Light-Controlled Microbots in Biomedical Application: A Review
Abstract
1. Introduction
2. Prospective Applications
2.1. Targeted Drug Delivery
2.2. Micro Manipulation
2.3. Micro Force Measurements and Mechanical Characterization of Bio-Cells
2.4. Localized Micro Mixing of Fluids
2.5. Cell Manipulation and Diagnosis
2.6. Light for Cell Penetration
3. Challenges
4. Aspects of Design
4.1. Manipulation of Microrobots
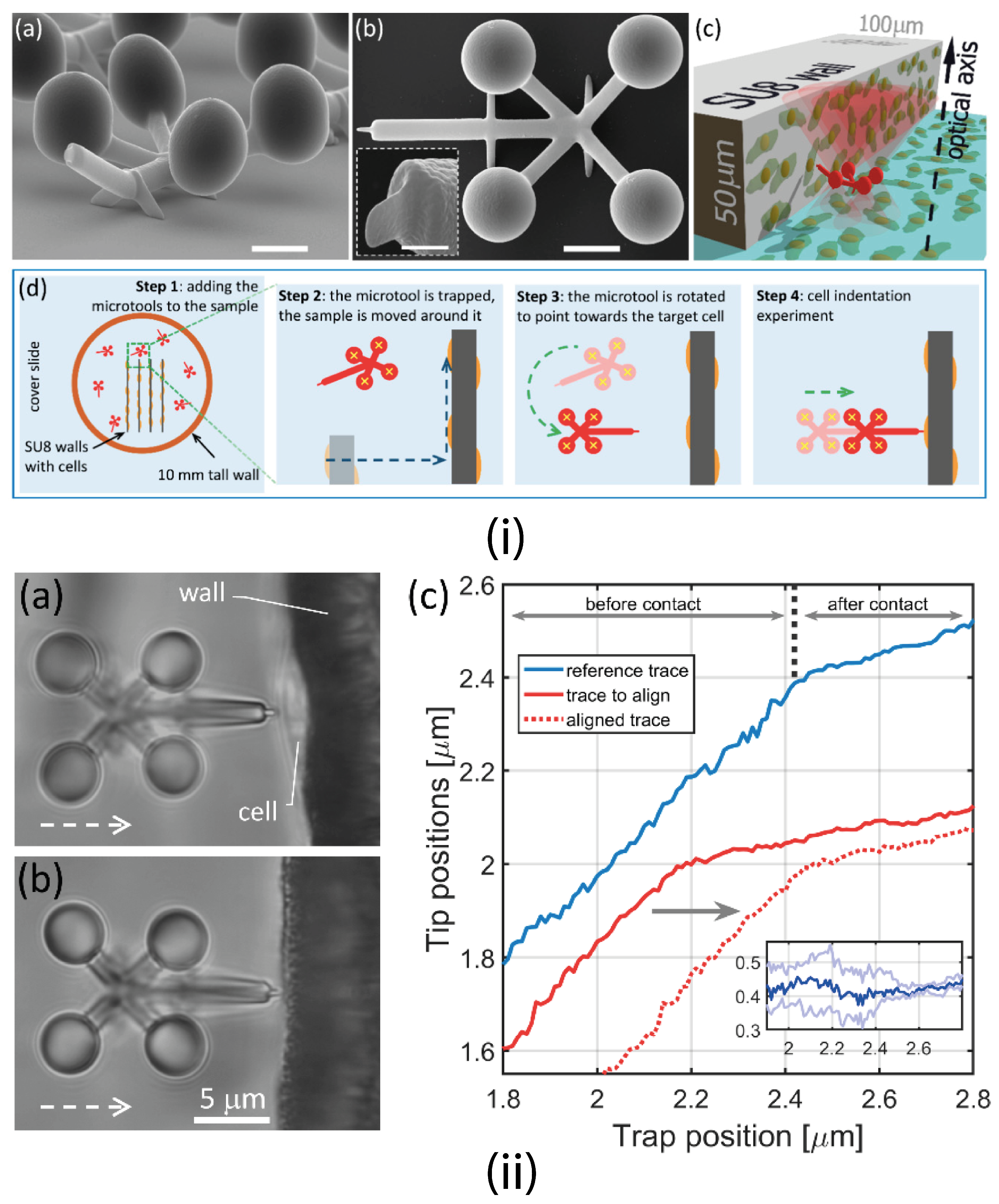
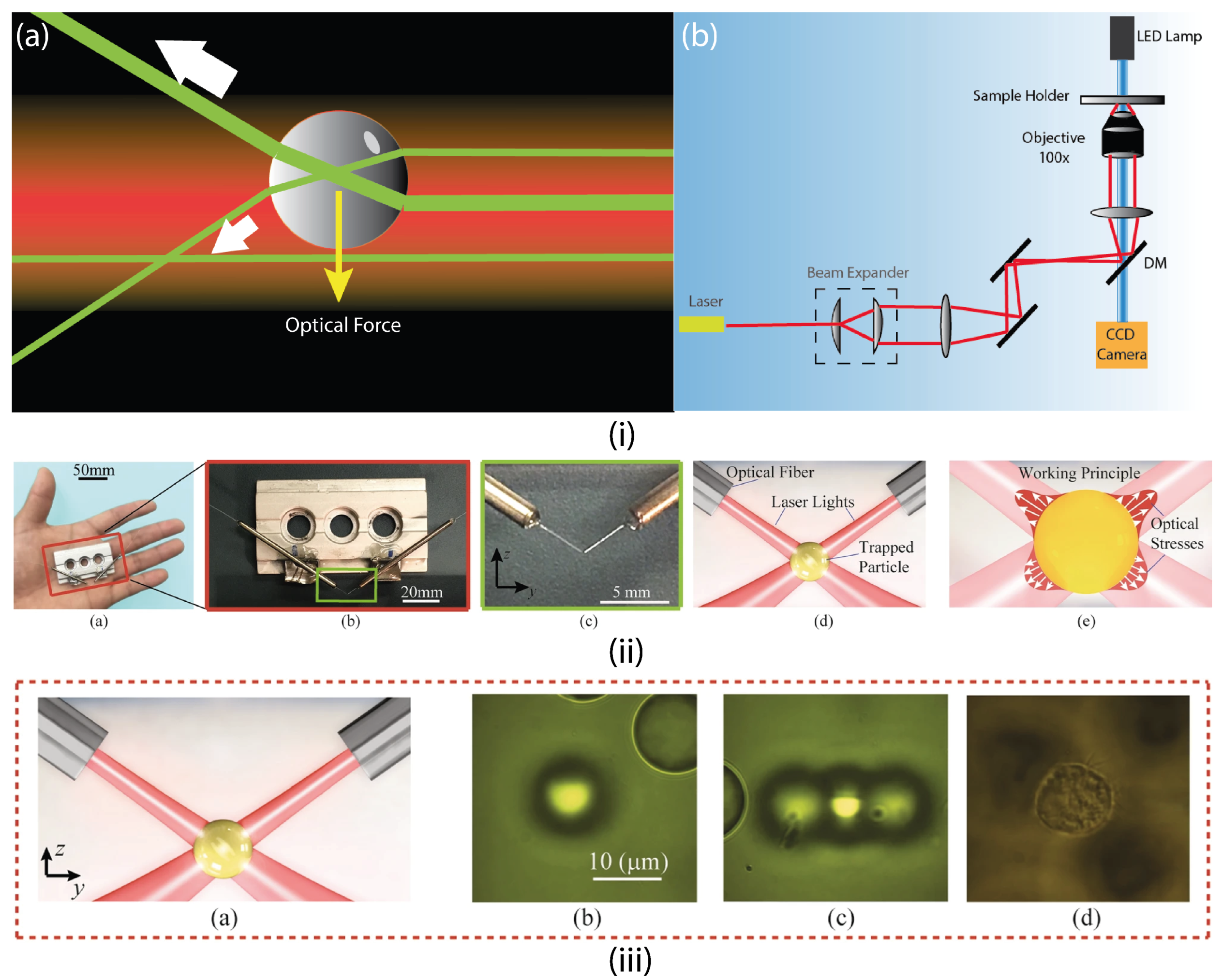
4.1.1. Optical Tweezers (OT)
4.1.2. Optoelectronic Tweezers (OET)
4.1.3. Electrothermoplasmonic Nanotweezers
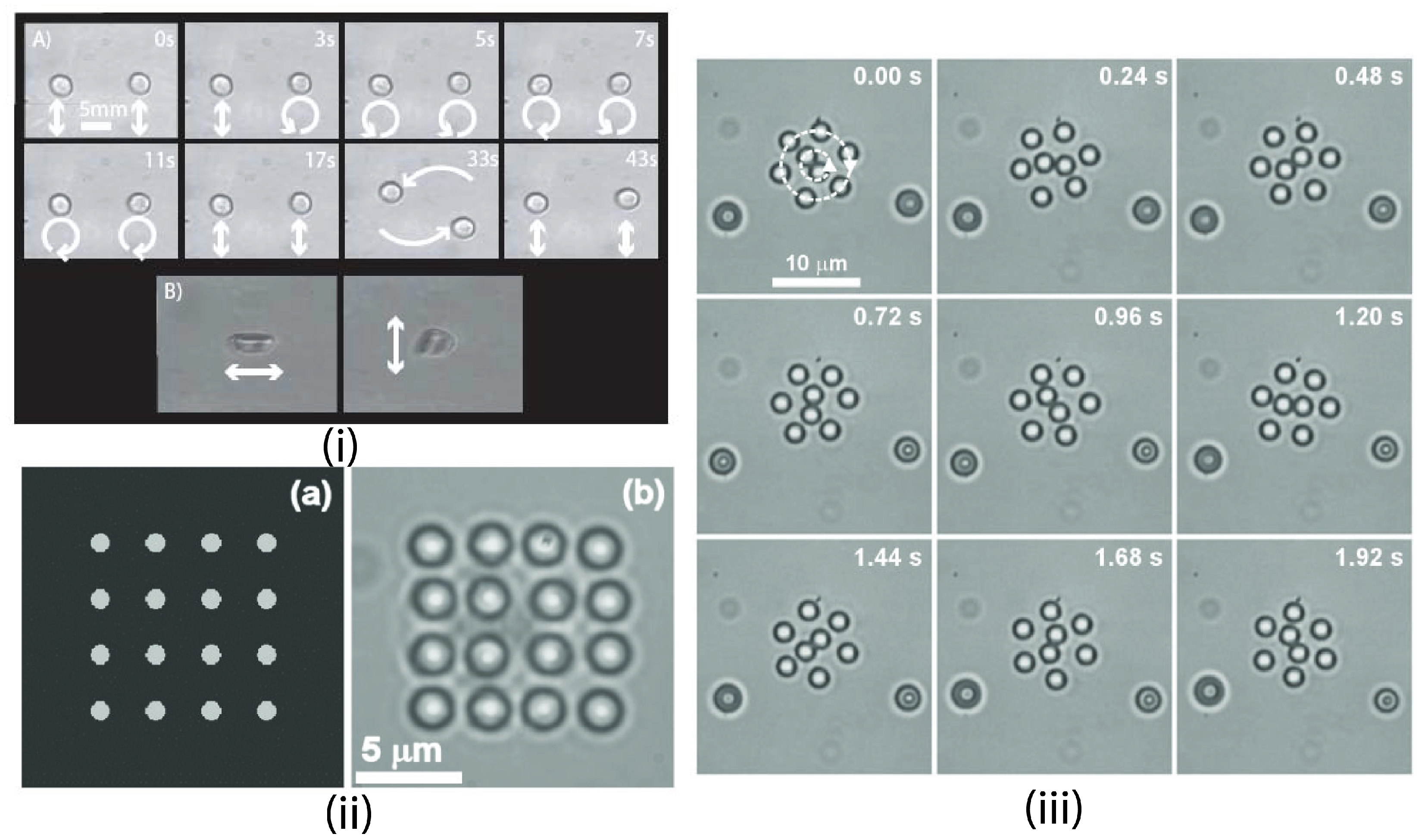
4.1.4. Trapping Multiple Particles
4.1.5. Passive Trapping Manipulation
4.2. Fabrication Process
4.2.1. Material Selection
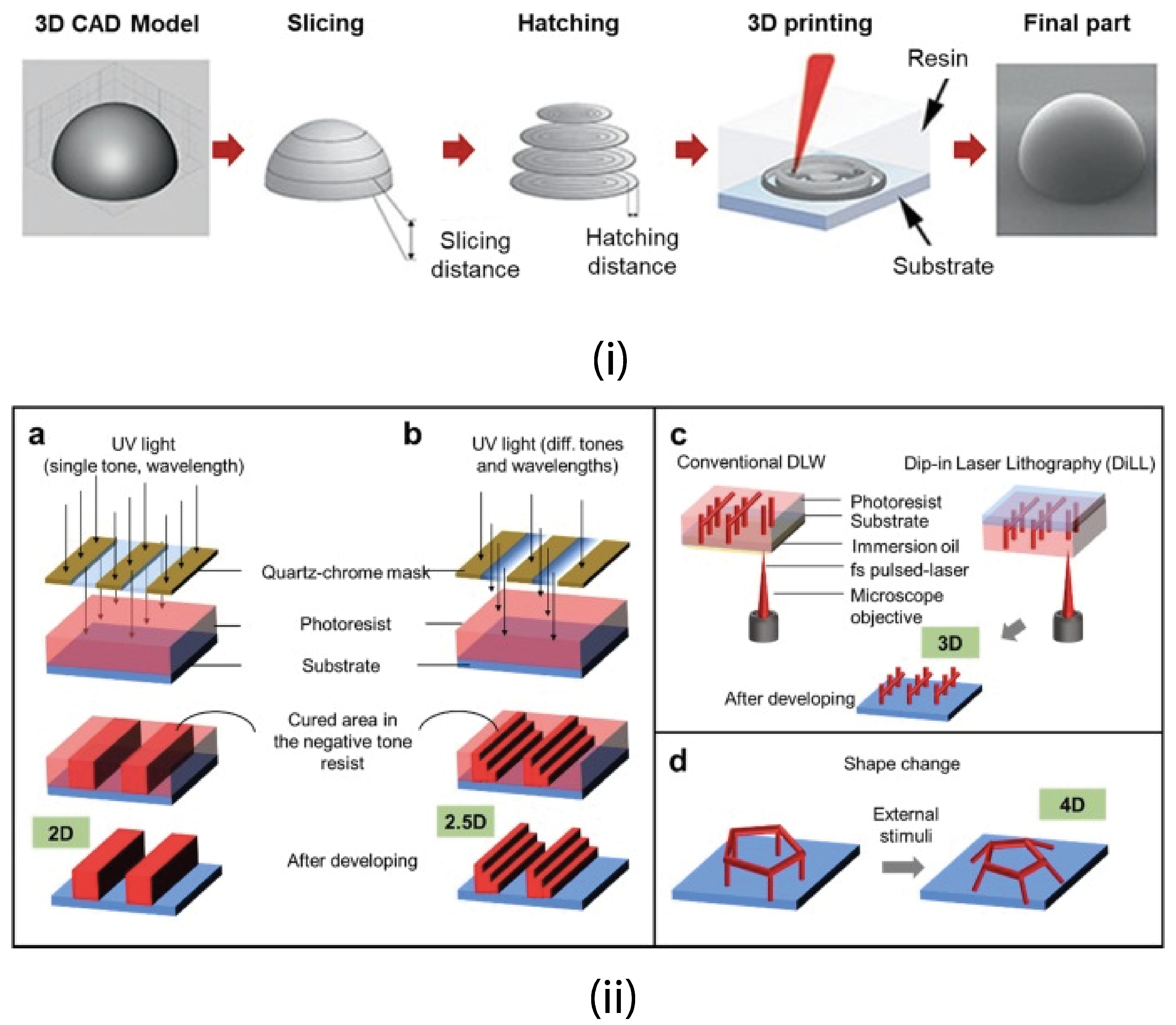
4.2.2. 3D Printing
4.2.3. Solid Free Form Fabrication Techniques (Rapid Prototyping)
4.2.4. Fused Deposition Modeling (FDM)
4.2.5. Stereolithography (SLA)
4.2.6. Selective Laser Sintering (SLS)
4.2.7. Direct Laser Writing
| Fabrication Technique | Applications | Resolution | Limitations | References |
|---|---|---|---|---|
| Fused Deposition Modelling | Transport, sensing, self-propelling vehicles | 100 μm | Limited dimensional accuracy Slower printing speeds | [156,181,182] |
| SLS | Scaffold base tissue engineering, drug delivery vehicles | 45 μm | Limited SLS materials, poor mechanical properties, low surface quality | [183,184,185] |
| SLA | Actuators, lab on a chip, microtissue models, microbots | 3 μm | Expensive, printing larger prints is difficult | [174,175,176] |
| DLW (or 2PP/MPP) | Microbots, microfluidic channels, drug delivery vehicles, scaffolds | 70–100 nm | Varying mechanical strength, material specificity | [12,157,186,187,188] |
4.3. Possible Workspace
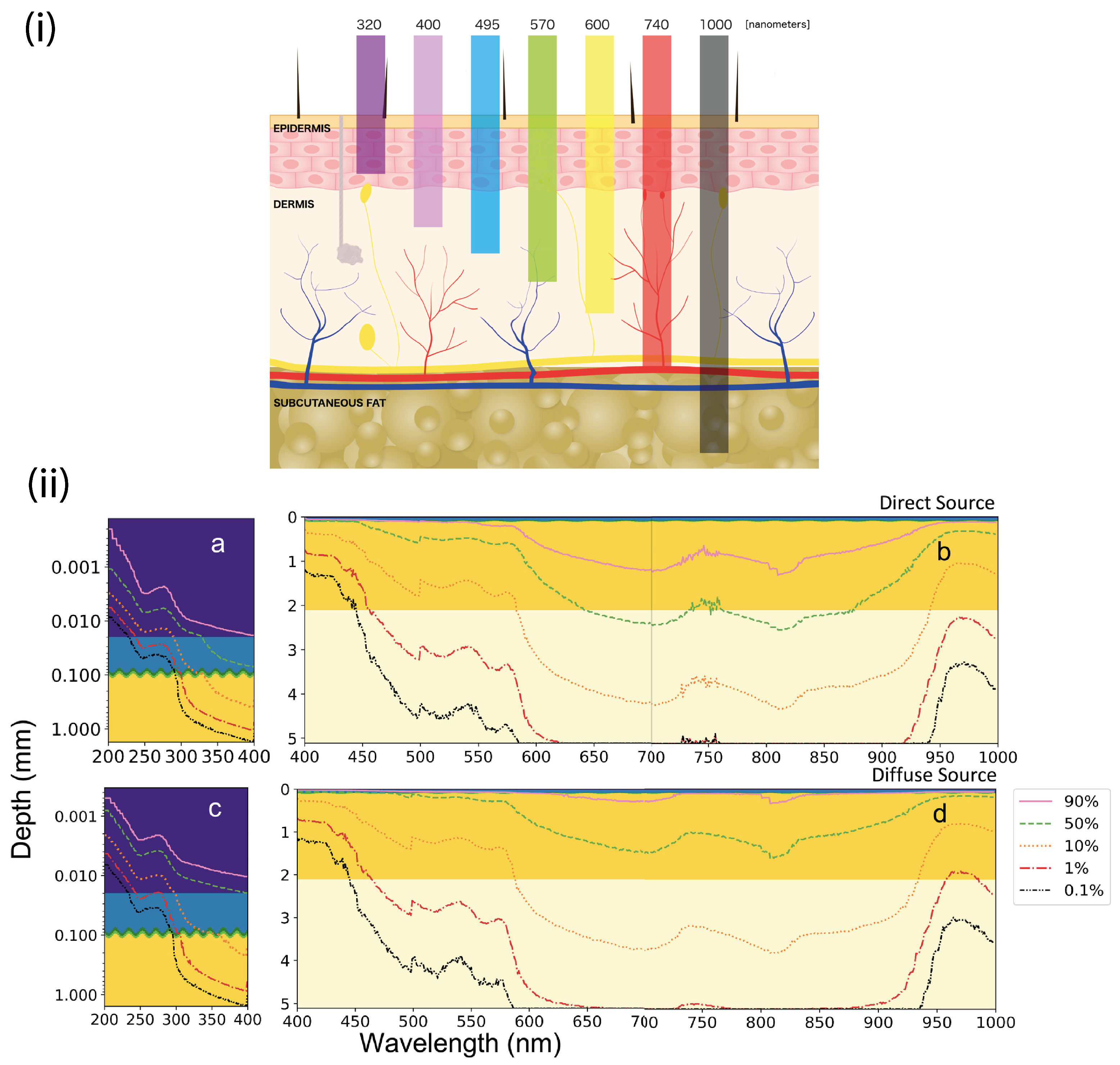
4.4. Wavelength Dependency and Trapping Efficiency
5. Imaging
5.1. Optical Imaging
5.2. Photoacoustic Imaging
5.3. Magnetic Imaging
5.4. Ultrasonic Imaging
5.5. Radiography Imaging
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia, E.; Jimenez, M.A.; De Santos, P.G.; Armada, M. The evolution of robotics research. IEEE Robot. Autom. Mag. 2007, 14, 90–103. [Google Scholar] [CrossRef]
- Halder, A.; Sun, Y. Biocompatible propulsion for biomedical micro/nano robotics. Biosens. Bioelectron. 2019, 139, 111334. [Google Scholar] [CrossRef] [PubMed]
- Koleoso, M.; Feng, X.; Xue, Y.; Li, Q.; Munshi, T.; Chen, X. Micro/nanoscale magnetic robots for biomedical applications. Mater. Today Bio 2020, 8, 100085. [Google Scholar] [CrossRef]
- Li, J.; de Ávila, B.E.F.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Zhang, L. Recent progress on micro-and nano-robots: Towards in vivo tracking and localization. Quant. Imaging Med. Surg. 2018, 8, 461. [Google Scholar] [CrossRef]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef]
- Bunea, A.I.; Engay, E.L.; Chouliara, M.; Bañas, A.R.; Glückstad, J. Rational design of light-controlled microrobots. In Advanced Manufacturing Technologies for Micro-and Nanosystems in Security and Defence; SPIE: Geneva, Switzerland, 2018; Volume 10804, p. 1080406. [Google Scholar]
- Suh, K.Y.; Seong, J.; Khademhosseini, A.; Laibinis, P.E.; Langer, R. A simple soft lithographic route to fabrication of poly (ethylene glycol) microstructures for protein and cell patterning. Biomaterials 2004, 25, 557–563. [Google Scholar] [CrossRef]
- Liu, C.C.; Jin, Z. Applications of microfabrication and micromachining techniques to biotechnology. Trends Biotechnol. 1997, 15, 213–216. [Google Scholar] [CrossRef]
- Yang, L.; Chang, L.; Hu, Y.; Huang, M.; Ji, Q.; Lu, P.; Liu, J.; Chen, W.; Wu, Y. An autonomous soft actuator with light-driven self-sustained wavelike oscillation for phototactic self-locomotion and power generation. Adv. Funct. Mater. 2020, 30, 1908842. [Google Scholar] [CrossRef]
- Sánchez, S.; Soler, L.; Katuri, J. Chemically powered micro-and nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef]
- Huang, Z.; Tsui, G.C.P.; Deng, Y.; Tang, C.Y. Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications. Nanotechnol. Rev. 2020, 9, 1118–1136. [Google Scholar] [CrossRef]
- Behkam, B.; Sitti, M. Bacterial flagella-based propulsion and on/off motion control of microscale objects. Appl. Phys. Lett. 2007, 90, 023902. [Google Scholar] [CrossRef]
- Martel, S. Bacterial microsystems and microrobots. Biomed. Microdevices 2012, 14, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Xuan, M.; Zhang, H.; Lin, X.; Wu, Z.; He, Q. Chemotaxis-guided hybrid neutrophil micromotors for targeted drug transport. Angew. Chem. 2017, 129, 13115–13119. [Google Scholar] [CrossRef]
- Kim, S.; Qiu, F.; Kim, S.; Ghanbari, A.; Moon, C.; Zhang, L.; Nelson, B.J.; Choi, H. Fabrication and characterization of magnetic microrobots for three-dimensional cell culture and targeted transportation. Adv. Mater. 2013, 25, 5863–5868. [Google Scholar] [CrossRef] [PubMed]
- Diller, E.; Giltinan, J.; Sitti, M. Independent control of multiple magnetic microrobots in three dimensions. Int. J. Robot. Res. 2013, 32, 614–631. [Google Scholar] [CrossRef]
- Sul, O.; Falvo, M.; Taylor, R.; Washburn, S.; Superfine, R. Thermally actuated untethered impact-driven locomotive microdevices. Appl. Phys. Lett. 2006, 89, 203512. [Google Scholar] [CrossRef]
- Erdem, E.Y.; Chen, Y.M.; Mohebbi, M.; Suh, J.W.; Kovacs, G.T.; Darling, R.B.; Böhringer, K.F. Thermally actuated omnidirectional walking microrobot. J. Microelectromechanical Syst. 2010, 19, 433–442. [Google Scholar] [CrossRef]
- Donald, B.R.; Levey, C.G.; McGray, C.D.; Paprotny, I.; Rus, D. An untethered, electrostatic, globally controllable MEMS micro-robot. J. Microelectromechanical Syst. 2006, 15, 1–15. [Google Scholar] [CrossRef]
- Pawashe, C.; Floyd, S.; Sitti, M. Multiple magnetic microrobot control using electrostatic anchoring. Appl. Phys. Lett. 2009, 94, 164108. [Google Scholar] [CrossRef]
- Nocentini, S.; Parmeggiani, C.; Martella, D.; Wiersma, D.S. Optically driven soft micro robotics. Adv. Opt. Mater. 2018, 6, 1800207. [Google Scholar] [CrossRef]
- Villangca, M.J.; Palima, D.; Banas, A.R.; Glückstad, J. Light-driven micro-tool equipped with a syringe function. Light. Sci. Appl. 2016, 5, e16148. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, S. Enzymatically induced motion at nano-and micro-scales. Nanoscale 2014, 6, 7757–7763. [Google Scholar] [CrossRef] [PubMed]
- Nourhani, A.; Crespi, V.H.; Lammert, P.E.; Borhan, A. Self-electrophoresis of spheroidal electrocatalytic swimmers. Phys. Fluids 2015, 27, 092002. [Google Scholar] [CrossRef]
- Ding, X.; Lin, S.C.S.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.K.; Shi, J.; Benkovic, S.J.; Huang, T.J. On-chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef]
- Ren, L.; Wang, W.; Mallouk, T.E. Two forces are better than one: Combining chemical and acoustic propulsion for enhanced micromotor functionality. Accounts Chem. Res. 2018, 51, 1948–1956. [Google Scholar] [CrossRef]
- Go, G.; Nguyen, V.D.; Jin, Z.; Park, J.O.; Park, S. A thermo-electromagnetically actuated microrobot for the targeted transport of therapeutic agents. Int. J. Control. Autom. Syst. 2018, 16, 1341–1354. [Google Scholar] [CrossRef]
- Sitti, M.; Wiersma, D.S. Pros and cons: Magnetic versus optical microrobots. Adv. Mater. 2020, 32, 1906766. [Google Scholar] [CrossRef]
- Erkoc, P.; Yasa, I.C.; Ceylan, H.; Yasa, O.; Alapan, Y.; Sitti, M. Mobile microrobots for active therapeutic delivery. Adv. Ther. 2019, 2, 1800064. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Xu, H.; Schmidt, O.G. Micro-and nano-motors: The new generation of drug carriers. Ther. Deliv. 2018, 9, 303–316. [Google Scholar] [CrossRef]
- Yamamoto, D.; Shioi, A. Self-propelled nano/micromotors with a chemical reaction: Underlying physics and strategies of motion control. KONA Powder Part. J. 2015, 2015005. [Google Scholar] [CrossRef]
- Soler, L.; Sánchez, S. Catalytic nanomotors for environmental monitoring and water remediation. Nanoscale 2014, 6, 7175–7182. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, I.C.; Renteria, L.; Burd, H.; Pliskin, N.H.; Thulborn, K.R. Safety of human MRI at static fields above the FDA 8T guideline: Sodium imaging at 9.4 T does not affect vital signs or cognitive ability. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2007, 26, 1222–1227. [Google Scholar] [CrossRef]
- Amara, S.; Abdelmelek, H.; Salem, M.B.; Abidi, R.; Sakly, M. Effects of static magnetic field exposure on hematological and biochemical parameters in rats. Braz. Arch. Biol. Technol. 2006, 49, 889–895. [Google Scholar] [CrossRef]
- Ahmad, B.; Gauthier, M.; Laurent, G.J.; Bolopion, A. Mobile microrobots for in vitro biomedical applications: A survey. IEEE Trans. Robot. 2021, 38, 646–663. [Google Scholar] [CrossRef]
- Ergeneman, O.; Chatzipirpiridis, G.; Pokki, J.; Marin-Suárez, M.; Sotiriou, G.A.; Medina-Rodriguez, S.; Sanchez, J.F.F.; Fernandez-Gutiérrez, A.; Pane, S.; Nelson, B.J. In vitro oxygen sensing using intraocular microrobots. IEEE Trans. Biomed. Eng. 2012, 59, 3104–3109. [Google Scholar] [CrossRef]
- Hosney, A.; Abdalla, J.; Amin, I.S.; Hamdi, N.; Khalil, I.S. In vitro validation of clearing clogged vessels using microrobots. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 272–277. [Google Scholar]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef]
- Banerjee, H.; Shen, S.; Ren, H. Magnetically actuated minimally invasive microbots for biomedical applications. In Electromagnetic Actuation and Sensing in Medical Robotics; Springer: Berlin/Heidelberg, Germany, 2018; pp. 11–41. [Google Scholar]
- Glückstad, J.; Villangca, M.J.; Palima, D.Z.; Bañas, A. Light-actuated microrobots for biomedical science. SPIE Newsroom 2017. [Google Scholar] [CrossRef]
- Yan, Y.; Mehrmohammadi, M.; Jing, W. Non-invasive Photoacoustic Imaging of Magnetic Microrobot through Deep Non-Transparent Tissue. In Proceedings of the 2020 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Toronto, ON, Canada, 13–17 July 2020; pp. 1–6. [Google Scholar]
- Glückstad, J. Sculpting the object. Nat. Photonics 2011, 5, 7–8. [Google Scholar] [CrossRef]
- Palima, D.; Glückstad, J. Gearing up for optical microrobotics: Micromanipulation and actuation of synthetic microstructures by optical forces. Laser Photonics Rev. 2013, 7, 478–494. [Google Scholar] [CrossRef]
- Rodrigo, P.J.; Gammelgaard, L.; Bøggild, P.; Perch-Nielsen, I.R.; Glückstad, J. Actuation of microfabricated tools using multiple GPC-based counterpropagating-beam traps. Opt. Express 2005, 13, 6899–6904. [Google Scholar] [CrossRef]
- Ikin, L.; Carberry, D.; Gibson, G.; Padgett, M.; Miles, M.a. Assembly and force measurement with SPM-like probes in holographic optical tweezers. New J. Phys. 2009, 11, 023012. [Google Scholar] [CrossRef]
- Carberry, D.M.; Simpson, S.; Grieve, J.A.; Wang, Y.; Schäfer, H.; Steinhart, M.; Bowman, R.; Gibson, G.M.; Padgett, M.J.; Hanna, S.; et al. Calibration of optically trapped nanotools. Nanotechnology 2010, 21, 175501. [Google Scholar] [CrossRef]
- Ashkin, A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett. 1970, 24, 156. [Google Scholar] [CrossRef]
- Guix, M.; Weiz, S.M.; Schmidt, O.G.; Medina-Sánchez, M. Self-propelled micro/nanoparticle motors. Part. Part. Syst. Charact. 2018, 35, 1700382. [Google Scholar] [CrossRef]
- Mills, J.K.; Needham, D. Targeted drug delivery. Expert Opin. Ther. Patents 1999, 9, 1499–1513. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198. [Google Scholar] [CrossRef]
- Deckert, P. Current constructs and targets in clinical development for antibody-based cancer therapy. Curr. Drug Targets 2009, 10, 158–175. [Google Scholar] [CrossRef]
- Hong, M.; Zhu, S.; Jiang, Y.; Tang, G.; Pei, Y. Efficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferrin. J. Control. Release 2009, 133, 96–102. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Galvin, P.; Thompson, D.; Ryan, K.B.; McCarthy, A.; Moore, A.C.; Burke, C.S.; Dyson, M.; MacCraith, B.D.; Gun’ko, Y.K.; Byrne, M.T.; et al. Nanoparticle-based drug delivery: Case studies for cancer and cardiovascular applications. Cell. Mol. Life Sci. 2012, 69, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Noyhouzer, T.; L’Homme, C.; Beaulieu, I.; Mazurkiewicz, S.; Kuss, S.; Kraatz, H.B.; Canesi, S.; Mauzeroll, J. Ferrocene-modified phospholipid: An innovative precursor for redox-triggered drug delivery vesicles selective to cancer cells. Langmuir 2016, 32, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Kummer, M.P.; Abbott, J.J.; Kratochvil, B.E.; Borer, R.; Sengul, A.; Nelson, B.J. OctoMag: An electromagnetic system for 5-DOF wireless micromanipulation. IEEE Trans. Robot. 2010, 26, 1006–1017. [Google Scholar] [CrossRef]
- Diller, E.; Giltinan, J.; Lum, G.Z.; Ye, Z.; Sitti, M. Six-degree-of-freedom magnetic actuation for wireless microrobotics. Int. J. Robot. Res. 2016, 35, 114–128. [Google Scholar] [CrossRef]
- Cheang, U.K.; Meshkati, F.; Kim, H.; Lee, K.; Fu, H.C.; Kim, M.J. Versatile microrobotics using simple modular subunits. Sci. Rep. 2016, 6, 30472. [Google Scholar] [CrossRef]
- Roper, M.; Dreyfus, R.; Baudry, J.; Fermigier, M.; Bibette, J.; Stone, H.A. On the dynamics of magnetically driven elastic filaments. J. Fluid Mech. 2006, 554, 167–190. [Google Scholar] [CrossRef]
- Kim, D.; Liu, A.; Diller, E.; Sitti, M. Chemotactic steering of bacteria propelled microbeads. Biomed. Microdevices 2012, 14, 1009–1017. [Google Scholar] [CrossRef]
- Zhuang, J.; Wright Carlsen, R.; Sitti, M. pH-taxis of biohybrid microsystems. Sci. Rep. 2015, 5, 11403. [Google Scholar] [CrossRef]
- Weibel, D.B.; Garstecki, P.; Ryan, D.; DiLuzio, W.R.; Mayer, M.; Seto, J.E.; Whitesides, G.M. Microoxen: Microorganisms to move microscale loads. Proc. Natl. Acad. Sci. USA 2005, 102, 11963–11967. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, W.; Mair, L.O.; Fraleigh, R.D.; Li, S.; Castro, L.A.; Hoyos, M.; Huang, T.J.; Mallouk, T.E. Steering acoustically propelled nanowire motors toward cells in a biologically compatible environment using magnetic fields. Langmuir 2013, 29, 16113–16118. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Zhang, J.; McNeill, J.M.; Kuenen, T.; Linde, F.P.; Rouwkema, J.; Misra, S. Acoustically-actuated bubble-powered rotational micro-propellers. Sens. Actuators B Chem. 2021, 347, 130589. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.y.; Pane, S.; Nelson, B.J.; Choi, H. Acoustically mediated controlled drug release and targeted therapy with degradable 3D porous magnetic microrobots. Adv. Healthc. Mater. 2021, 10, 2001096. [Google Scholar] [CrossRef]
- Gao, W.; Uygun, A.; Wang, J. Hydrogen-bubble-propelled zinc-based microrockets in strongly acidic media. J. Am. Chem. Soc. 2012, 134, 897–900. [Google Scholar] [CrossRef]
- Mou, F.; Chen, C.; Ma, H.; Yin, Y.; Wu, Q.; Guan, J. Self-propelled micromotors driven by the magnesium–water reaction and their hemolytic properties. Angew. Chem. Int. Ed. 2013, 52, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.K.; Medina-Sánchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Fortuna, L.; Buscarino, A. Microrobots in Micromachines. Micromachines 2022, 13, 1207. [Google Scholar] [CrossRef]
- Köhler, J.; Ksouri, S.I.; Esen, C.; Ostendorf, A. Optical screw-wrench for microassembly. Microsyst. Nanoeng. 2017, 3, 16083. [Google Scholar] [CrossRef]
- Kim, J.D.; Hwang, S.U.; Lee, Y.G. Traceable assembly of microparts using optical tweezers. J. Micromechanics Microengineering 2012, 22, 105003. [Google Scholar] [CrossRef]
- Baglio, S.; Castorina, S.; Fortuna, L.; Savalli, N. Modeling and design of novel photo-thermo-mechanical microactuators. Sens. Actuators A Phys. 2002, 101, 185–193. [Google Scholar] [CrossRef]
- Zhang, S.; Scott, E.Y.; Singh, J.; Chen, Y.; Zhang, Y.; Elsayed, M.; Chamberlain, M.D.; Shakiba, N.; Adams, K.; Yu, S.; et al. The optoelectronic microrobot: A versatile toolbox for micromanipulation. Proc. Natl. Acad. Sci. USA 2019, 116, 14823–14828. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.R.; Huang, H.; Kwon, R.Y. Introduction to Cell Mechanics and Mechanobiology; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Hao, Y.; Cheng, S.; Tanaka, Y.; Hosokawa, Y.; Yalikun, Y.; Li, M. Mechanical properties of single cells: Measurement methods and applications. Biotechnol. Adv. 2020, 45, 107648. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Galea, G.L.; Lanyon, L.E.; Price, J.S. Sclerostin’s role in bone’s adaptive response to mechanical loading. Bone 2017, 96, 38–44. [Google Scholar] [CrossRef]
- Sundh, D.; Nilsson, M.; Zoulakis, M.; Pasco, C.; Yilmaz, M.; Kazakia, G.J.; Hellgren, M.; Lorentzon, M. High-impact mechanical loading increases bone material strength in postmenopausal women—A 3-month intervention study. J. Bone Miner. Res. 2018, 33, 1242–1251. [Google Scholar] [CrossRef]
- Min, E.; Schwartz, M.A. Translocating transcription factors in fluid shear stress-mediated vascular remodeling and disease. Exp. Cell Res. 2019, 376, 92–97. [Google Scholar] [CrossRef]
- Roy Choudhury, A.; Gupta, S.; Chaturvedi, P.K.; Kumar, N.; Pandey, D. Mechanobiology of cancer stem cells and their niche. Cancer Microenviron. 2019, 12, 17–27. [Google Scholar] [CrossRef]
- Boyd, N.F.; Rommens, J.M.; Vogt, K.; Lee, V.; Hopper, J.L.; Yaffe, M.J.; Paterson, A.D. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005, 6, 798–808. [Google Scholar] [CrossRef]
- Daniels, C.E.; Jett, J.R. Does interstitial lung disease predispose to lung cancer? Curr. Opin. Pulm. Med. 2005, 11, 431–437. [Google Scholar] [CrossRef]
- Grexa, I.; Fekete, T.; Molnár, J.; Molnár, K.; Vizsnyiczai, G.; Ormos, P.; Kelemen, L. Single-cell elasticity measurement with an optically actuated microrobot. Micromachines 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Sánchez, P.; Bodensiek, K.; Li, S.; Simons, M.; Schaap, I.A. Cell visco-elasticity measured with AFM and optical trapping at sub-micrometer deformations. PLoS ONE 2012, 7, e45297. [Google Scholar] [CrossRef] [PubMed]
- Dy, M.C.C.; Kanaya, S.; Sugiura, T. Localized cell stiffness measurement using axial movement of an optically trapped microparticle. J. Biomed. Opt. 2013, 18, 111411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ndoye, F.; Yousafzai, M.S.; Coceano, G.; Bonin, S.; Scoles, G.; Ka, O.; Niemela, J.; Cojoc, D. The influence of lateral forces on the cell stiffness measurement by optical tweezers vertical indentation. Int. J. Optomechatronics 2016, 10, 53–62. [Google Scholar] [CrossRef]
- Yousafzai, M.S.; Coceano, G.; Mariutti, A.; Ndoye, F.; Amin, L.; Niemela, J.; Bonin, S.; Scoles, G.; Cojoc, D. Effect of neighboring cells on cell stiffness measured by optical tweezers indentation. J. Biomed. Opt. 2016, 21, 057004. [Google Scholar] [CrossRef]
- Vargas-Pinto, R.; Gong, H.; Vahabikashi, A.; Johnson, M. The effect of the endothelial cell cortex on atomic force microscopy measurements. Biophys. J. 2013, 105, 300–309. [Google Scholar] [CrossRef]
- Li, M.; Arlt, J. Trapping multiple particles in single optical tweezers. Opt. Commun. 2008, 281, 135–140. [Google Scholar] [CrossRef]
- Rodríguez-Oliveros, R.; Sánchez-Gil, J.A. Gold nanostars as thermoplasmonic nanoparticles for optical heating. Opt. Express 2012, 20, 621–626. [Google Scholar] [CrossRef]
- Mezeme, M.E.; Brosseau, C. Engineering nanostructures with enhanced thermoplasmonic properties for biosensing and selective targeting applications. Phys. Rev. E 2013, 87, 012722. [Google Scholar] [CrossRef]
- Braun, M.; Cichos, F. Optically controlled thermophoretic trapping of single nano-objects. ACS Nano 2013, 7, 11200–11208. [Google Scholar] [CrossRef]
- Ndukaife, J.C.; Kildishev, A.V.; Nnanna, A.G.A.; Shalaev, V.M.; Wereley, S.T.; Boltasseva, A. Long-range and rapid transport of individual nano-objects by a hybrid electrothermoplasmonic nanotweezer. Nat. Nanotechnol. 2016, 11, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Engay, E.; Bunea, A.I.; Chouliara, M.; Bañas, A.; Glückstad, J. Natural convection induced by an optically fabricated and actuated microtool with a thermoplasmonic disk. Opt. Lett. 2018, 43, 3870–3873. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Schiavo, R.; Pedrazzoli, P.; Carlo-Stella, C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J. Clin. Oncol. 2000, 18, 1360–1377. [Google Scholar] [CrossRef]
- Hristov, M.; Weber, C. Endothelial progenitor cells: Cellular biomarkers in vascular disease. Drug Discov. Today Dis. Mech. 2008, 5, e267–e271. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Fatoyinbo, H.; Li, X.J. Microfluidic devices for cell manipulation. In Microfluidic Devices for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–389. [Google Scholar]
- Steager, E.B.; Selman Sakar, M.; Magee, C.; Kennedy, M.; Cowley, A.; Kumar, V. Automated biomanipulation of single cells using magnetic microrobots. Int. J. Robot. Res. 2013, 32, 346–359. [Google Scholar] [CrossRef]
- Brufau, J.; Puig-Vidal, M.; Lopez-Sanchez, J.; Samitier, J.; Snis, N.; Simu, U.; Johansson, S.; Driesen, W.; Breguet, J.M.; Gao, J.; et al. MICRON: Small autonomous robot for cell manipulation applications. In Proceedings of the 2005 IEEE International Conference on Robotics and Automation, Barcelona, Spain, 18–22 April 2005; pp. 844–849. [Google Scholar]
- Sanchez, S.; Solovev, A.A.; Schulze, S.; Schmidt, O.G. Controlled manipulation of multiple cells using catalytic microbots. Chem. Commun. 2011, 47, 698–700. [Google Scholar] [CrossRef]
- Sakar, M.S.; Steager, E.B.; Cowley, A.; Kumar, V.; Pappas, G.J. Wireless manipulation of single cells using magnetic microtransporters. In Proceedings of the 2011 IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; pp. 2668–2673. [Google Scholar]
- Kim, H.; Ali, J.; Cheang, U.K.; Jeong, J.; Kim, J.S.; Kim, M.J. Micro manipulation using magnetic microrobots. J. Bionic Eng. 2016, 13, 515–524. [Google Scholar] [CrossRef]
- Lin, Z.; Fan, X.; Sun, M.; Gao, C.; He, Q.; Xie, H. Magnetically actuated peanut colloid motors for cell manipulation and patterning. ACS Nano 2018, 12, 2539–2545. [Google Scholar] [CrossRef]
- Buican, T.N.; Smyth, M.J.; Crissman, H.A.; Salzman, G.C.; Stewart, C.C.; Martin, J.C. Automated single-cell manipulation and sorting by light trapping. Appl. Opt. 1987, 26, 5311–5316. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J. Internal cell manipulation using infrared laser traps. Proc. Natl. Acad. Sci. USA 1989, 86, 7914–7918. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Sato-Maeda, M.; Tashiro, H. Micromanipulation: Whole-cell manipulation by optical trapping. Curr. Biol. 1995, 5, 380–382. [Google Scholar] [CrossRef][Green Version]
- Eriksson, E.; Sott, K.; Lundqvist, F.; Sveningsson, M.; Scrimgeour, J.; Hanstorp, D.; Goksör, M.; Granéli, A. A microfluidic device for reversible environmental changes around single cells using optical tweezers for cell selection and positioning. Lab Chip 2010, 10, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Keloth, A.; Anderson, O.; Risbridger, D.; Paterson, L. Single cell isolation using optical tweezers. Micromachines 2018, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, H.W.; Yu, P.P.; Zhang, S.H.; Zhou, J.H.; Li, Y.M.; Gong, L. Trapping and manipulation of single cells in crowded environments. Front. Bioeng. Biotechnol. 2020, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Torres-Mapa, M.L.; Lee, W.M.; Čižmár, T.; Campbell, P.; Gunn-Moore, F.J.; Dholakia, K. Spatially optimized gene transfection by laser-induced breakdown of optically trapped nanoparticles. Appl. Phys. Lett. 2011, 98, 093702. [Google Scholar] [CrossRef]
- Waleed, M.; Hwang, S.U.; Kim, J.D.; Shabbir, I.; Shin, S.M.; Lee, Y.G. Single-cell optoporation and transfection using femtosecond laser and optical tweezers. Biomed. Opt. Express 2013, 4, 1533–1547. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Wegener, M. Three-dimensional optical laser lithography beyond the diffraction limit. Laser Photonics Rev. 2013, 7, 22–44. [Google Scholar] [CrossRef]
- Hu, M.; Ge, X.; Chen, X.; Mao, W.; Qian, X.; Yuan, W.E. Micro/nanorobot: A promising targeted drug delivery system. Pharmaceutics 2020, 12, 665. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tu, Y.; Wilson, D.A. Micro/nanomotors towards in vivo application: Cell, tissue and biofluid. Chem. Soc. Rev. 2017, 46, 5289–5310. [Google Scholar] [CrossRef]
- Duan, W.; Wang, W.; Das, S.; Yadav, V.; Mallouk, T.E.; Sen, A. Synthetic nano-and micromachines in analytical chemistry: Sensing, migration, capture, delivery, and separation. Annu. Rev. Anal. Chem. 2015, 8, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Ravve, A. Light-Associated Reactions of Synthetic Polymers; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Solovev, A.A.; Xi, W.; Gracias, D.H.; Harazim, S.M.; Deneke, C.; Sanchez, S.; Schmidt, O.G. Self-propelled nanotools. ACS Nano 2012, 6, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Nijemeisland, M.; Abdelmohsen, L.K.; Huck, W.T.; Wilson, D.A.; Van Hest, J.C. A compartmentalized out-of-equilibrium enzymatic reaction network for sustained autonomous movement. ACS Cent. Sci. 2016, 2, 843–849. [Google Scholar] [CrossRef]
- Hortelão, A.C.; Patiño, T.; Perez-Jiménez, A.; Blanco, À.; Sánchez, S. Enzyme-powered nanobots enhance anticancer drug delivery. Adv. Funct. Mater. 2018, 28, 1705086. [Google Scholar] [CrossRef]
- Pantarotto, D.; Browne, W.R.; Feringa, B.L. Autonomous propulsion of carbon nanotubes powered by a multienzyme ensemble. Chem. Commun. 2008, 1533–1535. [Google Scholar] [CrossRef]
- Gaffney, E.A.; Gadêlha, H.; Smith, D.J.; Blake, J.R.; Kirkman-Brown, J.C. Mammalian Sperm Motility: Observation and Theory; University of Oxford: Oxford, UK, 2011. [Google Scholar]
- Ladner, I.S.; Cullinan, M.A.; Saha, S.K. Tensile properties of polymer nanowires fabricated via two-photon lithography. RSC Adv. 2019, 9, 28808–28813. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Lee, J.; Nelson, B.J.; Zhang, L.; Choi, H. Fabrication and manipulation of ciliary microrobots with non-reciprocal magnetic actuation. Sci. Rep. 2016, 6, 30713. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Yesin, K.B.; Vollmers, K.; Nelson, B.J. Modeling and control of untethered biomicrorobots in a fluidic environment using electromagnetic fields. Int. J. Robot. Res. 2006, 25, 527–536. [Google Scholar] [CrossRef]
- Grady, M.; Howard Iii, M.; Molloy, J.; Ritter, R.; Quate, E.; Gillies, G. Nonlinear magnetic stereotaxis: Three-dimensional, in vivo remote magnetic manipulation of a small object in canine brain. Med. Phys. 1990, 17, 405–415. [Google Scholar] [CrossRef]
- Wadhams, G.H.; Armitage, J.P. Making sense of it all: Bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 2004, 5, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Jeong, J.; Song, H.; Chung, S.K. Targeted drug delivery technology using untethered microrobots: A review. J. Micromechanics Microengineering 2019, 29, 053002. [Google Scholar] [CrossRef]
- Ti, C.; Shen, Y.; Ho Thanh, M.T.; Wen, Q.; Liu, Y. Reliable and mobile all-fiber modular optical tweezers. Sci. Rep. 2020, 10, 20099. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Mei, Y.; Bermúdez Ureña, E.; Huang, G.; Schmidt, O.G. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 2009, 5, 1688–1692. [Google Scholar] [CrossRef]
- Magdanz, V.; Sanchez, S.; Schmidt, O.G. Development of a sperm-flagella driven micro-bio-robot. Adv. Mater. 2013, 25, 6581–6588. [Google Scholar] [CrossRef]
- Zhang, S.; Wheeler, A.R. Light-driven microrobotics and their applications for single-cell manipulation. In Optical Trapping and Optical Micromanipulation XVII; SPIE: Geneva, Switzerland, 2020; Volume 11463, pp. 73–78. [Google Scholar]
- Jamil, M.F.; Pokharel, M.; Park, K. Design and Fabrication of Untethered Light-Actuated Microbots in Fluid for Biomedical Applications. Appl. Mech. 2022, 3, 1240–1253. [Google Scholar] [CrossRef]
- Jamil, M.F.; Pokharel, M.; Park, K. Optical Manipulation of Microparticles in Fluids Using Modular Optical Tweezers. In Proceedings of the 2022 International Symposium on Medical Robotics (ISMR), Atlanta, GA, USA, 13–15 April 2022; pp. 1–7. [Google Scholar]
- Ohta, A.T.; Chiou, P.Y.; Jamshidi, A.; Hsu, H.Y.; Valley, J.K.; Neale, S.L.; Wu, M.C. Optoelectronic Tweezers for the Manipulation of Cells, Microparticles, and Nanoparticles; InTech: London, UK, 2010; Volume 2. [Google Scholar]
- Neale, S.; Kremer, C.; Barrett, M.; Cooper, J. Optoelectronic Tweezers as a Tool for Medical Diagnostics. In Optical Trapping Applications; Optica Publishing Group: Washington, DC, USA, 2011; p. OTTuC1. [Google Scholar]
- Zhang, Y.; Min, C.; Dou, X.; Wang, X.; Urbach, H.P.; Somekh, M.G.; Yuan, X. Plasmonic tweezers: For nanoscale optical trapping and beyond. Light. Sci. Appl. 2021, 10, 59. [Google Scholar] [CrossRef]
- Ndukaife, J.C.; Nnanna, A.G.A.; Kildishev, A.V.; Shalaev, V.M.; Wereley, S.T.; Boltasseva, A. On-demand rapid transport and stable trapping of nanoparticles of nanoparticles by a hybrid electrothermoplasmonic nanotweezer (Conference Presentation). In Optical Trapping and Optical Micromanipulation XIII; SPIE: Geneva, Switzerland, 2016; Volume 9922, p. 237. [Google Scholar]
- Visscher, K.; Brakenhoff, G.; Krol, J. Micromanipulation by “multiple” optical traps created by a single fast scanning trap integrated with the bilateral confocal scanning laser microscope. Cytom. J. Int. Soc. Anal. Cytol. 1993, 14, 105–114. [Google Scholar] [CrossRef]
- Vermeulen, K.C.; Van Mameren, J.; Stienen, G.J.; Peterman, E.J.; Wuite, G.J.; Schmidt, C.F. Calibrating bead displacements in optical tweezers using acousto-optic deflectors. Rev. Sci. Instrum. 2006, 77, 013704. [Google Scholar] [CrossRef]
- Preece, D.; Keen, S.; Botvinick, E.; Bowman, R.; Padgett, M.; Leach, J. Independent polarisation control of multiple optical traps. Opt. Express 2008, 16, 15897–15902. [Google Scholar] [CrossRef]
- Liesener, J.; Reicherter, M.; Haist, T.; Tiziani, H.J. Multi-functional optical tweezers using computer-generated holograms. Opt. Commun. 2000, 185, 77–82. [Google Scholar] [CrossRef]
- Paterson, L.; MacDonald, M.P.; Arlt, J.; Sibbett, W.; Bryant, P.; Dholakia, K. Controlled rotation of optically trapped microscopic particles. Science 2001, 292, 912–914. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.T.; Padgett, M.J. Rotational control within optical tweezers by use of a rotating aperture. Opt. Lett. 2002, 27, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, R.L.; Daria, V.R.; Glückstad, J. Fully dynamic multiple-beam optical tweezers. Opt. Express 2002, 10, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Swartzlander, G.A.; Peterson, T.J.; Artusio-Glimpse, A.B.; Raisanen, A.D. Stable optical lift. Nat. Photonics 2011, 5, 48–51. [Google Scholar] [CrossRef]
- Maruo, S.; Kawata, S. Two-photon-absorbed near-infrared photopolymerization for three-dimensional microfabrication. J. Microelectromechanical Syst. 1998, 7, 411–415. [Google Scholar] [CrossRef]
- Lu, W.E.; Dong, X.Z.; Chen, W.Q.; Zhao, Z.S.; Duan, X.M. Novel photoinitiator with a radical quenching moiety for confining radical diffusion in two-photon induced photopolymerization. J. Mater. Chem. 2011, 21, 5650–5659. [Google Scholar] [CrossRef]
- Juodkazis, S.; Mizeikis, V.; Seet, K.K.; Miwa, M.; Misawa, H. Two-photon lithography of nanorods in SU-8 photoresist. Nanotechnology 2005, 16, 846. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Chen, Q.D.; Xia, H.; Sun, H.B. Designable 3D nanofabrication by femtosecond laser direct writing. Nano Today 2010, 5, 435–448. [Google Scholar] [CrossRef]
- Rajabasadi, F.; Schwarz, L.; Medina-Sánchez, M.; Schmidt, O.G. 3D and 4D lithography of untethered microrobots. Prog. Mater. Sci. 2021, 120, 100808. [Google Scholar] [CrossRef]
- Li, J.; Pumera, M. 3D printing of functional microrobots. Chem. Soc. Rev. 2021, 50, 2794–2838. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Decanini, D.; Leroy, L.; Haghiri-Gosnet, A. Note: On-chip multifunctional fluorescent-magnetic Janus helical microswimmers. Rev. Sci. Instruments 2016, 87, 036104. [Google Scholar] [CrossRef] [PubMed]
- Teh, W.; Dürig, U.; Drechsler, U.; Smith, C.; Güntherodt, H.J. Effect of low numerical-aperture femtosecond two-photon absorption on (SU-8) resist for ultrahigh-aspect-ratio microstereolithography. J. Appl. Phys. 2005, 97, 054907. [Google Scholar] [CrossRef]
- Niedert, E.E.; Bi, C.; Adam, G.; Lambert, E.; Solorio, L.; Goergen, C.J.; Cappelleri, D.J. A tumbling magnetic microrobot system for biomedical applications. Micromachines 2020, 11, 861. [Google Scholar] [CrossRef]
- Striggow, F.; Medina-Sánchez, M.; Auernhammer, G.K.; Magdanz, V.; Friedrich, B.M.; Schmidt, O.G. Sperm-driven micromotors moving in oviduct fluid and viscoelastic media. Small 2020, 16, 2000213. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Walboomers, X.F.; Swieszkowski, W. Enhancing X-ray attenuation of 3D printed gelatin methacrylate (GelMA) hydrogels utilizing gold nanoparticles for bone tissue engineering applications. Polymers 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Li, S.; Yu, K.; He, B.; Hong, J.; Xu, T.; Meng, J.; Ye, C.; Chen, Y.; Shi, Z.; et al. A 3D-printed PRP-GelMA hydrogel promotes osteochondral regeneration through M2 macrophage polarization in a rabbit model. Acta Biomater. 2021, 128, 150–162. [Google Scholar] [CrossRef]
- Seol, Y.J.; Kang, T.Y.; Cho, D.W. Solid freeform fabrication technology applied to tissue engineering with various biomaterials. Soft Matter 2012, 8, 1730–1735. [Google Scholar] [CrossRef]
- Kai, H.; Wang, X.; Madhukar, K.S.; Qin, L.; Yan, Y.; Zhang, R.; Wang, X. Fabrication of a two-level tumor bone repair biomaterial based on a rapid prototyping technique. Biofabrication 2009, 1, 025003. [Google Scholar] [CrossRef]
- Crump, S.S. Apparatus and Method for Creating Three-Dimensional Objects. U.S. Patent US5,121,329, 9 June 1992. [Google Scholar]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Chen, G.; Chen, N.; Wang, Q. Fabrication and properties of poly (vinyl alcohol)/β-tricalcium phosphate composite scaffolds via fused deposition modeling for bone tissue engineering. Compos. Sci. Technol. 2019, 172, 17–28. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Fleming, J.W.; Player, D.J.; Baker, L.A.; Turner, M.C.; Jones, J.M.; Martin, N.R.; Ferguson, R.A.; Mudera, V.C.; et al. Scalable 3D printed molds for human tissue engineered skeletal muscle. Front. Bioeng. Biotechnol. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, R.; Chen, Y.C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.; Bertoldi, K.; Gabbrielli, R.; Velders, A.H.; Feijen, J.; Grijpma, D.W. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials 2010, 31, 6909–6916. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly (propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef]
- Skoog, S.A.; Goering, P.L.; Narayan, R.J. Stereolithography in tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 845–856. [Google Scholar] [CrossRef]
- Lermusiaux, P.; Leroux, C.; Tasse, J.C.; Castellani, L.; Martinez, R. Aortic aneurysm: Construction of a life-size model by rapid prototyping. Ann. Vasc. Surg. 2001, 15, 131–135. [Google Scholar] [CrossRef]
- Ge, Z.; Dai, L.; Zhao, J.; Yu, H.; Yang, W.; Liao, X.; Tan, W.; Jiao, N.; Wang, Z.; Liu, L. Bubble-based microrobots enable digital assembly of heterogeneous microtissue modules. Biofabrication 2022, 14, 025023. [Google Scholar] [CrossRef]
- Chan, V.; Jeong, J.H.; Bajaj, P.; Collens, M.; Saif, T.; Kong, H.; Bashir, R. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip 2012, 12, 88–98. [Google Scholar] [CrossRef]
- Zhan, Z.; Chen, L.; Duan, H.; Chen, Y.; He, M.; Wang, Z. 3D printed ultra-fast photothermal responsive shape memory hydrogel for microrobots. Int. J. Extrem. Manuf. 2021, 4, 015302. [Google Scholar] [CrossRef]
- Tan, K.; Chua, C.; Leong, K.; Cheah, C.; Gui, W.; Tan, W.; Wiria, F. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Bio-Med. Mater. Eng. 2005, 15, 113–124. [Google Scholar]
- Yeong, W.; Sudarmadji, N.; Yu, H.; Chua, C.; Leong, K.; Venkatraman, S.; Boey, Y.; Tan, L. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Lee, M.Y.; Tsai, W.W.; Wang, H.C.; Lu, W.C. Osteogenesis of adipose-derived stem cells on polycaprolactone–β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J. Tissue Eng. Regen. Med. 2016, 10, E337–E353. [Google Scholar] [CrossRef]
- Malinauskas, M.; Farsari, M.; Piskarskas, A.; Juodkazis, S. Ultrafast laser nanostructuring of photopolymers: A decade of advances. Phys. Rep. 2013, 533, 1–31. [Google Scholar] [CrossRef]
- Adam, G.; Benouhiba, A.; Rabenorosoa, K.; Clévy, C.; Cappelleri, D.J. 4D printing: Enabling technology for microrobotics applications. Adv. Intell. Syst. 2021, 3, 2000216. [Google Scholar] [CrossRef]
- Shabaniverki, S.; Alvarez-Valdivia, A.; Juárez, J.J. 3D printed self-propelled composite floaters. Smart Mater. Struct. 2021, 30, 075015. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M. Selective laser sintering and its application in biomedical engineering. MRS Bull. 2011, 36, 998–1005. [Google Scholar] [CrossRef]
- Gu, D.; Hagedorn, Y.C.; Meiners, W.; Meng, G.; Batista, R.J.S.; Wissenbach, K.; Poprawe, R. Densification behavior, microstructure evolution, and wear performance of selective laser melting processed commercially pure titanium. Acta Mater. 2012, 60, 3849–3860. [Google Scholar] [CrossRef]
- Schmid, M.; Ludescher, D.; Giessen, H. Optical properties of photoresists for femtosecond 3D printing: Refractive index, extinction, luminescence-dose dependence, aging, heat treatment and comparison between 1-photon and 2-photon exposure. Opt. Mater. Express 2019, 9, 4564–4577. [Google Scholar] [CrossRef]
- Accardo, A.; Blatché, M.C.; Courson, R.; Loubinoux, I.; Thibault, C.; Malaquin, L.; Vieu, C. Multiphoton direct laser writing and 3D imaging of polymeric freestanding architectures for cell colonization. Small 2017, 13, 1700621. [Google Scholar] [CrossRef] [PubMed]
- Knoška, J.; Adriano, L.; Awel, S.; Beyerlein, K.R.; Yefanov, O.; Oberthuer, D.; Peña Murillo, G.E.; Roth, N.; Sarrou, I.; Villanueva-Perez, P.; et al. Ultracompact 3D microfluidics for time-resolved structural biology. Nat. Commun. 2020, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Douplik, A.; Saiko, G.; Schelkanova, I.; Tuchin, V.V. The response of tissue to laser light. In Lasers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 47–109. [Google Scholar]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.C.; Wei, X.B.; Zhou, J.H.; Wang, Z.Q.; Li, Y.M. Trapping red blood cells in living animals using optical tweezers. Nat. Commun. 2013, 4, 1768. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, D.; Sonek, G.; Berns, M.; Chapman, C.; Tromberg, B. Evidence for localized cell heating induced by infrared optical tweezers. Biophys. J. 1995, 68, 2137–2144. [Google Scholar] [CrossRef]
- Gross, S.P. Chapter 8. Application of optical trapsin Vivo. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 361, pp. 162–174. [Google Scholar]
- Rasmussen, M.; Oddershede, L.; Siegumfeldt, H. Optical tweezers cause physiological damage to Escherichia coli and Listeria bacteria. Appl. Environ. Microbiol. 2008, 74, 2441–2446. [Google Scholar] [CrossRef]
- Liang, H.; Vu, K.T.; Krishnan, P.; Trang, T.C.; Shin, D.; Kimel, S.; Berns, M.W. Wavelength dependence of cell cloning efficiency after optical trapping. Biophys. J. 1996, 70, 1529–1533. [Google Scholar] [CrossRef]
- Ayano, S.; Wakamoto, Y.; Yamashita, S.; Yasuda, K. Quantitative measurement of damage caused by 1064-nm wavelength optical trapping of Escherichia coli cells using on-chip single cell cultivation system. Biochem. Biophys. Res. Commun. 2006, 350, 678–684. [Google Scholar] [CrossRef]
- Neuman, K.C.; Chadd, E.H.; Liou, G.F.; Bergman, K.; Block, S.M. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 1999, 77, 2856–2863. [Google Scholar] [CrossRef]
- Leitz, G.; Fällman, E.; Tuck, S.; Axner, O. Stress response in Caenorhabditis elegans caused by optical tweezers: Wavelength, power, and time dependence. Biophys. J. 2002, 82, 2224–2231. [Google Scholar] [CrossRef]
- Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of different wavelengths of laser irradiation on the skin cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, L.; Barnard, I.R.; McMillan, L.; Ibbotson, S.H.; Brown, C.T.A.; Eadie, E.; Wood, K. Depth penetration of light into skin as a function of wavelength from 200 to 1000 nm. Photochem. Photobiol. 2022, 98, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sonek, G.; Berns, M.; Tromberg, B. Physiological monitoring of optically trapped cells: Assessing the effects of confinement by 1064-nm laser tweezers using microfluorometry. Biophys. J. 1996, 71, 2158–2167. [Google Scholar] [CrossRef]
- Nourhashemi, M.; Mahmoudzadeh, M.; Wallois, F. Thermal impact of near-infrared laser in advanced noninvasive optical brain imaging. Neurophotonics 2016, 3, 015001. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, L.; Mahgerefteh, S.Y.; Sosna, J.; Goldberg, S.N. Image-guided fusion and navigation: Applications in tumor ablation. Tech. Vasc. Interv. Radiol. 2013, 16, 287–295. [Google Scholar] [CrossRef]
- Walter, A.; Paul-Gilloteaux, P.; Plochberger, B.; Sefc, L.; Verkade, P.; Mannheim, J.G.; Slezak, P.; Unterhuber, A.; Marchetti-Deschmann, M.; Ogris, M.; et al. Correlated multimodal imaging in life sciences: Expanding the biomedical horizon. Front. Phys. 2020, 8, 47. [Google Scholar] [CrossRef]
- Dhawan, A.P.; D’Alessandro, B.; Fu, X. Optical imaging modalities for biomedical applications. IEEE Rev. Biomed. Eng. 2010, 3, 69–92. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Wang, Q.; Chan, K.F.; Zhang, L. Automated control of magnetic spore-based microrobot using fluorescence imaging for targeted delivery with cellular resolution. IEEE Trans. Autom. Sci. Eng. 2019, 17, 490–501. [Google Scholar] [CrossRef]
- Xing, J.; Yin, T.; Li, S.; Xu, T.; Ma, A.; Chen, Z.; Luo, Y.; Lai, Z.; Lv, Y.; Pan, H.; et al. Sequential Magneto-Actuated and Optics-Triggered Biomicrorobots for Targeted Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2008262. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Yang, Y.; Hu, P.; Li, Y.; Yang, S.Y.; Wang, L.V.; Gao, W. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 2019, 4, eaax0613. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Ma, C.; Lin, L.; Yao, J.; Wang, L.; Maslov, K.; Zhang, R.; Chen, W.; Shi, J.; et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 2017, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Kaberniuk, A.A.; Li, L.; Shcherbakova, D.M.; Zhang, R.; Wang, L.; Li, G.; Verkhusha, V.V.; Wang, L.V. Multiscale photoacoustic tomography using reversibly switchable bacterial phytochrome as a near-infrared photochromic probe. Nat. Methods 2016, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, J.; Maurudis, A.; Aguirre, A.; Huang, F.; Guo, P.; Wang, L.V.; Zhu, Q. A real-time photoacoustic tomography system for small animals. Opt. Express 2009, 17, 10489–10498. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xia, J.; Li, G.; Garcia-Uribe, A.; Sheng, Q.; Anastasio, M.A.; Wang, L.V. Label-free photoacoustic tomography of whole mouse brain structures ex vivo. Neurophotonics 2016, 3, 035001. [Google Scholar] [CrossRef]
- Wong, T.T.; Zhang, R.; Zhang, C.; Hsu, H.C.; Maslov, K.I.; Wang, L.; Shi, J.; Chen, R.; Shung, K.K.; Zhou, Q.; et al. Label-free automated three-dimensional imaging of whole organs by microtomy-assisted photoacoustic microscopy. Nat. Commun. 2017, 8, 1386. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhu, L.; Maslov, K.; Shi, J.; Hu, P.; Bo, E.; Yao, J.; Liang, J.; Wang, L.; et al. Snapshot photoacoustic topography through an ergodic relay for high-throughput imaging of optical absorption. Nat. Photonics 2020, 14, 164–170. [Google Scholar] [CrossRef]
- Luís Deán-Ben, X.; Razansky, D. Adding fifth dimension to optoacoustic imaging: Volumetric time-resolved spectrally enriched tomography. Light. Sci. Appl. 2014, 3, e137. [Google Scholar] [CrossRef]
- Lauterbur, P.C. Image formation by induced local interactions: Examples employing nuclear magnetic resonance. Nature 1973, 242, 190–191. [Google Scholar] [CrossRef]
- Dahmen, C.; Belharet, K.; Folio, D.; Ferreira, A.; Fatikow, S. MRI-based dynamic tracking of an untethered ferromagnetic microcapsule navigating in liquid. Int. J. Optomechatronics 2016, 10, 73–96. [Google Scholar] [CrossRef]
- Sharif, S.; Nguyen, K.T.; Bang, D.; Park, J.O.; Choi, E. Optimization of field-free point position, gradient field and ferromagnetic polymer ratio for enhanced navigation of magnetically controlled polymer-based microrobots in blood vessel. Micromachines 2021, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, F.; Niehoff, D.; Mohanty, S.; Misra, S. A contactless and biocompatible approach for 3D active microrobotic targeted drug delivery. Micromachines 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, L.; Yu, J.; Vong, C.I.; Chiu, P.W.Y.; Zhang, L. Magnetic navigation of a rotating colloidal swarm using ultrasound images. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 5380–5385. [Google Scholar]
- Wang, Q.; Chan, K.F.; Schweizer, K.; Du, X.; Jin, D.; Yu, S.C.H.; Nelson, B.J.; Zhang, L. Ultrasound Doppler-guided real-time navigation of a magnetic microswarm for active endovascular delivery. Sci. Adv. 2021, 7, eabe5914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L. Ultrasound imaging and tracking of micro/nanorobots: From individual to collectives. IEEE Open J. Nanotechnol. 2020, 1, 6–17. [Google Scholar] [CrossRef]
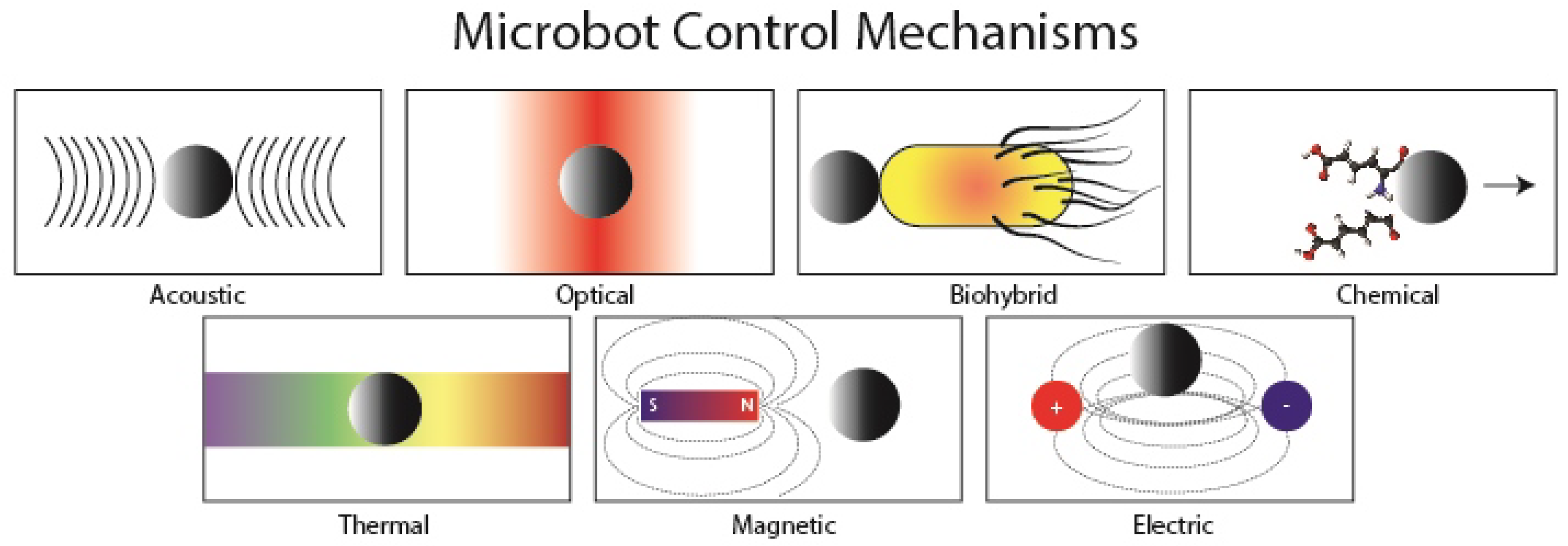
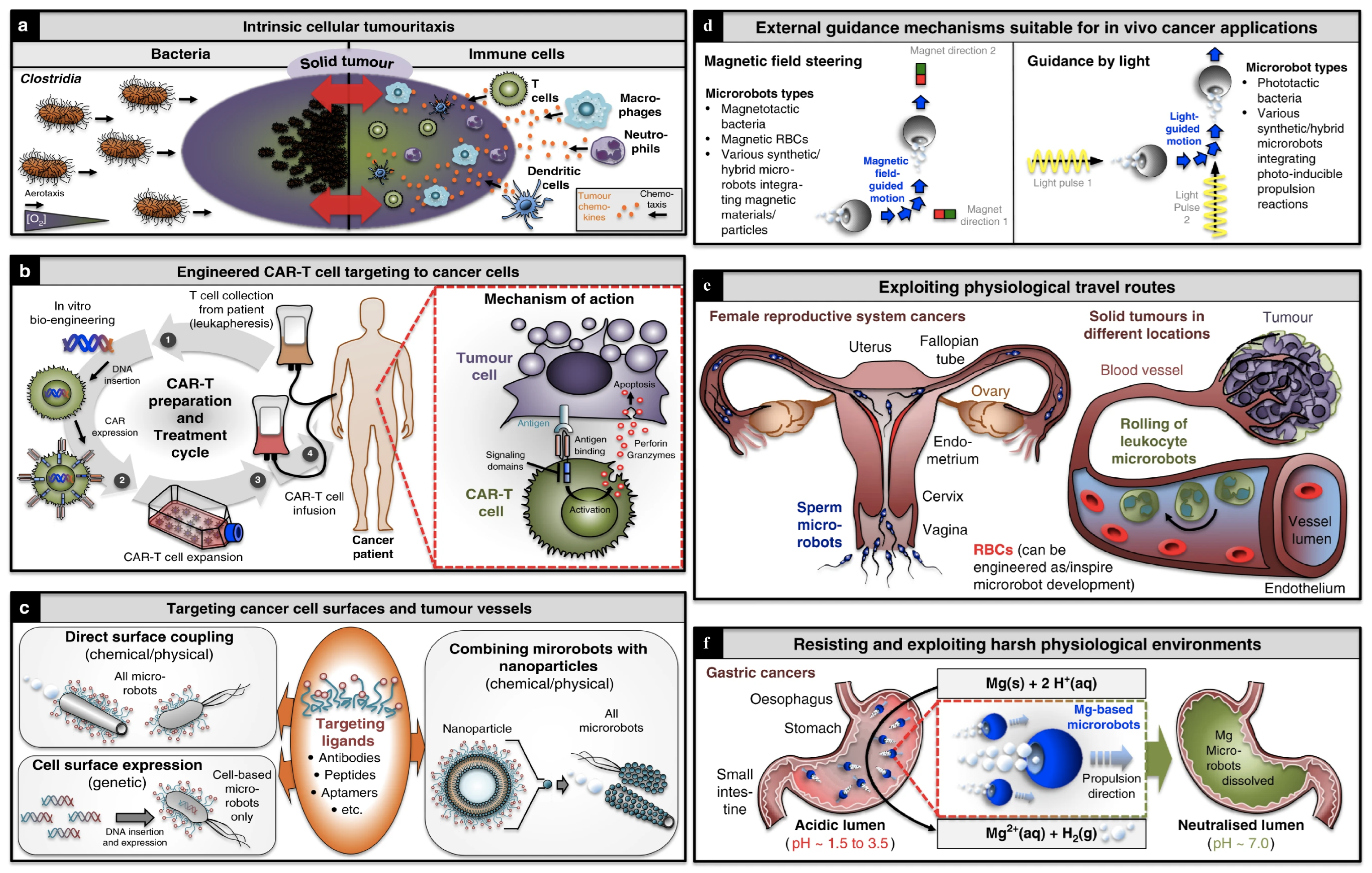

| Optical | Magnetic | Chemical | Biological | |
|---|---|---|---|---|
| Used Materials | Light Responsive Polymers [120] i.e., IP-Dip, IP-S, IP-L, IP-Visio, etc. | NdFeB microparticles SPIONs nanoparticles | InGaAs/GaAs/(Cr)Pt tubes [121] Catalysts i.e., Pd/Pt/Ag Enzymes i.e., glucose oxidase [122], urease [123], catalase [124] NO [39] | Bacterium [14] Micro organisms [125] |
| Material Characteristics | Usually soft Young’s modulus in the range of a few GPa [126] | Hard or soft Usually metal coated | Chemically activated surface | Mainly protein, Lipids, and DNA/RNA |
| Fabrication Methods | DLW (or 2PP/TPP) and Photolithography | 3D Laser Lithography [127] and metal deposition | – | Cell culture |
| Surface Functionalization | Not required unless a special functionality | Sputtered with different magnetic metals | Responsive chemical outer-layer | – |
| Biocompatibility | Many biocompatible materials are available | Metal coating makes it hard to be biocompatible | Use of chemicals makes it difficult to use in in vivo applications | – |
| Sensitivity to the working medium | Biocompatibility prevents from reacting with the biological medium | Possibility of side effects caused by the residual metals [128] Possible reaction between the medium and the metal | Responsive to Light, Oxygen Gradients, pH, etc. | Similar to chemically actuated microbots |
| Control | Optical Tweezers Optoelectronic Tweezers Electrothermoplasmonic Nanotweezer | Helmholtz and Maxwell coils [129] Single Position Controlled Electromagnetic coil [130] | – | Chemotaxis [131] |
| Precision of control | Highly accurate and precise | Precise with gradient, rotating, and oscillating magnetic fields [132] | Easy targeting using enzyme surface functionalization | Less predictable and less accurate |
| Limitation of control | Low penetration without photo damaging | Strong Magnetic Field results in heat in the microbots | – | |
| Self-propulsion capabilities | – | Not possible without external magnetic fields | Possible self-propulsion in fluid medium | – |
| Medical Applications | Single-cell manipulation, Live RBC capture, etc. [133] | Precision manipulation of cells e.g., Yeast, Mouse Embryonic STEM cell, Neuron Transport, etc. [102] | Catalytic microtubular jet [134] | Sperm cell manipulation [135] |
| Imaging | Optical Imaging PACT, PATER | MRI | Radiography/Ultrasonic etc. | Optically |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, M.F.; Pokharel, M.; Park, K. Light-Controlled Microbots in Biomedical Application: A Review. Appl. Sci. 2022, 12, 11013. https://doi.org/10.3390/app122111013
Jamil MF, Pokharel M, Park K. Light-Controlled Microbots in Biomedical Application: A Review. Applied Sciences. 2022; 12(21):11013. https://doi.org/10.3390/app122111013
Chicago/Turabian StyleJamil, Md Faiyaz, Mishal Pokharel, and Kihan Park. 2022. "Light-Controlled Microbots in Biomedical Application: A Review" Applied Sciences 12, no. 21: 11013. https://doi.org/10.3390/app122111013
APA StyleJamil, M. F., Pokharel, M., & Park, K. (2022). Light-Controlled Microbots in Biomedical Application: A Review. Applied Sciences, 12(21), 11013. https://doi.org/10.3390/app122111013








