Improving the Analysis of Sulfur Content and Calorific Values of Blended Coals with Data Processing Methods in Laser-Induced Breakdown Spectroscopy
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
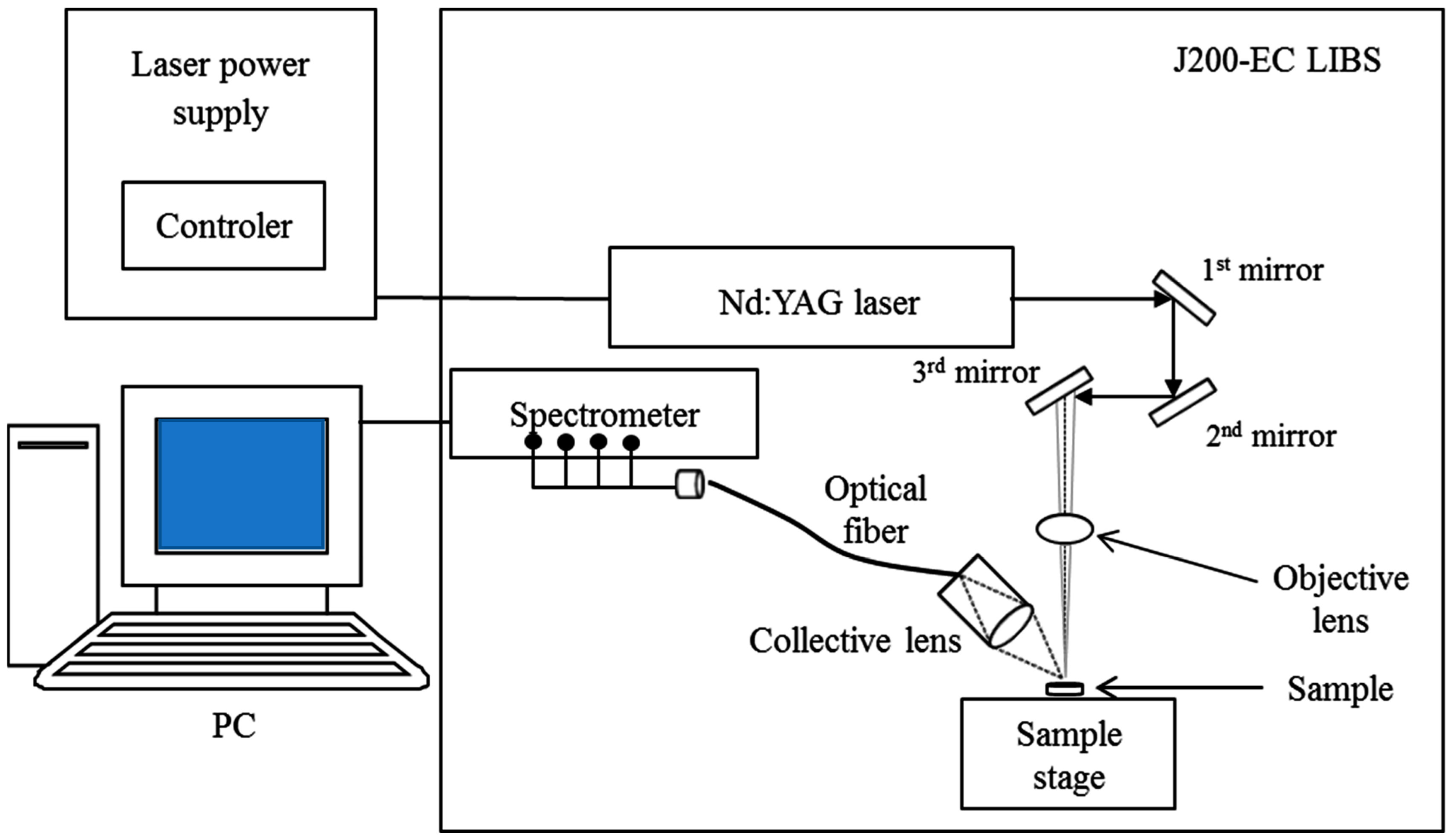
2.1. Materials and Measuring Systems
2.2. Statistical Analysis
2.2.1. Relative Standard Deviation (RSD)
2.2.2. Partial Least Square Regression (PLSR)
2.2.3. Root Mean Square Error (RSME) Average
3. Results and Discussion
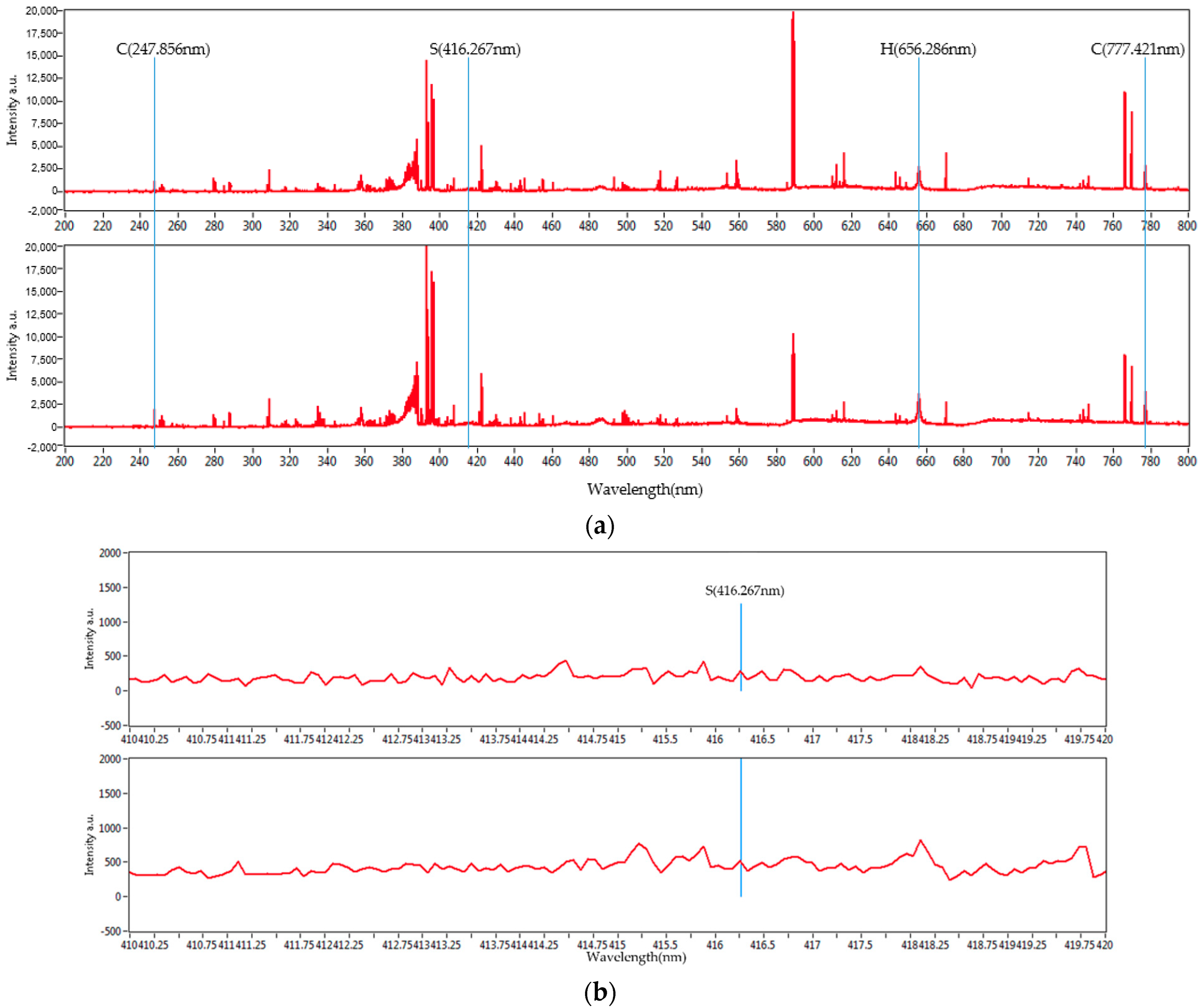
3.1. Sulfur Analysis
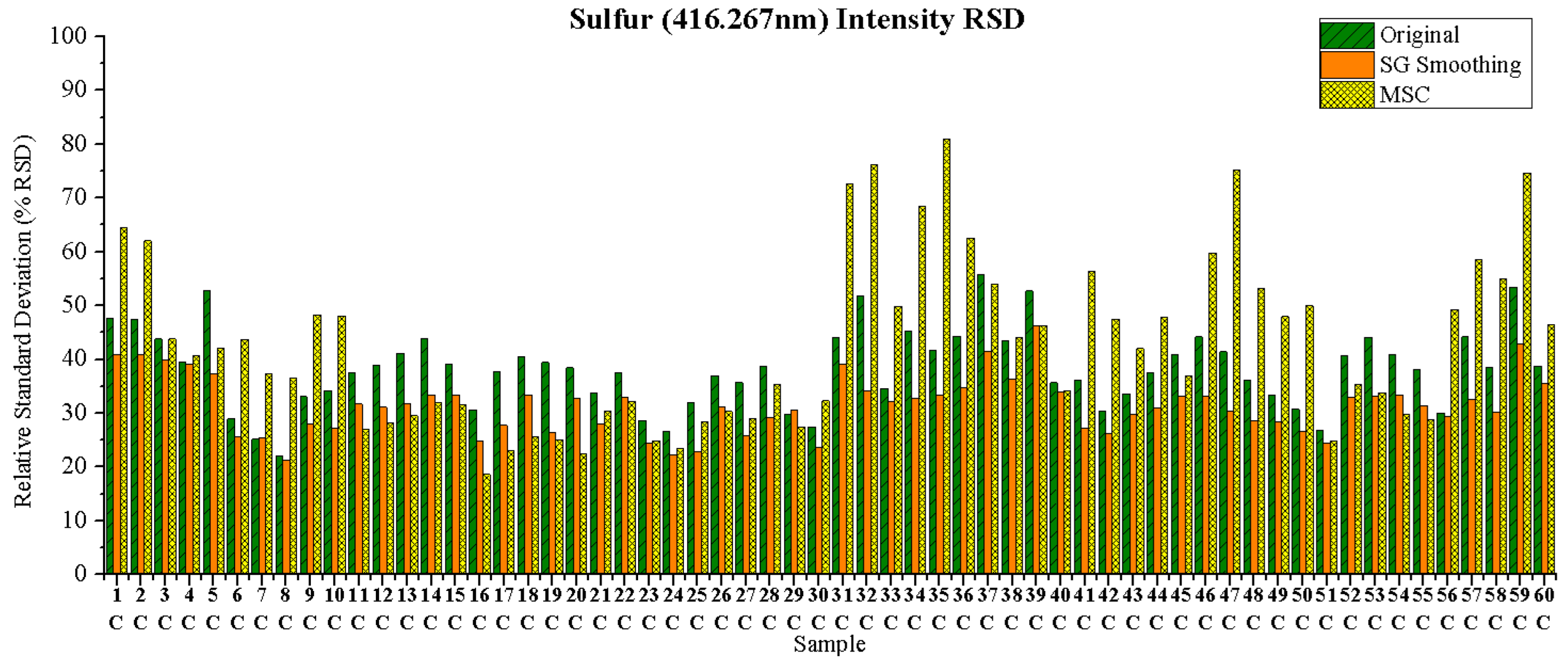
3.1.1. Relative Standard Deviation (RSD)
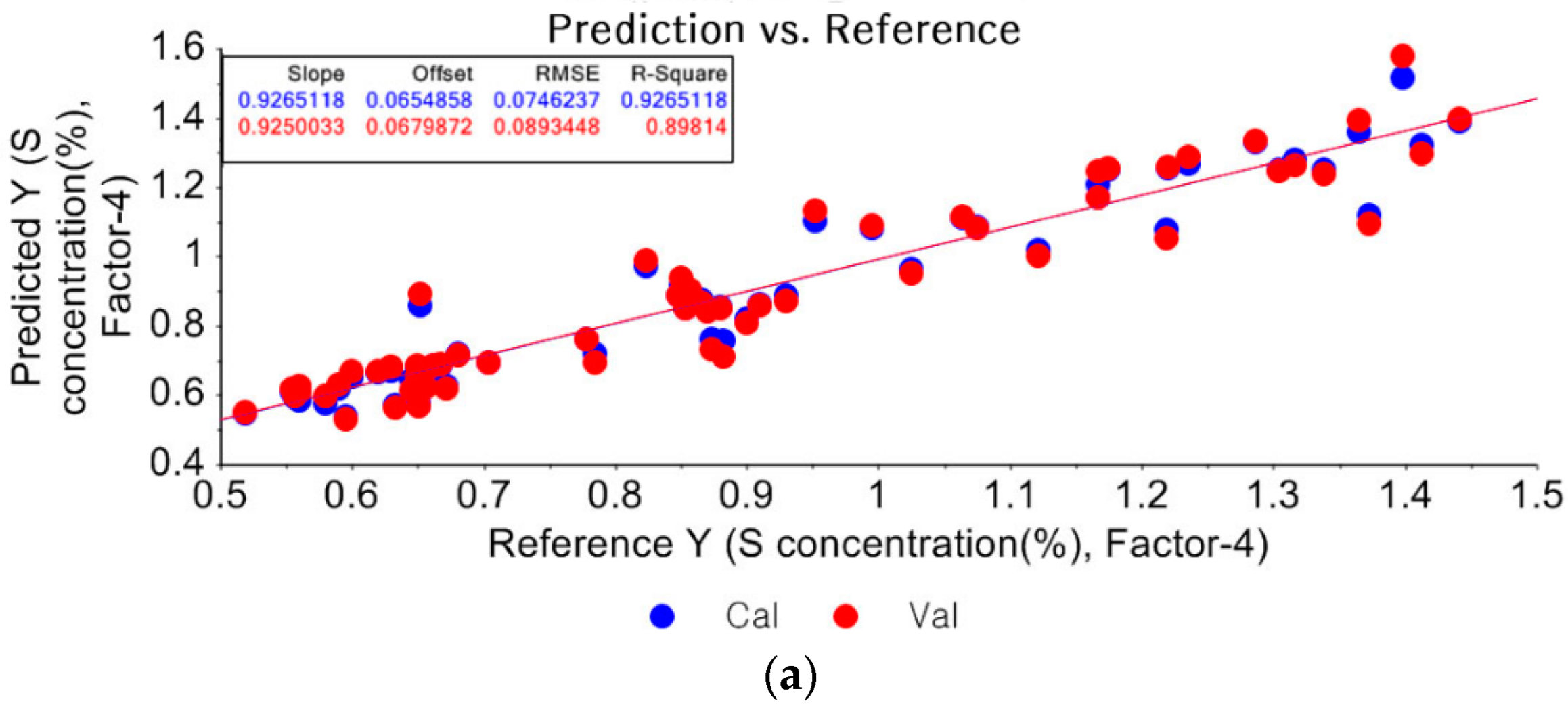
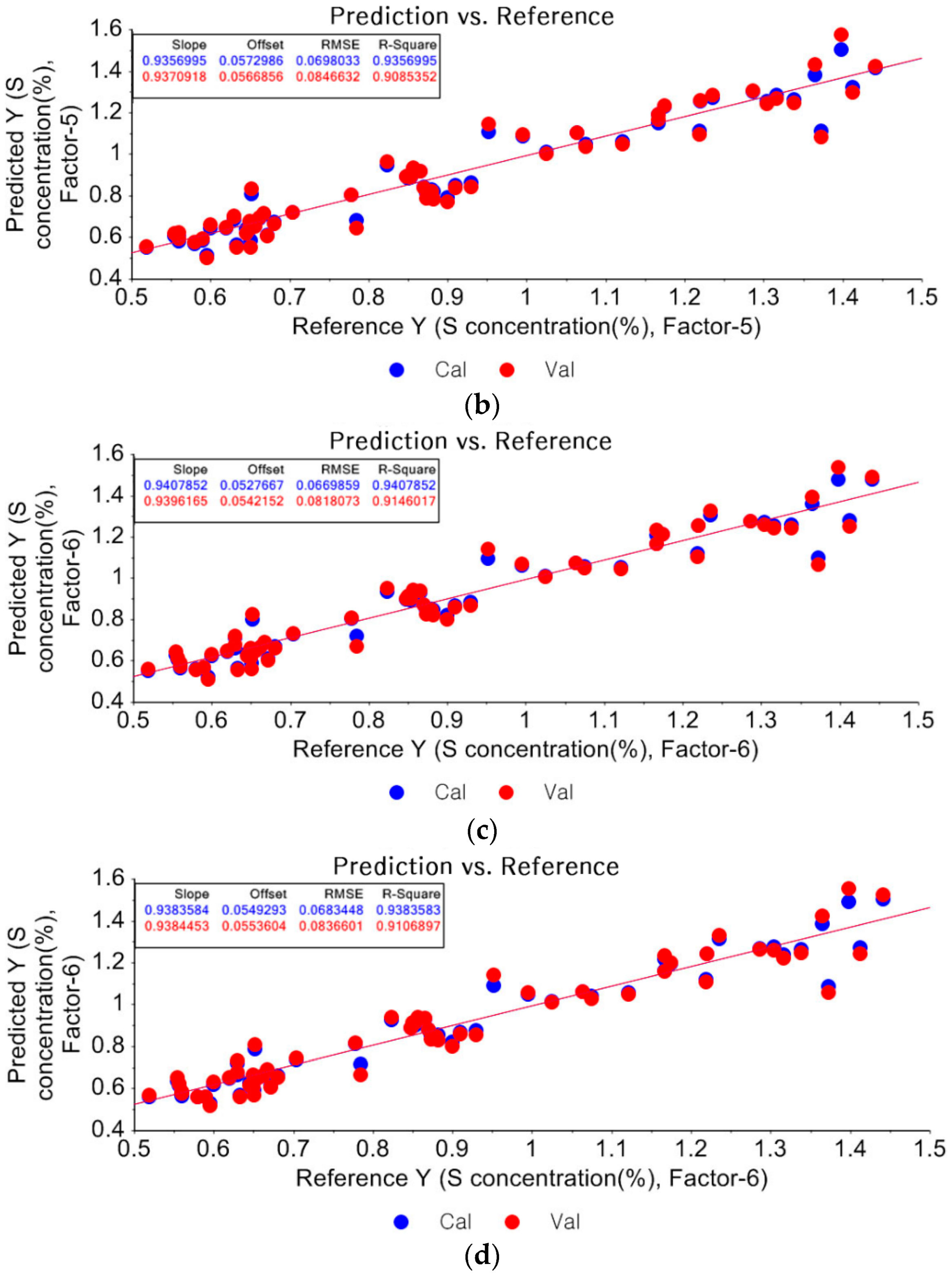
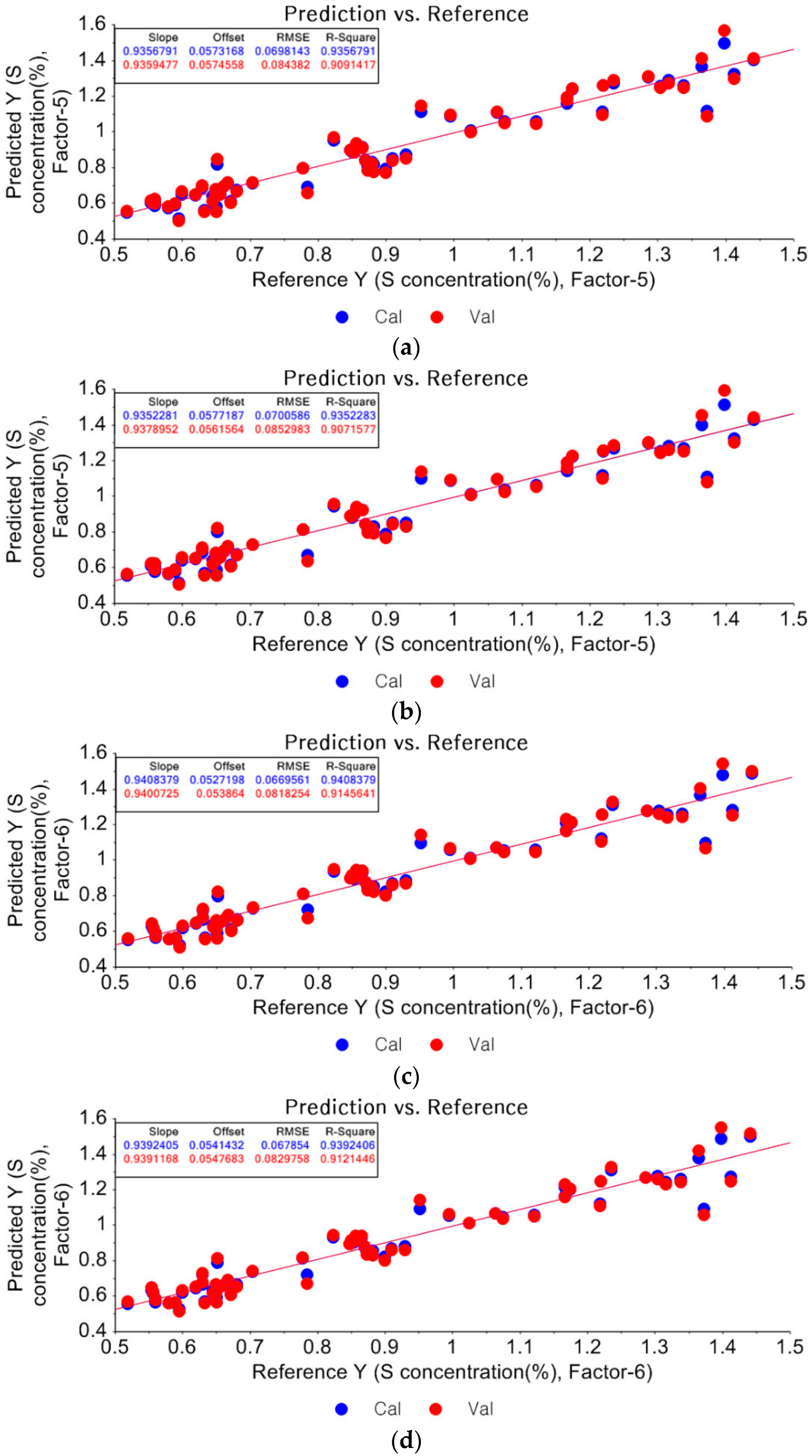
3.1.2. Partial Least Square Regression (PLSR)
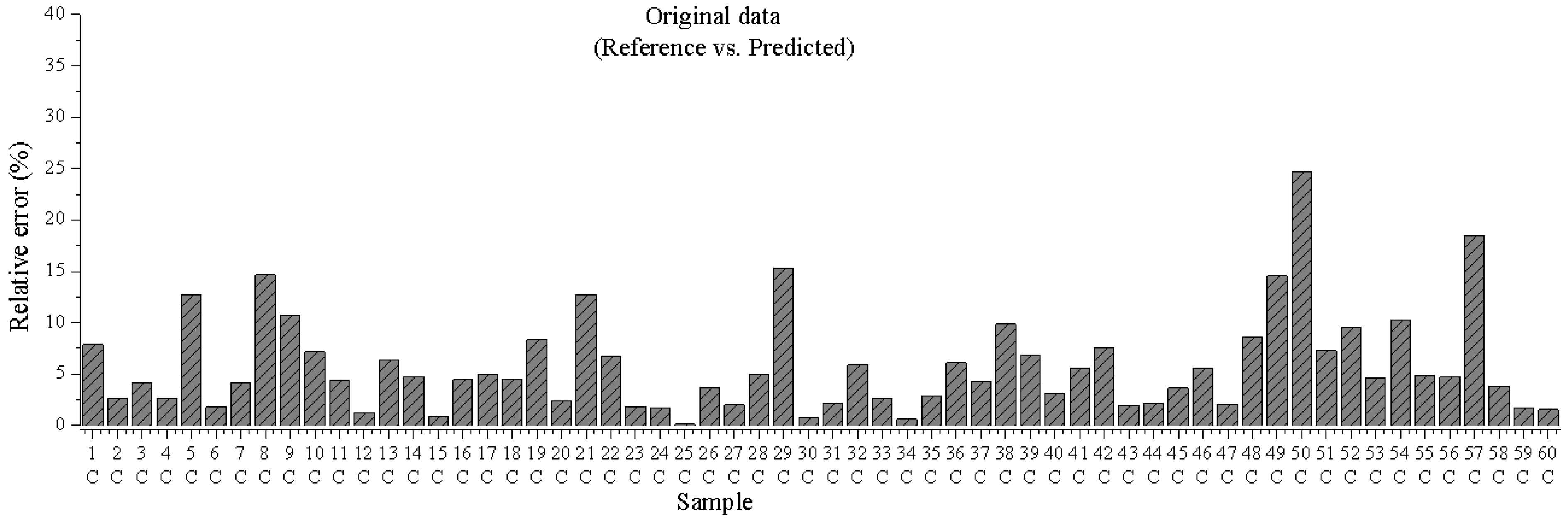
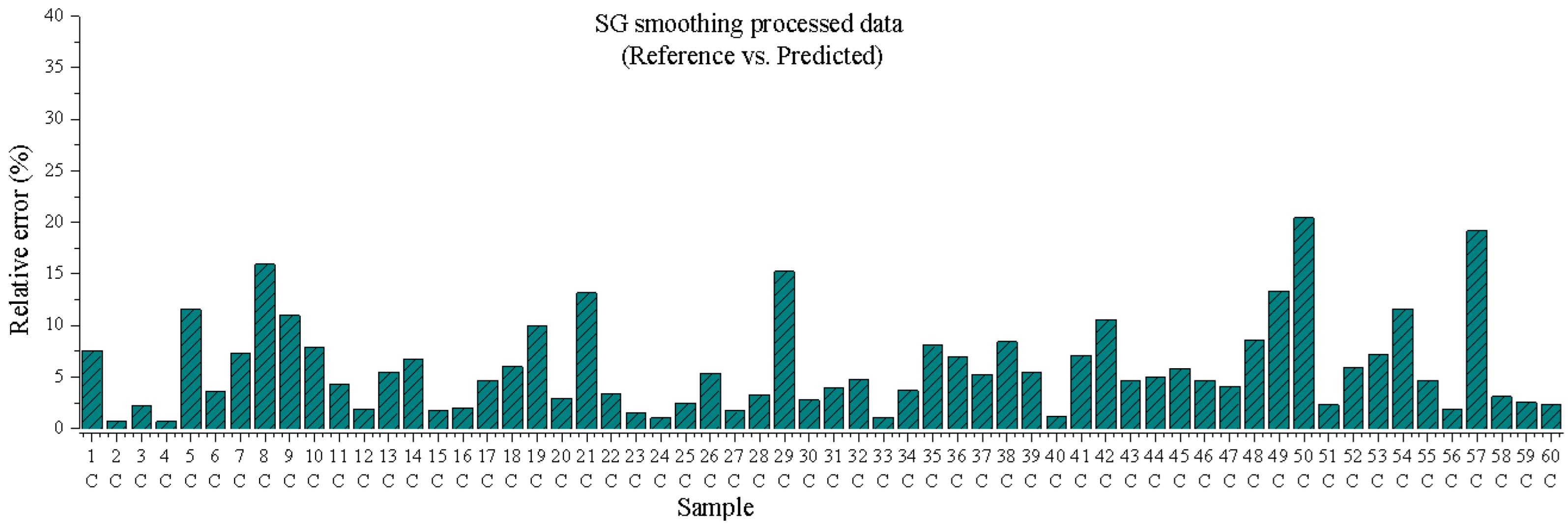
3.1.3. Prediction of Unknown Samples
3.1.4. Root Mean Square Error (RSME) Average
3.2. Calorific Value Analysis
3.2.1. Partial Least Square Regression (PLSR)
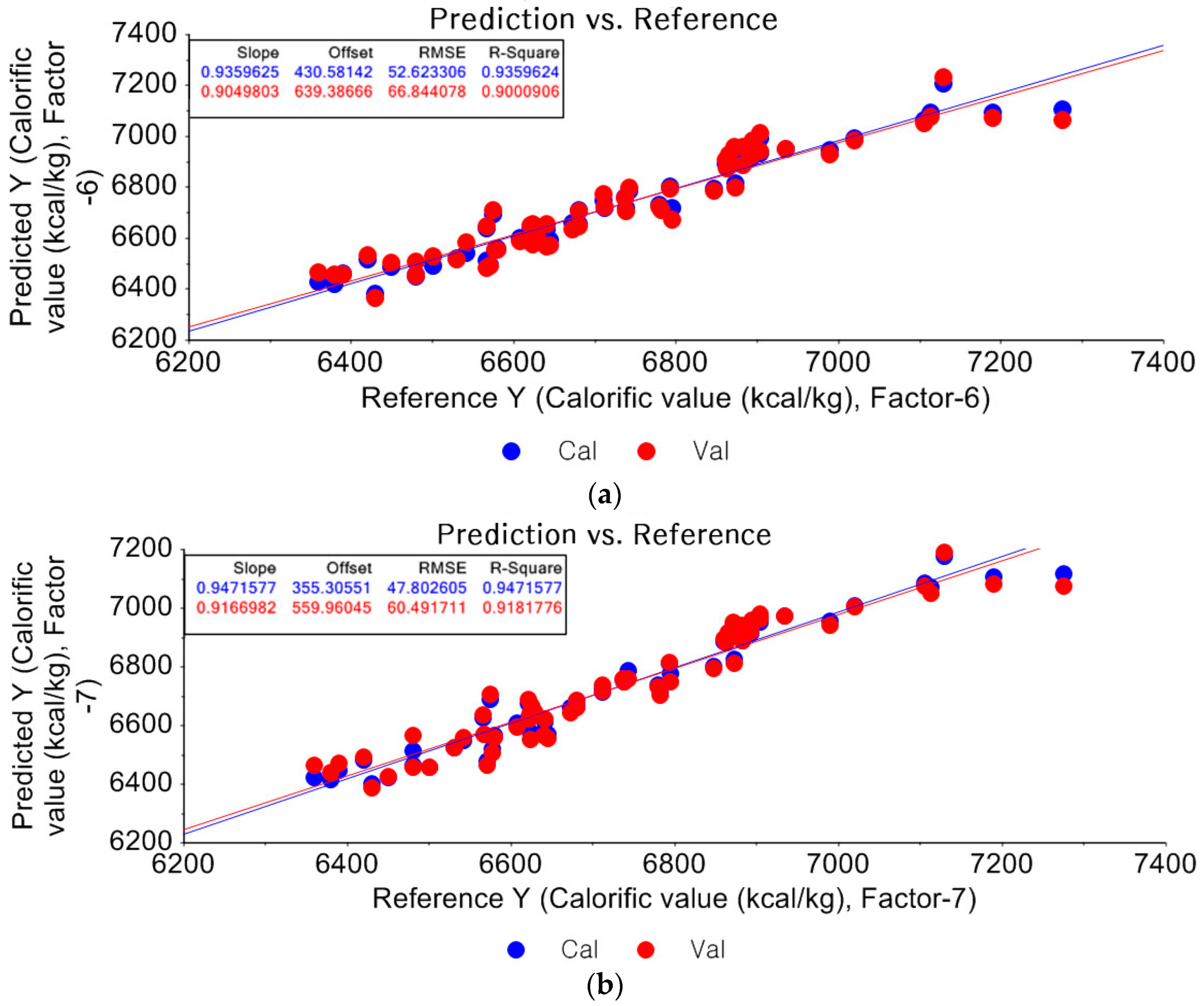
3.2.2. Prediction of Unknown Samples
3.2.3. Root Mean Square Error (RSME) Average
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- National Institute of Environmental Research. National Air Pollutants Emission 2013; National Institute of Environmental Research: Incheon, Republic of Korea, 2013. [Google Scholar]
- International Finance Corporation (IFC). Environmental, Health and Safety Guidelines. Thermal Power Plants; International Finance Corporation (IFC): Washington, DC, USA, 2008. [Google Scholar]
- Burakov, V.; Tarasenko, N.; Nedelko, M.; Kononov, V.; Vasilev, N.; Isakov, S. Analysis of lead and sulfur in environmental samples by double pulse laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 141–146. [Google Scholar] [CrossRef]
- Bona, M.; Andrés, J.M. Coal analysis by diffuse reflectance near-infrared spectroscopy: Hierarchical cluster and linear discriminant analysis. Talanta 2007, 72, 1423–1431. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Wei, G.; Hu, R.; Luo, Z.; Li, G. Improved PLS regression based on SVM classification for rapid analysis of coal properties by near-infrared reflectance spectroscopy. Sens. Actuators B Chem. 2014, 193, 723–729. [Google Scholar] [CrossRef]
- Russo, R.E.; Mao, X.L.; Liu, H.C.; Yoo, J.H.; Mao, S.S. Time-resolved plasma diagnostics and mass removal during single-pulse laser ablation. Appl. Phys. A 1999, 69, S887–S894. [Google Scholar] [CrossRef]
- Martin, M.Z.; Labbé, N.; Rials, T.G.; Wullschleger, S.D. Analysis of preservative-treated wood by multivariate analysis of laser-induced breakdown spectroscopy spectra. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 1179–1185. [Google Scholar] [CrossRef]
- Yao, S.; Lu, J.; Li, J.; Chen, K.; Li, J.; Dong, M. Multi-elemental analysis of fertilizer using laser-induced breakdown spectroscopy coupled with partial least squares regression. J. Anal. At. Spectrom. 2010, 25, 1733–1738. [Google Scholar] [CrossRef]
- Gaft, M.; Nagli, L.; Fasaki, I.; Kompitsas, M.; Wilsch, G. Laser-induced breakdown spectroscopy for on-line sulfur analyses of minerals in ambient conditions. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 1098–1104. [Google Scholar] [CrossRef]
- Yao, S.; Lu, J.; Dong, M.; Chen, K.; Li, J.; Li, J. Extracting Coal Ash Content from Laser-Induced Breakdown Spectroscopy (LIBS) Spectra by Multivariate Analysis. Appl. Spectrosc. 2011, 65, 1197–1201. [Google Scholar] [CrossRef]
- Gaft, M.; Dvir, E.; Modiano, H.; Schone, U. Laser Induced Breakdown Spectroscopy machine for online ash analyses in coal. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1177–1182. [Google Scholar] [CrossRef]
- Dong, M.; Lu, J.; Yao, S.; Li, J.; Li, J.; Zhong, Z.; Lu, W. Application of LIBS for direct determination of volatile matter content in coal. J. Anal. At. Spectrom. 2011, 26, 2183–2188. [Google Scholar] [CrossRef]
- Yuan, T.; Wang, Z.; Lui, S.-L.; Fu, Y.; Li, Z.; Liu, J.; Ni, W. Coal property analysis using laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2013, 28, 1045–1053. [Google Scholar] [CrossRef]
- Gazeli, O.; Stefas, D.; Couris, S. Sulfur Detection in Soil by Laser Induced Breakdown Spectroscopy Assisted by Multivariate Analysis. Materials 2021, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Dyar, M.D.; Tucker, J.M.; Humphries, S.; Clegg, S.M.; Wiens, R.C.; Lane, M.D. Strategies for Mars remote Laser-Induced Breakdown Spectroscopy analysis of sulfur in geological samples. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 39–56. [Google Scholar] [CrossRef]
- Hemmerlin, M.; Meilland, R.; Falk, H.; Wintjens, P.; Paulard, L. Application of vacuum ultraviolet laser-induced breakdown spectrometry for steel analysis—Comparison with spark-optical emission spectrometry figures of merit. Spectrochim. Acta Part B At. Spectrosc. 2001, 56, 661–669. [Google Scholar] [CrossRef]
- Weritz, F.; Ryahi, S.; Schaurich, D.; Taffe, A.; Wilsch, G. Quantitative determination of sulfur content in concrete with la-ser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 1121–1131. [Google Scholar] [CrossRef]
- Lal, B.; Zheng, H.; Yueh, F.-Y.; Singh, J.P. Parametric study of pellets for elemental analysis with laser-induced breakdown spectroscopy. Appl. Opt. 2004, 43, 2792–2797. [Google Scholar] [CrossRef]
- Noll, R. Laser-Induced Breakdown Spectroscopy: Fundamentals and Applications; Springer: Berlin,/Heidelberg, Germany, 2012; ISBN 9783642206689. [Google Scholar]
- Chen, H.; Song, Q.; Tang, G.; Feng, Q.; Lin, L. The Combined Optimization of Savitzky-Golay Smoothing and Multiplicative Scatter Correction for FT-NIR PLS Models. ISRN Spectrosc. 2013, 2013, 642190. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Lin, Z.; Gong, S.; Xie, C.; Chang, L.; Yang, L.; Li, P. Effects of experimental parameters on elemental analysis of coal by laser-induced breakdown spectroscopy. Opt. Laser Technol. 2009, 41, 907–913. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuo, Z.; Lu, P.; Tang, J.; Tang, H.; Lu, J.; Xing, T.; Wang, Y. LIBS analysis of the ash content, volatile matter, and calorific value in coal by partial least squares regression based on ash classification. J. Anal. At. Spectrom. 2020, 35, 1621–1631. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, J.M.; Kim, J.S.; Kim, H.J. The Technology for On-line Measurement of Coal Properties by using Near-Infrared. Korean Chem. Eng. Res. 2007, 5, 596–603. [Google Scholar] [CrossRef]
- Kathiravale, S. Modeling the heating value of Municipal Solid Waste. Fuel 2003, 82, 1119–1125. [Google Scholar] [CrossRef]
- Sanghapi, H.K.; Jain, J.; Bol’Shakov, A.; Lopano, C.; McIntyre, D.; Russo, R. Determination of elemental composition of shale rocks by laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2016, 122, 9–14. [Google Scholar] [CrossRef]




| Reference Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | S (%) | Calorific Value (kcal/kg) | Sample | S (%) | Calorific Value (kcal/kg) | Sample | S (%) | Calorific Value (kcal/kg) |
| C 1 | 1.3983 | 6567 | C 21 | 0.5600 | 6743 | C 41 | 0.5546 | 6861 |
| C 2 | 1.2866 | 6624 | C 22 | 0.5900 | 6866 | C 42 | 0.6291 | 6872 |
| C 3 | 1.1749 | 6681 | C 23 | 0.6200 | 6990 | C 43 | 0.7037 | 6882 |
| C 4 | 1.0632 | 6738 | C 24 | 0.6500 | 7113 | C 44 | 0.7782 | 6893 |
| C 5 | 0.9515 | 6795 | C 25 | 0.6800 | 7129 | C 45 | 0.8528 | 6904 |
| C 6 | 0.5183 | 6935 | C 26 | 0.6300 | 6580 | C 46 | 1.3383 | 6567 |
| C 7 | 0.5567 | 7020 | C 27 | 0.5900 | 6530 | C 47 | 1.1667 | 6623 |
| C 8 | 0.5950 | 7105 | C 28 | 0.5500 | 6480 | C 48 | 0.9950 | 6680 |
| C 9 | 0.6333 | 7190 | C 29 | 0.5000 | 6430 | C 49 | 0.8233 | 6737 |
| C 10 | 0.6717 | 7275 | C 30 | 0.4600 | 6380 | C 50 | 0.6517 | 6793 |
| C 11 | 0.5600 | 6621 | C 31 | 1.3284 | 6480 | C 51 | 0.7900 | 6500 |
| C 12 | 0.5800 | 6623 | C 32 | 1.1469 | 6450 | C 52 | 0.8000 | 6571 |
| C 13 | 0.6000 | 6625 | C 33 | 0.9653 | 6420 | C 53 | 0.8100 | 6641 |
| C 14 | 0.6300 | 6627 | C 34 | 0.7837 | 6390 | C 54 | 0.8200 | 6711 |
| C 15 | 0.6500 | 6629 | C 35 | 0.6022 | 6360 | C 55 | 0.8300 | 6782 |
| C 16 | 0.8500 | 6863 | C 36 | 1.4129 | 6577 | C 56 | 1.4413 | 6543 |
| C 17 | 0.8700 | 6873 | C 37 | 1.3158 | 6645 | C 57 | 1.3726 | 6575 |
| C 18 | 0.8800 | 6883 | C 38 | 1.2187 | 6712 | C 58 | 1.3039 | 6608 |
| C 19 | 0.9000 | 6894 | C 39 | 1.1215 | 6780 | C 59 | 1.2352 | 6640 |
| C 20 | 0.9100 | 6904 | C 40 | 1.0244 | 6847 | C 60 | 1.1665 | 6673 |
| Sample Number | Measured Value (%) | Predicted Value (%) | Absolute Error (%) | Relative Error (%) |
|---|---|---|---|---|
| UO1 | 0.880 | 0.8878 | 0.0078 | 0.8864 |
| UO2 | 0.880 | 0.9798 | 0.0998 | 11.3409 |
| UB1 | 0.985 | 0.8851 | 0.0999 | 10.1421 |
| UB2 | 1.090 | 1.2115 | 0.1215 | 11.1468 |
| UB3 | 1.195 | 1.3009 | 0.1059 | 8.8619 |
| UB4 | 1.300 | 1.4492 | 0.1492 | 11.4769 |
| UB5 | 1.405 | 1.2706 | 0.1344 | 9.5658 |
| Property | Data Process | RMSEC Average (%) | RMSECV Average (%) |
|---|---|---|---|
| Sulfur concentration | Original | 7.6019 | 9.1453 |
| SG smoothing | 7.5097 | 9.0301 |
| Sample Number | Measured Value (kcal/kg) | Predicted Value (kcal/kg) | Absolute Error (kcal/kg) | Relative Error (%) |
|---|---|---|---|---|
| UO1 | 6350.000 | 6284.033 | 65.967 | 1.0389 |
| UO2 | 6350.000 | 6279.933 | 70.067 | 1.1034 |
| UB1 | 6376.667 | 6248.880 | 127.787 | 2.0040 |
| UB2 | 6403.333 | 6315.346 | 87.987 | 1.3741 |
| UB3 | 6430.000 | 6434.265 | 4.265 | 0.0663 |
| UB4 | 6456.667 | 6450.932 | 5.735 | 0.0888 |
| UB5 | 6483.333 | 6449.053 | 34.280 | 0.5287 |
| Property | Data Process | RMSEC Average (%) | RMSECV Average (%) |
|---|---|---|---|
| Calorific value | Original | 0.7826 | 0.9941 |
| SG smoothing | 0.7109 | 0.8997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.S.; Ryu, C.M.; Choi, J.H.; Moon, S.J. Improving the Analysis of Sulfur Content and Calorific Values of Blended Coals with Data Processing Methods in Laser-Induced Breakdown Spectroscopy. Appl. Sci. 2022, 12, 12410. https://doi.org/10.3390/app122312410
Choi JS, Ryu CM, Choi JH, Moon SJ. Improving the Analysis of Sulfur Content and Calorific Values of Blended Coals with Data Processing Methods in Laser-Induced Breakdown Spectroscopy. Applied Sciences. 2022; 12(23):12410. https://doi.org/10.3390/app122312410
Chicago/Turabian StyleChoi, Jae Seung, Choong Mo Ryu, Jung Hyun Choi, and Seung Jae Moon. 2022. "Improving the Analysis of Sulfur Content and Calorific Values of Blended Coals with Data Processing Methods in Laser-Induced Breakdown Spectroscopy" Applied Sciences 12, no. 23: 12410. https://doi.org/10.3390/app122312410
APA StyleChoi, J. S., Ryu, C. M., Choi, J. H., & Moon, S. J. (2022). Improving the Analysis of Sulfur Content and Calorific Values of Blended Coals with Data Processing Methods in Laser-Induced Breakdown Spectroscopy. Applied Sciences, 12(23), 12410. https://doi.org/10.3390/app122312410







