]
- Fries, T.; Claudel, A.; Weber, H.; Johnson, L.; Leupin, O. The Swiss concept for the disposal of spent fuel and vitrified HLW. In Proceedings of the International Technical Conference on the Practical Aspects of Deep Geological Disposal of Radioactive Waste, Prague, Czech Republic, 16–18 June 2008; pp. 45–53. [Google Scholar]
- Kaufhold, S.; Dohrmann, R. Distinguishing between more and less suitable bentonites for storage of high-level radioactive waste. Clay Miner. 2016, 51, 289–302. [Google Scholar] [CrossRef]
- Birgersson, M.; Hedström, M.; Karnland, O.; Sjöland, A. Bentonite buffer: Macroscopic performance from nanoscale properties. In Geological Repository Systems for Safe Disposal of Spent Nuclear Fuels and Radioactive Waste; Apted, M.J., Joonhong, A., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 319–364. ISBN 9781845699789. [Google Scholar]
- Krupskaya, V.V.; Zakusin, S.V.; Lekhov, V.A.; Dorzhieva, O.V.; Belousov, P.E.; Tyupina, E.A. Buffer Properties of Bentonite Barrier Systems for Radioactive Waste Isolation in Geological Repository in the Nizhnekanskiy Massif. Radioact. Waste 2020, 1, 35–55. [Google Scholar] [CrossRef]
- Karnland, O.; Olsson, S.; Nilsson, U. Mineralogy and Sealing Properties of Various Bentonites and Smectite-Rich Clay Materials; SKB Technical Report TR-06-30; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 2006; pp. 1–112. [Google Scholar]
- A Review of the Development of Bentonite Barriers in KBS-3V Disposal Concept; Harwell: Oxford, UK, 2014.
- Krupskaya, V.V.; Biryukov, D.V.; Belousov, P.E.; Lekhov, V.A.; Romanchuk, A.Y.; Kalmykov, S.N. The use of natural clay materials to increase the nuclear and radiation safety level of nuclear legacy facilities. Radioact. Waste 2018, 2, 30–43. [Google Scholar]
- Dorofeev, A.N.; Bolshov, L.A.; Linge, I.I.; Utkin, S.S.; Saveleva, E.A. Strategic Master Plan for R&D Demonstrating the Safety of Construction, Operation and Closure of a Deep Geological Disposal Facility for Radioactive Waste. Radioact. Waste 2017, 1, 33–41. [Google Scholar]
- Bogatov, S.A.; Kryuchkov, D.V.; Pavlov, D.I.; Sychenko, D.V. Analysis of Various Concepts for RW Class 1 Disposal in Crystalline Rocks. Radioact. Waste 2020, 12, 66–77. [Google Scholar] [CrossRef]
- Alonso, M.C.; García Calvo, J.L.; Cuevas, J.; Turrero, M.J.; Fernández, R.; Torres, E.; Ruiz, A.I. Interaction processes at the concrete-bentonite interface after 13 years of FEBEX-Plug operation. Part I: Concrete alteration. Phys. Chem. Earth 2017, 99, 38–48. [Google Scholar] [CrossRef]
- Fernández, R.; Torres, E.; Ruiz, A.I.; Cuevas, J.; Alonso, M.C.; García Calvo, J.L.; Rodríguez, E.; Turrero, M.J. Interaction processes at the concrete-bentonite interface after 13 years of FEBEX-Plug operation. Part II: Bentonite contact. Phys. Chem. Earth 2017, 99, 49–63. [Google Scholar] [CrossRef]
- Robineau, M.; Deydier, V.; Crusset, D.; Bellefleur, A.; Neff, D.; Vega, E.; Sabot, R.; Jeannin, M.; Refait, P. Formation of iron sulfides on carbon steel in a specific cement grout designed for radioactive waste repository and associated corrosion mechanisms. Materials 2021, 14, 3563. [Google Scholar] [CrossRef]
- The Scientific Foundations of Deep Geological Disposal; United Kingdom Nirex Limited: Harwell, UK, 2001; p. 118.
- IAEA. The Behaviours of Cementitious Materials in Long Term Storage and Disposal of Radioactive Waste: Results of a Coordinated Research Project; IAEA: Vienna, Austria, 2013; ISBN 9789201393104. [Google Scholar]
- Bogatov, S.A. Disposal of in Verticale Deposition Holes with Cement Backfill—Pro and Contra with Regard to Long Term Safety of Geological Disposal Facility. Radioact. Waste 2018, 1, 21–33. [Google Scholar]
- Dickinson, M.; Holton, D.; Cairns, M. Development of Understanding of Disposability of High-Heat-Generating Wastes in the UK. In Proceedings of the WM2015 Conference, Phoenix, AR, USA, 15–19 March 2015; p. 13. [Google Scholar]
- García Calvo, J.L.; Hidalgo, A.; Alonso, C.; Fernández Luco, L. Development of low-pH cementitious materials for HLRW repositories: Resistance against ground waters aggression. Cem. Concr. Res. 2010, 40, 1290–1297. [Google Scholar] [CrossRef]
- Kozlov, P.V.; Gorbunova, O.A. Cementing as a Method of Radioactive Waste Immobilization; Kozlov, P.V., Gorbunova, O.A., Eds.; PO “Mayak”: Ozersk, Russia, 2011; ISBN 978-5-903159-30-7. [Google Scholar]
- Ochs, M.; Mallants, D.; Wang, L. Radionuclide and Metal Sorption on Cement and Concrete (Topics in Safety, Risk, Reliability and Quality); Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-23650-6. [Google Scholar]
- Jacques, D.; Wang, L.; Martens, E.; Mallants, D. Time Dependence of the Geochemical Boundary Conditions for the Cementitious Engineered Barriers of the Belgian Surface Disposal Facility. Project Near Surface Disposal of Category A Waste at Dessel NIRAS-MP5-03 DATA-LT(NF). Version 1.; NIROND-TR. 2009. Available online: https://www.researchgate.net/profile/Dirk-Mallants/publication/248708031_Time_dependence_of_the_geochemical_boundary_conditions_for_the_cementitious_engineered_barriers_of_the_Belgian_surface_disposal_facility/links/0deec51e155c435821000000/Time-dependence-of-the-geochemical-boundary-conditions-for-the-cementitious-engineered-barriers-of-the-Belgian-surface-disposal-facility.pdf (accessed on 19 December 2021).
- Zakusin, S.V.; Krupskaya, V.V.; Dorzhieva, O.V.; Tyupina, E.A.; Zhuhlistov, A.P. Modification of the adsorption properties of montmorillonite by the thermochemical treatment. Sorption Chromatogr. Process. 2015, 15, 874–883. [Google Scholar]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Chernov, M.S.; Bychkova, Y. V Transformation of Structure and Adsorption Properties of Montmorillonite under Thermochemical Treatment. Geochemistry Int. 2019, 57, 314–330. [Google Scholar] [CrossRef]
- Krupskaya, V.; Novikova, L.; Tyupina, E.; Belousov, P.; Dorzhieva, O.; Zakusin, S.; Kim, K.; Roessner, F.; Badetti, E.; Brunelli, A.; et al. The influence of acid modification on the structure of montmorillonites and surface properties of bentonites. Appl. Clay Sci. 2019, 172, 1–10. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R.; Ufer, K.; Kober, F. Interactions of bentonite with metal and concrete from the FEBEX experiment: Mineralogical and geochemical investigations of selected sampling sites. Clay Miner. 2018, 53, 745–763. [Google Scholar] [CrossRef]
- Sánchez, L.; Cuevas, J.; Ramírez, S.; Riuiz De León, D.; Fernández, R.; Vigil Dela Villa, R.; Leguey, S. Reaction kinetics of FEBEX bentonite in hyperalkaline conditions resembling the cement-bentonite interface. Appl. Clay Sci. 2006, 33, 125–141. [Google Scholar] [CrossRef]
- Ramírez, S.; Cuevas, J.; Vigil, R.; Leguey, S. Hydrothermal alteration of “La Serrata” bentonite (Almeria, Spain) by alkaline solutions. Appl. Clay Sci. 2002, 21, 257–269. [Google Scholar] [CrossRef]
- Savage, D.; Bateman, K.; Hill, P.; Hughes, C.; Milodowski, A.; Pearce, J.; Rae, E.; Rochelle, C. Rate and mechanism of the reaction of silicates with cement pore fluids. Appl. Clay Sci. 1992, 7, 33–45. [Google Scholar] [CrossRef]
- Liu, L.N.; Chen, Y.G.; Ye, W.M.; Cui, Y.J.; Wu, D.B. Effects of hyperalkaline solutions on the swelling pressure of compacted Gaomiaozi (GMZ) bentonite from the viewpoint of Na+ cations and OH– anions. Appl. Clay Sci. 2018, 161, 334–342. [Google Scholar] [CrossRef]
- Kaufhold, S.; Dohrmann, R. Stability of bentonites in salt solutions III—Calcium hydroxide. Appl. Clay Sci. 2011, 51, 300–307. [Google Scholar] [CrossRef]
- Karnland, O.; Olsson, S.; Nilsson, U.; Sellin, P. Experimentally determined swelling pressures and geochemical interactions of compacted Wyoming bentonite with highly alkaline solutions. Phys. Chem. Earth 2007, 32, 275–286. [Google Scholar] [CrossRef]
- Fernández, R.; Rodríguez, M.; de la Villa, R.V.; Cuevas, J. Geochemical constraints on the stability of zeolites and C-S-H in the high pH reaction of bentonite. Geochim. Cosmochim. Acta 2010, 74, 890–906. [Google Scholar] [CrossRef]
- Fernández, R.; Ruiz, A.I.; Cuevas, J. The role of smectite composition on the hyperalkaline alteration of bentonite. Appl. Clay Sci. 2014, 95, 83–94. [Google Scholar] [CrossRef]
- Cuisinier, O.; Masrouri, F.; Pelletier, M.; Villieras, F.; Mosser-Ruck, R. Microstructure of a compacted soil submitted to an alkaline PLUME. Appl. Clay Sci. 2008, 40, 159–170. [Google Scholar] [CrossRef]
- Cuevas, J.; Fernández, R.; Sánchez, L.; Vigil de la Villa, R.; Rodríguez, M.; Leguey, S. Reactive Diffusion Front Driven by an Alkaline Plume in Compacted Mg—Homoionic Bentonite. pp. 1–2. 2007. Available online: https://www.andra.fr/mini-sites/lille2007/abstract_lille2007/donnees/pdf/509_510_P_MTPM_44.pdf (accessed on 24 December 2021).
- Claret, F.; Bauer, A.; Schäfer, T.; Griffault, L.; Lanson, B. Experimental investigation of the interaction of clays with high-pH solutions: A case study from the Callovo-Oxfordian formation, Meuse-Haute Marne underground laboratory (France). Clays Clay Miner. 2002, 50, 633–646. [Google Scholar] [CrossRef]
- Bauer, A.; Lanson, B.; Ferrage, E.; Emmerich, K.; Taubald, H.; Schild, D.; Velde, B. The fate of smectite in KOH solutions. Am. Mineral. 2006, 91, 1313–1322. [Google Scholar] [CrossRef][Green Version]
- Anh, H.N.; Ahn, H.; Jo, H.Y.; Kim, G.Y. Effect of alkaline solutions on bentonite properties. Environ. Earth Sci. 2017, 76, 1–10. [Google Scholar] [CrossRef]
- Savage, D.; Benbow, S.; Watson, C.; Takase, H.; Ono, K.; Oda, C.; Honda, A. Natural systems evidence for the alteration of clay under alkaline conditions: An example from Searles Lake, California. Appl. Clay Sci. 2010, 47, 72–81. [Google Scholar] [CrossRef]
- Pusch, R.; Zwahr, H.; Gerber, R.; Schomburg, J. Interaction of cement and smectitic clay—Theory and practice. Appl. Clay Sci. 2003, 23, 203–210. [Google Scholar] [CrossRef]
- Mosser-Ruck, R.; Cathelineau, M. Experimental transformation of Na,Ca-smectite under basic conditions at 150 °C. Appl. Clay Sci. 2004, 26, 259–273. [Google Scholar] [CrossRef]
- Savage, D.; Noy, D.; Mihara, M. Modelling the interaction of bentonite with hyperalkaline fluids. Appl. Geochem. 2002, 17, 207–223. [Google Scholar] [CrossRef]
- Savage, D.; Walker, C.; Arthur, R.; Rochelle, C.; Oda, C.; Takase, H. Alteration of bentonite by hyperalkaline fluids: A review of the role of secondary minerals. Phys. Chem. Earth 2007, 32, 287–297. [Google Scholar] [CrossRef]
- Eberl, D.D.; Velde, B.; Mccormick, T. Synthesis of illite-smectite from smectite at Earth surface temperatures and high pH. Clay Miner. 1993, 28, 49–60. [Google Scholar] [CrossRef]
- Dauzeres, A.; Le Bescop, P.; Sardini, P.; Cau Dit Coumes, C. Physico-chemical investigation of clayey/cement-based materials interaction in the context of geological waste disposal: Experimental approach and results. Cem. Concr. Res. 2010, 40, 1327–1340. [Google Scholar] [CrossRef]
- Carlson, L.; Karnland, O.; Oversby, V.M.V.M.; Rance, A.P.; Smart, N.R.; Snellman, M.; Vähänen, M.; Werme, M.; Werme, L.O. Experimental studies of the interactions between anaerobically corroding iron and bentonite. Phys. Chem. Earth 2007, 32, 334–345. [Google Scholar] [CrossRef]
- González-Santamaría, D.E.; Fernández, R.; Ruiz, A.I.; Ortega, A.; Cuevas, J. High-pH/low pH ordinary Portland cement mortars impacts on compacted bentonite surfaces: Application to clay barriers performance. Appl. Clay Sci. 2020, 193, 105672. [Google Scholar] [CrossRef]
- Marty, N.C.M.; Claret, F.; Lassin, A.; Tremosa, J.; Blanc, P.; Madé, B.; Giffaut, E.; Cochepin, B.; Tournassat, C. A database of dissolution and precipitation rates for clay-rocks minerals. Appl. Geochem. 2015, 55, 108–118. [Google Scholar] [CrossRef]
- Belousov, P.E.; Krupskaya, V.V.; Zakusin, S.V.; Zhigarev, V.V. Bentonite Clays From 10Th Khutor Deposite: Features of Genesis, Composition and Adsorption Properties. Rudn. J. Eng. Res. 2017, 18, 135–143. [Google Scholar] [CrossRef]
- Belousov, P.E.; Krupskaya, V.V. Bentonite clays of Russia and neighboring countries. Georesursy 2019, 21, 79–90. [Google Scholar] [CrossRef]
- Belousov, P.; Chupalenkov, N.; Christidis, G.; Zakusina, O.; Zakusin, S.; Morozov, I.; Chernov, M.; Zaitseva, T.; Tyupina, E.; Krupskaya, V. Carboniferous Bentonites of 10th Khutor Deposit (Russia): Geology, Mineralogy, Genesis and Properties. Appl. Clay Sci. 2021, 215, 106308. [Google Scholar] [CrossRef]
- Rozov, K.B.; Rumynin, V.G.; Nikulenkov, A.M.; Leskova, P.G. Sorption of 137Cs, 90Sr, Se, 99Tc, 152(154)Eu, 239(240)Pu on fractured rocks of the Yeniseysky site (Nizhne-Kansky massif, Krasnoyarsk region, Russia). J. Environ. Radioact. 2018, 192, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Meleshyn, A.Y.A.Y.; Zakusin, S.V.; Krupskaya, V.V. Swelling pressure and permeability of compacted bentonite from 10th khutor deposit (Russia). Minerals 2021, 11, 742. [Google Scholar] [CrossRef]
- Sokolov, V.N.; Yurkovets, D.I.; Razgulina, O.V. Study of soil microstructure using computer analysis of SEM images. Geoekologia. Inzhenernaya Geol. Gidrogeol. Geokriol. 2008, 4, 377–382. [Google Scholar]
- Moore, D.M.; Reynolds, R.C.J. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. Rev. Mineral. 1989, 20, 277–308. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2): A computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey: Reston, VA, USA, 1999.
- Sokolov, V.N.; Yurkovets, D.I.; Razgulina, O.V.; Melnik, V.N. Method for evaluating the roundness of micro-objects from SEM images. Izv. RAN. Ser. Fiz. 2005, 69, 533–536. [Google Scholar]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Hayenes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974; p. 539. [Google Scholar]
- Madejova, J.; Komadel, P. Information available from infrared spectra of the fine fractions of bentonites. Appl. Vib. Spectrosc. to Clay Miner. Layer. Double Hydroxides 2005, 13, 65–98. [Google Scholar]
- Madejová, J.; Gates, W.P.; Petit, S. IR Spectra of Clay Minerals. In Infrared and Raman Spectroscopies of Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, ISBN 9780081003558. [Google Scholar]
- Zviagina, B.B.; McCarty, D.K.; Środoń, J.; Drits, V.A. Interpretation of infrared spectra of dioctahedral smectites in the region of OH-stretching vibrations. Clays Clay Miner. 2004, 52, 399–410. [Google Scholar] [CrossRef]
- Reka, A.; Anovski, T.; Bogoevski, S.; Pavlovski, B.; Boškovski, B. Physical-chemical and mineralogical-petrographic examinations of diatomite from deposit near village of Rožden, Republic of Macedonia. Geol. Maced. 2014, 28, 121–126. [Google Scholar]
- Fontaine, F.; Christidis, G.E.; Yans, J.; Hollanders, S.; Hoffman, A.; Fagel, N. Characterization and origin of two Fe-rich bentonites from Westerwald (Germany). Appl. Clay Sci. 2020, 187, 105444. [Google Scholar] [CrossRef]
- Rozalén, M.L.; Huertas, F.J.; Brady, P.V.; Cama, J.; García-Palma, S.; Linares, J. Experimental study of the effect of pH on the kinetics of montmorillonite dissolution at 25 °C. Geochim. Cosmochim. Acta 2008, 72, 4224–4253. [Google Scholar] [CrossRef]
- Huertas, F.J.; Caballero, E.; Jiménez de Cisneros, C.; Huertas, F.; Linares, J. Kinetics of montmorillonite dissolution in granitic solutions. Appl. Geochem. 2001, 16, 397–407. [Google Scholar] [CrossRef]
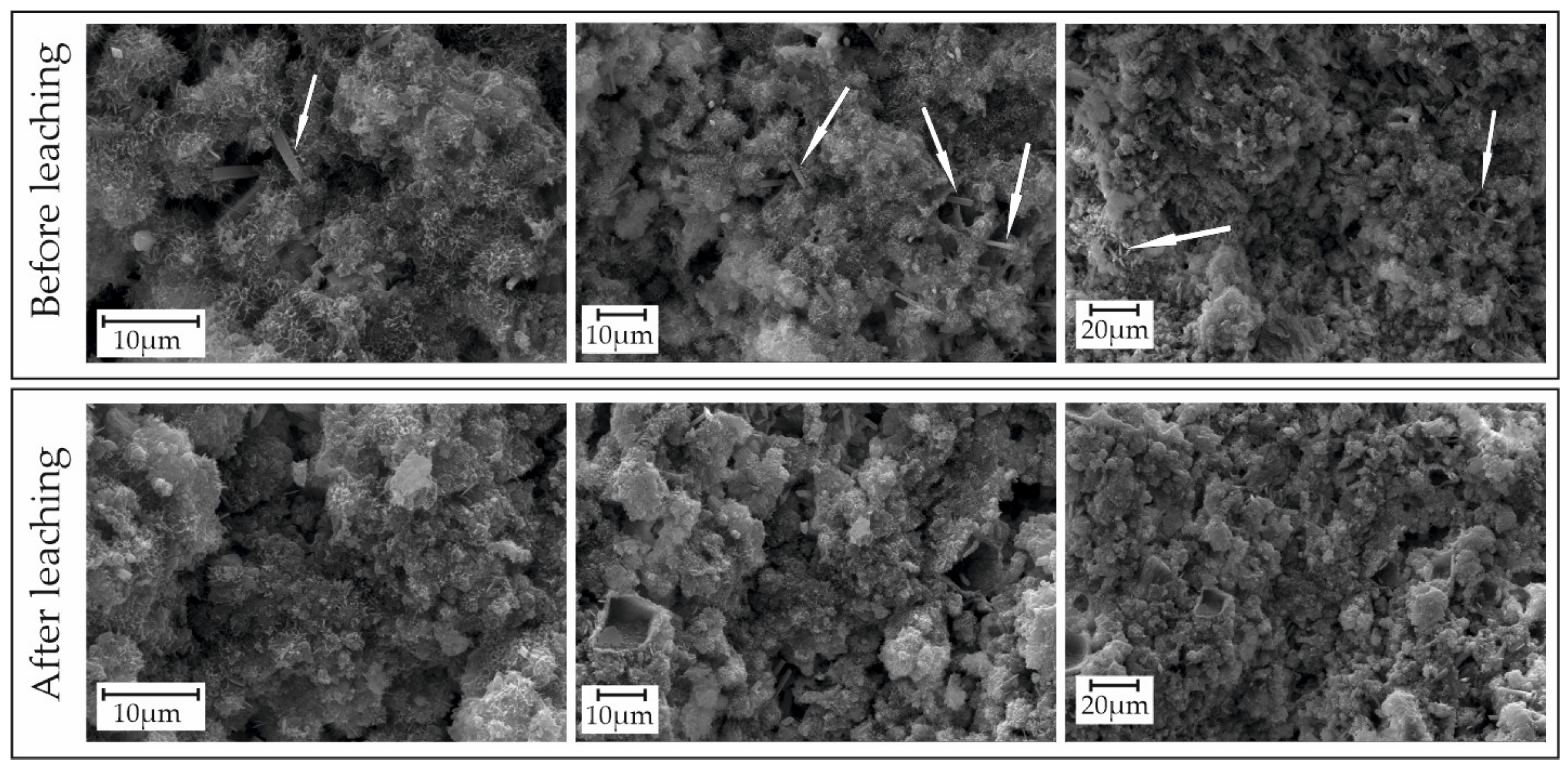
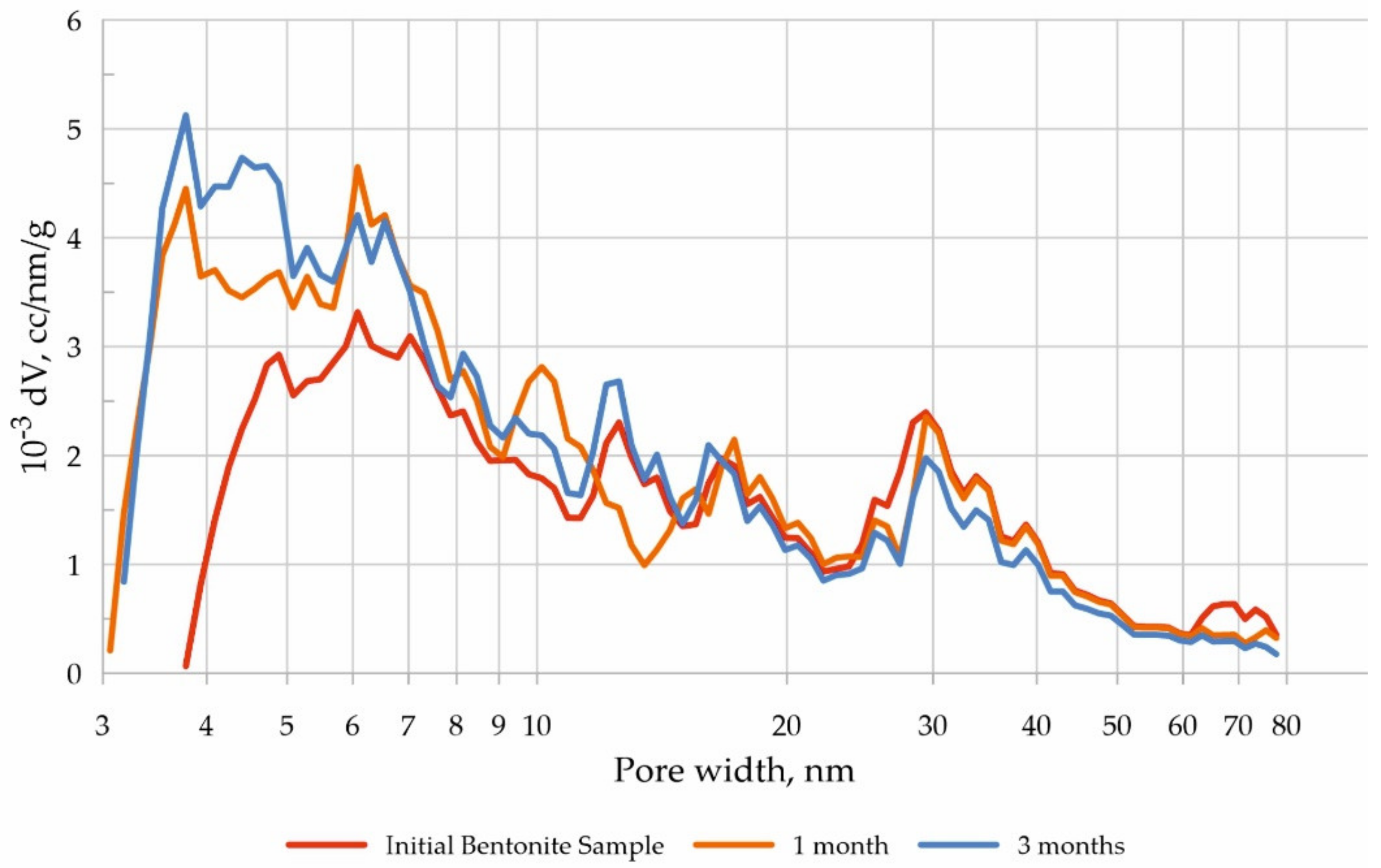
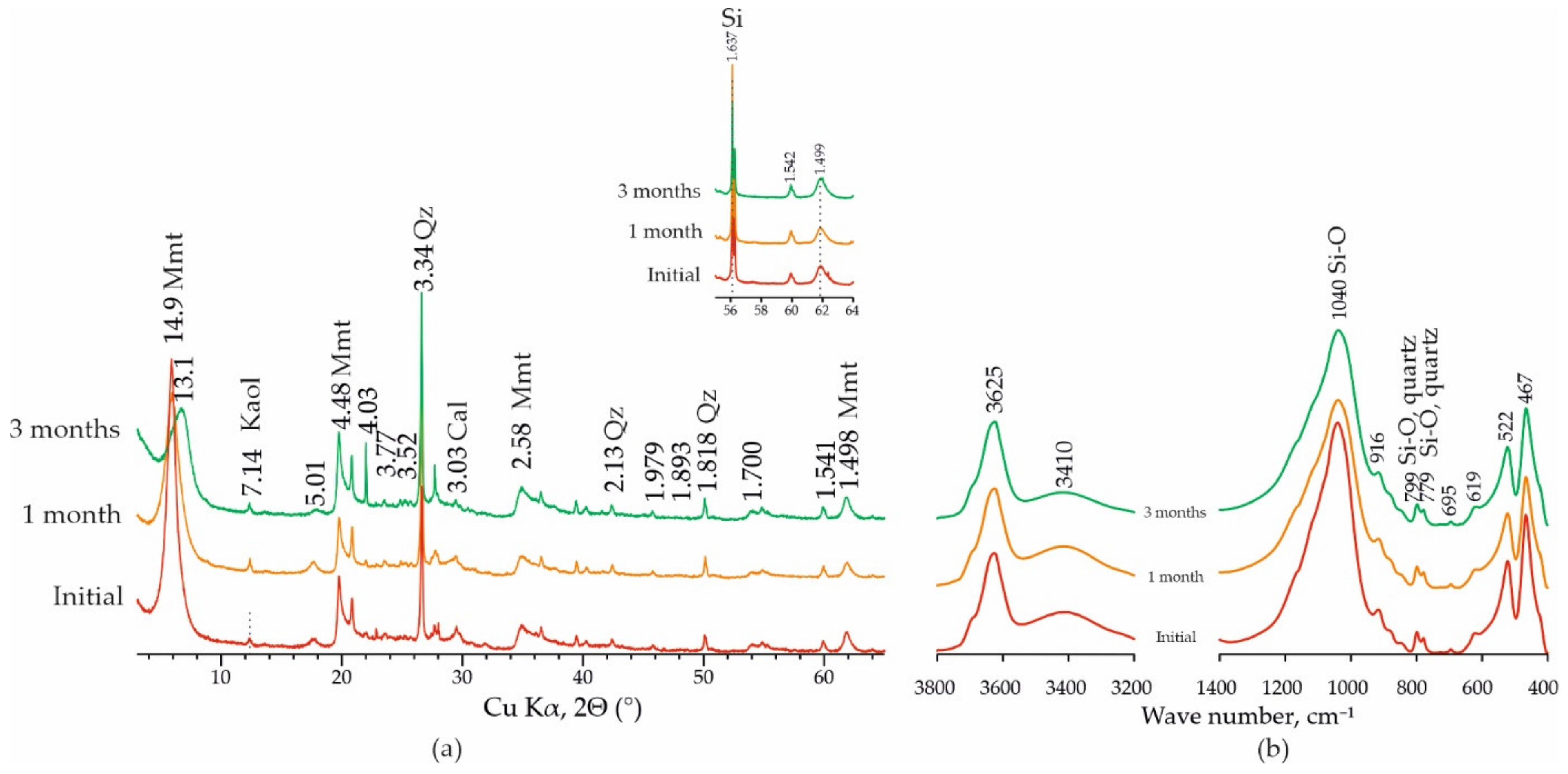

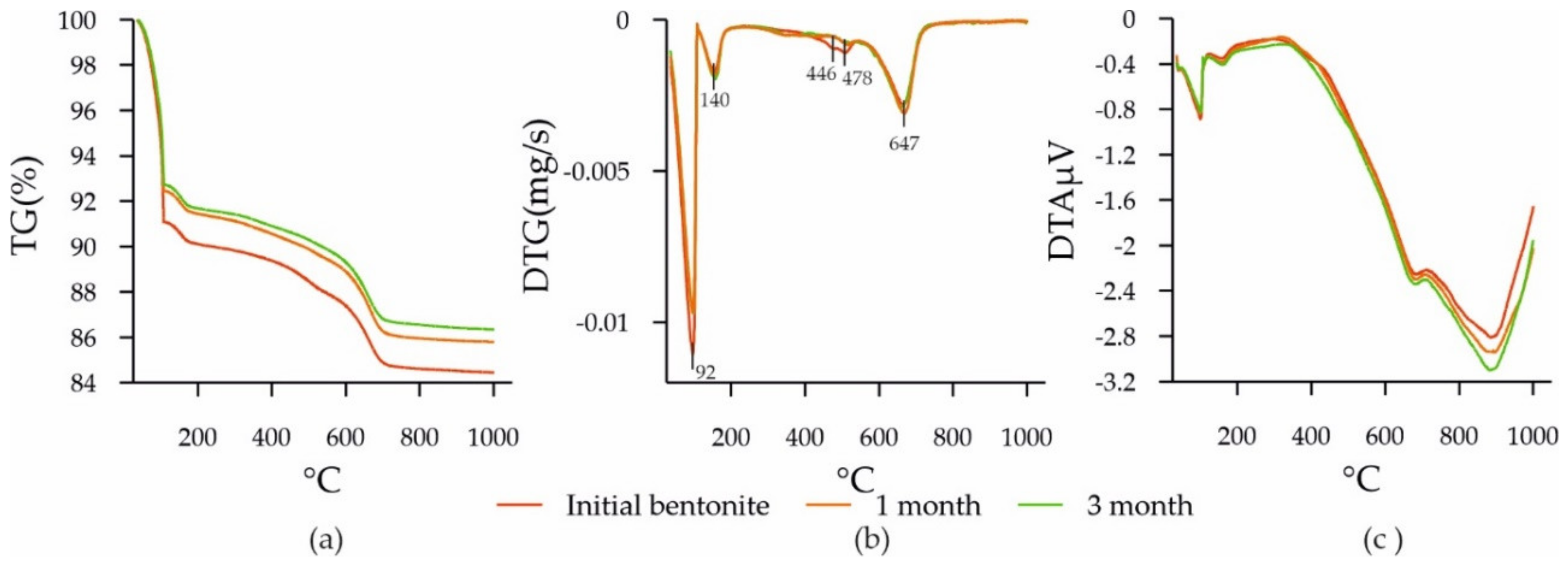

| Sample | Surface Area S, m2/g | Pore Volume, g·cm−3/Average Pore Size, nm |
|---|---|---|
| Concrete | ||
| Initial | 12.1 | 0.089/29.4 |
| 1 month | 16.9 | 0.111/12.6 |
| Bentonite | ||
| Initial | 22.0 | 0.084/6.0 |
| 1 month | 32.0 | 0.080/4.0 |
| 3 months | 30.0 | 0.086/4.0 |
| Na+ | Al3+ | Si4+ | K+ | Mg2+ | Ca2+ | Cl | SO42 | pH | |
|---|---|---|---|---|---|---|---|---|---|
| Synthetic water | 8.87 | - | - | 1.15 | 4.95 | 12.2 | 25.6 | 4.95 | 6.1 |
| Solution after leaching | 67.8 | 0.01938 | 0.3877 | 119.82 | 0.000411 | 0.8034 | - | - | 12.1 |
| Sample | LOI 110–1000 | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | Fe2O3 | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Before leaching | 18.15 | 0.12 | 4.64 | 3.58 | 20.59 | 0.33 | 40.49 | 0.22 | 9.99 | 1.29 |
| After leaching | 17.23 | 0.06 | 4.67 | 3.69 | 21.16 | 0.07 | 40.95 | 0.23 | 10.15 | 1.18 |
| Phase (Ideal Formula) | Concrete before Leaching | Concrete after Leaching |
|---|---|---|
| Quartz (SiO2) | 3.1 | 5.7 |
| Dolomite (CaMg(CO3)2) | 1.4 | - |
| Calcite (CaCO3) | 12.9 | 15.5 |
| Vaterite (CaCO3) | 20.0 | 2.8 |
| Magnesite (MgCO3) | 1.4 | 0.2 |
| Afwillite (Ca3(HSiO4)2·2H2O) | - | 8.5 |
| Oldhamite ((Ca, Mg)S) | 0.9 | 1.3 |
| Chabazite ((K2,Ca,Na2,Sr,Mg)2[Al2Si4O12]2·12H2O) | 3.7 | 4.1 |
| Gismondine (CaAl2Si2O8·4H2O) | 9.4 | 10.1 |
| Wairakite (Ca(Al2Si4O12)·2H2O) | 1.4 | 1.0 |
| Alite (Ca3SiO5) | 3.9 | 6.8 |
| Belite (Ca2SiO4) | 4.0 | 6.3 |
| α-C2SH (Ca2[SiO3(OH)](OH)) | 5.4 | 14.9 |
| Portlandite (Ca(OH)2) | 13.0 | 7.8 |
| Alunite (KAl3(SO4)2(OH)6) | <0.5 * | 2.3 |
| Nordstrandite (Al(OH)3) | - | 0.9 |
| Hydrocalumite (Ca4Al2(OH)12(Cl,CO3,OH)2·4H2O) | 1.5 | 2.1 |
| Larnite (Ca2SiO4) | 6.5 | 6.3 |
| Magnetite (Fe2+Fe3+2O3) | 1.2 | 1.4 |
| Wuestite (FeO) | 3.5 | 2.0 |
| Gypsum (CaSO4·2H2O) | 6.5 | - |
| Sample | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | Fe2O3 |
|---|---|---|---|---|---|---|---|---|
| Initial sample | 1.13 | 3.2 | 19.66 | 67.1 | 1.18 | 2.4 | 0.86 | 4.26 |
| 1 month | 1.04 | 3.05 | 19.81 | 66.77 | 1.45 | 2.72 | 0.81 | 4.13 |
| 3 month | 1.2 | 3.03 | 19.56 | 66.4 | 1.49 | 2.72 | 0.84 | 4.1 |
| Mineral | Initial Sample | 1 Month | 3 Month |
|---|---|---|---|
| Quartz | 13.3 | 12.3 | 10.8 |
| Albite | 5.3 | 6.5 | 6.7 |
| Microcline | 6.7 | 6.8 | 4.8 |
| Calcite | 1.4 | 1.2 | 1.1 |
| Siderite | 0.7 | - * | - * |
| Anatase | 0.7 | 0.5 | 0.9 |
| Kaolinite | 2 | 1 | 1.1 |
| Illite | 4.5 | 5.9 | 4.6 |
| Smectite | 64.3 | 64.8 | 68.5 |
| Chlorite | 1.1 | 0.9 | 1.3 |
| Doublets of Quadrupole Splitting | G, mm/s | δ, mm/s | Δfit, mm/s | Sfit, % | Fe2+/Fe3+ |
|---|---|---|---|---|---|
| initial | |||||
| (1) Fe3+ | 0.34 | 0.35 | 0.51 | 11 | |
| (2) Fe3+ | 1.04 | 0.33 | 0.67 | 72 | 0.19 |
| (3) Fe2+ | 0.43 | 1.16 | 2.78 | 16 | |
| 1 month | |||||
| (1) Fe3+ | 0.27 | 0.37 | 0.55 | 10 | |
| (2) Fe3+ | 0.86 | 0.34 | 0.70 | 80 | |
| (3) Fe3+ | 0.40 | 1.19 | 2.60 | 10 | 0.11 |
| 3 month | |||||
| (1) Fe3+ | 0.32 | 0.34 | 0.47 | 13 | |
| (2) Fe3+ | 0.79 | 0.36 | 0.73 | 78 | |
| (3) Fe3+ | 0.37 | 1.14 | 2.62 | 9 | 0.10 |
| Mineral | S, m2/g | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Albite | 2 | 5.1 × 10−20 | 57 | 8.5 × 10−11 | 58 | 0.34 | 1.4 × 10−10 | 56 | 0.32 |
| Chlorite | 1 | 6.4 × 10−17 1.6 × 10−17 | 16 | 8.2 × 10−9 2.1 × 10−9 | 17 | 0.28 | 6.9 × 10−9 1.75 × 10−9 * | 16 | 0.34 |
| Illite | 30 | 3.3 × 10−17 | 35 | 9.8 × 10−12 | 36 | 0.52 | 3.1 × 10−12 | 48 | 0.38 |
| Kaolinite | 5 | 1.1 × 10−14 | 38 | 7.5 × 10−12 | 43 | 0.51 | 2.5 × 10−11 1.3 × 10−11 * | 46 | 0.58 |
| Microcline | 1 | 1.0 × 10−14 | 31 | 1.7 × 10−11 | 31 | 0.27 | 1.4 × 10−10 | 31 | 0.35 |
| Quartz | 0.03 | 6.4 × 10−14 | 77 | 1.9 × 10−10 | 80 | 0.34 | |||
| Smectite | 40 | 9.3 × 10−15 4.7 × 10−16 * | 63 | 5.3 × 10−11 2.7 × 10−12 * | 54 | 0.69 | 2.9 × 10−12 3.6 × 10−15 * | 61 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozov, I.; Zakusin, S.; Kozlov, P.; Zakusina, O.; Roshchin, M.; Chernov, M.; Boldyrev, K.; Zaitseva, T.; Tyupina, E.; Krupskaya, V. Bentonite–Concrete Interactions in Engineered Barrier Systems during the Isolation of Radioactive Waste Based on the Results of Short-Term Laboratory Experiments. Appl. Sci. 2022, 12, 3074. https://doi.org/10.3390/app12063074
Morozov I, Zakusin S, Kozlov P, Zakusina O, Roshchin M, Chernov M, Boldyrev K, Zaitseva T, Tyupina E, Krupskaya V. Bentonite–Concrete Interactions in Engineered Barrier Systems during the Isolation of Radioactive Waste Based on the Results of Short-Term Laboratory Experiments. Applied Sciences. 2022; 12(6):3074. https://doi.org/10.3390/app12063074
Chicago/Turabian StyleMorozov, Ivan, Sergey Zakusin, Pavel Kozlov, Olga Zakusina, Marat Roshchin, Michail Chernov, Kirill Boldyrev, Tatiana Zaitseva, Ekaterina Tyupina, and Victoria Krupskaya. 2022. "Bentonite–Concrete Interactions in Engineered Barrier Systems during the Isolation of Radioactive Waste Based on the Results of Short-Term Laboratory Experiments" Applied Sciences 12, no. 6: 3074. https://doi.org/10.3390/app12063074
APA StyleMorozov, I., Zakusin, S., Kozlov, P., Zakusina, O., Roshchin, M., Chernov, M., Boldyrev, K., Zaitseva, T., Tyupina, E., & Krupskaya, V. (2022). Bentonite–Concrete Interactions in Engineered Barrier Systems during the Isolation of Radioactive Waste Based on the Results of Short-Term Laboratory Experiments. Applied Sciences, 12(6), 3074. https://doi.org/10.3390/app12063074








